Fabrication of r-GO/GO/α-Fe2O3/Fe2TiO5 Nanocomposite Using Natural Ilmenite and Graphite for Efficient Photocatalysis in Visible Light
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Dissolution of Ilmenite Sand and Precipitation
2.3. Preparation of Graphene Oxide
2.4. Synthesis of Photocatalysts
2.5. Photocatalytic Measurements
2.6. Materials Characterization
2.7. Determination of Antibacterial Activity against Escherichia coli
2.7.1. Microbial Strain and Inoculum Preparation
2.7.2. Broth Dilution Assay
- (O.D.) control = Absorbance of the control sample
- (O.D.) test = Absorbance of the test sample with the composites.
3. Results
3.1. Morphological Analysis
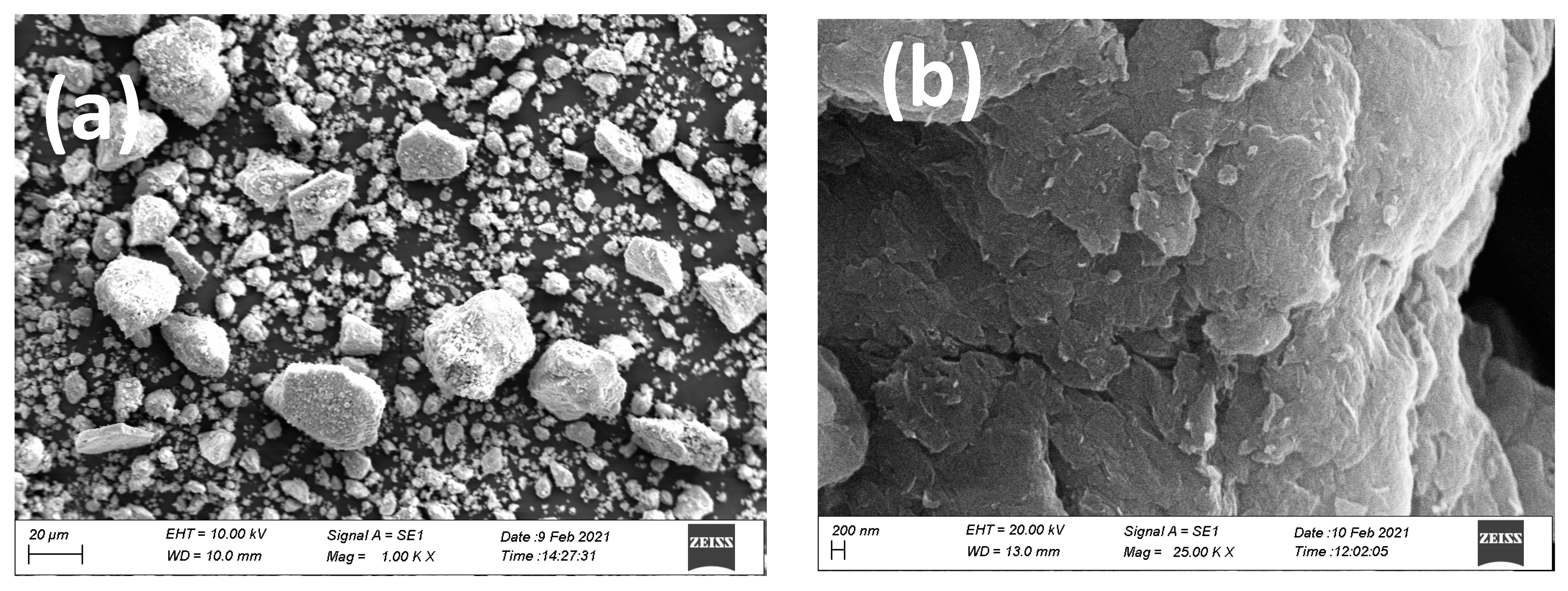
3.1.1. SEM and EDX Analysis
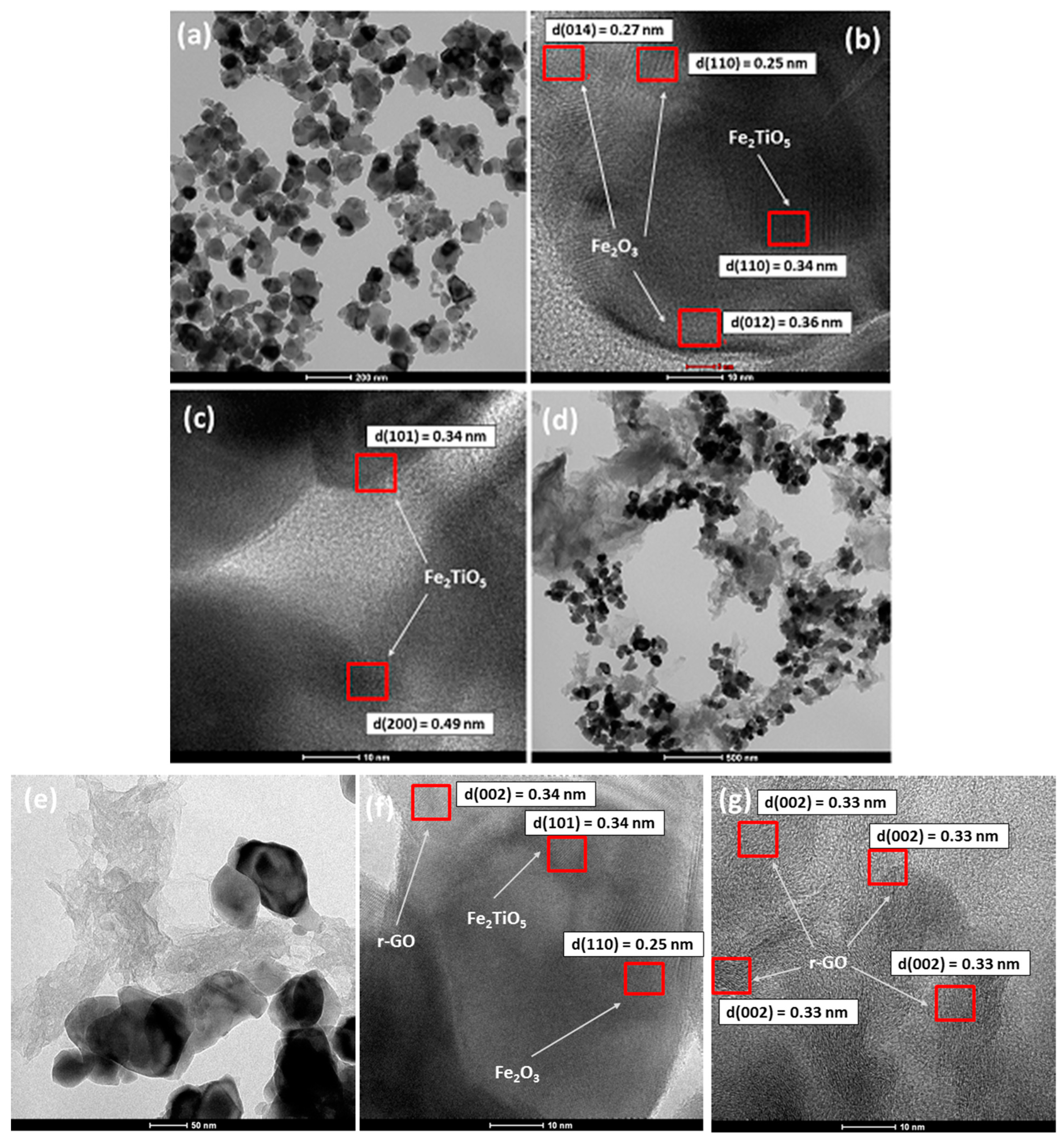
3.1.2. TEM Analysis
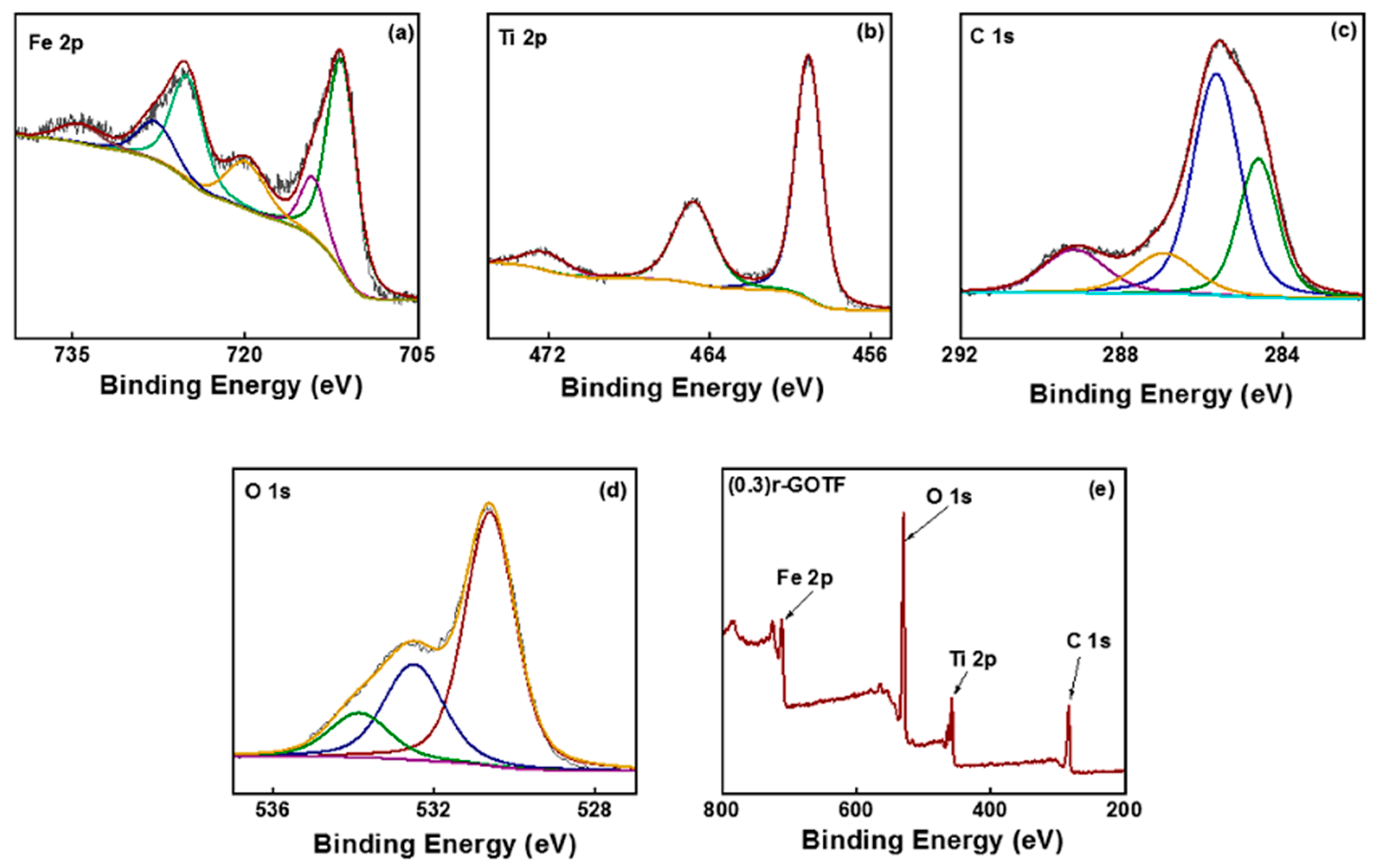
3.2. XPS Analysis
3.3. Raman Analysis
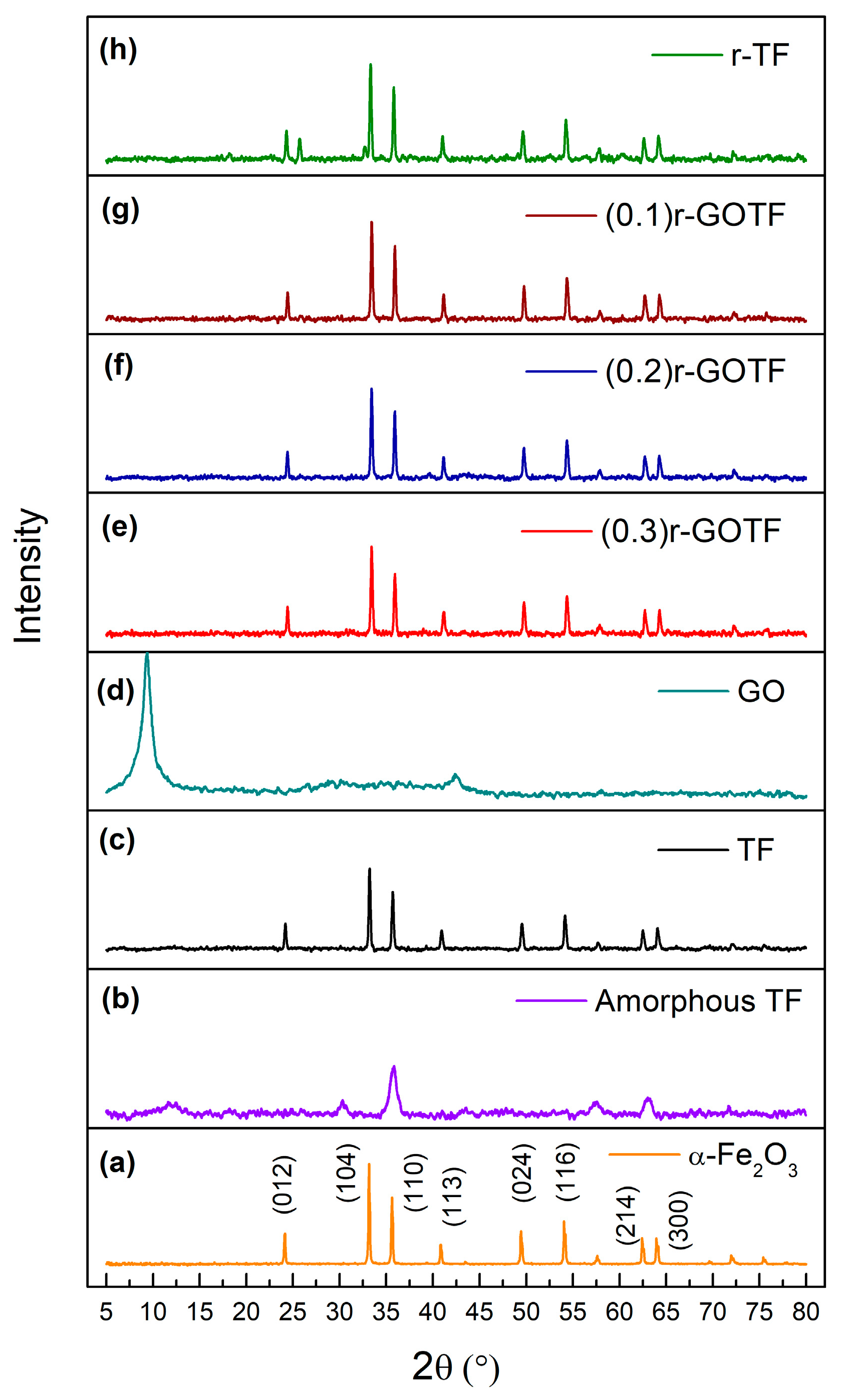
3.4. XRD Analysis
3.5. XRF Analysis
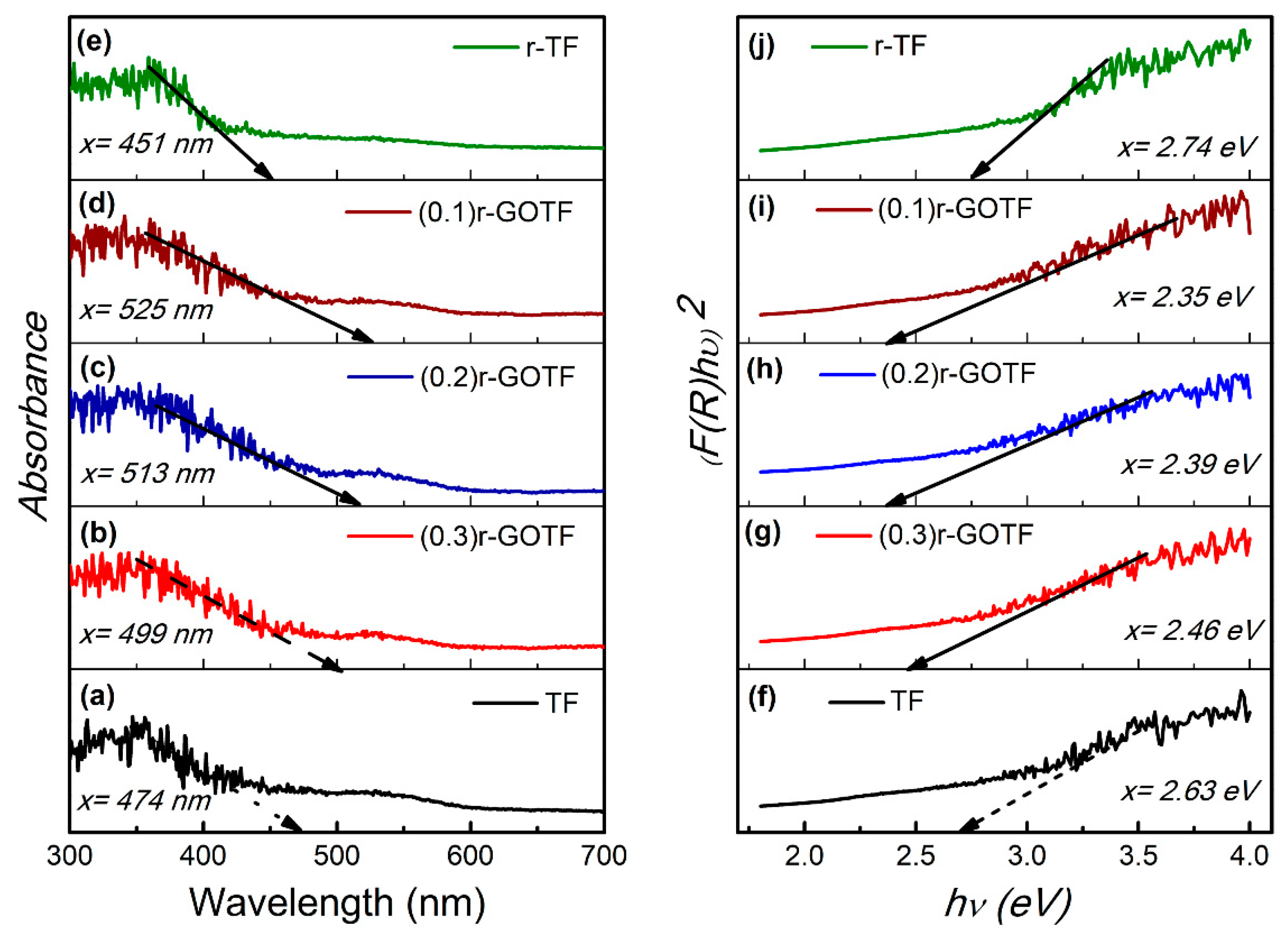
3.6. Optical Adsorption Properties (DRS Analysis)
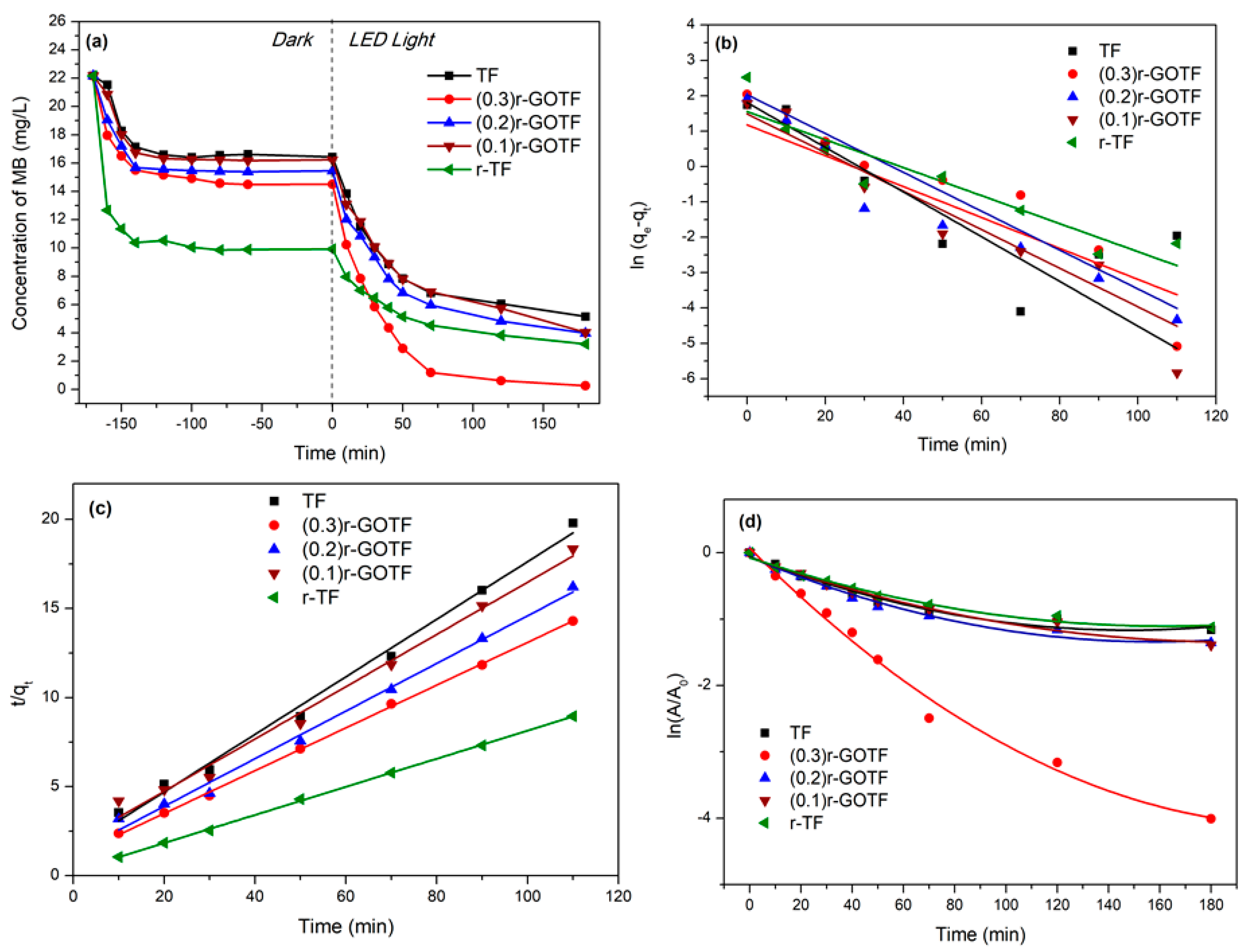
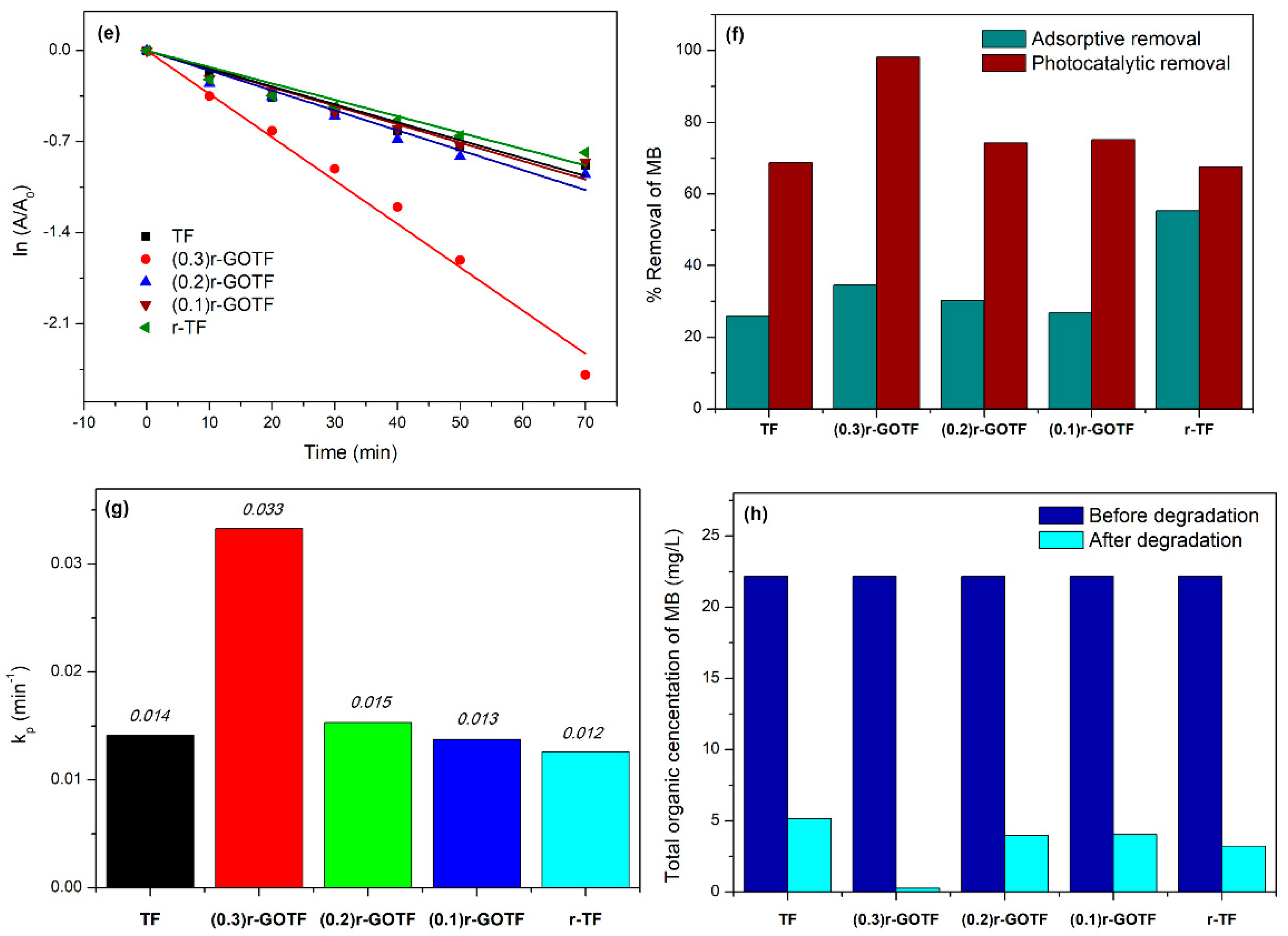
3.7. Photocatalytic Degradation
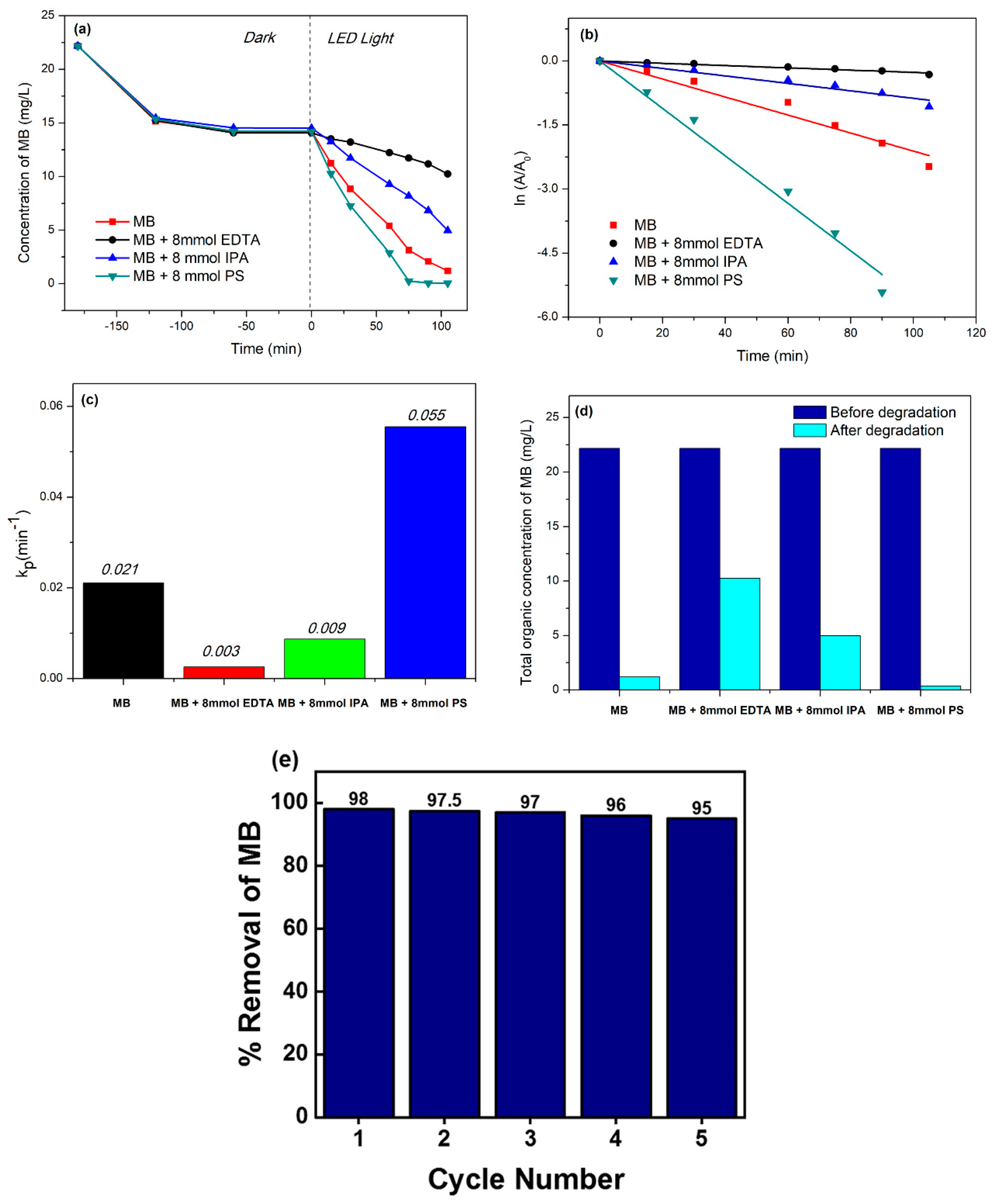
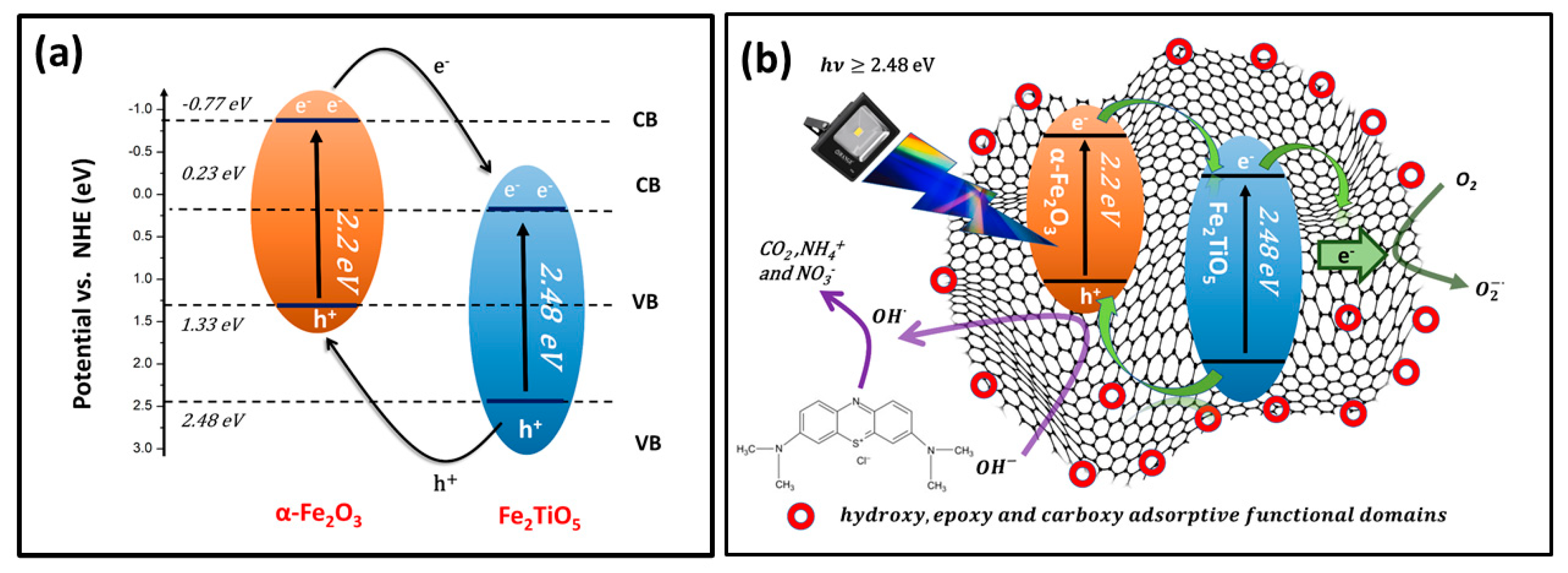
3.8. Mechanism of the Photocatalytic Activity
- X—Absolute electronegativity of the semiconductor, which is defined as the geometric mean of the absolute electronegativity of the constituent atoms
- EC—Energy of free electrons on the hydrogen scale
- Eg—The band gap of the semiconductor
- ECB—Conduction band (CB) position
- EVB—Valance band (VB) position
- Absorption of efficient photons
- 2.
- Oxygen ionosorption:
- 3.
- Neutralization of groups by photo holes to produce OH• radicals:
- 4.
- Neutralization of by protons:
- 5.
- Transient hydrogen peroxide formation and dismutation of oxygen:
- 6.
- Decomposition of and the second reduction of oxygen:
- 7.
- Oxidation of the MB by radicals:
- 8.
- Direct oxidation by reaction with holes:
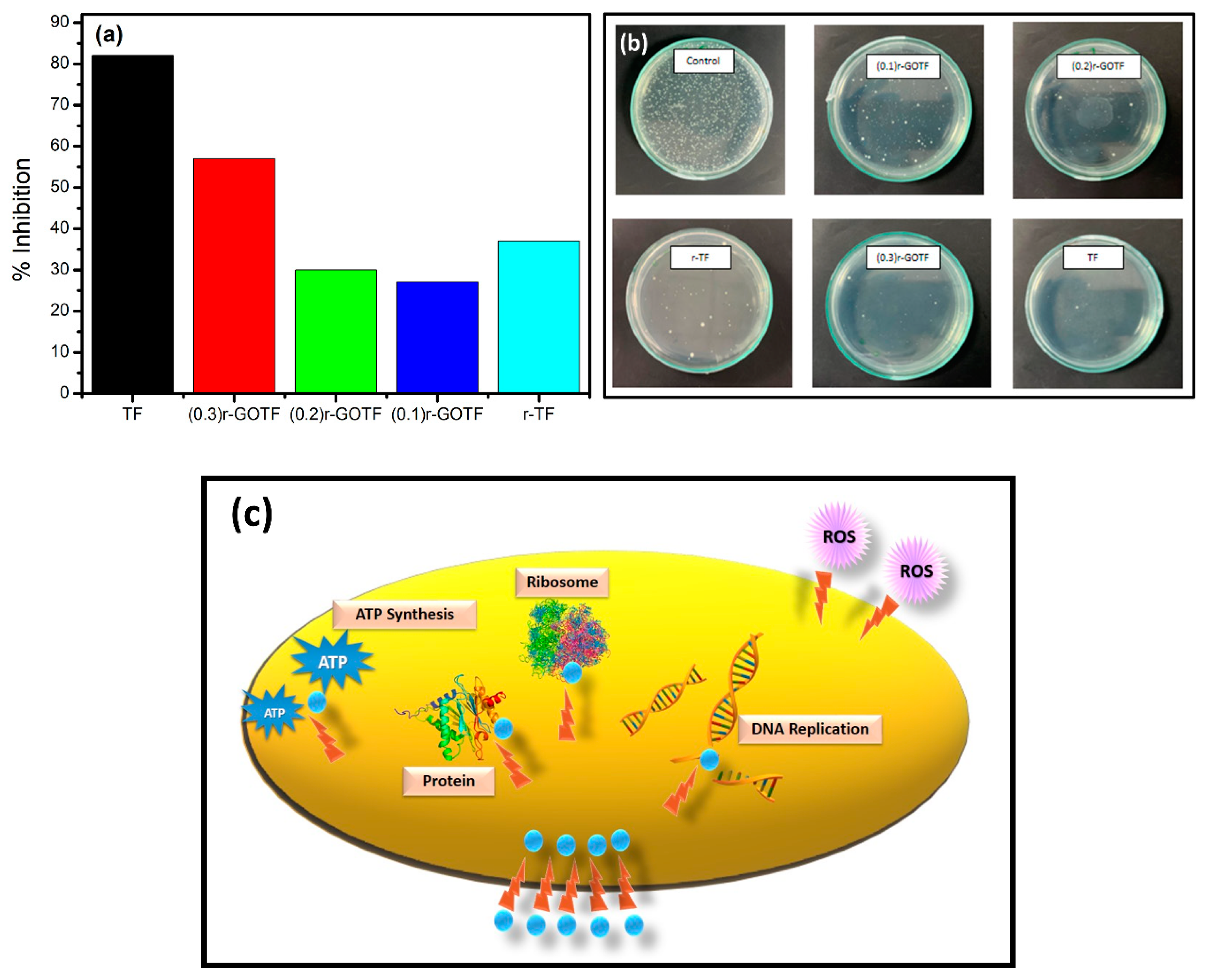
3.9. Antibacterial Activity against Escherichia coli
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Li, X.; Zhao, X.; Li, C.; Song, X.; Zhang, P.; Huo, P. A review on heterogeneous photocatalysis for environmental remediation: From semiconductors to modification strategies. Chin. J. Catal. 2022, 43, 178–214. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Liu, H.; Liu, C.; Wan, Y.; Long, Y.; Cai, Z. Recent Advances and Applications of Semiconductor Photocatalytic Technology. Appl. Sci. 2019, 9, 2489. [Google Scholar] [CrossRef] [Green Version]
- Qi, M.-Y.; Conte, M.; Anpo, M.; Tang, Z.-R.; Xu, Y.-J. Cooperative Coupling of Oxidative Organic Synthesis and Hydrogen Production over Semiconductor-Based Photocatalysts. Chem. Rev. 2021, 121, 13051–13085. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Seo, J.-H.; Singisetti, U.; Ma, Z. Recent advances in free-standing single crystalline wide band-gap semiconductors and their applications: GaN, SiC, ZnO, β-Ga2O3, and diamond. J. Mater. Chem. C 2017, 5, 8338–8354. [Google Scholar] [CrossRef]
- Sharma, S.; Dutta, V.; Singh, P.; Raizada, P.; Rahmani-Sani, A.; Hosseini-Bandegharaei, A.; Thakur, V.K. Carbon quantum dot supported semiconductor photocatalysts for efficient degradation of organic pollutants in water: A review. J. Clean. Prod. 2019, 228, 755–769. [Google Scholar] [CrossRef]
- Reddy, K.R.; Reddy, C.V.; Nadagouda, M.N.; Shetti, N.P.; Jaesool, S.; Aminabhavi, T.M. Polymeric graphitic carbon nitride (g-C3N4)-based semiconducting nanostructured materials: Synthesis methods, properties and photocatalytic applications. J. Environ. Manag. 2019, 238, 25–40. [Google Scholar] [CrossRef]
- Gong, M.; Xiao, S.; Yu, X.; Dong, C.; Ji, J.; Zhang, D.; Xing, M. Research progress of photocatalytic sterilization over semiconductors. RSC Adv. 2019, 9, 19278–19284. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Tan, C.F.; Lu, W.; Zeng, K.; Ho, G.W. Spectrum Tailored Defective 2D Semiconductor Nanosheets Aerogel for Full-Spectrum-Driven Photothermal Water Evaporation and Photochemical Degradation. Adv. Funct. Mater. 2020, 30, 2004460. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Subhan, A.; Saha, P.C.; Sumon, S.A.; Ahmed, J.; Asiri, A.M.; Rahman, M.M.; Al-Mamun, M. Enhanced photocatalytic activity and ultra-sensitive benzaldehyde sensing performance of a SnO2·ZnO·TiO2 nanomaterial. RSC Adv. 2018, 8, 33048–33058. [Google Scholar] [CrossRef]
- Elmaslmane, A.R.; Watkins, M.B.; McKenna, K.P. First-Principles Modeling of Polaron Formation in TiO2 Polymorphs. J. Chem. Theory Comput. 2018, 14, 3740–3751. [Google Scholar] [CrossRef]
- Tee, S.Y.; Win, K.Y.; Teo, W.S.; Koh, L.-D.; Liu, S.; Teng, C.P.; Han, M.-Y. Recent Progress in Energy-Driven Water Splitting. Adv. Sci. 2017, 4, 1600337. [Google Scholar] [CrossRef] [Green Version]
- Zunger, A.; Malyi, O.I. Understanding Doping of Quantum Materials. Chem. Rev. 2021, 121, 3031–3060. [Google Scholar] [CrossRef]
- Chang, K.; Hai, X.; Ye, J. Transition Metal Disulfides as Noble-Metal-Alternative Co-Catalysts for Solar Hydrogen Production. Adv. Energy Mater. 2016, 6, 1502555. [Google Scholar] [CrossRef]
- Anas, M.; Han, D.S.; Mahmoud, K.; Park, H.; Abdel-Wahab, A. Photocatalytic degradation of organic dye using titanium dioxide modified with metal and non-metal deposition. Mater. Sci. Semicond. Process. 2016, 41, 209–218. [Google Scholar] [CrossRef]
- Thalluri, S.M.; Bai, L.; Lv, C.; Huang, Z.; Hu, X.; Liu, L. Strategies for Semiconductor/Electrocatalyst Coupling toward Solar-Driven Water Splitting. Adv. Sci. 2020, 7, 1902102. [Google Scholar] [CrossRef] [Green Version]
- Su, Q.; Li, Y.; Hu, R.; Song, F.; Liu, S.; Guo, C.; Zhu, S.; Liu, W.; Pan, J. Heterojunction Photocatalysts Based on 2D Materials: The Role of Configuration. Adv. Sustain. Syst. 2020, 4, 2000130. [Google Scholar] [CrossRef]
- Yu, M.; Yuan, X.; Guo, J.; Tang, N.; Ye, S.; Liang, J.; Jiang, L. Selective graphene-like metal-free 2D nanomaterials and their composites for photocatalysis. Chemosphere 2021, 284, 131254. [Google Scholar] [CrossRef]
- Gao, L.; Gan, W.; Qiu, Z.; Zhan, X.; Qiang, T.; Li, J. Preparation of heterostructured WO3/TiO2 catalysts from wood fibers and its versatile photodegradation abilities. Sci. Rep. 2017, 7, 1102. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Schaak, R.E. Size- and Interface-Modulated Metal–Insulator Transition in Solution-Synthesized Nanoscale VO2-TiO2-VO2 Heterostructures. Angew. Chem. Int. Ed. 2017, 56, 15550–15554. [Google Scholar] [CrossRef]
- Sun, Q.; Li, Y.; Hao, J.; Zheng, S.; Zhang, T.; Wang, T.; Wu, R.; Fang, H.; Wang, Y. Increased Active Sites and Charge Transfer in the SnS2/TiO2 Heterostructure for Visible-Light-Assisted NO2 Sensing. ACS Appl. Mater. Interfaces 2021, 13, 54152–54161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lu, X.F.; Luan, D.; Lou, X.W. Fabrication of Heterostructured Fe 2 TiO 5 –TiO2 Nanocages with Enhanced Photoelectrochemical Performance for Solar Energy Conversion. Angew. Chem. 2020, 132, 8205–8209. [Google Scholar] [CrossRef]
- Zhang, P.; Yu, L.; Lou, X.W. Construction of Heterostructured Fe2O3-TiO2 Microdumbbells for Photoelectrochemical Water Oxidation. Angew. Chem. Int. Ed. 2018, 57, 15076–15080. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Liu, Z.; Li, X.; You, W.; Shao, Z.; Che, R. Enhanced dielectric polarization from disorder-engineered Fe3O4@black TiO2-x heterostructure for broadband microwave absorption. Chem. Eng. J. 2021, 419, 130020. [Google Scholar] [CrossRef]
- Manjunath, K.; Souza, V.S.; Ramakrishnappa, T.; Nagaraju, G.; Scholten, J.D.; Dupont, J. Heterojunction CuO-TiO2 nanocomposite synthesis for significant photocatalytic hydrogen production. Mater. Res. Express 2016, 3, 115904. [Google Scholar] [CrossRef]
- Xing, C.; Zhang, Y.; Liu, Y.; Wang, X.; Li, J.; Martínez-Alanis, P.; Spadaro, M.; Guardia, P.; Arbiol, J.; Llorca, J.; et al. Photodehydrogenation of Ethanol over Cu2O/TiO2 Heterostructures. Nanomaterials 2021, 11, 1399. [Google Scholar] [CrossRef]
- Wu, S.; Li, Z.; Li, M.-Q.; Diao, Y.; Lin, F.; Liu, T.; Zhang, J.; Tieu, P.; Gao, W.; Qi, F.; et al. 2D metal–organic framework for stable perovskite solar cells with minimized lead leakage. Nat. Nanotechnol. 2020, 15, 934–940. [Google Scholar] [CrossRef]
- Wu, J.; Chen, J.; Gao, J.; Chen, Z.; Li, L.; Wang, W. Recent Progress and Perspectives on Nonlead Halide Perovskites in Photocatalytic Applications. Energy Fuels 2022, 36, 14613–14624. [Google Scholar] [CrossRef]
- Fan, L.; Yu, Q.; Chen, J.; Khan, U.; Wang, X.; Gao, J. Achievements and Perspectives in Metal–Organic Framework-Based Materials for Photocatalytic Nitrogen Reduction. Catalysts 2022, 12, 1005. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, X.; Zhang, G.; Wei, G.; Su, Z. Electrospinning design of functional nanostructures for biosensor applications. J. Mater. Chem. B 2017, 5, 1699–1711. [Google Scholar] [CrossRef]
- Pascariu, P.; Olaru, L.; Matricala, A.L.; Olaru, N. Photocatalytic activity of ZnO nanostructures grown on electrospun CAB ultrafine fibers. Appl. Surf. Sci. 2018, 455, 61–69. [Google Scholar] [CrossRef]
- Usgodaarachchi, L.; Thambiliyagodage, C.; Wijesekera, R.; Vigneswaran, S.; Kandanapitiye, M. Fabrication of TiO2 Spheres and a Visible Light Active α-Fe2O3/TiO2-Rutile/TiO2-Anatase Heterogeneous Photocatalyst from Natural Ilmenite. ACS Omega 2022, 7, 27617–27637. [Google Scholar] [CrossRef]
- Jia, X.; Cao, J.; Lin, H.; Zhang, M.; Guo, X.; Chen, S. Transforming type-I to type-II heterostructure photocatalyst via energy band engineering: A case study of I-BiOCl/I-BiOBr. Appl. Catal. B Environ. 2017, 204, 505–514. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, P.; Liao, Q.; Kang, Z.; Si, H.; Zhang, Y. Graphene-Based Mixed-Dimensional van der Waals Heterostructures for Advanced Optoelectronics. Adv. Mater. 2019, 31, e1806411. [Google Scholar] [CrossRef]
- Solís-Fernández, P.; Bissett, M.; Ago, H. Synthesis, structure and applications of graphene-based 2D heterostructures. Chem. Soc. Rev. 2017, 46, 4572–4613. [Google Scholar] [CrossRef] [Green Version]
- Banszerus, L.; Janssen, H.; Otto, M.; Epping, A.; Taniguchi, T.; Watanabe, K.; Beschoten, B.; Neumaier, D.; Stampfer, C. Identifying suitable substrates for high-quality graphene-based heterostructures. 2D Mater. 2017, 4, 025030. [Google Scholar] [CrossRef] [Green Version]
- Gnanamoorthy, G.; Karthikeyan, V.; Ali, D.; Kumar, G.; Yadav, V.K.; Narayanan, V. Global popularization of CuNiO2 and their rGO nanocomposite loveabled to the photocatalytic properties of methylene blue. Environ. Res. 2022, 204, 112338. [Google Scholar] [CrossRef]
- Thambiliyagodage, C.; Usgodaarachchi, L.; Jayanetti, M.; Liyanaarachchi, C.; Kandanapitiye, M.; Vigneswaran, S. Efficient Visible-Light Photocatalysis and Antibacterial Activity of TiO2-Fe3C-Fe-Fe3O4/Graphitic Carbon Composites Fabricated by Catalytic Graphitization of Sucrose Using Natural Ilmenite. ACS Omega 2022, 7, 25403–25421. [Google Scholar] [CrossRef]
- Charitha, T.; Leshan, U.; Shanitha, M.; Ramanee, W.; Buddi, L.; Martin, B. Efficient photodegradation activity of α-Fe2O3/Fe2TiO5/TiO2 and Fe2TiO5/TiO2 nanocomposites synthesized from natural ilmenite. Results Mater. 2021, 12, 100219. [Google Scholar] [CrossRef]
- Lou, Z.; Li, Y.; Song, H.; Ye, Z.; Zhu, L. Fabrication of Fe2TiO5/TiO2 nanoheterostructures with enhanced visible-light photocatalytic activity. RSC Adv. 2016, 6, 45343–45348. [Google Scholar] [CrossRef]
- De Marchi, L.; Pretti, C.; Gabriel, B.; Marques, P.A.; Freitas, R.; Neto, V. An overview of graphene materials: Properties, applications and toxicity on aquatic environments. Sci. Total. Environ. 2018, 631–632, 1440–1456. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Meng, L.; Prominski, A.; Schaumann, E.N.; Seebald, M.; Tian, B. Recent advances in bioelectronics chemistry. Chem. Soc. Rev. 2020, 49, 7978–8035. [Google Scholar] [CrossRef] [PubMed]
- Saba, N.; Alothman, O.Y.; Almutairi, Z.; Jawaid, M.; Asad, M. Introduction of graphene-based nanotechnologies. In Graphene-Based Nanotechnologies for Energy and Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–21. [Google Scholar] [CrossRef]
- Jain, V.P.; Chaudhary, S.; Sharma, D.; Dabas, N.; Lalji, R.S.K.; Singh, B.K.; Jaiswar, G. Advanced functionalized nanographene oxide as a biomedical agent for drug delivery and anti-cancerous therapy: A review. Eur. Polym. J. 2021, 142, 110124. [Google Scholar] [CrossRef]
- Shamaila, S.; Sajjad, A.K.L.; Iqbal, A. Modifications in development of graphene oxide synthetic routes. Chem. Eng. J. 2016, 294, 458–477. [Google Scholar] [CrossRef]
- Pendolino, F.; Armata, N. Synthesis, Characterization and Models of Graphene Oxide. In Graphene Oxide in Environmental Remediation Process; SpringerBriefs in Applied Sciences and Technology; Springer: Cham, Switzerland, 2017; pp. 5–21. [Google Scholar] [CrossRef]
- Habte, A.T.; Ayele, D.W. Synthesis and Characterization of Reduced Graphene Oxide (rGO) Started from Graphene Oxide (GO) Using the Tour Method with Different Parameters. Adv. Mater. Sci. Eng. 2019, 2019, 5058163. [Google Scholar] [CrossRef] [Green Version]
- Lei, B.; Xu, D.; Wei, B.; Xie, T.; Xiao, C.; Jin, W.; Xu, L. In Situ Synthesis of α-Fe2O3/Fe3O4 Heterojunction Photoanode via Fast Flame Annealing for Enhanced Charge Separation and Water Oxidation. ACS Appl. Mater. Interfaces 2021, 13, 4785–4795. [Google Scholar] [CrossRef]
- Enhessari, M.; Razi, M.K.; Etemad, L.; Parviz, A.; Sakhaei, M. Structural, optical and magnetic properties of the Fe2TiO5nanopowders. J. Exp. Nanosci. 2014, 9, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Zaaba, N.I.; Foo, K.L.; Hashim, U.; Tan, S.J.; Liu, W.W.; Voon, C.H. Synthesis of Graphene Oxide using Modified Hummers Method: Solvent Influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Meng, X.; Xiao, F. New Strategies for the Preparation of Sinter-Resistant Metal-Nanoparticle-Based Catalysts. Adv. Mater. 2019, 31, e1901905. [Google Scholar] [CrossRef]
- Wang, Y.L.; Li, Y.H.; Wang, X.L.; Hou, Y.; Chen, A.P.; Yang, H.G. Effects of redox mediators on α-Fe2O3 exposed by {012} and {104} facets for photocatalytic water oxidation. Appl. Catal. B Environ. 2017, 206, 216–220. [Google Scholar] [CrossRef]
- Xiao, C.; Lu, B.-A.; Xue, P.; Tian, N.; Zhou, Z.-Y.; Lin, X.; Lin, W.-F.; Sun, S.-G. High-Index-Facet- and High-Surface-Energy Nanocrystals of Metals and Metal Oxides as Highly Efficient Catalysts. Joule 2020, 4, 2562–2598. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, P.; Zhao, J.; Li, X.; Zhang, Y. Simple solution-combustion synthesis of Fe2TiO5 nanomaterials with enhanced lithium storage properties. Ceram. Int. 2019, 45, 11382–11387. [Google Scholar] [CrossRef]
- Cheng, G.; Xu, F.; Xiong, J.; Wei, Y.; Stadler, F.J.; Chen, R. A novel protocol to design TiO2-Fe2O3 hybrids with effective charge separation efficiency for improved photocatalysis. Adv. Powder Technol. 2017, 28, 665–670. [Google Scholar] [CrossRef]
- Sivasankaran, R.P.; Das, P.K.; Arunachalam, M.; Kanase, R.S.; Park, Y.I.; Seo, J.; Kang, S.H. TiO2 Nanotube Arrays Decorated with Reduced Graphene Oxide and Cu–Tetracyanoquinodimethane as Anode Materials for Photoelectrochemical Water Oxidation. ACS Appl. Nano Mater. 2021, 4, 13218–13233. [Google Scholar] [CrossRef]
- Ahmad, S.I.; Hamoudi, H.; Ponraj, J.; Youssef, K.M. In-situ growth of single-crystal plasmonic aluminum–lithium-graphene nanosheets with a hexagonal platelet-like morphology using ball-milling. Carbon 2021, 178, 657–665. [Google Scholar] [CrossRef]
- Bagus, P.S.; Nelin, C.J.; Brundle, C.R.; Lahiri, N.; Ilton, E.S.; Rosso, K.M. Analysis of the Fe 2p XPS for hematite α Fe2O3: Consequences of covalent bonding and orbital splittings on multiplet splittings. J. Chem. Phys. 2020, 152, 014704. [Google Scholar] [CrossRef]
- Qiu, Y.; Xing, Z.; Guo, M.; Zhao, T.; Wang, Y.; Chen, P.; Li, Z.; Pan, K.; Zhou, W. Cadmium sulfide quantum dots/dodecahedral polyoxometalates/oxygen-doped mesoporous graphite carbon nitride with Z-scheme and Type-II as tandem heterojunctions for boosting visible-light-driven photocatalytic performance. J. Colloid Interface Sci. 2021, 582, 752–763. [Google Scholar] [CrossRef]
- Regue, M.; Ahmet, I.Y.; Bassi, P.S.; Johnson, A.L.; Fiechter, S.; van de Krol, R.; Abdi, F.F.; Eslava, S. Zn-Doped Fe2TiO5 Pseudobrookite-Based Photoanodes Grown by Aerosol-Assisted Chemical Vapor Deposition. ACS Appl. Energy Mater. 2020, 3, 12066–12077. [Google Scholar] [CrossRef]
- Tang, P.; Xie, H.; Ros, C.; Han, L.; Biset-Peiró, M.; He, Y.; Kramer, W.; Rodríguez, A.P.; Saucedo, E.; Galán-Mascarós, J.R.; et al. Enhanced photoelectrochemical water splitting of hematite multilayer nanowire photoanodes by tuning the surface state via bottom-up interfacial engineering. Energy Environ. Sci. 2017, 10, 2124–2136. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Wang, X.; Fang, D. A review on C1s XPS-spectra for some kinds of carbon materials. Full-Nanotub. Carbon Nanostructures 2020, 28, 1048–1058. [Google Scholar] [CrossRef]
- Khazaee, Z.; Mahjoub, A.R.; Khavar, A.H.C.; Srivastava, V.; Sillanpää, M. Synthesis of layered perovskite Ag,F-Bi2MoO6/rGO: A surface plasmon resonance and oxygen vacancy promoted nanocomposite as a visible-light photocatalyst. J. Photochem. Photobiol. A Chem. 2019, 379, 130–143. [Google Scholar] [CrossRef]
- Rufus, A.; Sreeju, N.; Vilas, V.; Philip, D. Biosynthesis of hematite (α-Fe2O3) nanostructures: Size effects on applications in thermal conductivity, catalysis, and antibacterial activity. J. Mol. Liq. 2017, 242, 537–549. [Google Scholar] [CrossRef]
- Kumar, S.; Bhorolua, D.; Ojha, A.K.; Kumar, A. Onion juice assisted green reduction of graphene oxide with tunable structural and optical properties: Effect of onion juice concentration and reaction temperature. Adv. Mater. Lett. 2019, 10, 58–66. [Google Scholar] [CrossRef]
- Lavin-Lopez, M.P.; Paton-Carrero, A.P.; Sanchez-Silva, L.; Valverde, J.L.; Romero, A. Influence of the reduction strategy in the synthesis of reduced graphene oxide. Adv. Powder Technol. 2017, 28, 3195–3203. [Google Scholar] [CrossRef] [Green Version]
- Albers, R.F.; Bini, R.A.; Souza, J.B.; Machado, D.T.; Varanda, L.C. A general one-pot synthetic strategy to reduced graphene oxide (rGO) and rGO-nanoparticle hybrid materials. Carbon 2019, 143, 73–84. [Google Scholar] [CrossRef]
- Usgodaarachchi, L.; Thambiliyagodage, C. Photocatalytic activity of GO/Fe3O4 fabricated by Sri Lankan graphite under visible light irradiation. J. Sci. Univ. Kelaniya Sri Lanka 2021, 14, 51. [Google Scholar] [CrossRef]
- Abhilash, V.; Rajender, N.; Suresh, K. X-ray diffraction spectroscopy of polymer nanocomposites. In Spectroscopy of Polymer Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2016; pp. 410–451. [Google Scholar] [CrossRef]
- Vasiljević, Z.; Dojčinović, M.P.; Vujančević, J.D.; Spreitzer, M.; Kovač, J.; Bartolić, D.; Marković, S.; Janković-Čaštvan, I.; Tadić, N.B.; Nikolić, M.V. Exploring the impact of calcination parameters on the crystal structure, morphology, and optical properties of electrospun Fe2TiO5 nanofibers. RSC Adv. 2021, 11, 32358–32368. [Google Scholar] [CrossRef]
- Manzoor, S.; Husain, S. Analysis of Zn substitution on structure, optical absorption, magnetization, and high temperature specific heat anomaly of the nano-crystalline LaFeO3. J. Appl. Phys. 2018, 124, 065110. [Google Scholar] [CrossRef]
- Usgodaarachchi, L.; Thambiliyagodage, C.; Wijesekera, R.; Bakker, M.G. Synthesis of mesoporous silica nanoparticles derived from rice husk and surface-controlled amine functionalization for efficient adsorption of methylene blue from aqueous solution. Curr. Res. Green Sustain. Chem. 2021, 4, 100116. [Google Scholar] [CrossRef]
- Thambiliyagodage, C.; Usgodaarachchi, L. Efficient removal of methylene blue by turbostratic carbon/Fe3C/Fe composite synthesized by catalytic graphitization of sucrose. Mater. Today Proc. 2022, 56, 2189–2194. [Google Scholar] [CrossRef]
- Gunathilaka, H.; Thambiliyagodage, C.; Usgodaarchchi, L.; Angappan, S. Effect of surfactants on morphology and textural parameters of silica nanoparticles derived from paddy husk and their efficient removal of methylene blue. In Proceedings of the International Conference on Innovations in Energy Engineering & Cleaner Production (IEECP’21), San Francisco, CA, USA, 29–30 July 2021; Available online: https://sci-index.com/DAl/2021.99101/IEECP/14904873 (accessed on 10 October 2022).
- Mounir, C.; Ahlafi, H.; Aazza, M.; Moussout, H.; Mounir, S. Kinetics and Langmuir–Hinshelwood mechanism for the catalytic reduction of para-nitrophenol over Cu catalysts supported on chitin and chitosan biopolymers. React. Kinet. Mech. Catal. 2021, 134, 285–302. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Cao, M.; Xiong, D.-B.; Yang, L.; Li, S.; Xie, Y.; Guo, Q.; Li, Z.; Adams, H.; Gu, J.; Fan, T.; et al. Ultrahigh electrical conductivity of graphene embedded in metals. Adv. Funct. Mater. 2019, 29, 1806792. [Google Scholar] [CrossRef]
- Szunerits, S.; Boukherroub, R. Antibacterial activity of graphene-based materials. J. Mater. Chem. B 2016, 4, 6892–6912. [Google Scholar] [CrossRef] [Green Version]
- Thambiliyagodage, C.; Kumara, A.; Jayanetti, M.; Usgodaarachchi, L.; Liyanaarachchi, H.; Lansakara, B. Fabrication of dual Z-scheme g-C3N4/Fe2TiO5/Fe2O3 ternary nanocomposite using natural ilmenite for efficient photocatalysis and photosterilization under visible light. Appl. Surf. Sci. Adv. 2022, 12, 100337. [Google Scholar] [CrossRef]
- Díez-Pascual, A.; Luceño-Sánchez, J. Antibacterial Activity of Polymer Nanocomposites Incorporating Graphene and Its Derivatives: A State of Art. Polymers 2021, 13, 2105. [Google Scholar] [CrossRef]
- Alavi, M.; Rai, M. Recent advances in antibacterial applications of metal nanoparticles (MNPs) and metal nanocomposites (MNCs) against multidrug-resistant (MDR) bacteria. Expert Rev. Anti-Infect. Ther. 2019, 17, 419–428. [Google Scholar] [CrossRef]


| Material | Chemical Composition of Carbon (%) | ||||
|---|---|---|---|---|---|
| C=C (sp2) | C-C (sp3) | C-O | C=O | C/O Ratio | |
| TF | 55.18 | 32.21 | - | 12.59 | 0.39 |
| (0.3)r-GOTF | 13.24 | 49.82 | 19.77 | 17.15 | 0.21 |
| (0.2)r-GOTF | 12.01 | 49.39 | 25.72 | 12.86 | 0.45 |
| (0.1)r-GOTF | 22.54 | 43.07 | 22.56 | 11.82 | 0.23 |
| r-TG | 45.73 | 41.09 | - | 13.17 | 0.30 |
| Material | Peak Position (cm−1) | FWHM (cm−1) | Peak Intensity Ratio | La (nm) | LD (nm) | ||||
|---|---|---|---|---|---|---|---|---|---|
| D Band | G Band | D Band | G Band | D Band | G Band | ID/IG | |||
| GO | 1350.8 | 1589.6 | 149.1 | 84.2 | 806.6 | 777.5 | 1.0 | 38.5 | 10.1 |
| (0.3)r-GOTF | 1339.5 | 1588.5 | 135.1 | 83.6 | 229.7 | 186.4 | 1.2 | 32.1 | 9.2 |
| (0.2)r-GOTF | 1318.7 | 1572.7 | 134.4 | 78.1 | 277.1 | 138.6 | 2.0 | 19.3 | 7.1 |
| (0.1)r-GOTF | 1316.5 | 1585.5 | 133.9 | 73.1 | 352.7 | 100.4 | 3.5 | 11.1 | 5.4 |
| Nanocomposite | Component | Peak Position (2θ) | Crystalline Plane (hkl) | Full Width at Half Maximum (FWHM) (β) | Integrated Peak Area | Crystalline Size (nm) (Lc) | Interplanar Distance (nm) (d) |
|---|---|---|---|---|---|---|---|
| TF | Fe2O3 | 33.22 | (104) | 0.191 | 23.431 | 45.33 | 0.2694 |
| GO | C | 9.34 | (001) | 1.168 | 52.683 | 7.13 | 0.9461 |
| (0.3)r-GOTF | Fe2O3 | 33.44 | (104) | 0.184 | 26.132 | 47.09 | 0.2677 |
| (0.2)r-GOTF | Fe2O3 | 33.44 | (104) | 0.188 | 23.757 | 46.08 | 0.2677 |
| (0.1)r-GOTF | Fe2O3 | 33.45 | (104) | 0.182 | 25.601 | 47.60 | 0.2676 |
| r-TF | Fe2O3 | 33.33 | (104) | 0.186 | 24.712 | 46.57 | 0.2686 |
| Fe2TiO5 | 25.43 | (101) | 0.148 | 4.4415 | 57.47 | 0.349 |
| Material | Al2O3 (%) | V2O5 (%) | SiO2 (%) | P2O5 (%) | K2O (%) | CaO (%) | TiO2 (%) | Cr2O3 (%) | MnO2 (%) | FeO (%) | ZnO (%) | ZrO2 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ilmenite | 1.02 | 1.21 | 3.94 | 0.04 | 0.08 | 0.61 | 48.87 | 0.15 | 0.87 | 42.81 | 0.08 | 0.15 |
| TF | 1.04 | - | 1.21 | - | - | - | 11.80 | 0.17 | 1.48 | 84.21 | 0.04 | - |
| (0.3)r-GOTF | 1.07 | - | 1.35 | - | - | - | 11.37 | 0.17 | 1.77 | 82.23 | 0.04 | - |
| (0.2)r-GOTF | 0.73 | - | 1.07 | - | - | - | 11.46 | 0.16 | 1.24 | 85.31 | 0.03 | |
| (0.1)r-GOTF | 0.54 | - | 1.32 | - | - | - | 10.38 | - | 1.13 | 86.58 | - | - |
| r-TF | 0.91 | - | 0.98 | - | - | - | 11.66 | 0.16 | 1.24 | 85.72 | 0.05 | - |
| Material | qe, exp (mg g−1) | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|---|
| qe (mg g−1) | k1 (min−1) | qe (mg g−1) | k2 (g mg−1 min−1) | ||||
| TF | 5.713 | 0.163 | 0.044 | 0.611 | 6.190 | 0.018 | 0.992 |
| (0.3)r-GOTF | 7.724 | 0.708 | 0.055 | 0.909 | 8.329 | 0.013 | 0.999 |
| (0.2)r-GOTF | 6.813 | 0.396 | 0.054 | 0.942 | 7.481 | 0.015 | 0.992 |
| (0.1)r-GOTF | 6.011 | 0.591 | 0.063 | 0.939 | 6.817 | 0.012 | 0.987 |
| r-TF | 12.421 | 0.438 | 0.039 | 0.857 | 12.674 | 0.024 | 0.999 |
| Material | Polynomial Regression Analysis (180 min) | Linear regression Analysis (First 70 min) | |||
|---|---|---|---|---|---|
| Polynomial Equation | Polynomial Rate Constant kp (min−1) | Initial Rate Constant kp (min−1) | |||
| TF | 0.008 | 0.996 | 0.014 | 0.994 | |
| (0.3)r-GOTF | 0.025 | 0.987 | 0.033 | 0.998 | |
| (0.2)r-GOTF | 0.009 | 0.978 | 0.015 | 0.997 | |
| (0.1)r-GOTF | 0.009 | 0.989 | 0.013 | 0.991 | |
| r-TF | 0.008 | 0.987 | 0.012 | 0.995 | |
| Semiconductor Oxide | Electronegativity (X) | Eg (eV) | Calculated CB Position (eV) (NHE) | Calculated VB Position (eV) (NHE) |
|---|---|---|---|---|
| Fe2TiO5 | 4.78 | 2.25 | 0.23 | 2.48 |
| α-Fe2O3 | 4.78 | 2.20 | −0.77 | 1.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usgodaarachchi, L.; Jayanetti, M.; Thambiliyagodage, C.; Liyanaarachchi, H.; Vigneswaran, S. Fabrication of r-GO/GO/α-Fe2O3/Fe2TiO5 Nanocomposite Using Natural Ilmenite and Graphite for Efficient Photocatalysis in Visible Light. Materials 2023, 16, 139. https://doi.org/10.3390/ma16010139
Usgodaarachchi L, Jayanetti M, Thambiliyagodage C, Liyanaarachchi H, Vigneswaran S. Fabrication of r-GO/GO/α-Fe2O3/Fe2TiO5 Nanocomposite Using Natural Ilmenite and Graphite for Efficient Photocatalysis in Visible Light. Materials. 2023; 16(1):139. https://doi.org/10.3390/ma16010139
Chicago/Turabian StyleUsgodaarachchi, Leshan, Madara Jayanetti, Charitha Thambiliyagodage, Heshan Liyanaarachchi, and Saravanamuthu Vigneswaran. 2023. "Fabrication of r-GO/GO/α-Fe2O3/Fe2TiO5 Nanocomposite Using Natural Ilmenite and Graphite for Efficient Photocatalysis in Visible Light" Materials 16, no. 1: 139. https://doi.org/10.3390/ma16010139
APA StyleUsgodaarachchi, L., Jayanetti, M., Thambiliyagodage, C., Liyanaarachchi, H., & Vigneswaran, S. (2023). Fabrication of r-GO/GO/α-Fe2O3/Fe2TiO5 Nanocomposite Using Natural Ilmenite and Graphite for Efficient Photocatalysis in Visible Light. Materials, 16(1), 139. https://doi.org/10.3390/ma16010139






