Comparison of Hydrogen Embrittlement Susceptibility of Different Types of Advanced High-Strength Steels
Abstract
1. Introduction
2. Materials and Methods
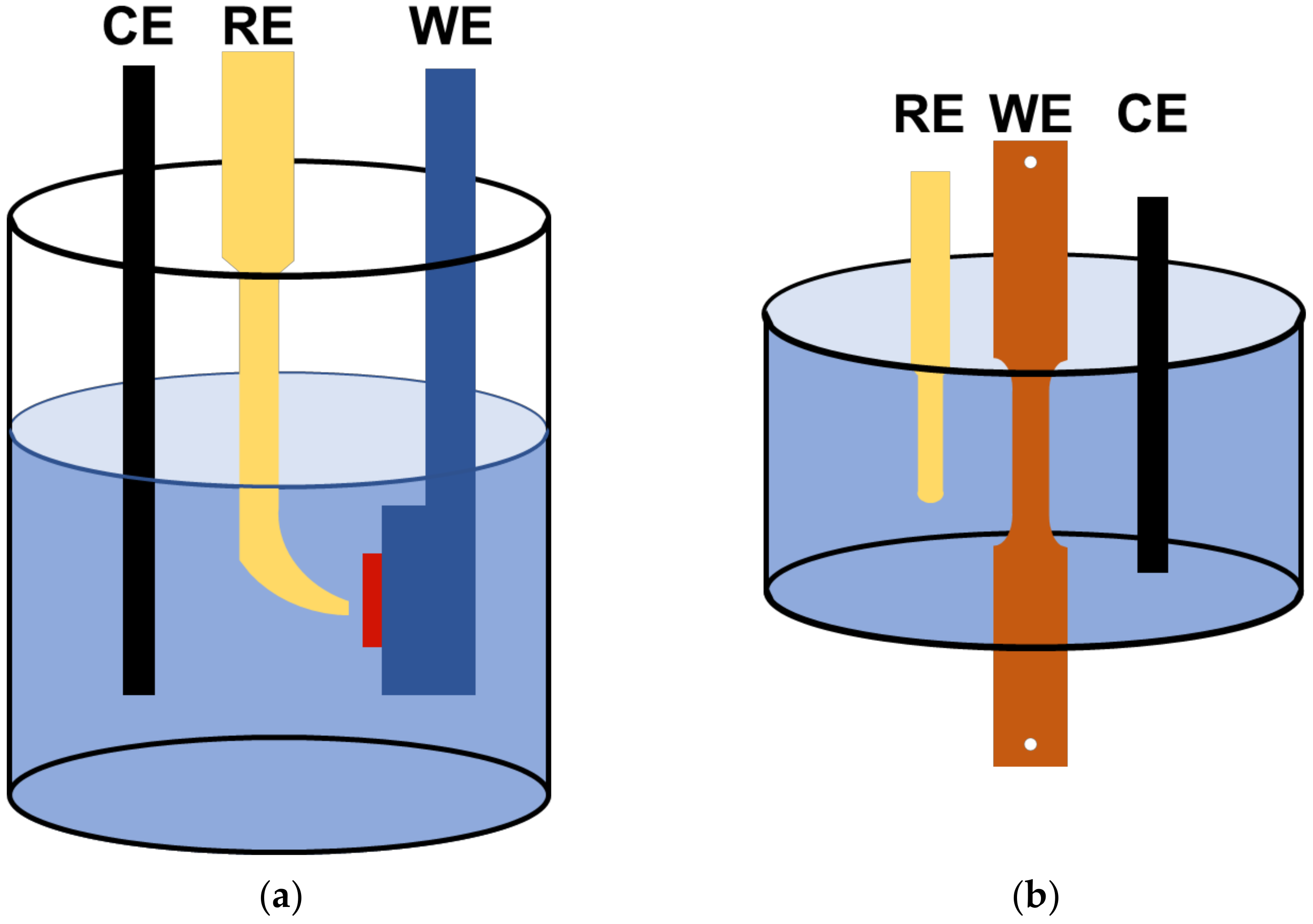
2.1. Electrochemical Tests
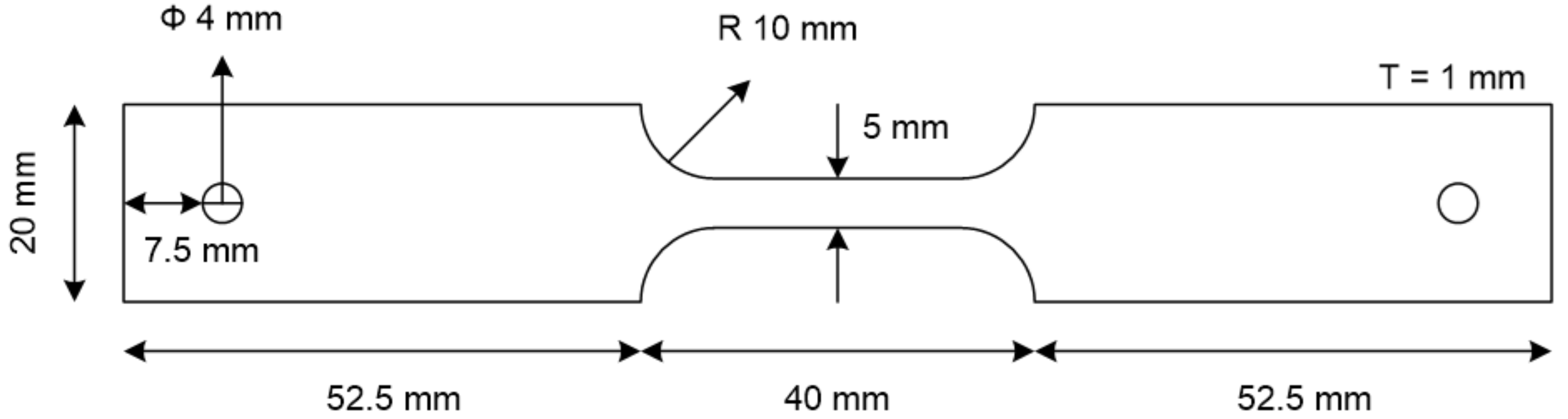
2.2. Slow Strain Rate Tests
2.3. Analyses for Hydrogen-Trapping Sites
3. Results and Discussion
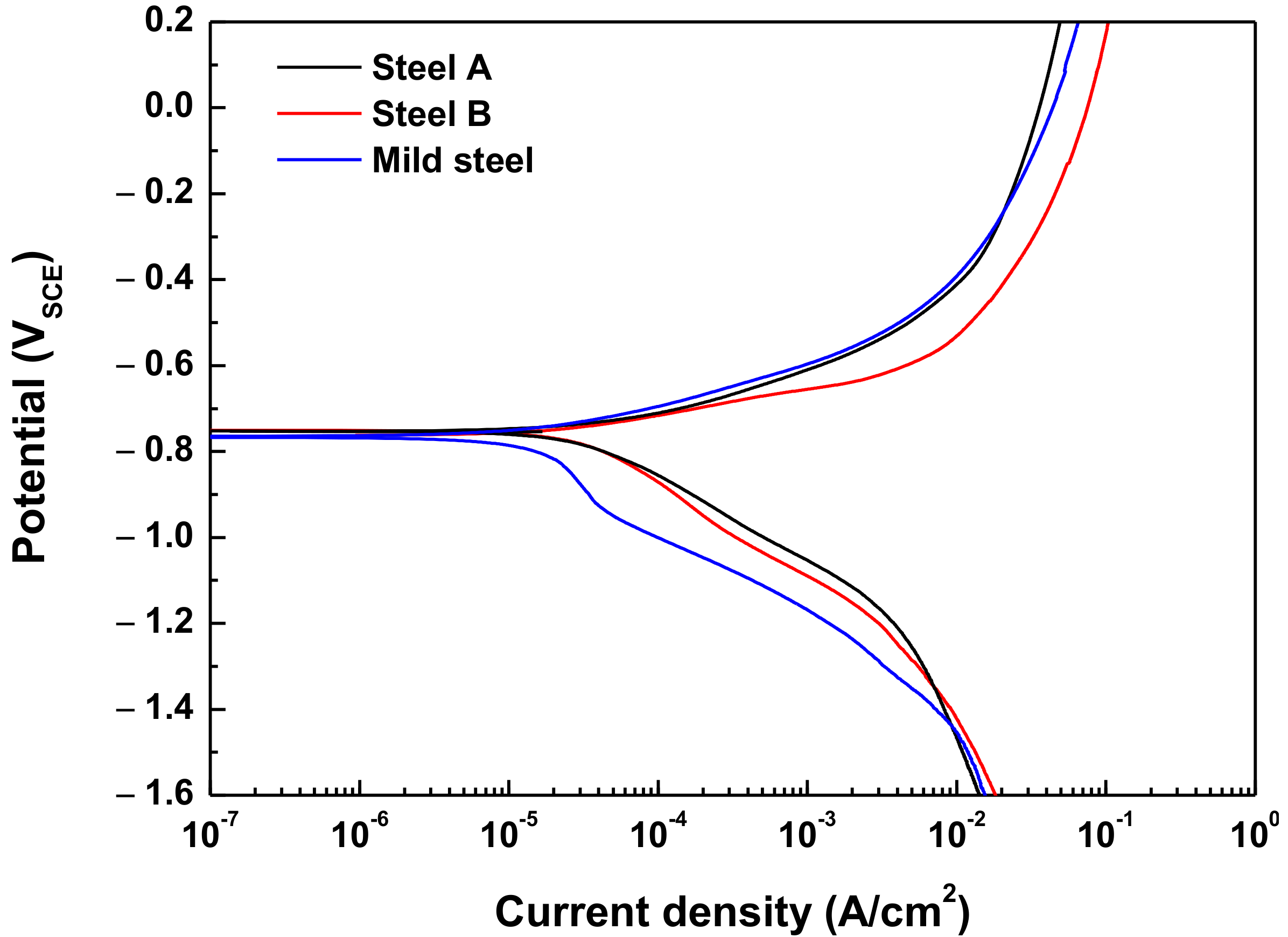
3.1. Potentiodynamic Polarization Test
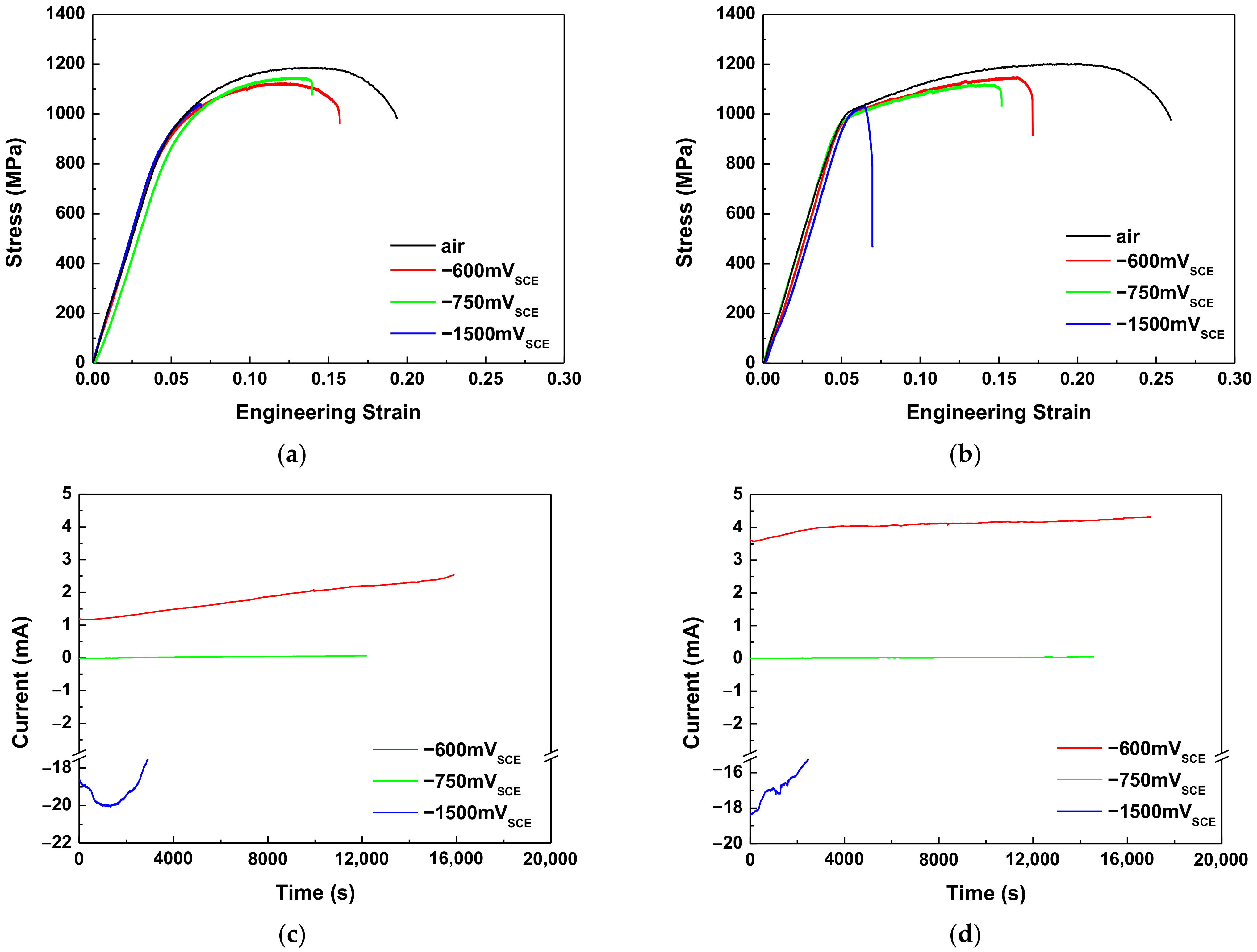
3.2. Slow Strain Rate Tests
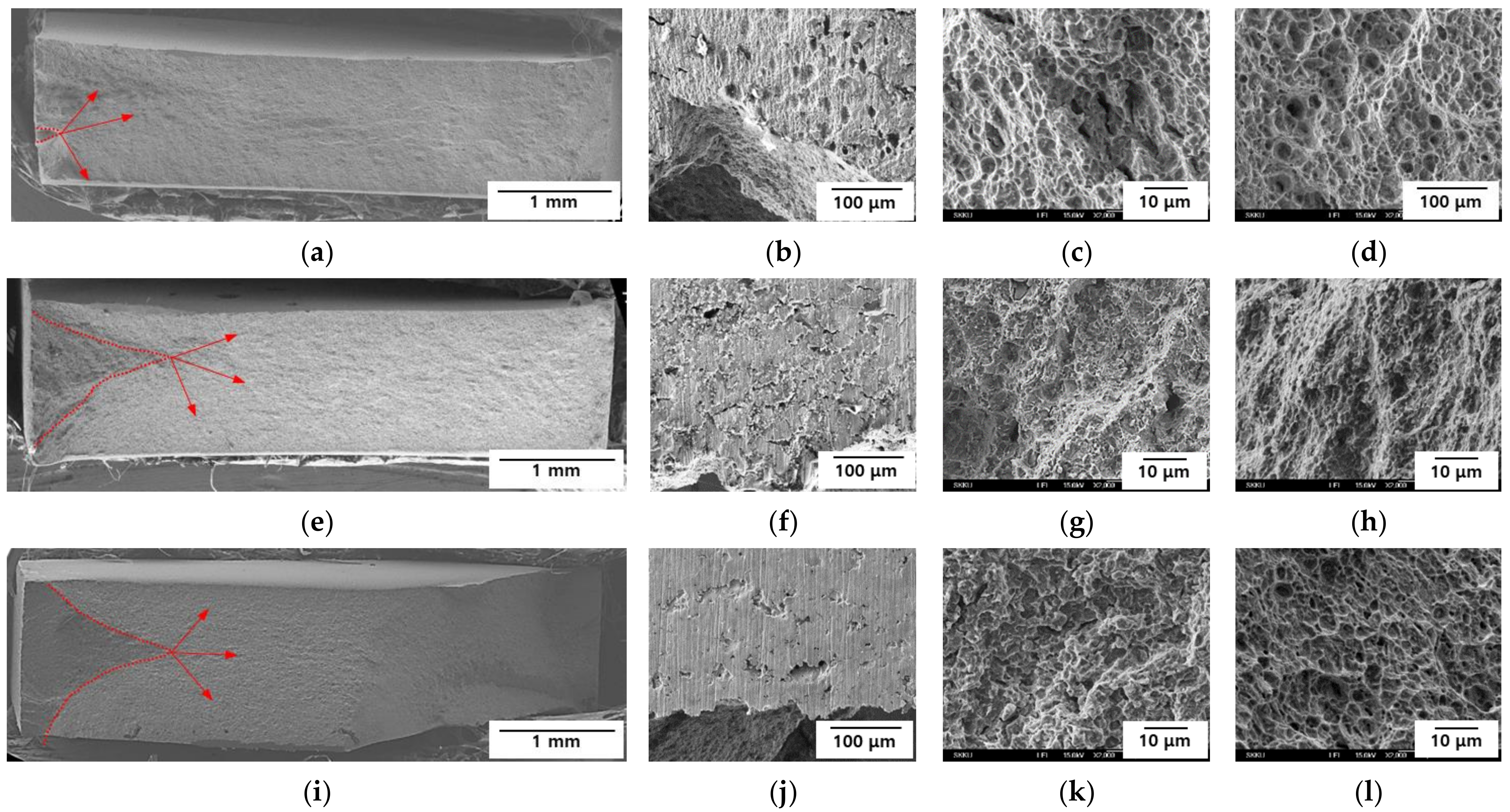
3.3. Fractography
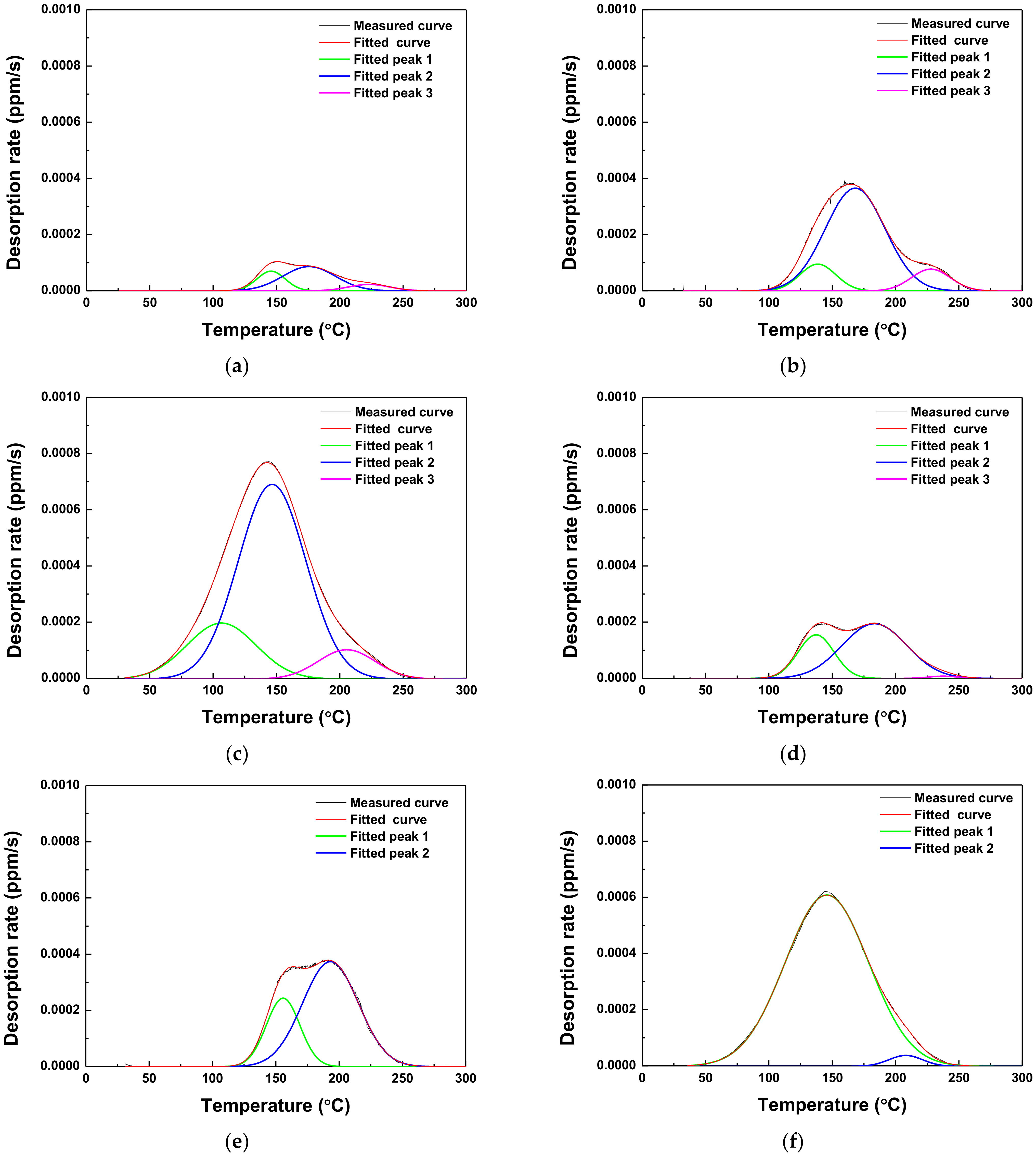
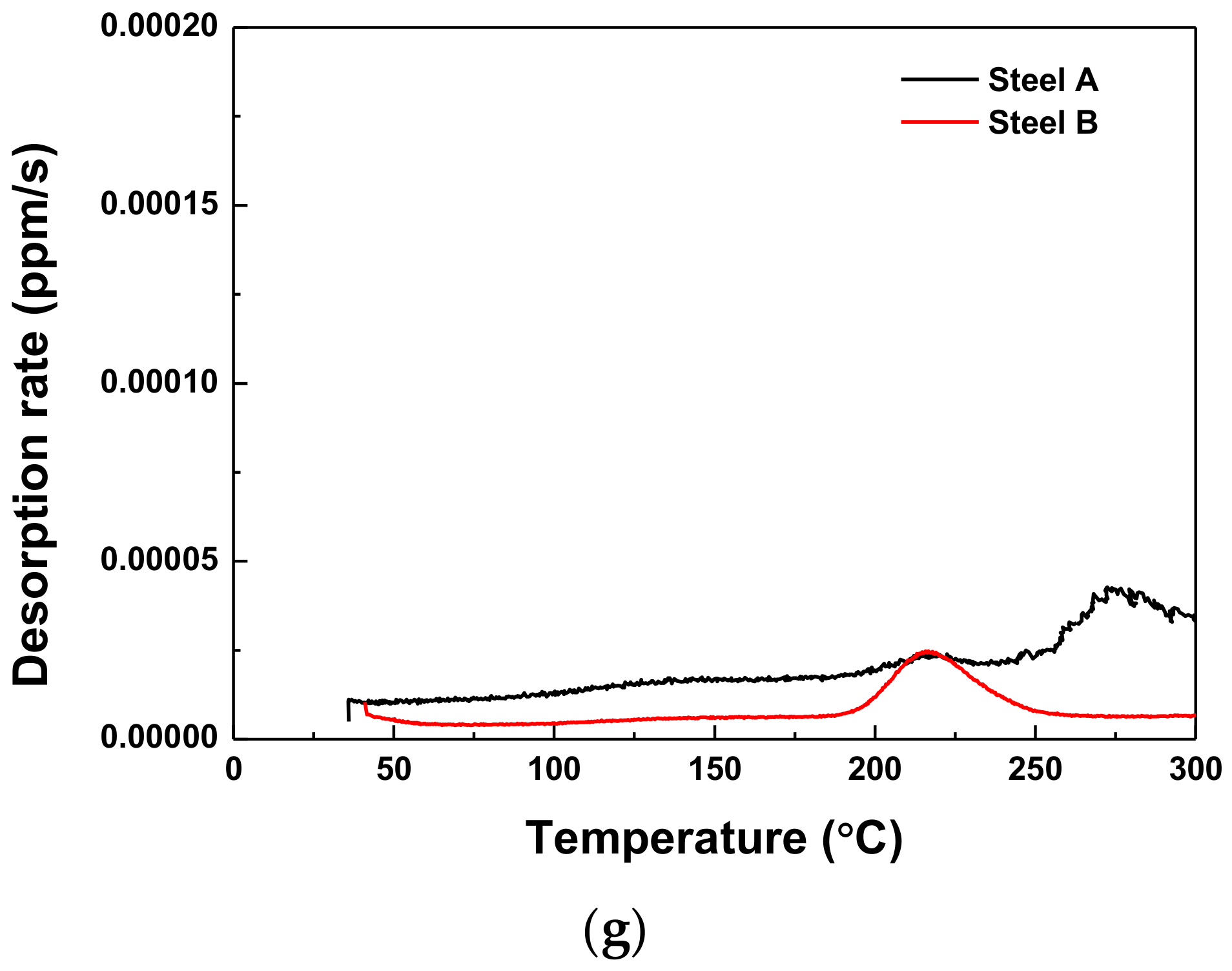
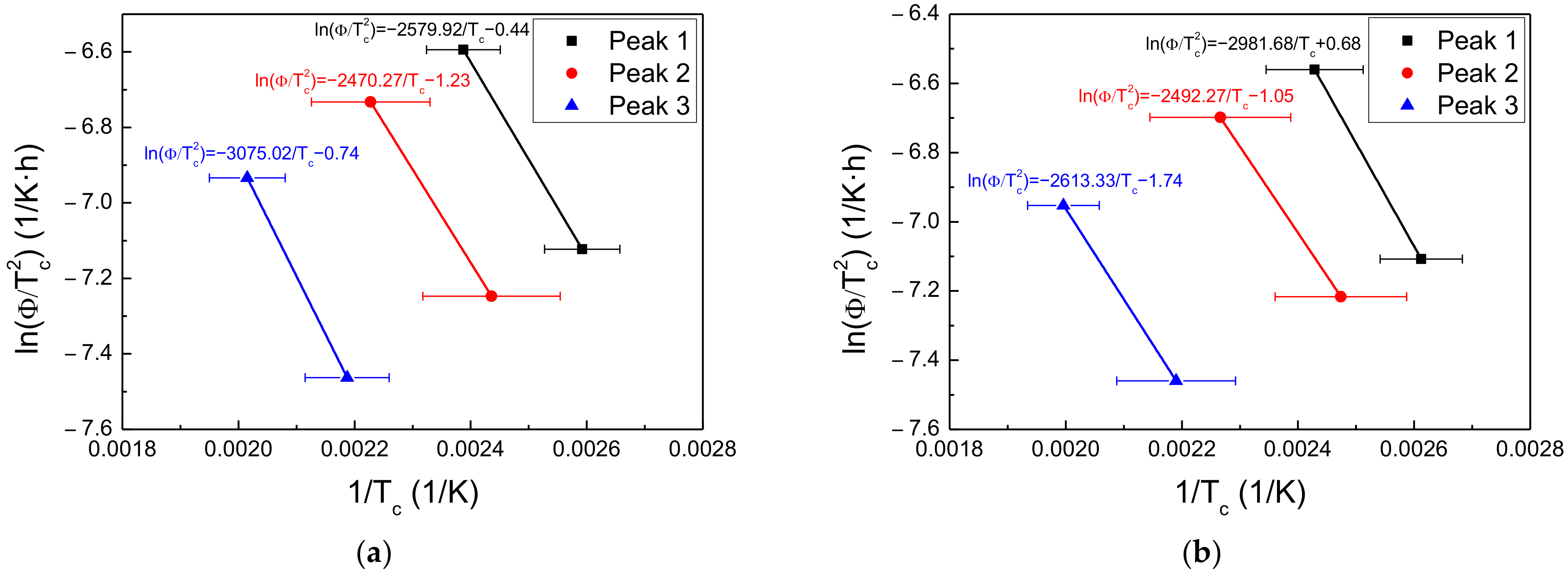
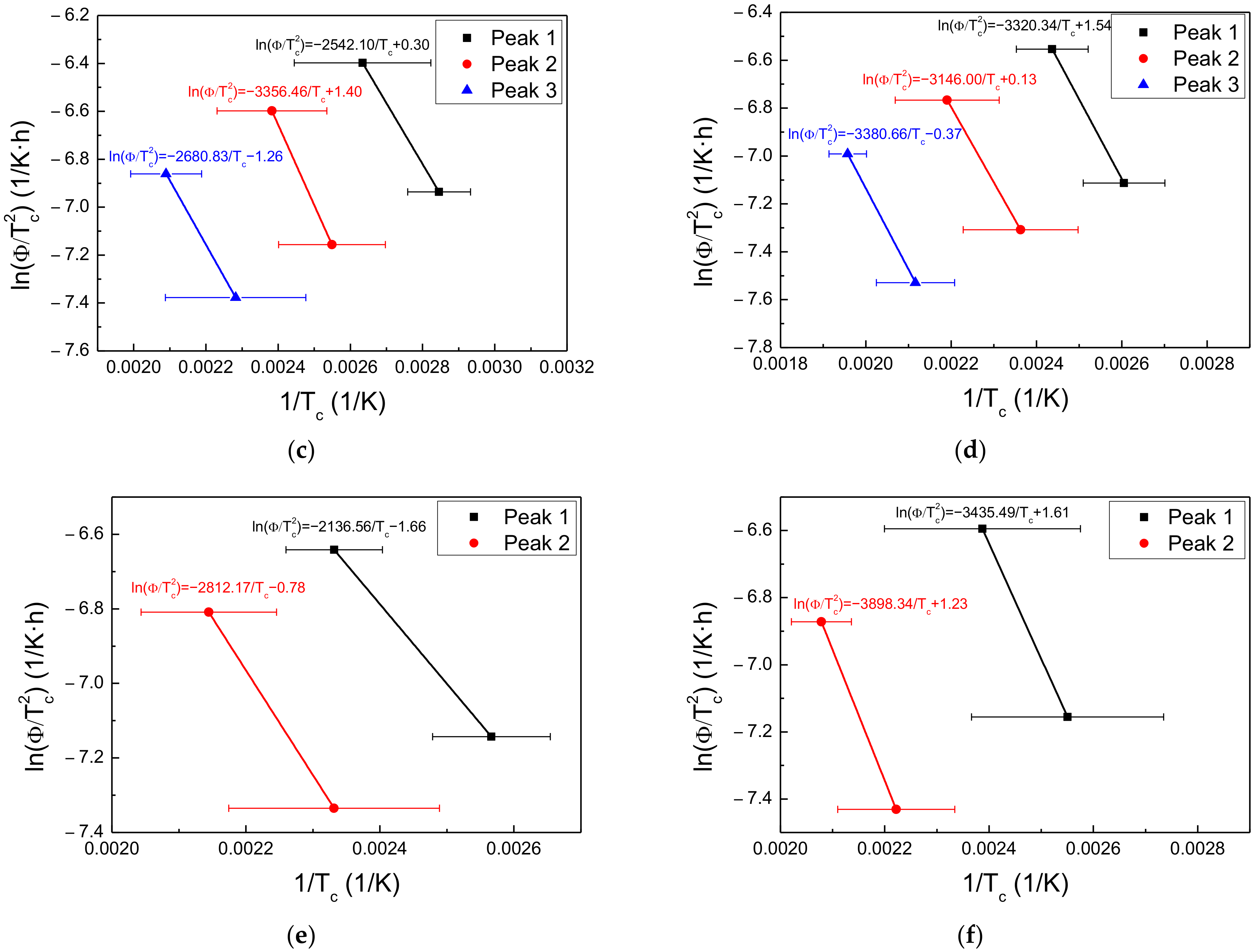
3.4. Hydrogen Trapping and Desorption Behaviors
3.5. Analysis of Defects Acting as Hydrogen Trapping Sites
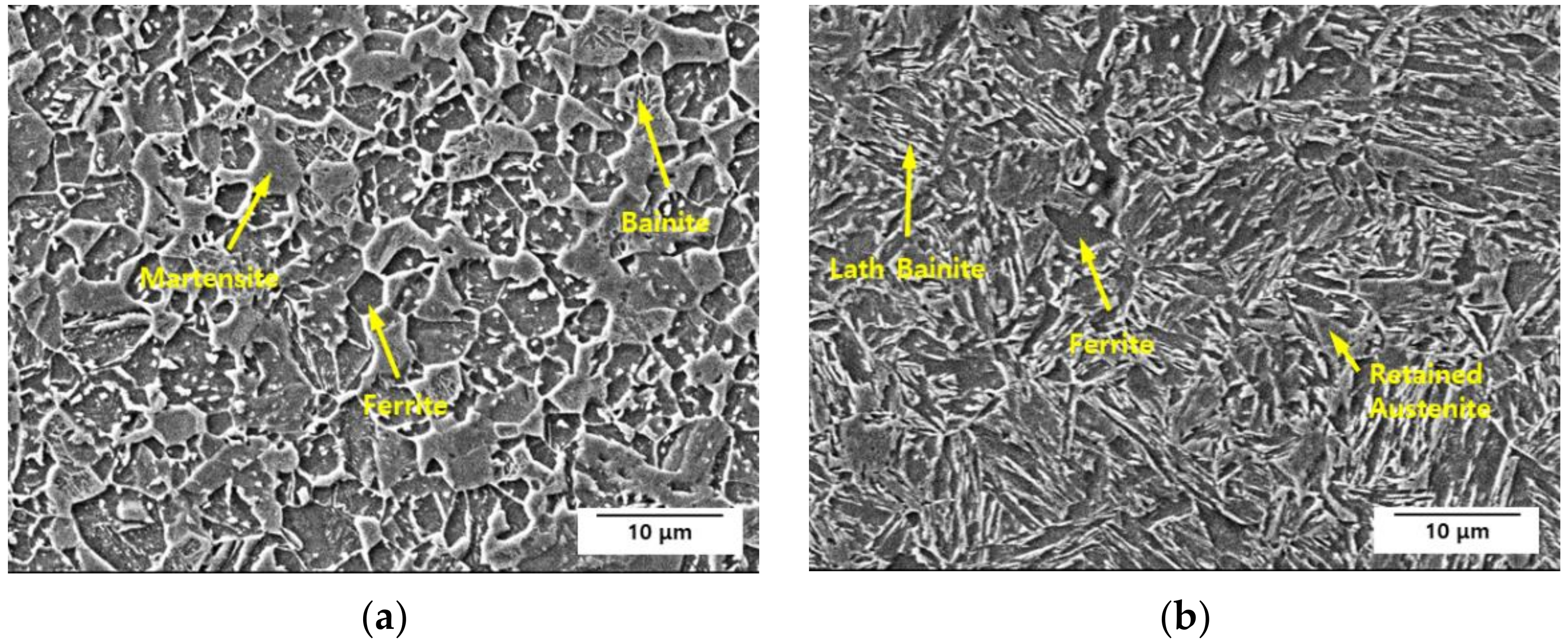
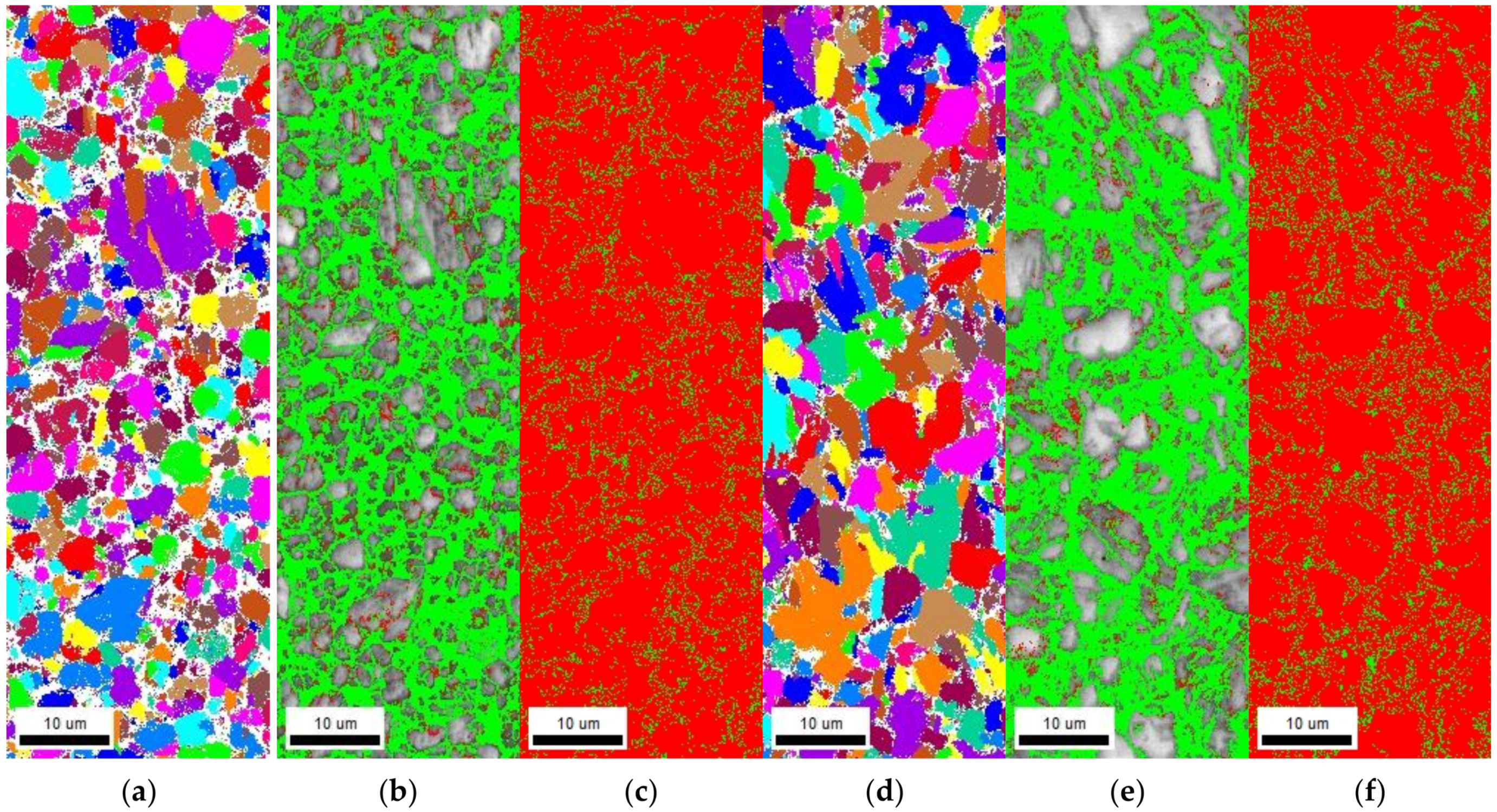
3.5.1. Electron Backscattered Diffraction Analysis
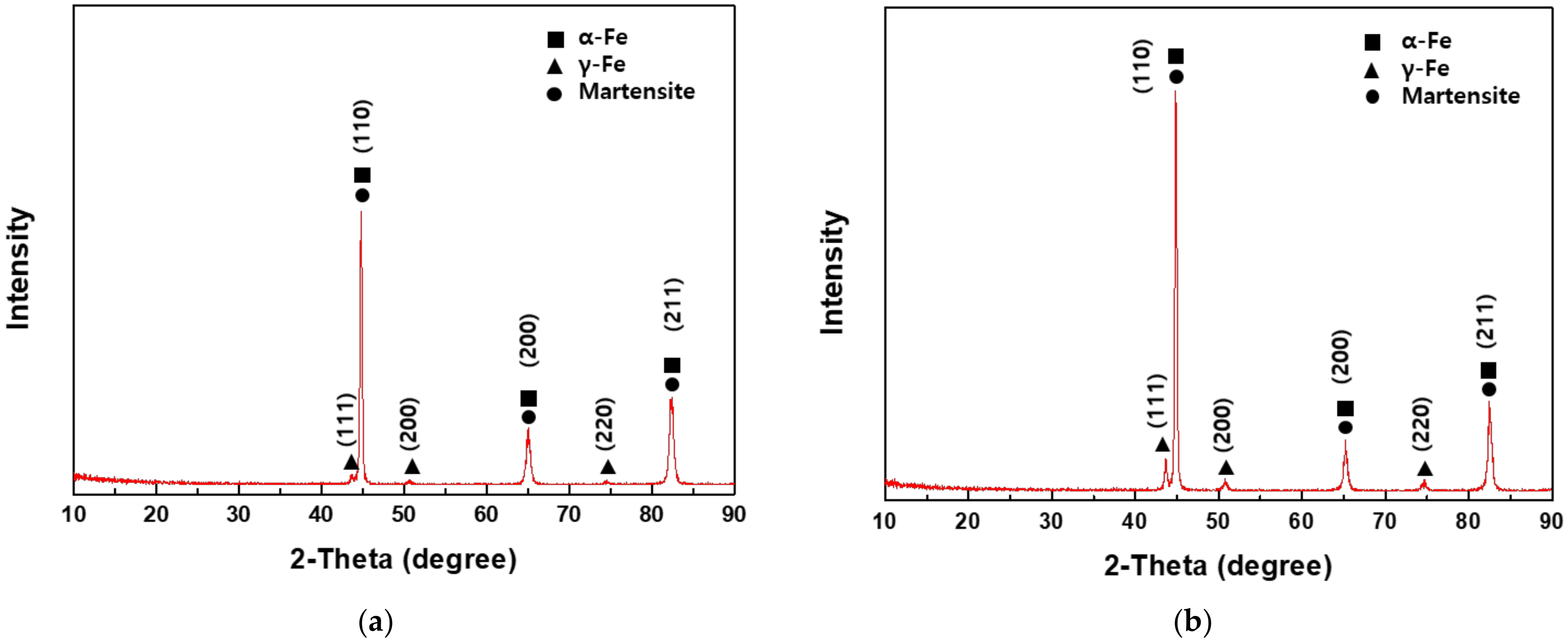
3.5.2. X-ray Diffraction
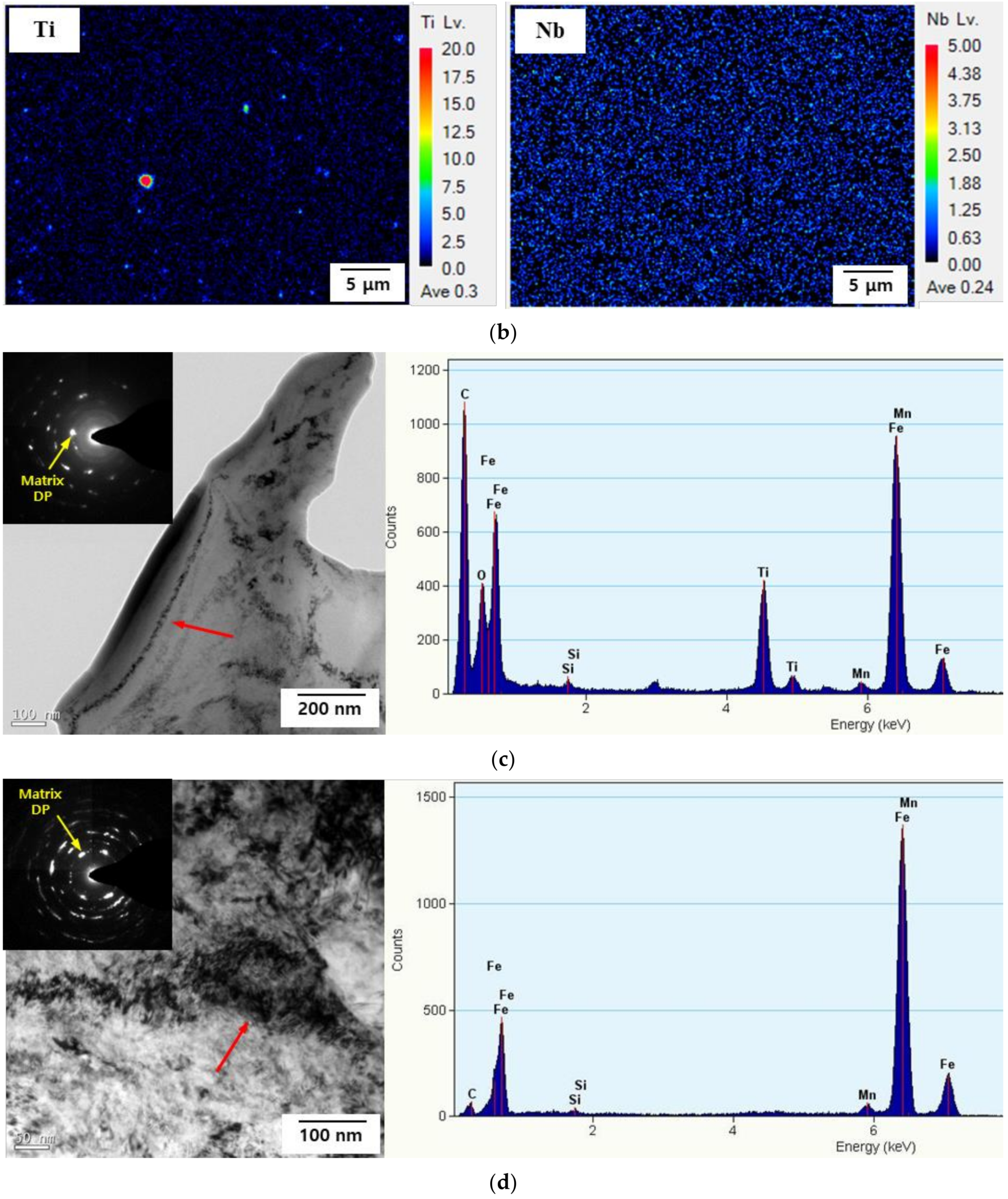
3.5.3. Characterization of Precipitates
4. Conclusions
- For both AHSSs, elongation decreased as the cathodic overvoltage increased, i.e., both types of AHSSs were fractured by the mechanism of HE. Even when the anodic potential was applied, HE was more dominant than SCC. Although the HE sensitivity of Steel B was higher than that of Steel A, both AHSSs were more sensitive to HE than SCC.
- In both AHSSs, the lower Eapp was associated with a strong brittle fracture behavior. However, the center of each specimen exhibited ductile fracture behavior, because the hydrogen did not diffuse into that region. It was clear that the fracture surface of Steel B was more brittle than that of Steel A.
- The lower Eapp was associated with the higher rate of hydrogen desorption. In both AHSSs, diffusible hydrogen was trapped mainly at the grain boundary and dislocation.
- The density of the irreversible hydrogen-trapping sites (high-angle grain boundaries and TiC precipitates) was higher in Steel A than in Steel B. However, the density of the reversible hydrogen-trapping sites (low-angle grain boundaries and dislocations) was lower in Steel A than in Steel B. Therefore, compared to Steel A, Steel B was more susceptible to HE.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, W.; Zhuang, L.; Bottema, J.; Wittebrood, A.J.; De Smet, P.; Haszler, A.; Vieregge, A. Recent development in aluminium alloys for the automotive industry. Mater. Sci. Eng. A 2000, 280, 37–49. [Google Scholar] [CrossRef]
- Kulekci, M.K. Magnesium and its alloys applications in automotive industry. Int. J. Adv. Manuf. Technol. 2008, 39, 851–865. [Google Scholar] [CrossRef]
- Cole, G.; Sherman, A. Light weight materials for automotive applications. Mater. Charact. 1995, 35, 3–9. [Google Scholar] [CrossRef]
- Friedrich, H.; Schumann, S. Research for a “new age of magnesium” in the automotive industry. J. Mater. Process. Technol. 2001, 117, 276–281. [Google Scholar] [CrossRef]
- Musfirah, A.; Jaharah, A. Magnesium and aluminum alloys in automotive industry. J. Appl. Sci. Res 2012, 8, 4865–4875. [Google Scholar]
- Gándara, M.J.F. Recent growing demand for magnesium in the automotive industry. Mater. Technol. 2011, 45, 633–637. [Google Scholar]
- Tisza, M.; Czinege, I. Comparative study of the application of steels and aluminium in lightweight production of automotive parts. Int. J. Lightweight Mater. Manuf. 2018, 1, 229–238. [Google Scholar] [CrossRef]
- Hirsch, J. Recent development in aluminium for automotive applications. Trans. Nonferrous Met. Soc. China 2014, 24, 1995–2002. [Google Scholar] [CrossRef]
- Baluch, N.; Udin, Z.M.; Abdullah, C.S. Advanced high strength steel in auto industry: An overview. Eng. Technol. Appl. Sci. Res. 2014, 4, 686–689. [Google Scholar] [CrossRef]
- Galán, J.; Samek, L.; Verleysen, P.; Verbeken, K.; Houbaert, Y. Advanced high strength steels for automotive industry. Rev. Metal. 2012, 48, 118. [Google Scholar] [CrossRef]
- Funakawa, Y.; Nagataki, Y. High Strength Steel Sheets for Weight Reduction of Automotives. JFE Tech. Rep. 2019, 24, 1–5. [Google Scholar]
- Lovicu, G.; Bottazzi, M.; D’aiuto, F.; De Sanctis, M.; Dimatteo, A.; Santus, C.; Valentini, R. Hydrogen embrittlement of automotive advanced high-strength steels. Metall. Mater. Trans. A 2012, 43, 4075–4087. [Google Scholar] [CrossRef]
- Khanzhin, V.; Nikulin, S.; Belov, V.; Rogachev, S.; Turilina, V.Y. Hydrogen embrittlement of steels: III. Influence of secondary-phase particles. Russ. Metall. (Met.) 2013, 2013, 790–796. [Google Scholar] [CrossRef]
- Khanzhin, V.; Turilina, V.Y.; Rogachev, S.; Nikitin, A.; Belov, V. Effect of various factors on hydrogen embrittlement of structural steels. Met. Sci. Heat Treat. 2015, 57, 197–204. [Google Scholar] [CrossRef]
- Hagi, H.; Hayashi, Y.; Ohtani, N. Diffusion coefficient of hydrogen in pure iron between 230 and 300 K. Trans. Jpn. Inst. Met. 1979, 20, 349–357. [Google Scholar] [CrossRef]
- Choo, W.; Lee, J.Y. Thermal analysis of trapped hydrogen in pure iron. Metall. Mater. Trans. A 1982, 13, 135–140. [Google Scholar] [CrossRef]
- Katiyar, P.K.; Misra, S.; Mondal, K. Comparative corrosion behavior of five microstructures (pearlite, bainite, spheroidized, martensite, and tempered martensite) made from a high carbon steel. Metall. Mater. Trans. A 2019, 50, 1489–1501. [Google Scholar] [CrossRef]
- Jones, D.A. Principles and Prevention of Corrosion, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1996. [Google Scholar]
- Yoo, J.; Xian, G.; Lee, M.; Kim, Y.; Kang, N. Hydrogen embrittlement resistance and diffusible hydrogen desorption behavior of multipass FCA weld metals. J. Weld. Join. 2014, 31, 112–118. [Google Scholar] [CrossRef][Green Version]
- Hurley, C.; Martin, F.; Marchetti, L.; Chêne, J.; Blanc, C.; Andrieu, E. Numerical modeling of thermal desorption mass spectroscopy (TDS) for the study of hydrogen diffusion and trapping interactions in metals. Int. J. Hydrog. Energy 2015, 40, 3402–3414. [Google Scholar] [CrossRef]
- Kirchheim, R. Bulk diffusion-controlled thermal desorption spectroscopy with examples for hydrogen in iron. Metall. Mater. Trans. A 2016, 47, 672–696. [Google Scholar] [CrossRef]
- Ryu, J.H.; Chun, Y.S.; Lee, C.S.; Bhadeshia, H.; Suh, D.W. Effect of deformation on hydrogen trapping and effusion in TRIP-assisted steel. Acta Mater. 2012, 60, 4085–4092. [Google Scholar] [CrossRef]
- Krieger, W.; Merzlikin, S.V.; Bashir, A.; Szczepaniak, A.; Springer, H.; Rohwerder, M. Spatially resolved localization and characterization of trapped hydrogen in zero to three dimensional defects inside ferritic steel. Acta Mater. 2018, 144, 235–244. [Google Scholar] [CrossRef]
- Hong, G.-W.; Lee, J.-Y. The interaction of hydrogen and the cementite-ferrite interface in carbon steel. J. Mater. Sci. 1983, 18, 271–277. [Google Scholar] [CrossRef]
- Wang, M.; Akiyama, E.; Tsuzaki, K. Effect of hydrogen and stress concentration on the notch tensile strength of AISI 4135 steel. Mater. Sci. Eng. A 2005, 398, 37–46. [Google Scholar] [CrossRef]
- Hong, G.-W.; Lee, J.-Y. The interaction of hydrogen with dislocation in iron. In Perspectives in Hydrogen in Metals; Elsevier: Amsterdam, The Netherlands, 1986; pp. 427–435. [Google Scholar]
- Yamasaki, S.; Takahashi, T. Evaluation method of delayed fracture property of high strength steels. J. Iron Steel Inst. Jpn. 1997, 83, 454–459. [Google Scholar] [CrossRef]
- Wei, F.; Hara, T.; Tsuzaki, K. Precise determination of the activation energy for desorption of hydrogen in two Ti-added steels by a single thermal-desorption spectrum. Metall. Mater. Trans. B 2004, 35, 587–597. [Google Scholar] [CrossRef]
- Pressouyre, G. A classification of hydrogen traps in steel. Metall. Mater. Trans. A 1979, 10, 1571–1573. [Google Scholar] [CrossRef]
- Wallaert, E.; Depover, T.; Arafin, M.; Verbeken, K. Thermal desorption spectroscopy evaluation of the hydrogen-trapping capacity of NbC and NbN precipitates. Metall. Mater. Trans. A 2014, 45, 2412–2420. [Google Scholar] [CrossRef]
- Jangir, D.K.; Verma, A.; Sankar, K.M.; Khanna, A.; Singla, A. Influence of grain size on corrosion resistance and electrochemical behaviour of mild steel. Int. J. Res. Appl. Sci. Eng. Technol. 2018, 6, 2875–2881. [Google Scholar] [CrossRef]
- Oger, L.; Malard, B.; Odemer, G.; Peguet, L.; Blanc, C. Influence of dislocations on hydrogen diffusion and trapping in an Al-Zn-Mg aluminium alloy. Mater. Des. 2019, 180, 107901. [Google Scholar] [CrossRef]
- Kang, H.-J.; Kang, N.-H.; Park, S.-J.; Chang, W.-S. Evaluation of hydrogen delayed fracture for high strength steels and weldments. J. Weld. Join. 2011, 29, 33–39. [Google Scholar] [CrossRef][Green Version]
- Szost, B.A.; Vegter, R.H.; Rivera-Díaz-del-Castillo, P.E. Hydrogen-trapping mechanisms in nanostructured steels. Metall. Mater. Trans. A 2013, 44, 4542–4550. [Google Scholar] [CrossRef]
- Shahmoradi, Y.; Souri, D.; Khorshidi, M. Glass-ceramic nanoparticles in the Ag2O–TeO2–V2O5 system: Antibacterial and bactericidal potential, their structural and extended XRD analysis by using Williamson–Smallman approach. Ceram. Int. 2019, 45, 6459–6466. [Google Scholar] [CrossRef]
- Bykkam, S.; Ahmadipour, M.; Narisngam, S.; Kalagadda, V.R.; Chidurala, S.C. Extensive studies on X-ray diffraction of green synthesized silver nanoparticles. Adv. Nanopart 2015, 4, 1–10. [Google Scholar] [CrossRef]
- Yamasaki, S.; Manabe, T.; Hirakami, D. Analysis of Hydrogen State in Steel and Trapping Using Thermal Desorption Method. Nippon Steel & Sumitomo Metal Technical Report. 2017, pp. 38–43. Available online: https://www.nipponsteel.com/en/tech/report/nssmc/pdf/116-08.pdf (accessed on 18 January 2022).
- Turk, A.; San Martín, D.; Rivera-Díaz-del-Castillo, P.E.; Galindo-Nava, E.I. Correlation between vanadium carbide size and hydrogen trapping in ferritic steel. Scr. Mater. 2018, 152, 112–116. [Google Scholar] [CrossRef]




| Component | C | Si | Mn | Cr | Ti | Nb | Fe |
|---|---|---|---|---|---|---|---|
| Steel A | 0.11~0.18 | 0.4~0.7 | 2.2~2.7 | 0.0~0.1 | 0.01~0.02 | 0.01~0.02 | Bal. |
| Steel B | 0.11~0.18 | 1.2~1.9 | 2.4~2.7 | 0.20~0.45 | 0.01~0.02 | - | Bal. |
| NaCl | CaCl2 | NaHCO3 | (NH4)2SO4 | pH |
|---|---|---|---|---|
| 0.5 | 0.1 | 0.075 | 0.35 | 7.3 |
| Specimen | Ecorr (VSCE) | Icorr (A/cm2) | βa (mV/Decade) | βc (mV/Decade) | Corrosion Rate (mm/y) |
|---|---|---|---|---|---|
| Steel A | −0.748 | 3.54 × 10−5 | 92 | 205 | 0.41 |
| Steel B | −0.755 | 3.38 × 10−5 | 61 | 241 | 0.39 |
| Mild steel | −0.765 | 1.70 × 10−5 | 89 | 419 | 0.20 |
| Steel | Applied Potential (mVSCE) | Yield Strength (MPa) | Tensile Strength (MPa) | Elongation (%) | HE Susceptibility Index, IHE (%) | Hydrogen Evolution Rate (g) |
|---|---|---|---|---|---|---|
| Steel A | Air | 781 | 1186 | 16.0 | - | - |
| −600 | 703 | 1124 | 14.1 | 11.9 | - | |
| −750 (Ecorr) | 699 | 1146 | 11.2 | 30.1 | 5.76 × 10−6 | |
| −1500 | 690 | 1041 | 4.6 | 71.0 | 1.23 × 10−3 | |
| Steel B | Air | 800 | 1202 | 21.6 | - | - |
| −600 | 906 | 1150 | 16.4 | 24.0 | - | |
| −750 (Ecorr) | 852 | 1119 | 13.3 | 38.3 | 2.62 × 10−6 | |
| −1500 | 851 | 1034 | 3.7 | 82.9 | 9.76 × 10−4 |
| Steel | Applied Potential (mVSCE) | Peak 1 (ppm) | Peak 2 (ppm) | Peak 3 (ppm) | Sum of Peaks (ppm) |
|---|---|---|---|---|---|
| Steel A | As-received | 0.0569 | - | - | 0.0569 ± 0.0323 |
| −600 | 0.0622 | 0.1511 | 0.0311 | 0.2485 ± 0.1262 | |
| −750 | 0.0579 | 0.3693 | 0.0514 | 0.4787 ± 0.0145 | |
| −1500 | 0.2264 | 0.7735 | 0.0978 | 1.0977 ± 0.0968 | |
| Steel B | As-received | 0.0506 | - | - | 0.0506 ± 0.0268 |
| −600 | 0.1388 | 0.3122 | 0.0057 | 0.4568 ± 0.2070 | |
| −750 | 0.1748 | 0.4445 | - | 0.6193 ± 0.1280 | |
| −1500 | 0.9622 | 0.0246 | - | 0.9868 ± 0.0052 |
| Steel | Applied Potential (mVSCE) | Peak 1 (kJ/mol) | Peak 2 (kJ/mol) | Peak 3 (kJ/mol) |
|---|---|---|---|---|
| Steel A | −600 | 21.5 | 20.5 | 25.6 |
| −750 | 24.8 | 20.7 | 21.7 | |
| −1500 | 21.1 | 27.9 | 22.3 | |
| Steel B | −600 | 27.6 | 26.1 | 28.1 |
| −750 | 17.8 | 23.4 | - | |
| −1500 | 28.6 | 32.4 | - |
| Type of Trap | Activation Energy (kJ/mol) | References |
|---|---|---|
| Reversible hydrogen-trapping sites | ||
| Ferrite/Fe3C | 10.9 | [16] |
| Grain boundary | 17.2 | [16] |
| Ferrite/Fe3C interface | 18.4 | [16,24] |
| Grain boundary, Dislocation | 21–29 | [25,26,27,28] |
| Deformation field around dislocation | 29 ± 5 | [23] |
| Irreversible hydrogen-trapping sites | ||
| Semi-coherent TiC | 49.9 | [28] |
| High-angle grain boundary | 53–59 | [29] |
| NbC interface | 63–68 | [30] |
| Incoherent TiC | 85.7, 86.9 | [28] |
| Specimen | Average Grain Size | High-Angle Grain Boundary Length | Low-Angle Grain Boundary Length | Retained Austenite Fraction |
|---|---|---|---|---|
| Steel A | 2.79 μm | 15.73 mm | 1.91 mm | 9.5% |
| Steel B | 4.03 μm | 13.93 mm | 1.99 mm | 10.9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.; Kim, G.-I.; Ko, S.-J.; Yoo, J.-S.; Jung, Y.-S.; Yoo, Y.-H.; Kim, J.-G. Comparison of Hydrogen Embrittlement Susceptibility of Different Types of Advanced High-Strength Steels. Materials 2022, 15, 3406. https://doi.org/10.3390/ma15093406
Cho S, Kim G-I, Ko S-J, Yoo J-S, Jung Y-S, Yoo Y-H, Kim J-G. Comparison of Hydrogen Embrittlement Susceptibility of Different Types of Advanced High-Strength Steels. Materials. 2022; 15(9):3406. https://doi.org/10.3390/ma15093406
Chicago/Turabian StyleCho, Sangwon, Geon-Il Kim, Sang-Jin Ko, Jin-Seok Yoo, Yeon-Seung Jung, Yun-Ha Yoo, and Jung-Gu Kim. 2022. "Comparison of Hydrogen Embrittlement Susceptibility of Different Types of Advanced High-Strength Steels" Materials 15, no. 9: 3406. https://doi.org/10.3390/ma15093406
APA StyleCho, S., Kim, G.-I., Ko, S.-J., Yoo, J.-S., Jung, Y.-S., Yoo, Y.-H., & Kim, J.-G. (2022). Comparison of Hydrogen Embrittlement Susceptibility of Different Types of Advanced High-Strength Steels. Materials, 15(9), 3406. https://doi.org/10.3390/ma15093406







