Impact of Precursor Concentration on Perovskite Crystallization for Efficient Wide-Bandgap Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of PSCs
2.3. Characterization of Films and Devices
3. Results
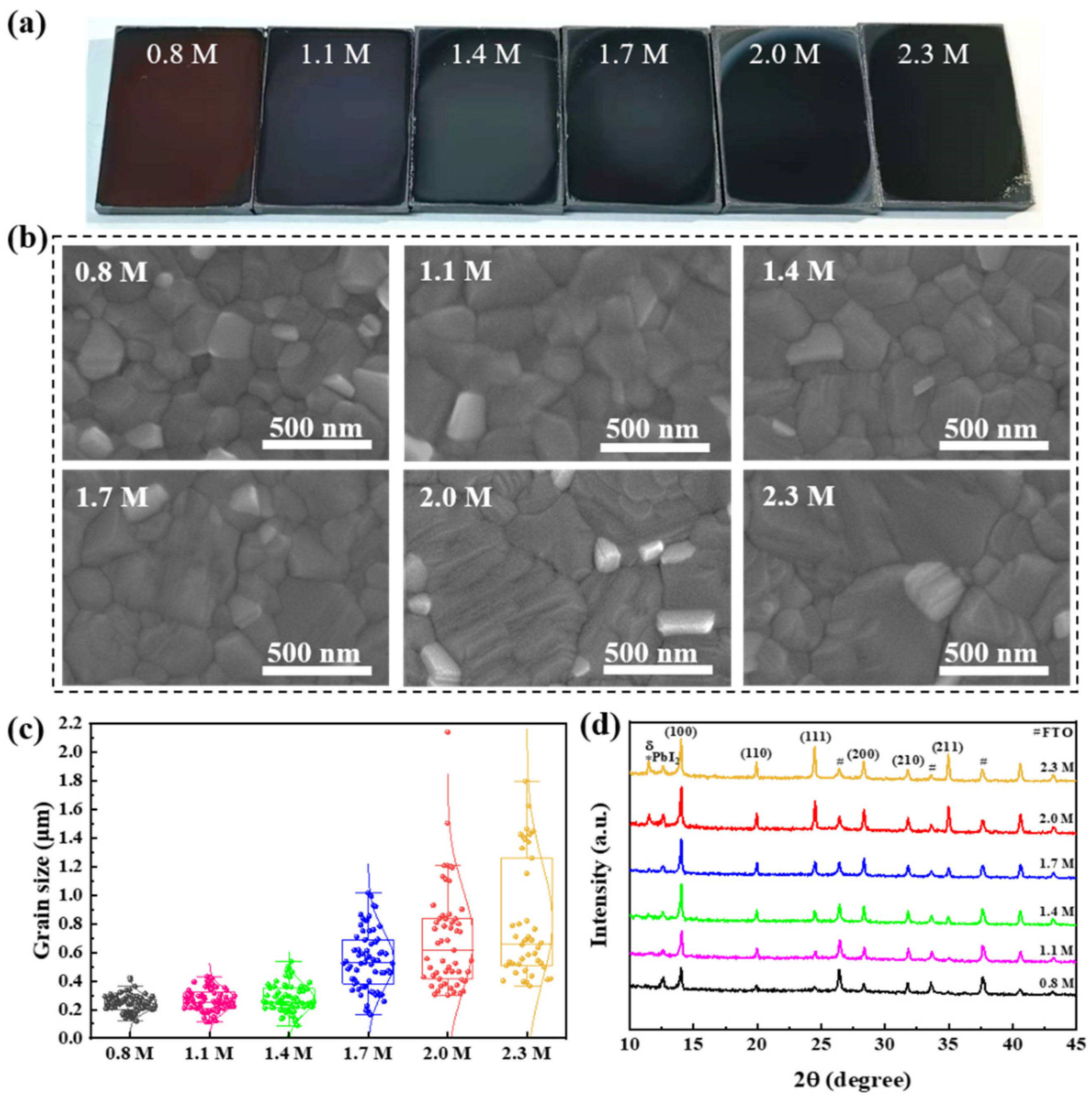
3.1. Morphology and Crystallization of the Wide-Bandgap Perovskite Films
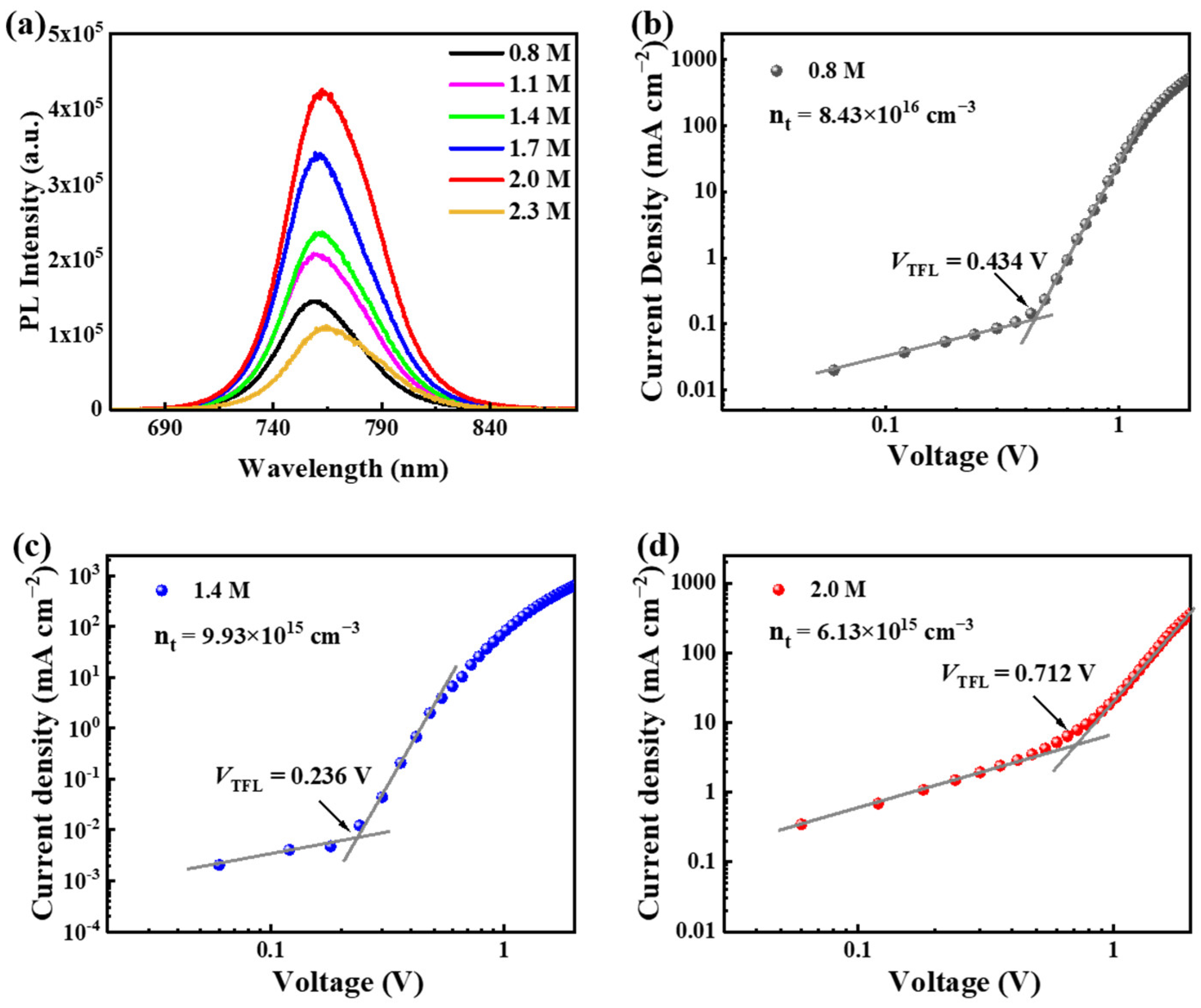
3.2. Optical Properties of the Wide-Bandgap Perovskite Films
3.3. Trap States of the Wide-Bandgap Perovskite Films
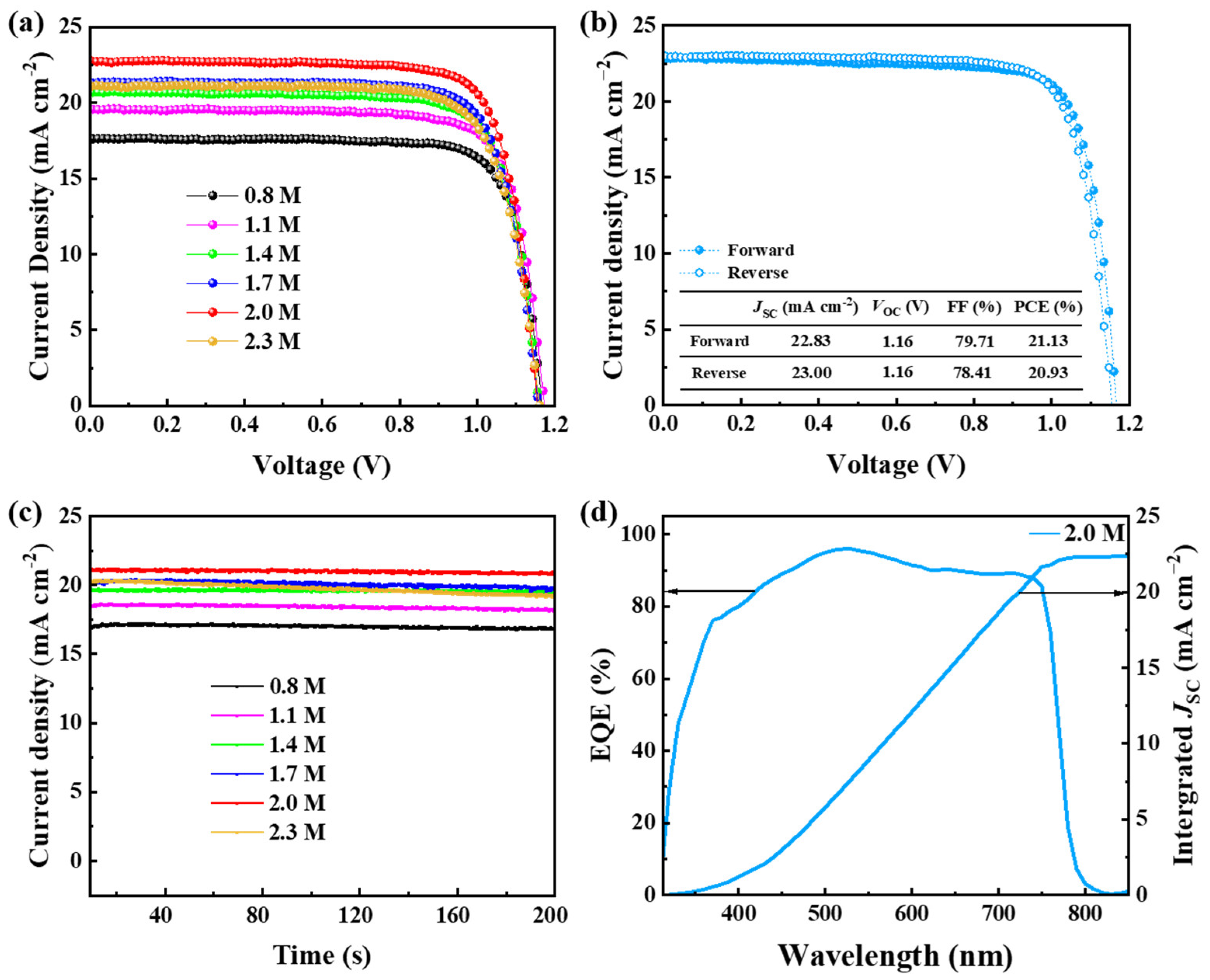
3.4. Performance of Wide-Bandgap PSCs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Best Research-Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 18 March 2022).
- Shockley, W.; Queisser, H.J. Detailed Balance Limit of Efficiency of p-n Junction Solar Cells. J. Appl. Phys. 1961, 32, 510–519. [Google Scholar] [CrossRef]
- Liu, J.; Aydin, E.; Yin, J.; De Bastiani, M.; Isikgor, F.H.; Rehman, A.U.; Yengel, E.; Ugur, E.; Harrison, G.T.; Wang, M.; et al. 28.2%-efficient, outdoor-stable perovskite/silicon tandem solar cell. Joule 2021, 5, 3169–3186. [Google Scholar] [CrossRef]
- Zhu, S.; Yao, X.; Ren, Q.; Zheng, C.; Li, S.; Tong, Y.; Shi, B.; Guo, S.; Fan, L.; Ren, H.; et al. Transparent electrode for monolithic perovskite/silicon-heterojunction two-terminal tandem solar cells. Nano Energy 2018, 45, 280–286. [Google Scholar] [CrossRef]
- Bailie, C.D.; Christoforo, M.G.; Mailoa, J.P.; Bowring, A.R.; Unger, E.L.; Nguyen, W.H.; Burschka, J.; Pellet, N.; Lee, J.Z.; Grätzel, M.; et al. Semi-transparent perovskite solar cells for tandems with silicon and CIGS. Energy Environ. Sci. 2015, 8, 956–963. [Google Scholar] [CrossRef]
- Lang, F.; Jost, M.; Frohna, K.; Kohnen, E.; Al-Ashouri, A.; Bowman, A.R.; Bertram, T.; Morales-Vilches, A.B.; Koushik, D.; Tennyson, E.M.; et al. Proton Radiation Hardness of Perovskite Tandem Photovoltaics. Joule 2020, 4, 1054–1069. [Google Scholar] [CrossRef]
- Lang, K.; Guo, Q.; He, Z.; Bai, Y.; Yao, J.; Wakeel, M.; Alhodaly, M.S.; Hayat, T.; Tan, Z. High Performance Tandem Solar Cells with Inorganic Perovskite and Organic Conjugated Molecules to Realize Complementary Absorption. J. Phys. Chem. Lett. 2020, 11, 9596–9604. [Google Scholar] [CrossRef]
- Xiao, K.; Lin, R.; Han, Q.; Hou, Y.; Qin, Z.; Nguyen, H.T.; Wen, J.; Wei, M.; Yeddu, V.; Saidaminov, M.I.; et al. All-perovskite tandem solar cells with 24.2% certified efficiency and area over 1 cm2 using surface-anchoring zwitterionic antioxidant. Nat. Energy 2020, 5, 870–880. [Google Scholar] [CrossRef]
- Heo, J.H.; Im, S.H. CH3NH3PbBr3-CH3NH3PbI3 Perovskite-Perovskite Tandem Solar Cells with Exceeding 2.2 V Open Circuit Voltage. Adv. Mater. 2016, 28, 5121–5125. [Google Scholar] [CrossRef]
- Oliver, R.D.J.; Caprioglio, P.; Peña-Camargo, F.; Buizza, L.R.V.; Zu, F.; Ramadan, A.J.; Motti, S.G.; Mahesh, S.; McCarthy, M.M.; Warby, J.H.; et al. Understanding and suppressing non-radiative losses in methylammonium-free wide-bandgap perovskite solar cells. Energy Environ. Sci. 2022, 15, 714–726. [Google Scholar] [CrossRef]
- Ahmad, M.; Shahzad, N.; Tariq, M.A.; Sattar, A.; Pugliese, D. Investigating the Sequential Deposition Route for Mixed Cation Mixed Halide Wide Bandgap Perovskite Absorber Layer. Energies 2021, 14, 8401. [Google Scholar] [CrossRef]
- Wang, D.; Guo, H.; Wu, X.; Deng, X.; Li, F.; Li, Z.; Lin, F.; Zhu, Z.; Zhang, Y.; Xu, B.; et al. Interfacial Engineering of Wide-Bandgap Perovskites for Efficient Perovskite/CZTSSe Tandem Solar Cells. Adv. Funct. Mater. 2021, 32, 2107359. [Google Scholar] [CrossRef]
- Noh, J.H.; Im, S.H.; Heo, J.H.; Mandal, T.N.; Seok, S.I. Chemical management for colorful, efficient, and stable inorganic-organic hybrid nanostructured solar cells. Nano Lett. 2013, 13, 1764–1769. [Google Scholar] [CrossRef]
- Ryu, S.; Noh, J.H.; Jeon, N.J.; Chan Kim, Y.; Yang, W.S.; Seo, J.; Seok, S.I. Voltage output of efficient perovskite solar cells with high open-circuit voltage and fill factor. Energy Environ. Sci. 2014, 7, 2614–2618. [Google Scholar] [CrossRef]
- Li, M.-H.; Yum, J.-H.; Moon, S.-J.; Chen, P. Inorganic p-Type Semiconductors: Their Applications and Progress in Dye-Sensitized Solar Cells and Perovskite Solar Cells. Energies 2016, 9, 331. [Google Scholar] [CrossRef] [Green Version]
- Slotcavage, D.J.; Karunadasa, H.I.; McGehee, M.D. Light-Induced Phase Segregation in Halide-Perovskite Absorbers. ACS Energy Lett. 2016, 1, 1199–1205. [Google Scholar] [CrossRef]
- Zhou, Y.; Poli, I.; Meggiolaro, D.; De Angelis, F.; Petrozza, A. Defect activity in metal halide perovskites with wide and narrow bandgap. Nat. Rev. Mater. 2021, 6, 986–1002. [Google Scholar] [CrossRef]
- Unger, E.L.; Kegelmann, L.; Suchan, K.; Sörell, D.; Korte, L.; Albrecht, S. Correction: Roadmap and roadblocks for the band gap tunability of metal halide perovskites. J. Mater. Chem. A 2017, 5, 15983. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Song, Z.; Xiao, C.; Zhao, D.; Shrestha, N.; Li, C.; Yang, G.; Yao, F.; Zheng, X.; Ellingson, R.J.; et al. Achieving a high open-circuit voltage in inverted wide-bandgap perovskite solar cells with a graded perovskite homojunction. Nano Energy 2019, 61, 141–147. [Google Scholar] [CrossRef]
- Kim, J.; Saidaminov, M.I.; Tan, H.; Zhao, Y.; Kim, Y.; Choi, J.; Jo, J.W.; Fan, J.; Quintero-Bermudez, R.; Yang, Z.; et al. Amide-Catalyzed Phase-Selective Crystallization Reduces Defect Density in Wide-Bandgap Perovskites. Adv. Mater. 2018, 30, e1706275. [Google Scholar] [CrossRef]
- Wilk, B.; Öz, S.; Radicchi, E.; Ünlü, F.; Ahmad, T.; Herman, A.P.; Nunzi, F.; Mathur, S.; Kudrawiec, R.; Wojciechowski, K. Green Solvent-Based Perovskite Precursor Development for Ink-Jet Printed Flexible Solar Cells. ACS Sustain. Chem. Eng. 2021, 9, 3920–3930. [Google Scholar] [CrossRef]
- Abdi-Jalebi, M.; Andaji-Garmaroudi, Z.; Cacovich, S.; Stavrakas, C.; Philippe, B.; Richter, J.M.; Alsari, M.; Booker, E.P.; Hutter, E.M.; Pearson, A.J.; et al. Maximizing and stabilizing luminescence from halide perovskites with potassium passivation. Nature 2018, 555, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Ling, T.; Zou, X.; Cheng, J.; Yang, Y.; Ren, H.; Chen, D. Modulating Surface Morphology Related to Crystallization Speed of Perovskite Grain and Optical Semiconductor and Crystallization Properties of the Absorber Layer Under Controlled Doping of Potassium Ions for Solar Cells. Materials 2018, 11, 1605. [Google Scholar] [CrossRef] [Green Version]
- Cui, P.; Wei, D.; Ji, J.; Huang, H.; Jia, E.; Dou, S.; Wang, T.; Wang, W.; Li, M. Planar p–n homojunction perovskite solar cells with efficiency exceeding 21.3%. Nat. Energy 2019, 4, 150–159. [Google Scholar] [CrossRef]
- Huang, H.; Yan, H.; Duan, M.; Ji, J.; Liu, X.; Jiang, H.; Liu, B.; Sajid, S.; Cui, P.; Li, Y.; et al. TiO2 surface oxygen vacancy passivation towards mitigated interfacial lattice distortion and efficient perovskite solar cell. Appl. Surf. Sci. 2021, 544, 148583. [Google Scholar] [CrossRef]
- Ji, J.; Liu, B.; Huang, H.; Wang, X.; Yan, L.; Qu, S.; Liu, X.; Jiang, H.; Duan, M.; Li, Y.; et al. Nondestructive passivation of the TiO2 electron transport layer in perovskite solar cells by the PEIE-2D MOF interfacial modified layer. J. Mater. Chem. C 2021, 9, 7057–7064. [Google Scholar] [CrossRef]
- Lewis, A.E.; Zhang, Y.; Gao, P.; Nazeeruddin, M.K. Unveiling the Concentration-Dependent Grain Growth of Perovskite Films from One- and Two-Step Deposition Methods: Implications for Photovoltaic Application. ACS Appl. Mater. Interfaces 2017, 9, 25063–25066. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Ji, S.G.; Kim, G.; Seok, S.I. Perovskite precursor solution chemistry: From fundamentals to photovoltaic applications. Chem. Soc. Rev. 2019, 48, 2011–2038. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, T.; Schulz, P.; Li, Z.; Li, G.; Kim, D.H.; Guo, N.; Berry, J.J.; Zhu, K.; Zhao, Y. Facile fabrication of large-grain CH3NH3PbI3-xBrx films for high-efficiency solar cells via CH3NH3Br-selective Ostwald ripening. Nat. Commun. 2016, 7, 12305. [Google Scholar] [CrossRef]
- Pham, N.D.; Tiong, V.T.; Yao, D.; Martens, W.; Guerrero, A.; Bisquert, J.; Wang, H. Guanidinium thiocyanate selective Ostwald ripening induced large grain for high performance perovskite solar cells. Nano Energy 2017, 41, 476–487. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Shoyama, K.; Sato, W.; Matsuo, Y.; Inoue, K.; Harano, K.; Liu, C.; Tanaka, H.; Nakamura, E. Chemical Pathways Connecting Lead(II) Iodide and Perovskite via Polymeric Plumbate(II) Fiber. J. Am. Chem. Soc. 2015, 137, 15907–15914. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, D.; Wang, S.; Zhang, T. Highly Efficient and Stable MAPbI(3) Perovskite Solar Cell Induced by Regulated Nucleation and Ostwald Recrystallization. Materials 2018, 11, 778. [Google Scholar] [CrossRef] [Green Version]
- Wieghold, S.; Correa-Baena, J.-P.; Nienhaus, L.; Sun, S.; Shulenberger, K.E.; Liu, Z.; Tresback, J.S.; Shin, S.S.; Bawendi, M.G.; Buonassisi, T. Precursor Concentration Affects Grain Size, Crystal Orientation, and Local Performance in Mixed-Ion Lead Perovskite Solar Cells. ACS Appl. Energy Mater. 2018, 1, 6801–6808. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Y.; Shin, I.; Hwang, I.W.; Jung, Y.K.; Jeong, J.H.; Park, S.H.; Kim, K.H. Understanding and Tailoring Grain Growth of Lead-Halide Perovskite for Solar Cell Application. ACS Appl. Mater. Interfaces 2017, 9, 33925–33933. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhou, W.; Tang, H.; Fu, P.; Ning, Z. Supersaturation controlled growth of MAFAPbI3 perovskite film for high efficiency solar cells. Sci. China Chem. 2018, 61, 1278–1284. [Google Scholar] [CrossRef]
- Zhou, Q.; Qiu, J.; Wang, Y.; Yu, M.; Liu, J.; Zhang, X. Multifunctional Chemical Bridge and Defect Passivation for Highly Efficient Inverted Perovskite Solar Cells. ACS Energy Lett. 2021, 6, 1596–1606. [Google Scholar] [CrossRef]
- Park, B.W.; Kedem, N.; Kulbak, M.; Lee, D.Y.; Yang, W.S.; Jeon, N.J.; Seo, J.; Kim, G.; Kim, K.J.; Shin, T.J.; et al. Understanding how excess lead iodide precursor improves halide perovskite solar cell performance. Nat. Commun. 2018, 9, 3301. [Google Scholar] [CrossRef] [Green Version]
- Qin, M.; Xue, H.; Zhang, H.; Hu, H.; Liu, K.; Li, Y.; Qin, Z.; Ma, J.; Zhu, H.; Yan, K.; et al. Precise Control of Perovskite Crystallization Kinetics via Sequential A-Site Doping. Adv. Mater. 2020, 32, e2004630. [Google Scholar] [CrossRef]
- Lu, H.; Liu, Y.; Ahlawat, P.; Mishra, A.; Tress, W.R.; Eickemeyer, F.T.; Yang, Y.; Fu, F.; Wang, Z.; Avalos, C.E.; et al. Vapor-assisted deposition of highly efficient, stable black-phase FAPbI3 perovskite solar cells. Science 2020, 370, eabb8985. [Google Scholar] [CrossRef]
- Chen, X.; Xia, Y.; Zheng, Z.; Xiao, X.; Ling, C.; Xia, M.; Hu, Y.; Mei, A.; Cheacharoen, R.; Rong, Y.; et al. In Situ Formation of δ-FAPbI3 at the Perovskite/Carbon Interface for Enhanced Photovoltage of Printable Mesoscopic Perovskite Solar Cells. Chem. Mater. 2022, 34, 728–735. [Google Scholar] [CrossRef]
- Wang, S.; Jin, J.; Qi, Y.; Liu, P.; Xia, Y.; Jiang, Y.; He, R.X.; Chen, B.; Liu, Y.; Zhao, X.Z. δ-CsPbI3 Intermediate Phase Growth Assisted Sequential Deposition Boosts Stable and High-Efficiency Triple Cation Perovskite Solar Cells. Adv. Funct. Mater. 2019, 30, 1908343. [Google Scholar] [CrossRef]


| Precursor Concentration | JSC (mA cm−2) | VOC (V) | FF (%) | PCEbest (%) | PCEaverage (%) |
|---|---|---|---|---|---|
| 0.8 M | 17.62 | 1.16 | 79.53 | 16.38 | 16.08 ± 0.35 |
| 1.1 M | 19.56 | 1.16 | 78.92 | 18.05 | 17.45 ± 0.67 |
| 1.4 M | 20.70 | 1.15 | 78.11 | 18.69 | 18.35 ± 0.59 |
| 1.7 M | 21.31 | 1.15 | 78.45 | 19.31 | 18.92 ± 0.46 |
| 2.0 M | 22.85 | 1.16 | 79.71 | 21.13 | 20.39 ± 0.74 |
| 2.3 M | 21.18 | 1.16 | 76.18 | 18.73 | 18.42 ± 0.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, S.; Yang, J.; Qu, S.; Lan, Z.; Sun, T.; Dong, Y.; Shang, Z.; Liu, D.; Yang, Y.; Yan, L.; et al. Impact of Precursor Concentration on Perovskite Crystallization for Efficient Wide-Bandgap Solar Cells. Materials 2022, 15, 3185. https://doi.org/10.3390/ma15093185
Du S, Yang J, Qu S, Lan Z, Sun T, Dong Y, Shang Z, Liu D, Yang Y, Yan L, et al. Impact of Precursor Concentration on Perovskite Crystallization for Efficient Wide-Bandgap Solar Cells. Materials. 2022; 15(9):3185. https://doi.org/10.3390/ma15093185
Chicago/Turabian StyleDu, Shuxian, Jing Yang, Shujie Qu, Zhineng Lan, Tiange Sun, Yixin Dong, Ziya Shang, Dongxue Liu, Yingying Yang, Luyao Yan, and et al. 2022. "Impact of Precursor Concentration on Perovskite Crystallization for Efficient Wide-Bandgap Solar Cells" Materials 15, no. 9: 3185. https://doi.org/10.3390/ma15093185
APA StyleDu, S., Yang, J., Qu, S., Lan, Z., Sun, T., Dong, Y., Shang, Z., Liu, D., Yang, Y., Yan, L., Wang, X., Huang, H., Ji, J., Cui, P., Li, Y., & Li, M. (2022). Impact of Precursor Concentration on Perovskite Crystallization for Efficient Wide-Bandgap Solar Cells. Materials, 15(9), 3185. https://doi.org/10.3390/ma15093185






