Microstructure, Phase Formation and Heat-Treating of Novel Cast Al-Mg-Zn-Cu-Si Lightweight Complex Concentrated Aluminum Based Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results-Discussion
3.1. Thermo-Physical Parameters for Phase Formation in HEAs/CCAs
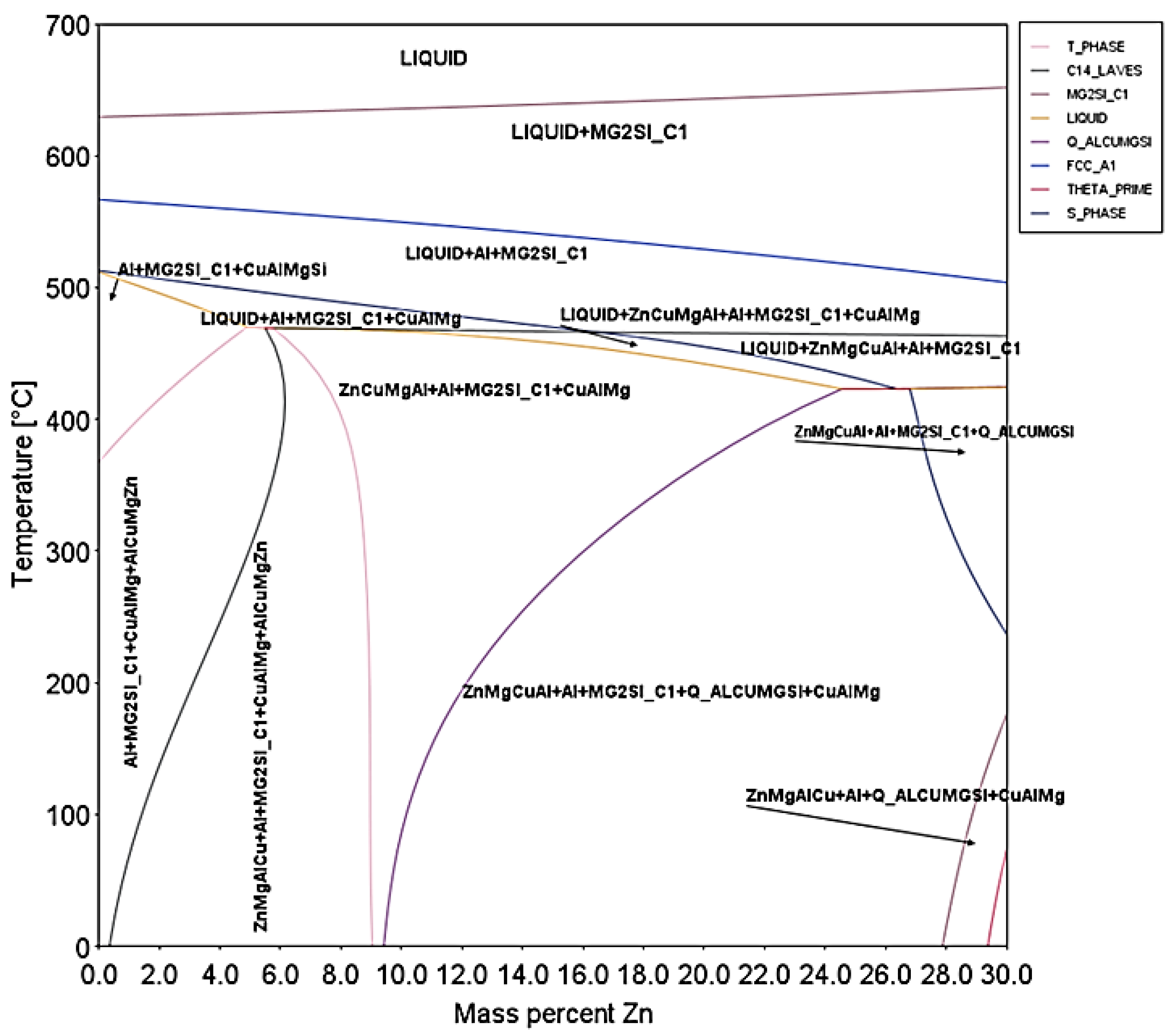
3.2. CALPHAD Methodology and Equilibrium Phase Diagram
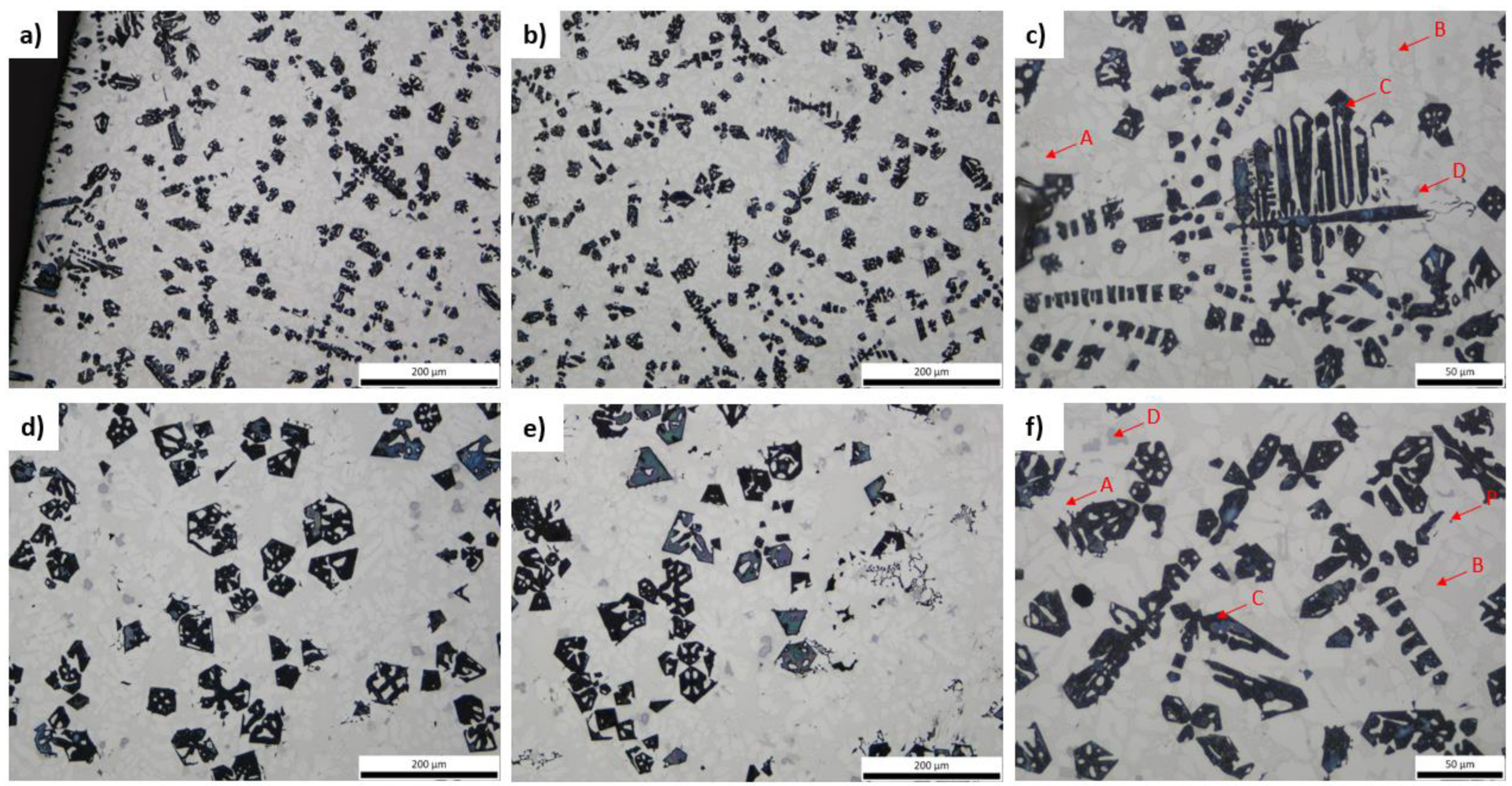
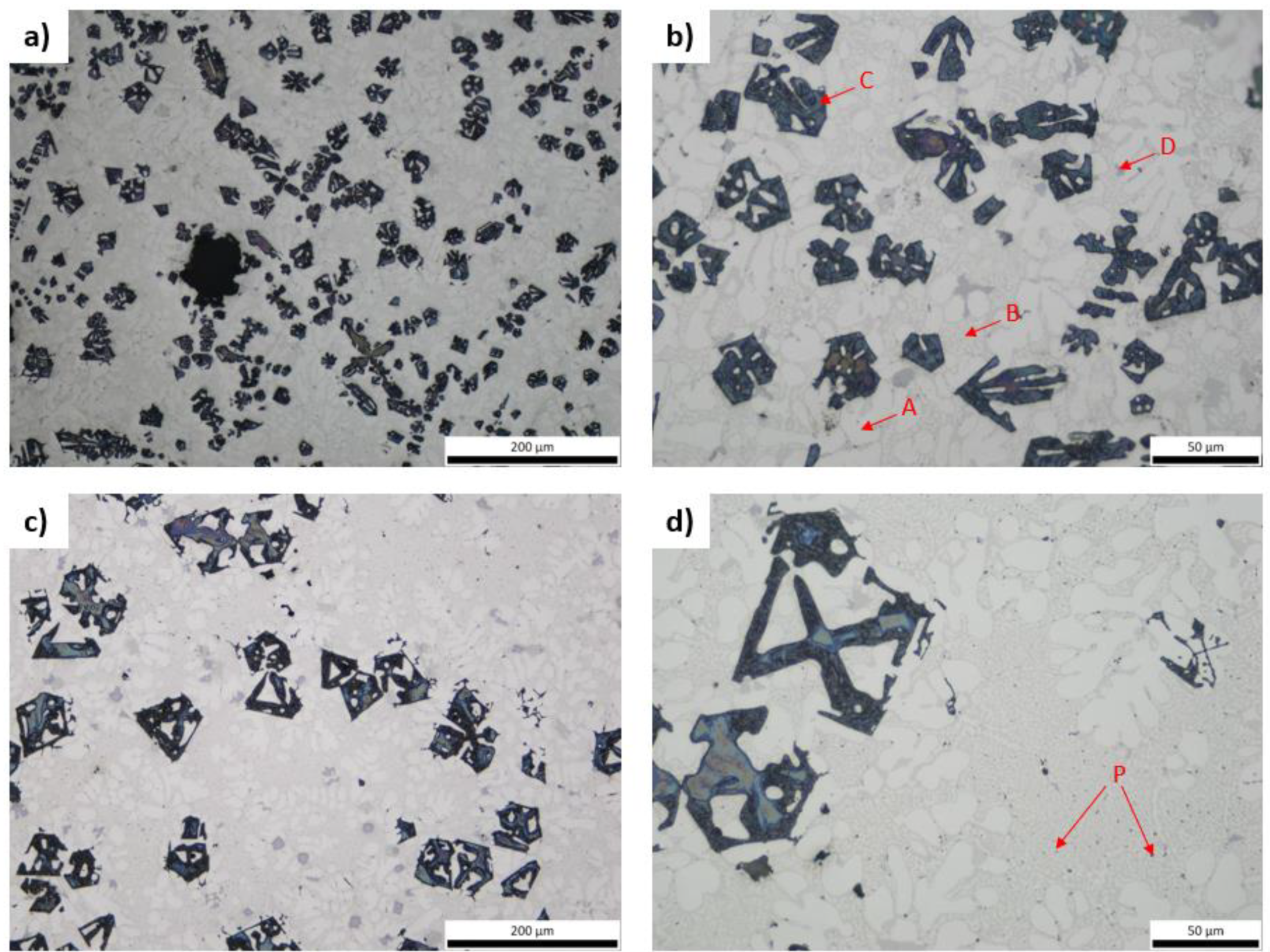
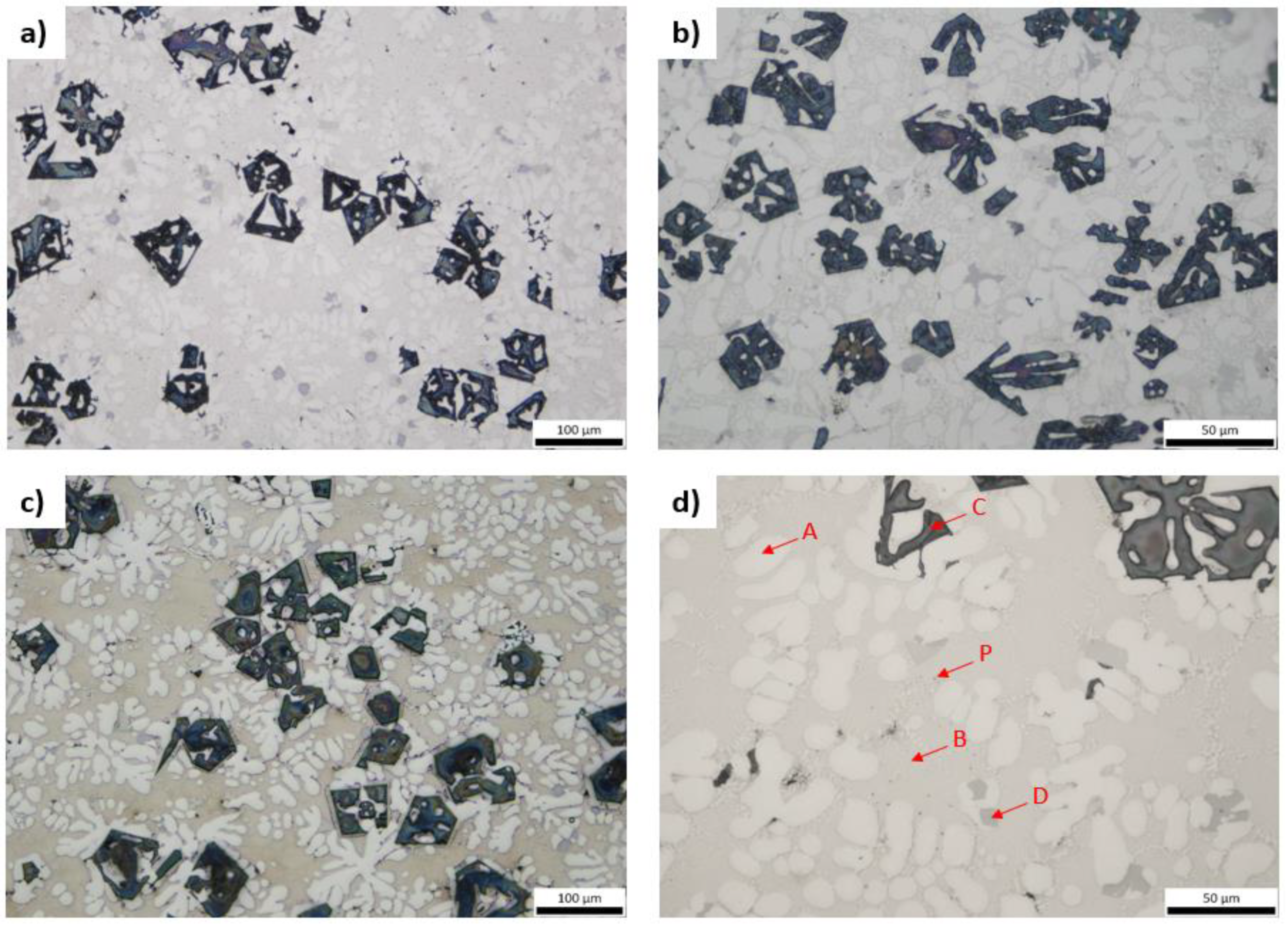
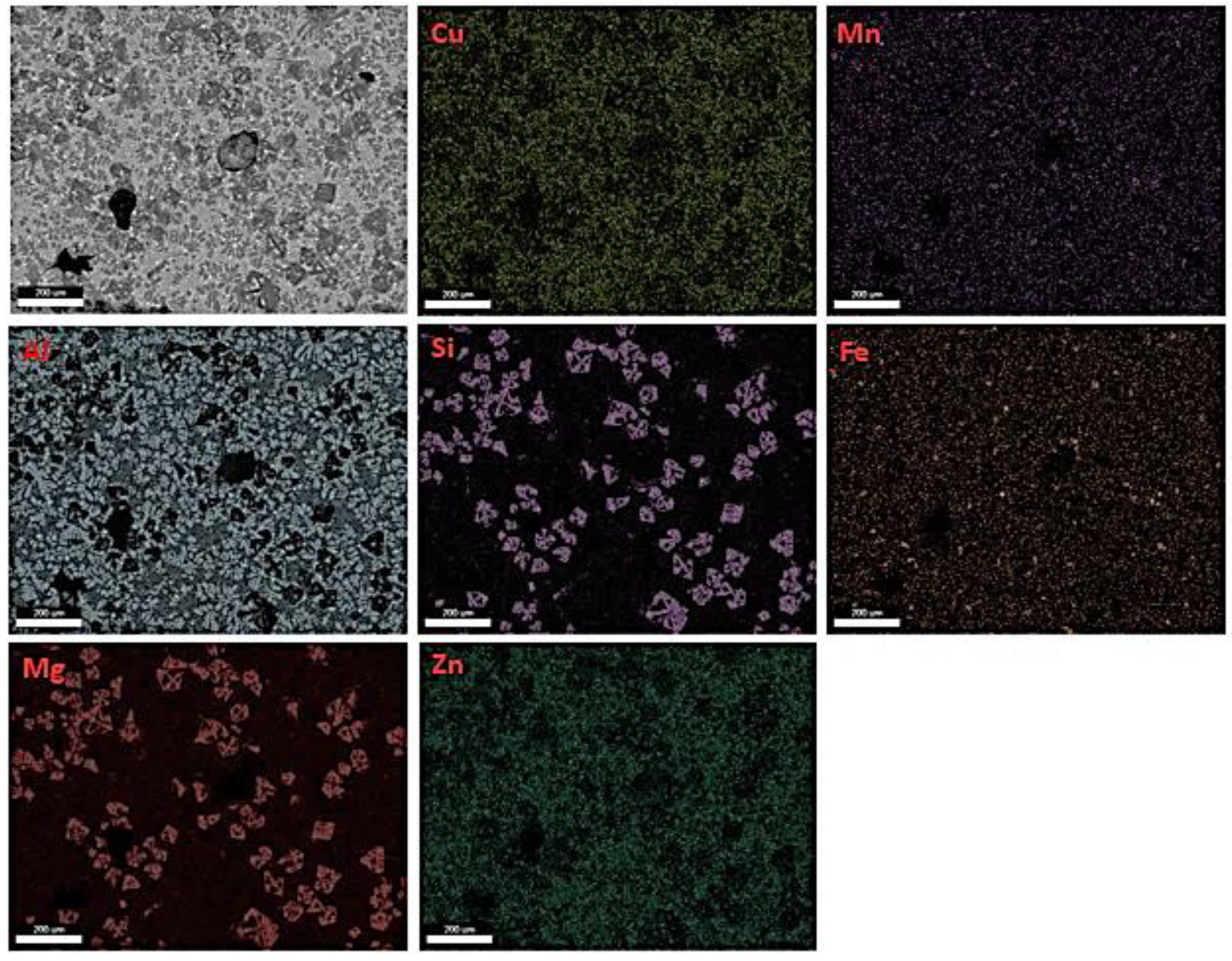
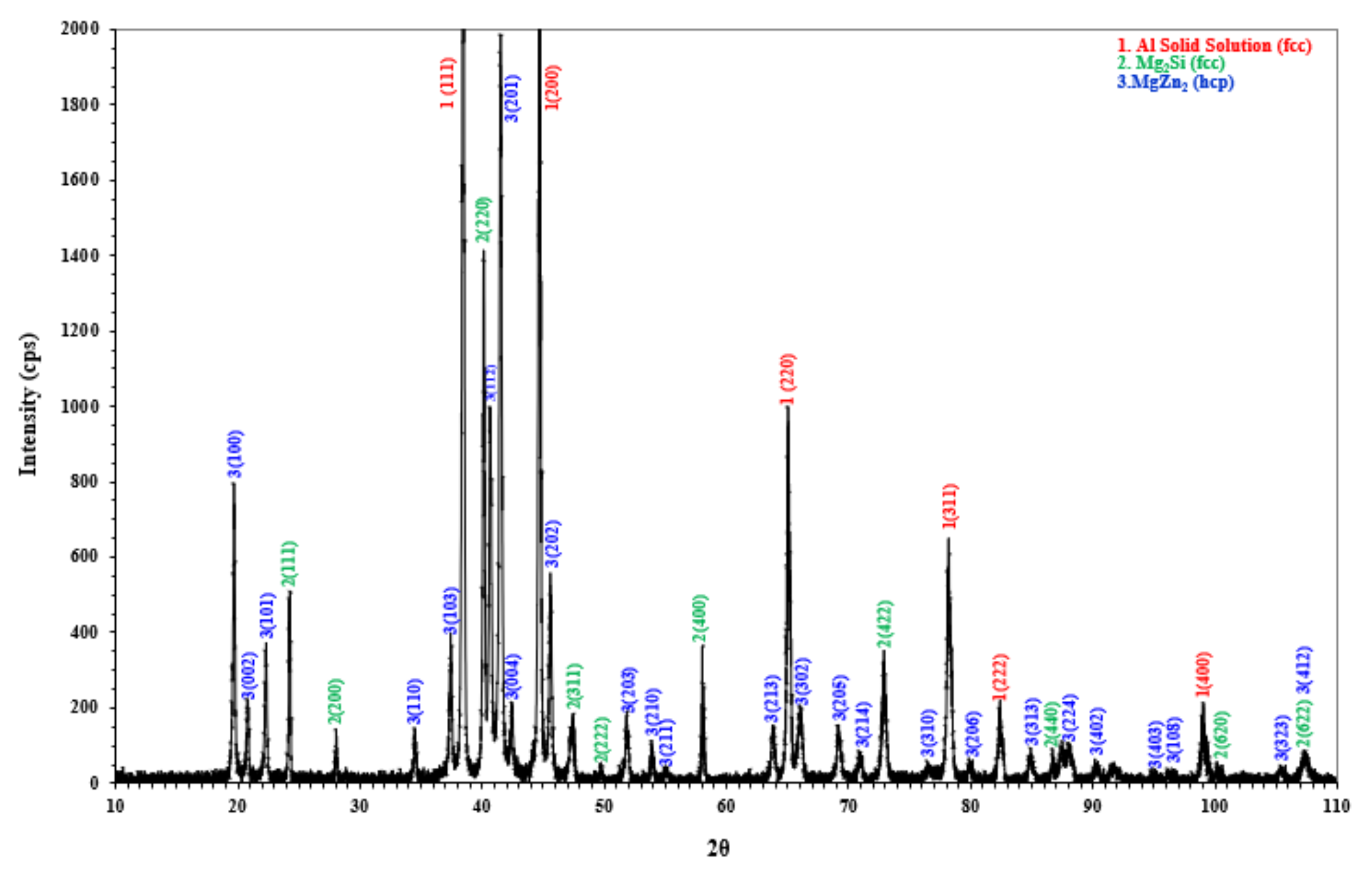
3.3. Microstructural Characterization and X-ray Diffraction Analysis
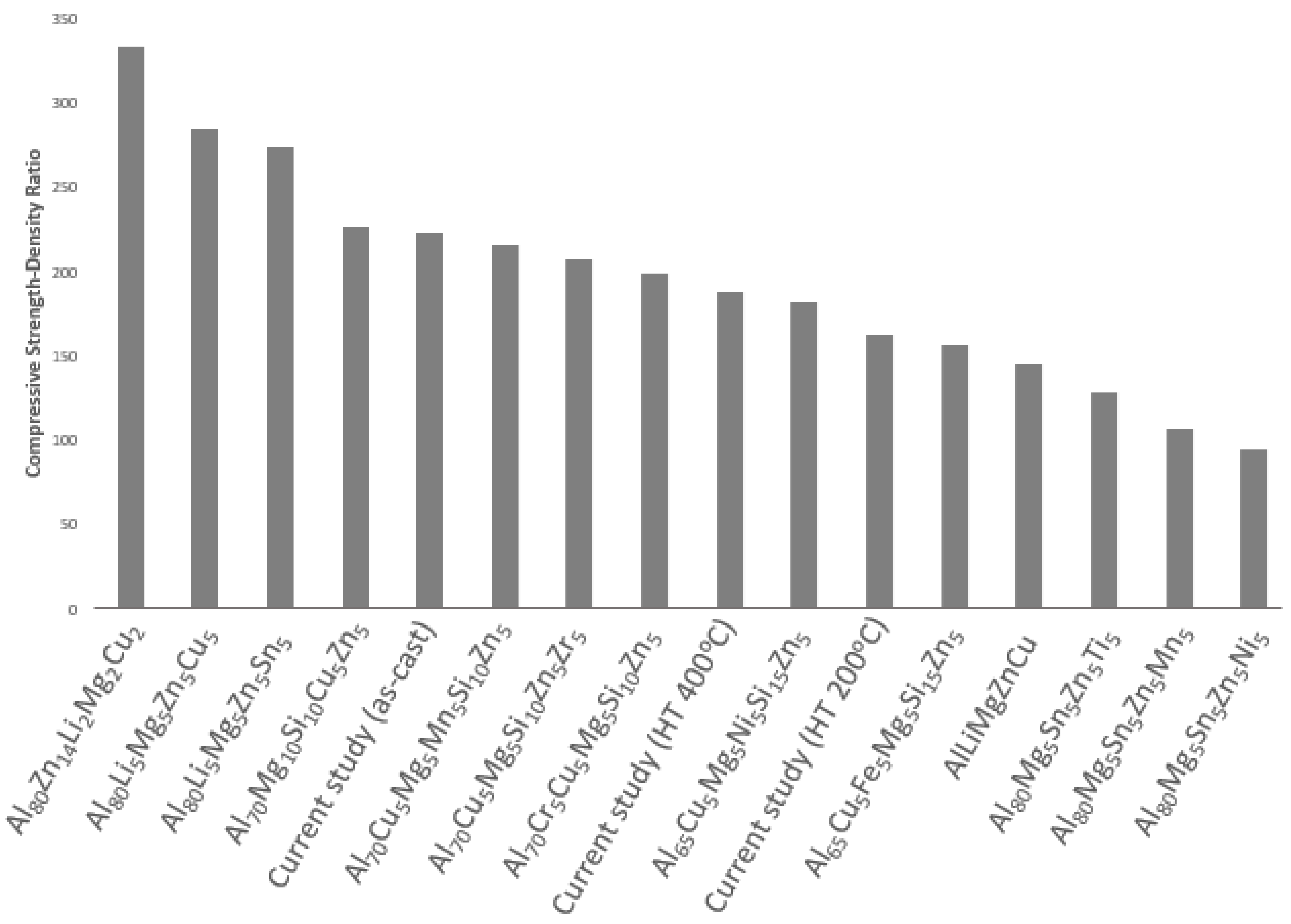
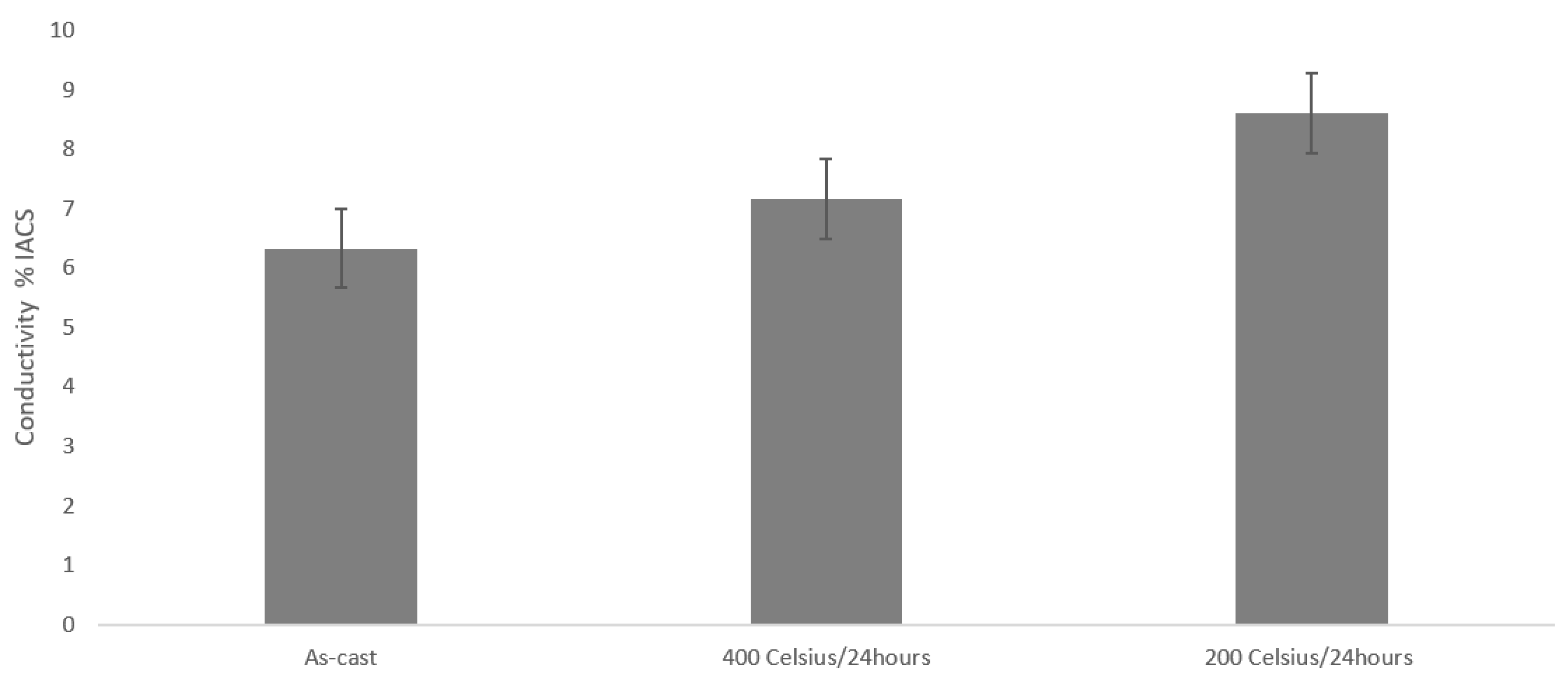
3.4. Mechanical & Physical Properties
4. Conclusions
- There is consistency of CALPHAD and thermo-physical parameters calculations regarding the major Al-base and Mg2Si and C14 Laves. CALPHAD approach overestimates the stability of S-phase and Q-phase.
- Scanning electron microscopy and X-ray diffraction analysis confirm the formation of three major phases.
- Mechanical properties achieved are 588 MPa compressive strength for the as-cast specimen, 495 MPa and 426 MPa for heat-treated at 400 °C and 200 °C respectively. Hardness for the as-cast specimen, heat-treated at 400 °C and 200 °C is 249, 200 and 171 Vickers respectively.
- Heat-treatment leads to decrease of hardness and compressive strength and an increase of electrical conductivity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cantor, B. Multicomponent and High Entropy Alloys. Entropy 2014, 16, 4749–4768. [Google Scholar] [CrossRef] [Green Version]
- Yeh, J.W. Recent progress in high-entropy alloys. Ann. Chim. Sci. Mater. 2006, 31, 633–648. [Google Scholar] [CrossRef]
- Gorsse, S.; Couzinié, J.P.; Miracle, D.B. From high-entropy alloys to complex concentrated alloys. Comptes Rendus Phys. 2018, 19, 721–736. [Google Scholar] [CrossRef]
- Sanchez, J.M.; Vicario, I.; Albizuri, J.; Guraya, T.; Garcia, J.C. Phase prediction, microstructure and high hardness of novel light-weight high entropy alloys. J. Mater. Res. Technol. 2019, 8, 795–803. [Google Scholar] [CrossRef]
- Sanchez, J.M.; Vicario, I.; Albizuri, J.; Guraya, T.; Acuña, E.M. Design, Microstructure and Mechanical Properties of Cast Medium Entropy Aluminium Alloys. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Oh, H.S.; Odbadrakh, K.; Ikeda, Y.; Mu, S.; Körmann, F.; Sun, C.-J.; Ahn, H.S.; Yoon, K.N.; Ma, D.; Tasan, C.C.; et al. Element-resolved local lattice distortion in complex concentrated alloys: An observable signature of electronic effects. Acta Mater. 2021, 216, 117135. [Google Scholar] [CrossRef]
- Gao, M.C.; Liaw, P.K.; Yeh, J.W.; Zhang, Y. High-Entropy Alloys: Fundamentals and Applications; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Jablonski, P.D.; Licavoli, J.J.; Gao, M.C.; Hawk, J.A. Manufacturing of High Entropy Alloys. JOM 2015, 67, 2278–2287. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, M. An insight into evolution of light weight high entropy alloys: A review. Metals 2016, 6, 199. [Google Scholar] [CrossRef] [Green Version]
- Maulik, O.; Kumar, D.; Kumar, S.; Dewangan, S.K.; Kumar, V. Structure and properties of lightweight high entropy alloys: A brief review. Mater. Res. Express 2018, 5, 052001. [Google Scholar] [CrossRef]
- Tsai, M.-H. Three Strategies for the Design of Advanced High-Entropy Alloys. Entropy 2016, 18, 252. [Google Scholar] [CrossRef]
- High Entropy Alloys: Innovations, Advances, and Applications, 1st ed; Srivatsan, T.S.; Gupta, M. (Eds.) CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Barnett, M.R.; Senadeera, M.; Fabijanic, D.; Shamlaye, K.F.; Joseph, J.; Kada, S.R.; Rana, S.; Gupta, S. Venkatesh, S. A scrap-tolerant alloying concept based on high entropy alloys. Acta Mater. 2020, 200, 735–744. [Google Scholar] [CrossRef]
- Mitrica, D.; Badea, I.C.; Serban, B.A.; Olaru, M.T.; Vonica, D.; Burada, M.; Piticescu, R.-R.; Popov, V.V. Complex Concentrated Alloys for Substitution of Critical Raw Materials in Applications for Extreme Conditions. Materials 2021, 14, 1197. [Google Scholar] [CrossRef] [PubMed]
- Chaskis, S.; Bouzouni, M.; Gavalas, E.; Loukadakis, V.; Papaefthymiou, S. Development of Complex Concentrated Alloys (CCAs) Utilizing Scrap to Preserve Critical Raw Materials. Mater. Proc. 2021, 5, 5109. [Google Scholar] [CrossRef]
- Kaufman, J.G.; Rooy, E.L. Aluminum Alloy Castings: Properties, Processes, and Applications; ASM International: Russell Township, OH, USA, 2004. [Google Scholar]
- Youssef, K.M.; Zaddach, A.J.; Niu, C.; Irving, D.L.; Koch, C.C. A novel low-density, high-hardness, high-entropy alloy with close-packed single-phase nanocrystalline structures. Mater. Res. Lett. 2014, 3, 95–99. [Google Scholar] [CrossRef] [Green Version]
- Tseng, K.K.; Yang, Y.C.; Juan, C.C.; Chin, T.S.; Tsai, C.W.; Yeh, J.W. A light-weight high-entropy alloy Al20Be20Fe10Si15Ti35. Sci. China Technol. Sci. 2017, 61, 184–188. [Google Scholar] [CrossRef]
- Gobernik, A.; Lemay, C.M.; Haddad, J.G. Modelling and Testing Aluminum Based High Entropy Alloys. 2018. Available online: https://digital.wpi.edu/concern/student_works/nv9354436?locale=en. (accessed on 25 January 2022).
- Li, R.; Ren, Z.; Wu, Y.; He, Z.; Liaw, P.K.; Ren, J.; Zhang, Y. Mechanical behaviors and precipitation transformation of the lightweight high-Zn-content Al–Zn–Li–Mg–Cu alloy. Mater. Sci. Eng. A 2021, 802, 140637. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y. Light-Weight and Flexible High-Entropy Alloys In Engineering Steels High Entropy-Alloys; Books on Demand: Norderstadt, Germany, 2019. [Google Scholar] [CrossRef] [Green Version]
- Gondhalekar, A.A. Design and Development of Light Weight High Entropy Alloys. 2019. Available online: http://urn.kb.se/resolve?urn=urn:nbn:se:hj:diva-45551. (accessed on 25 January 2022).
- Yang, X.; Chen, S.Y.; Cotton, J.D.; Zhang, Y. Phase Stability of Low-Density, Multiprincipal Component Alloys Containing Aluminum, Magnesium, and Lithium. JOM 2014, 66, 2009–2020. [Google Scholar] [CrossRef]
- Zhang, B.; Liaw, P.K.; Brechtl, J.; Ren, J.; Guo, X.; Zhang, Y. Effects of Cu and Zn on microstructures and mechanical behavior of the medium-entropy aluminum alloy. J. Alloys Compd. 2020, 820, 153092. [Google Scholar] [CrossRef]
- Li, Y.; Li, R.; Zhang, Y. Effects of Si Addition on Microstructure, Properties and Serration Behaviors of Lightweight Al-Mg-Zn-Cu Medium-entropy Alloys. Res. Appl. Mater. Sci. 2019, 1, 7–13. [Google Scholar] [CrossRef]
- Sanchez, J.M.; Vicario, I.; Albizuri, J.; Guraya, T.; Koval, N.E.; Garcia, J.C. Compound Formation and Microstructure of As-Cast High Entropy Aluminums. Metals 2018, 8, 167. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, J.M.; Pascual, A.; Vicario, I.; Albizuri, J.; Guraya, T.; Galarraga, H. Microstructure and Phase Formation of Novel Al80Mg5Sn5Zn5X5 Light-Weight Complex Concentrated Aluminum Alloys. Metals 2021, 11, 1944. [Google Scholar] [CrossRef]
- Baek, E.J.; Ahn, T.Y.; Jung, J.G.; Lee, J.M.; Cho, Y.R.; Euh, K. Effects of ultrasonic melt treatment and solution treatment on the microstructure and mechanical properties of low-density multicomponent Al70Mg10Si10Cu5Zn5 alloy. J. Alloys Compd. 2017, 696, 450–459. [Google Scholar] [CrossRef]
- Nagase, T.; Terayama, A.; Nagaoka, T.; Fuyama, N.; Sakamoto, T. Alloy Design and Fabrication of Ingots of Al–Mg–Li–Ca Light-Weight Medium Entropy Alloys. Mater. Trans. 2020, 61, 1369–1380. [Google Scholar]
- Miracle, D.B.; Miller, J.D.; Senkov, O.N.; Woodward, C.; Uchic, M.D.; Tiley, J. Exploration and development of high entropy alloys for structural applications. Entropy 2014, 16, 494–525. [Google Scholar] [CrossRef]
- Subedi, U.; Kunwar, A.; Coutinho, Y.A.; Gyanwali, K. pyMPEALab Toolkit for Accelerating Phase Design in Multi-principal Element Alloys. Met. Mater. Int. 2022, 28, 269–281. [Google Scholar] [CrossRef]
- Computational Materials Engineering—Thermo-Calc Software. Available online: https://thermocalc.com/ (accessed on 17 January 2022).
- Yeh, J.W. Alloy Design Strategies and Future Trends in High-Entropy Alloys. JOM 2013, 65, 1759–1771. [Google Scholar] [CrossRef]
- Ding, L.; Jia, Z.; Nie, J.-F.; Weng, Y.; Cao, L.; Chen, H.; Wu, X.; Liu, Q. The structural and compositional evolution of precipitates in Al-Mg-Si-Cu alloy. Acta Mater. 2018, 145, 437–450. [Google Scholar] [CrossRef]
- Mohamed, A.M.A.; Samuel, F.H.; Kahtani, S.A. Microstructure, tensile properties and fracture behavior of high temperature Al-Si-Mg-Cu cast alloys. Mater. Sci. Eng. A 2013, 577, 64–72. [Google Scholar] [CrossRef]
- Li, C.; Wu, Y.Y.; Li, H.; Liu, X.F. Morphological evolution and growth mechanism of primary Mg2Si phase in Al-Mg2Si alloys. Acta Mater. 2011, 59, 1058–1067. [Google Scholar] [CrossRef]
- Li, L.; Ji, S.; Zhu, Q.; Wang, Y.; Dong, X.; Yang, W.; Midson, S.; Kang, Y. Effect of Zn Concentration on the Microstructure and Mechanical Properties of Al-Mg-Si-Zn Alloys Processed by Gravity Die Casting. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2018, 49, 3247–3256. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Yang, H.; Dong, X.; Ji, S. The effects of varying Mg and Si levels on the microstructural inhomogeneity and eutectic Mg2Si morphology in die-cast Al–Mg–Si alloys. J. Mater. Sci. 2019, 54, 5773–5787. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulou, S.; Kontopoulou, A.; Gavalas, E.; Papaefthymiou, S. The effects of reduction and thermal treatment on the recrystallization and crystallographic texture evolution of 5182 aluminum alloy. Metals 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Lervik, A.; Marioara, C.D.; Kadanik, M.; Walmsley, J.C.; Milkereit, B.; Holmestad, R. Precipitation in an extruded AA7003 aluminium alloy: Observations of 6xxx-type hardening phases. Mater. Des. 2020, 186, 108204. [Google Scholar] [CrossRef]
- Ohmori, Y.; Doan, L.C.; Matsuura, Y.; Kobayashi, S.; Nakai, K. Morphology and crystallography of β-Mg2Si precipitation in Al-Mg-Si alloys. Mater. Trans. 2001, 42, 2576–2583. [Google Scholar] [CrossRef] [Green Version]
- Brunet, M.; Malard, B.; Ratel-Ramond, N.; Deshayes, C.; Joulié, S.; Warot-Fonrose, B.; Sciau, P.; Douin, J.; Geuser, F.D.; Deschamps, A. Precipitation in original Duralumin A-U4G versus modern 2017A alloy. Materialia 2019, 8, 100429. [Google Scholar] [CrossRef]
- Zupanič, F.; Steinacher, M.; Žist, S.; Bončina, T. Microstructure and properties of a novel Al-Mg-Si alloy aa 6086. Metals 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Weatherly, G.C.; Perovic, A.; Perovic, D.D.; Mukhopadhyay, N.K.; Lloyd, D.J. The precipitation of the Q phase in an AA6111 alloy. Metall. Mater. Trans. A 2001, 32, 213–218. [Google Scholar] [CrossRef]
- Ding, L.; Orekhov, A.; Weng, Y.; Jia, Z.; Idrissi, H.; Schryvers, D.; Muraishi, S.; Hao, L.; Liu, Q. Study of the Q′ (Q)-phase precipitation in Al–Mg–Si–Cu alloys by quantification of atomic-resolution transmission electron microscopy images and atom probe tomography. J. Mater. Sci. 2019, 54, 7943–7952. [Google Scholar] [CrossRef]
- Bobel, A.; Kim, K.; Wolverton, C.; Walker, M.; Olson, G.B. Equilibrium composition variation of Q-phase precipitates in aluminum alloys. Acta Mater. 2017, 138, 150–160. [Google Scholar] [CrossRef]
- Fiawoo, M.; Gao, X.; Bourgeois, L.; Parson, N.; Zhang, X.Q.; Couper, M.; Nie, J.F. Formation of multiple orientation relationships of Q precipitates in Al-Mg-Si-Cu alloys. Scr. Mater. 2014, 88, 53–56. [Google Scholar] [CrossRef]
- Andersen, S.J.; Marioara, C.D.; Friis, J.; Wenner, S.; Holmestad, R. Precipitates in aluminium alloys. Adv. Phys. X 2018, 3, 790–814. [Google Scholar] [CrossRef]
- Li, M.; Sun, Y.; Li, C.; Dong, J.; Yu, L.; Liu, Y. Effect of Cu addition on precipitation and age-hardening response of an Al-15%Mg2Si alloy. Mater. Charact. 2020, 169, 110611. [Google Scholar] [CrossRef]
- Thermo-Calc Software. TCS Al-based Alloy Database (TCAL7). 2020. Available online: https://www.engineering-eye.com/THERMOCALC/details/db/pdf/thermo-calc/02/TCAL7_technical_info.pdf (accessed on 25 April 2022).
- Wang, S.C.; Starink, M.J. Precipitates and intermetallic phases in precipitation hardening Al–Cu–Mg–(Li) based alloys. Int. Mater. Rev. 2013, 50, 193–215. [Google Scholar] [CrossRef] [Green Version]
- Krakow, R.; Johnstone, D.N.; Eggeman, A.S.; Hünert, D.; Hardy, M.C.; Rae, C.M.F.; Midgley, P.A. On the crystallography and composition of topologically close-packed phases in ATI 718Plus®. Acta Mater. 2017, 130, 271–280. [Google Scholar] [CrossRef]
- Aluminum 308.0-F, Permanent Mold Cast. Available online: http://www.matweb.com/search/DataSheet.aspx?MatGUID=d3ee2f7bb6e64391a16cdd15eb3f932e (accessed on 23 February 2022).
- Aluminum 707.0-T7, Permanent Mold Cast. Available online: http://www.matweb.com/search/DataSheet.aspx?MatGUID=cfce98401b7d449e911ca539ef72b312 (accessed on 23 February 2022).


| Alloying Elements | Melting Temperature (°C) | Boiling Temperature (°C) | Density (g/cm3) |
|---|---|---|---|
| Al | 660.3 | 2470 | 2.7 |
| Mg | 650 | 1091 | 1.738 |
| Si | 1410 | 2355 | 2.33 |
| Cu | 1085 | 2562 | 8.96 |
| Zn | 419.5 | 907 | 7.133 |
| AlSi12.5 | 577 | - | - |
| AlCu35 | 548.2 | - | - |
| ΔHmix (kJ/mol) | δ (%) | ΔSmix (J/K/mol) | Ω | Δχ | VEC | Τm (K) | ρtheoretical (g/cm3) |
|---|---|---|---|---|---|---|---|
| −4.94 | 7.64 | 10.1 | 2 | 0.16 | 4.37 | 976.06 | 2.63 |
| Phases | Chemical Composition (Mole Percent) | Lattice Type | Lattice Parameters(Å) | Symmetry/Space Group |
|---|---|---|---|---|
| FCC-A1 | Al0.99 | FCC | a = 4.05 | m |
| Mg2Si-C1 | Mg0.66Si0.33 | FCC | a = 6.351 | m |
| C14-laves | Zn0.57Mg0.33 | HCP | a = 4.9 c = 7.8 | /mmc |
| S-phase | Cu0.25Al0.5Mg0.25 | Orthorhombic | a = 4.01 b = 9.23 c = 7.14 | Cmcm |
| Q-phase | Mg0.38Si0.29Al0.24Cu0.09 | HCP | a = 10.39 c = 4.02 |
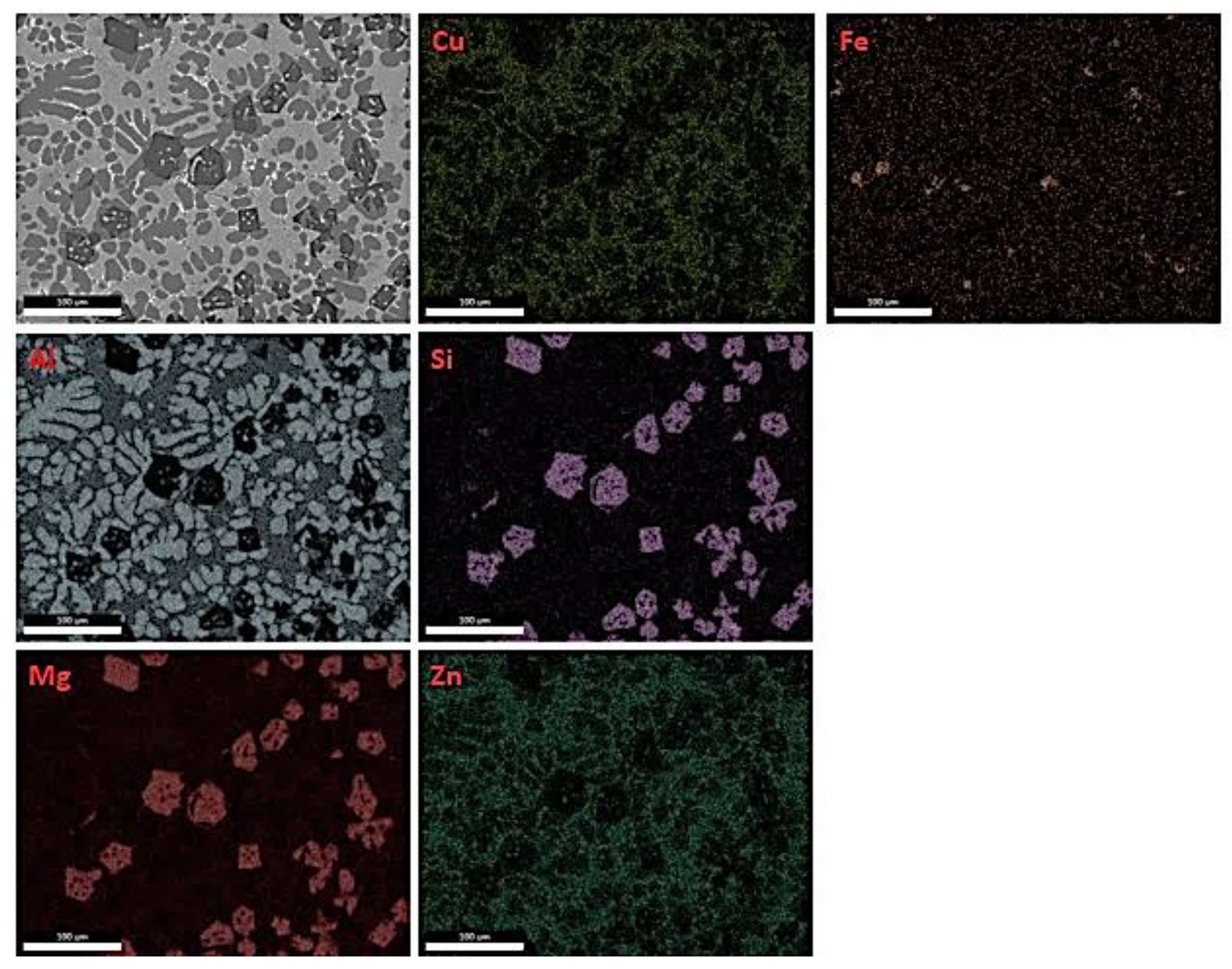
| Alloying Elements | Al | Mg | Zn | Cu | Si |
|---|---|---|---|---|---|
| Nominal (at.%) | 58 | 18 | 12 | 5 | 7 |
| Full Area (at.%) | 58.2 | 18.5 | 12.8 | 4.5 | 6.0 |
| Full Area (wt.%) | 47.4 | 13.6 | 25.3 | 8.6 | 5.1 |
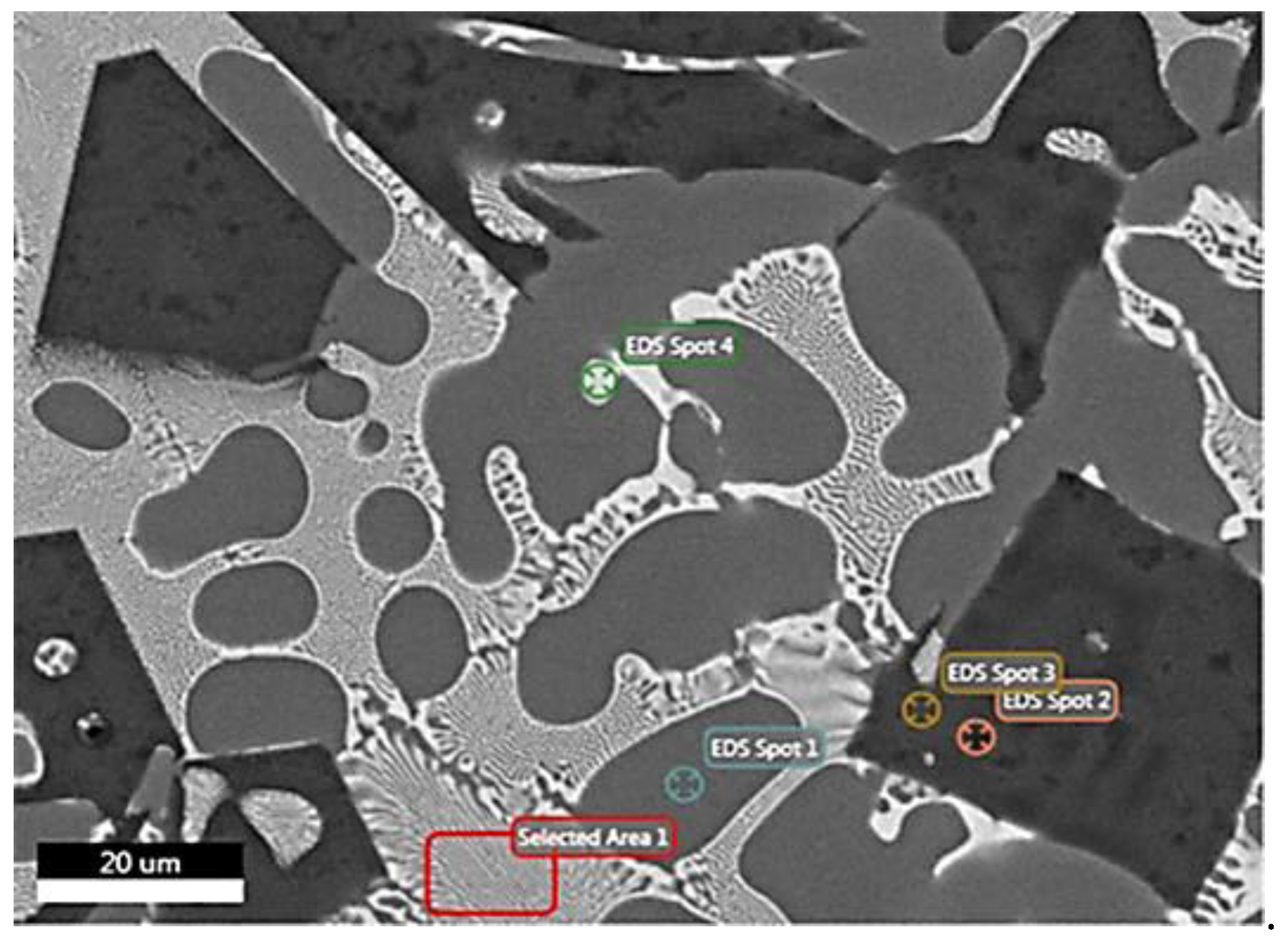
| Analysis/ Elements | Spot 1 (at.%) | Spot 1 (wt.%) | Spot 2 (at.%) | Spot 2 (wt.%) | Spot 3 (at.%) | Spot 3 (wt.%) | Spot 4 (at.%) | Spot 4 (wt.%) | Area 1 (at.%) | Area 1 (wt.%) |
|---|---|---|---|---|---|---|---|---|---|---|
| O | - | - | 0.4 | 0.2 | 0.2 | 0.1 | - | - | - | - |
| Mg | 3.8 | 3.2 | 67.1 | 62.8 | 66 | 61 | 19.3 | 9.8 | 13.6 | 8.2 |
| Al | 89.9 | 82.8 | - | - | 1.3 | 1.3 | 24.2 | 13.6 | 49.7 | 33 |
| Si | - | - | 31.2 | 33.7 | 30.4 | 32.5 | - | - | - | - |
| Cu | 0.9 | 2 | 0.3 | 0.7 | 0.6 | 1.4 | 15 | 19.9 | 10.3 | 16.2 |
| Zn | 5.4 | 12 | 1 | 2.6 | 1.5 | 3.7 | 41.5 | 56.7 | 26.4 | 42.6 |
| Elements/Phases | Al-Base (at.%) | Mg2Si (at.%) | Eutectic (at.%) |
|---|---|---|---|
| Mg | 3.5 ± 1.1 | 68 ± 2.3 | 14.4 ± 1.4 |
| Al | 90 ± 2 | - | 48.1 ± 1.8 |
| Si | - | 30.8 ± 1.9 | - |
| Cu | 1 ± 0.2 | 0.3 ± 0.3 | 9.6 ± 1.6 |
| Zn | 5.5 ± 1.9 | 0.9 ± 0.2 | 27.9 ± 1.7 |
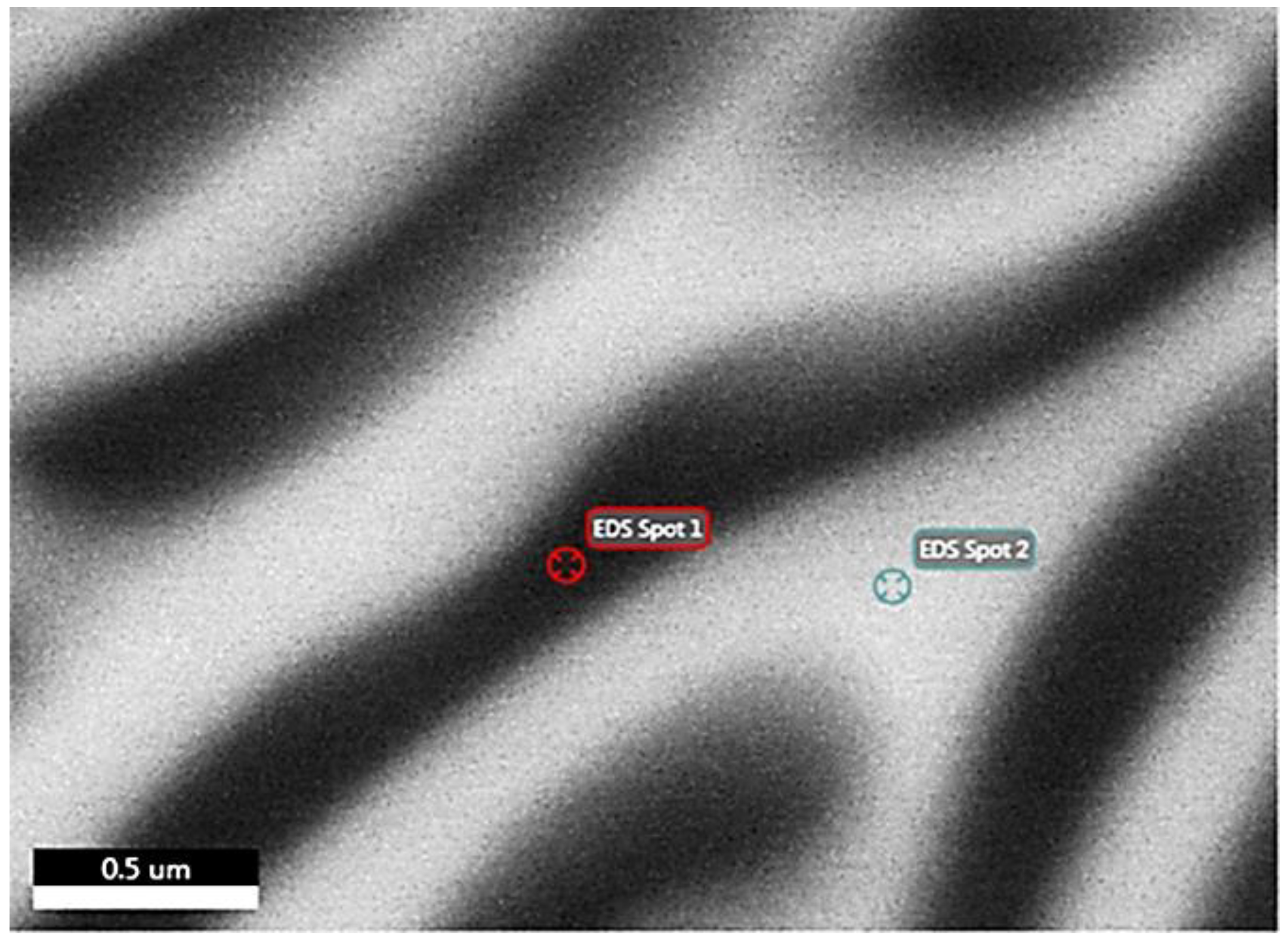
| Analysis/ Elements | Spot 1 (at.%) | Spot 1 (wt.%) | Spot 2 (at.%) | Spot 2 (wt.%) |
|---|---|---|---|---|
| Mg | 9.4 | 5.8 | 16.1 | 9.3 |
| Al | 56.4 | 38.4 | 43.5 | 28.1 |
| Cu | 9.2 | 14.7 | 11.5 | 17.5 |
| Zn | 24.9 | 41 | 28.9 | 45.1 |
| Si | 0.1 | 0.1 | - | - |
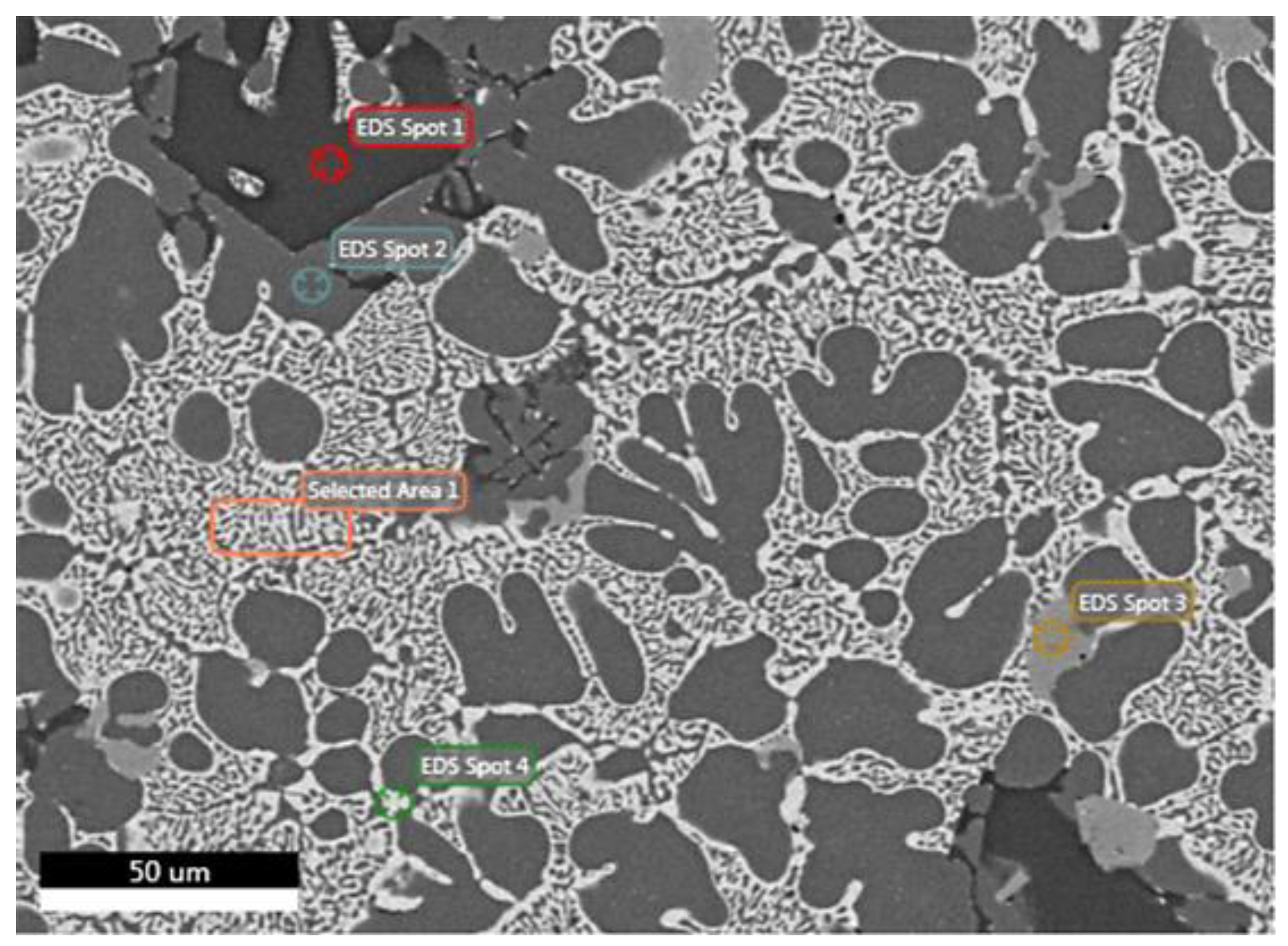
| Analysis/ Elements | Spot 1 (at.%) | Spot 1 (wt.%) | Spot 2 (at.%) | Spot 2 (wt.%) | Spot 3 (at.%) | Spot 3 (wt.%) | Spot 4 (at.%) | Spot 4 (wt.%) | Area 1 (at.%) | Area 1 (wt.%) |
|---|---|---|---|---|---|---|---|---|---|---|
| O | 1 | 0.6 | - | - | - | - | - | - | - | - |
| Mg | 60.8 | 57.1 | 2.9 | 2.5 | 2.7 | 2.2 | 25.8 | 15.5 | 10.8 | 6 |
| Al | 0.1 | 0.1 | 93.6 | 89.4 | 81.2 | 73.4 | 36.6 | 24.4 | 45.1 | 28 |
| Si | 37.5 | 40.7 | - | - | 7.1 | 6.7 | - | - | - | - |
| Mn | - | - | - | - | 1.9 | 3.5 | - | - | - | - |
| Fe | - | - | - | - | 3.9 | 7.3 | - | - | - | - |
| Cu | 0.2 | 0.5 | 0.5 | 1.1 | 2.2 | 4.7 | 10.4 | 16.3 | 11.8 | 17,4 |
| Zn | 0.4 | 1 | 3 | 7 | 1 | 2.2 | 27.2 | 43.8 | 32.3 | 48.6 |
| Elements/Phases | Mg2Si (at.%) | Al-Base (at.%) | Eutectic (at.%) |
|---|---|---|---|
| Mg | 61.7 ± 2.3 | 2.3 ± 1.4 | 11 ± 1 |
| Al | 0.1 ± 0 | 95.4 ± 2.1 | 44.2 ± 1.8 |
| Si | 37.6 ± 2.1 | - | - |
| Cu | 0.3 ±0.1 | 0.4 ± 0.2 | 10.7 ± 1.6 |
| Zn | 0.3 ± 0.1 | 1.9 ± 1.6 | 34.1 ± 1.9 |
| Specimen | Hardness (HV0.2) | σmax (MPa) |
|---|---|---|
| 1M | 249 ± 14 | 588 ± 3 |
| 1MH | 200 ± 11 | 495 ± 8 |
| 2M | 258 ± 11 | - |
| 2MH | 208 ± 15 | - |
| 1MH’ | 171 ± 7 | 426 ± 9 |
| Alloy | Density (g/cm3) | Hardness Vickers | Ratio (Hardness/Density) |
|---|---|---|---|
| UNS A03080 | 2.79 | 80 | 28.7 |
| UNS A07070 | 2.77 | 107 | 38.6 |
| Current study (as-cast) | 2.63 | 249 | 94.7 |
| Current study (heat-treated 400 °C) | 2.63 | 200 | 76 |
| Current study (heat-treated 200 °C) | 2.63 | 171 | 65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaskis, S.; Stachouli, E.; Gavalas, E.; Bouzouni, M.; Papaefthymiou, S. Microstructure, Phase Formation and Heat-Treating of Novel Cast Al-Mg-Zn-Cu-Si Lightweight Complex Concentrated Aluminum Based Alloy. Materials 2022, 15, 3169. https://doi.org/10.3390/ma15093169
Chaskis S, Stachouli E, Gavalas E, Bouzouni M, Papaefthymiou S. Microstructure, Phase Formation and Heat-Treating of Novel Cast Al-Mg-Zn-Cu-Si Lightweight Complex Concentrated Aluminum Based Alloy. Materials. 2022; 15(9):3169. https://doi.org/10.3390/ma15093169
Chicago/Turabian StyleChaskis, Spyridon, Eva Stachouli, Evangelos Gavalas, Marianthi Bouzouni, and Spyros Papaefthymiou. 2022. "Microstructure, Phase Formation and Heat-Treating of Novel Cast Al-Mg-Zn-Cu-Si Lightweight Complex Concentrated Aluminum Based Alloy" Materials 15, no. 9: 3169. https://doi.org/10.3390/ma15093169
APA StyleChaskis, S., Stachouli, E., Gavalas, E., Bouzouni, M., & Papaefthymiou, S. (2022). Microstructure, Phase Formation and Heat-Treating of Novel Cast Al-Mg-Zn-Cu-Si Lightweight Complex Concentrated Aluminum Based Alloy. Materials, 15(9), 3169. https://doi.org/10.3390/ma15093169








