High-Temperature Corrosion of Ni-Cr-Mo Cladding Layers with Different Si Contents in NaCl-KCl-Na2SO4-K2SO4 Mixed Salt Medium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Laser-Cladding Process and Preparation of Samples
2.3. High-Temperature Corrosion Experiment
2.4. Characterization Method
3. Results and Discussion
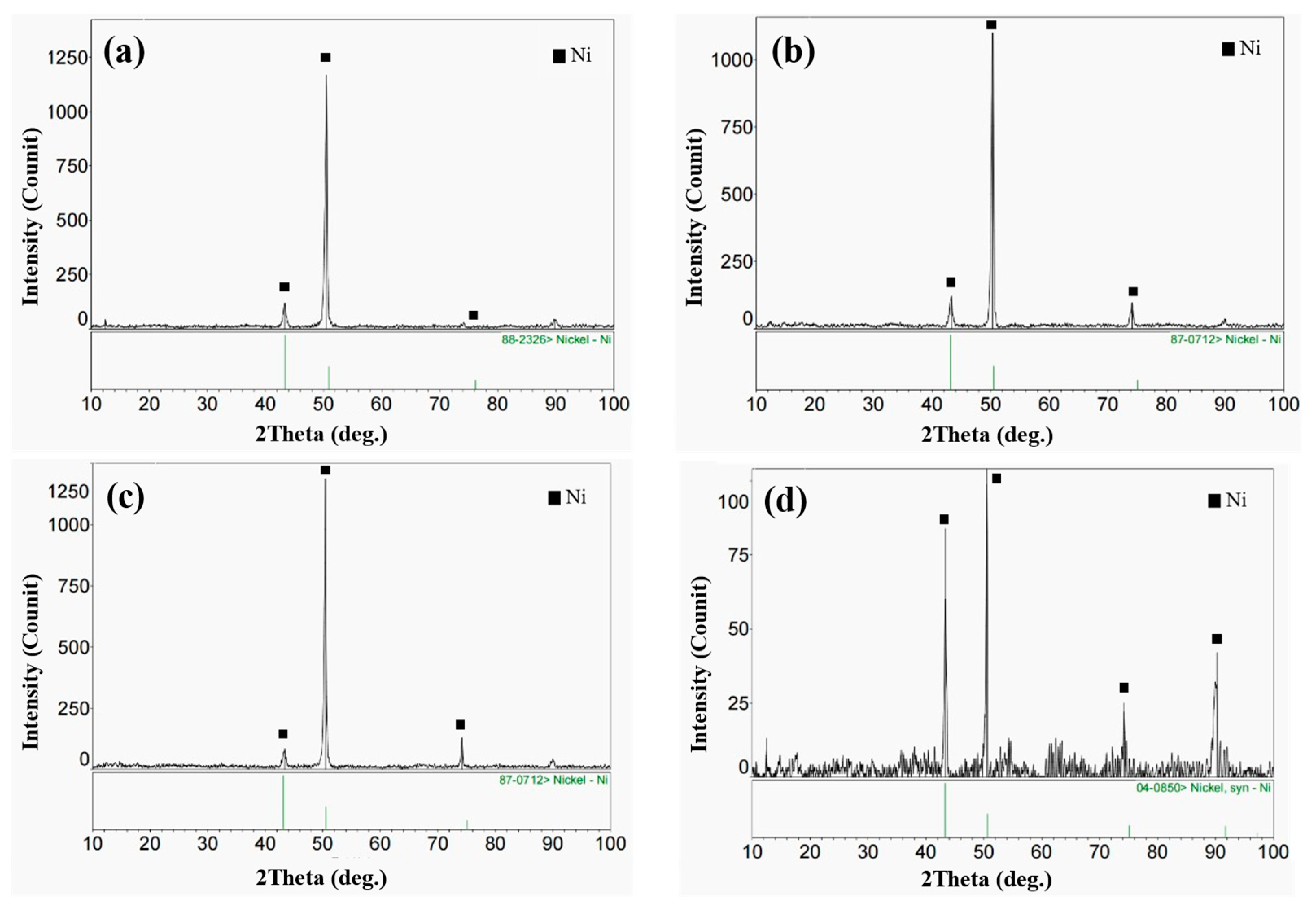
3.1. Microstructure of the Cladding Layer
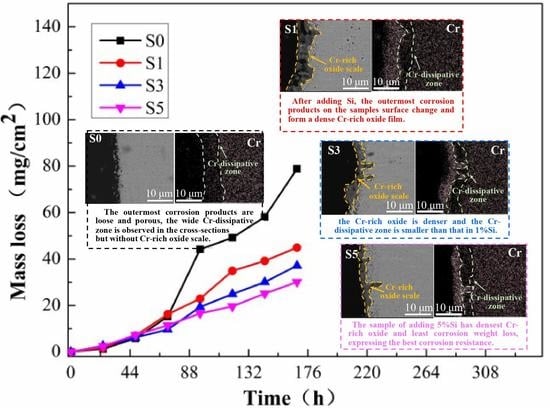
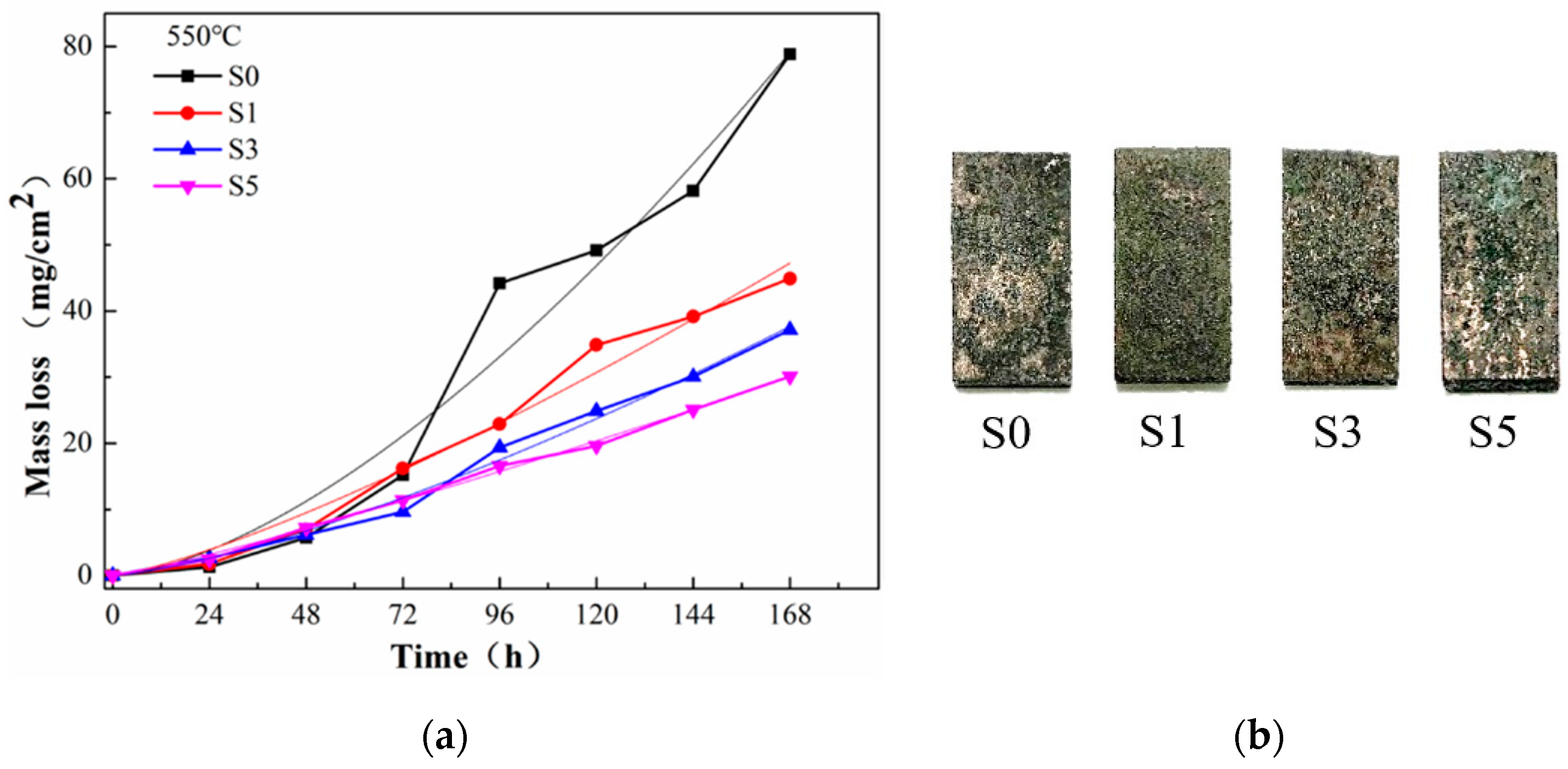
3.2. High-Temperature Corrosion Kinetics
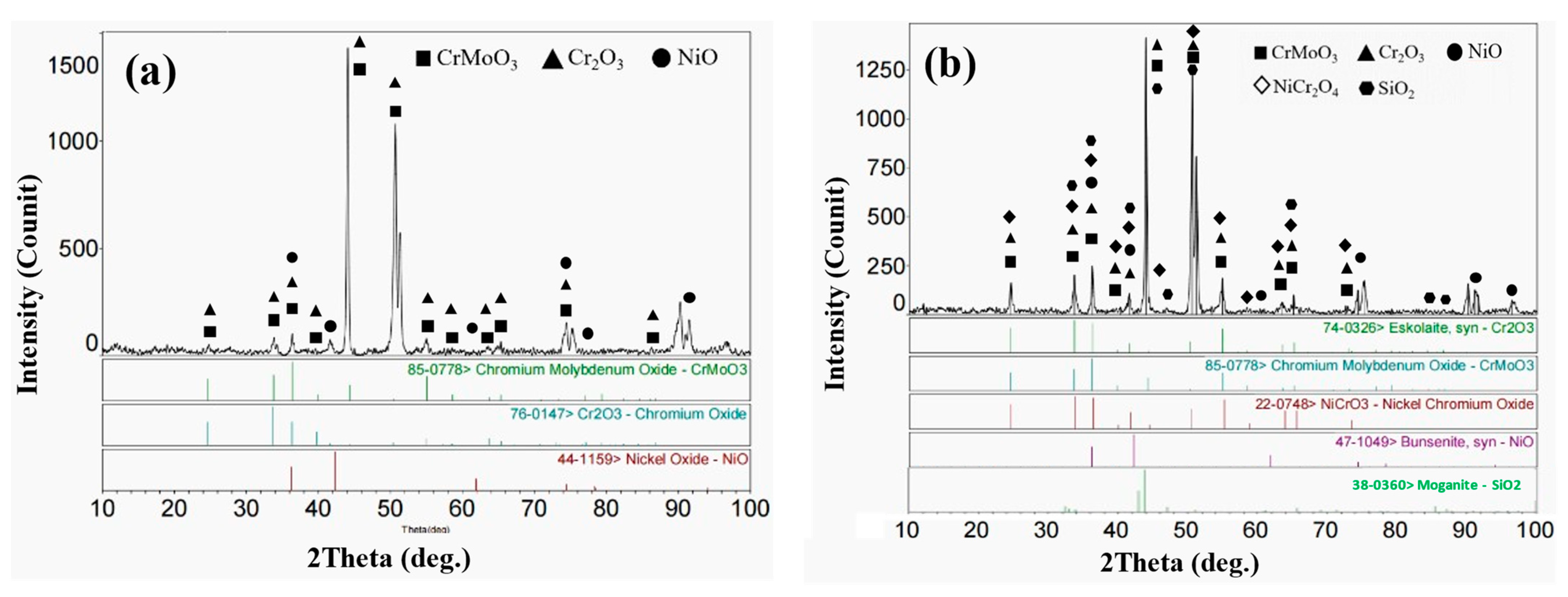
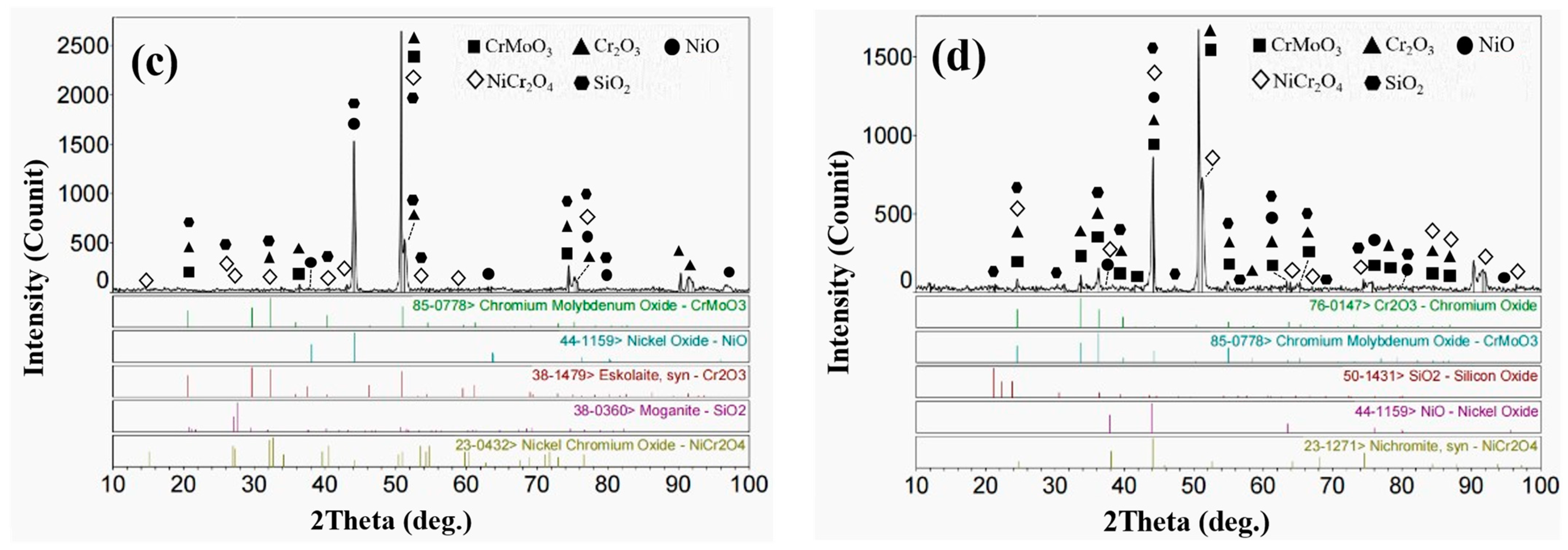
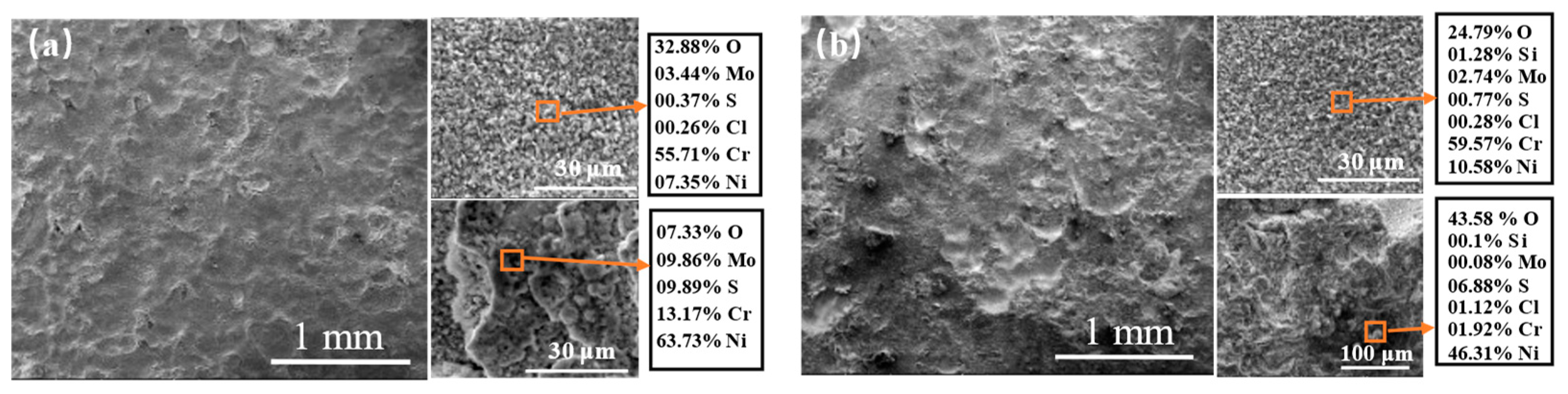
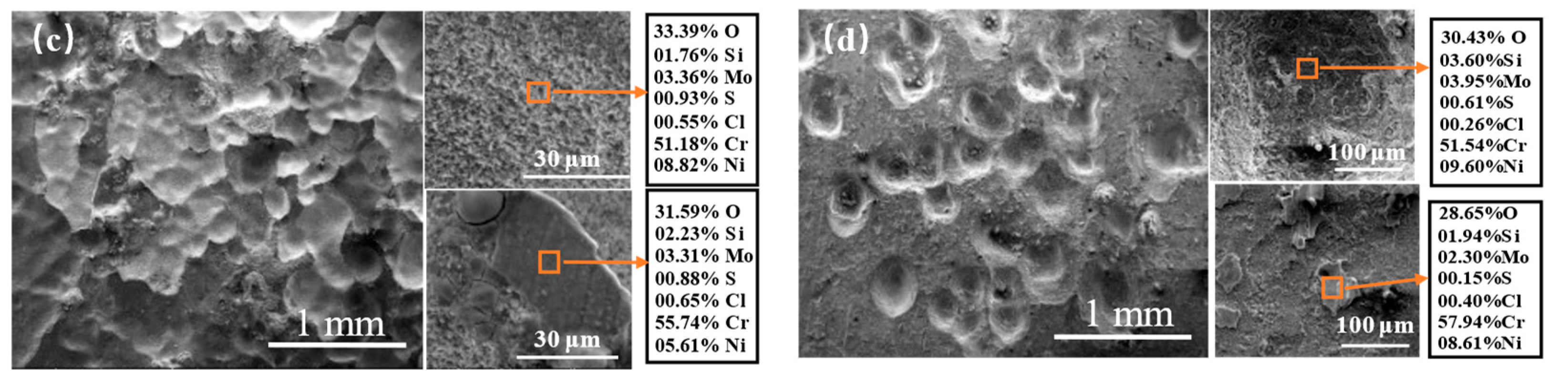
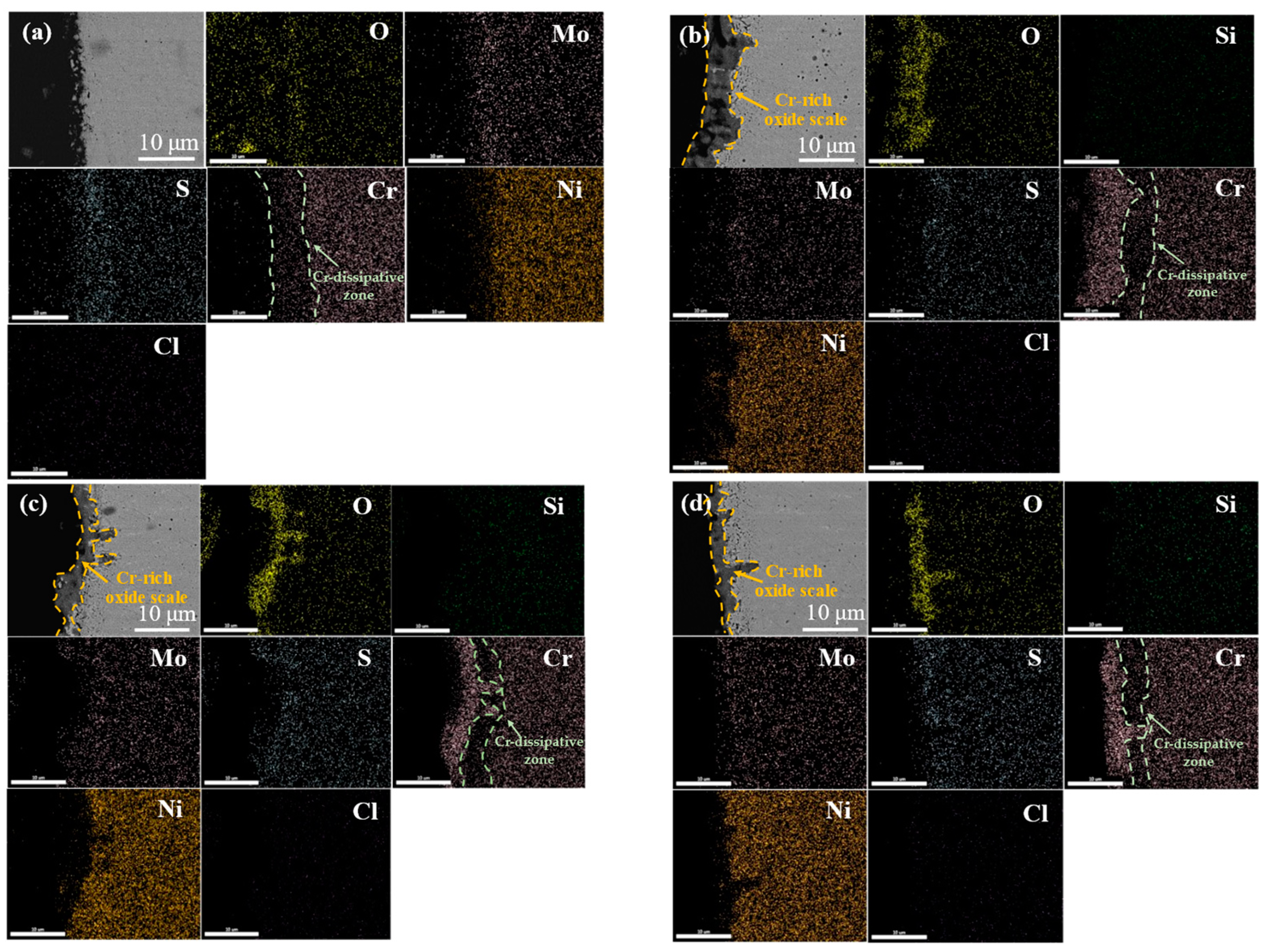
3.3. High-Temperature Corrosion Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Li, J.; Li, T.; Xu, G.; Jin, X.; Wang, M.; Liu, T. Performance assessment of a novel medical-waste-to-energy design based on plasma gasification and integrated with a municipal solid waste incineration plant. Energy 2022, 245, 123156. [Google Scholar] [CrossRef]
- Joseph, A.M.; Snellings, R.; Nielsen, P.; Matthys, S.; De Belie, N. Pre-treatment and utilisation of municipal solid waste incineration bottom ashes towards a circular economy. Constr. Build. Mater. 2020, 260, 14. [Google Scholar]
- Ma, W.C.; Wenga, T.; Frandsen, F.J.; Yan, B.B.; Chen, G.Y. The fate of chlorine during MSW incineration: Vaporization, transformation, deposition, corrosion and remedies. Prog. Energy Combust. Sci. 2020, 76, 39. [Google Scholar] [CrossRef]
- Otsuka, N. Chemistry and melting characteristics of fireside deposits taken from boiler tubes in waste incinerators. Corros. Sci. 2011, 53, 2269–2276. [Google Scholar] [CrossRef]
- Mudgal, D.; Ahuja, L.; Bhatia, D.; Singh, S.; Prakash, S. High temperature corrosion behaviour of superalloys under actual waste incinerator environment. Eng. Fail. Anal. 2016, 63, 160–171. [Google Scholar] [CrossRef]
- Qiu, Z.J.; Wu, B.T.; Wang, Z.Y.; Wexler, D.; Carpenter, K.; Zhu, H.L.; Muransky, O.; Zhang, J.R.; Li, H.J. Effects of post heat treatment on the microstructure and mechanical properties of wire arc additively manufactured Hastelloy C276 alloy. Mater. Charact. 2021, 177, 12. [Google Scholar] [CrossRef]
- Zhu, H.; Muránsky, O.; Wei, T.; Davis, J.; Budzakoska-Testone, E.; Huang, H.; Drew, M. The effect of applied stress on the high-temperature creep behaviour and microstructure of NiMoCr Hastelloy-N® alloy. Materialia 2021, 16, 101069. [Google Scholar] [CrossRef]
- Banoth, S.; Palleda, T.N.; Saito, T.; Murakami, H.; Kakehi, K. Effects of yttrium and silicon contents in Hastelloy-X built by selective laser melting process. J. Alloys Compd. 2022, 896, 18. [Google Scholar] [CrossRef]
- Liu, H.F.; Tan, C.K.I.; Meng, T.L.; Teo, S.L.; Liu, J.Y.; Cao, J.; Wei, Y.F.; Tan, D.C.C.; Lee, C.J.J.; Suwardi, A.; et al. Hot corrosion and internal spallation of laser-cladded Inconel 625 superalloy coatings in molten sulfate salts. Corros. Sci. 2021, 193, 10. [Google Scholar] [CrossRef]
- Li, X.Z.; Liu, Z.D.; Li, H.C.; Wang, Y.T.; Li, B. Investigations on the behavior of laser cladding Ni-Cr-Mo alloy coating on TP347H stainless steel tube in HCl rich environment. Surf. Coat. Technol. 2013, 232, 627–639. [Google Scholar] [CrossRef]
- Liu, S.N.; Liu, Z.D.; Wang, Y.T.; Tang, J. A comparative study on the high temperature corrosion of TP347H stainless steel, C22 alloy and laser-cladding C22 coating in molten chloride salts. Corros. Sci. 2014, 83, 396–408. [Google Scholar] [CrossRef]
- Liu, Z.D.; Liu, C.C.; Gao, Y.; Zheng, C. High Temperature Corrosion Behaviors of 20G Steel, Hastelloy C22 Alloy and C22 Laser Coating under Reducing Atmosphere with H2S. Coatings 2020, 10, 617. [Google Scholar] [CrossRef]
- Gines, M.J.L.; Benitez, G.J.; Perez, T.; Merli, E.; Firpo, M.A.; EgliI, W. Study of the picklability of 1.8 mm hot-rolled steel strip in hydrochloric acid. Lat. Am. Appl. Res. 2002, 32, 281–288. [Google Scholar]
- Abels, J.-M.; Strehblow, H.-H. A surface analytical approach to the high temperature chlorination behaviour of Inconel 600 at 700 °C. Corros. Sci. 1997, 39, 115–132. [Google Scholar] [CrossRef]
- Viklund, P.; Hjörnhede, A.; Henderson, P.; Stålenheim, A.; Pettersson, R. Corrosion of superheater materials in a waste-to-energy plant. Fuel Process. Technol. 2013, 105, 106–112. [Google Scholar] [CrossRef]
- Liu, B.; Wei, X.L.; Wang, W.L.; Lu, J.F.; Ding, J. Corrosion behavior of Ni-based alloys in molten NaCl-CaCl2-MgCl2 eutectic salt for concentrating solar power. Sol. Energy Mater. Sol. Cells 2017, 170, 77–86. [Google Scholar] [CrossRef]
- Skrifvars, B.J.; Westen-Karlsson, M.; Hupa, M.; Salmenoja, K. Corrosion of super-heater steel materials under alkali salt deposits. Part 2: SEM analyses of different steel materials. Corros. Sci. 2010, 52, 1011–1019. [Google Scholar] [CrossRef]
- Wang, S.; Wu, Y.; Ni, C.S.; Niu, Y. The effect of Si additions on the high temperature oxidation of a ternary Ni-10Cr-4Al alloy in 1 atm O2 at 1100 °C. Corros. Sci. 2009, 51, 511–517. [Google Scholar] [CrossRef]
- Wang, J.H.; Li, D.G.; Shao, T.M. Electrochemical study on the hot corrosion behavior of Ni16Cr13Co4Mo alloy in molten NaCl-KCl and NaCl-KCl-Na2SO4. Corros. Sci. 2022, 200, 110247. [Google Scholar] [CrossRef]
- Zahrani, E.M.; Alfantazi, A.M. High temperature corrosion and electrochemical behavior of INCONEL 625 weld overlay in PbSO4-Pb3O4-PbCl2-Cdo-ZnO molten salt medium. Corros. Sci. 2014, 85, 60–76. [Google Scholar] [CrossRef]
- Yun, D.W.; Seo, S.M.; Jeong, H.W.; Yoo, Y.S. The effects of the minor alloying elements Al, Si and Mn on the cyclic oxidation of Ni-Cr-W-Mo alloys. Corros. Sci. 2014, 83, 176–188. [Google Scholar] [CrossRef]


| Substrate | Element Content (wt.%) | ||||
|---|---|---|---|---|---|
| Fe | C | Cr | Mn | Si | |
| Q235 | Bal | 0.10–0.22 | ≤0.25 | 0.40–0.60 | 0.12–0.30 |
| Samples | Element Content (wt.%) | |||
|---|---|---|---|---|
| Ni | Cr | Mo | Si | |
| S0 | Bal | 24 | 13 | 0 |
| S1 | Bal | 24 | 13 | 1 |
| S3 | Bal | 24 | 13 | 3 |
| S5 | Bal | 24 | 13 | 5 |
| Sample Number and Location | Element Content (wt.%) | ||||
|---|---|---|---|---|---|
| Ni | Cr | Mo | Si | ||
| S0 | gray–black phase | 61.38 | 24.44 | 14.18 | 00.00 |
| gray–white phase | 59.72 | 23.98 | 16.30 | 00.00 | |
| S1 | gray–black phase | 61.68 | 23.36 | 13.61 | 01.35 |
| gray–white phase | 56.61 | 23.78 | 17.85 | 01.76 | |
| S3 | gray–black phase | 61.09 | 23.61 | 13.37 | 01.93 |
| gray–white phase | 56.70 | 22.48 | 17.47 | 03.35 | |
| S5 | gray–black phase | 61.54 | 23.29 | 12.42 | 02.75 |
| gray–white phase | 49.35 | 20.64 | 23.78 | 06.22 | |
| Sample | Equation | Anastomosis Degree | Derived Function | The Rate of Corrosion Change (168 h) |
|---|---|---|---|---|
| S0 | y = 0.027x1.558 | 0.94909 | y = 0.042x0.558 | 0.68 mg·cm−2·h−1 |
| S1 | y = 0.067x1.279 | 0.97504 | y = 0.085x0.279 | 0.34 mg·cm−2·h−1 |
| S3 | y = 0.033x1.372 | 0.98796 | y = 0.045x0.372 | 0.29 mg·cm−2·h−1 |
| S5 | y = 0.082x1.152 | 0.99618 | y = 0.945x0.152 | 0.20 mg·cm−2·h−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Liu, Z.; Shen, Y.; Liu, S. High-Temperature Corrosion of Ni-Cr-Mo Cladding Layers with Different Si Contents in NaCl-KCl-Na2SO4-K2SO4 Mixed Salt Medium. Materials 2022, 15, 3152. https://doi.org/10.3390/ma15093152
Chen S, Liu Z, Shen Y, Liu S. High-Temperature Corrosion of Ni-Cr-Mo Cladding Layers with Different Si Contents in NaCl-KCl-Na2SO4-K2SO4 Mixed Salt Medium. Materials. 2022; 15(9):3152. https://doi.org/10.3390/ma15093152
Chicago/Turabian StyleChen, Shanshan, Zongde Liu, Yue Shen, and Simin Liu. 2022. "High-Temperature Corrosion of Ni-Cr-Mo Cladding Layers with Different Si Contents in NaCl-KCl-Na2SO4-K2SO4 Mixed Salt Medium" Materials 15, no. 9: 3152. https://doi.org/10.3390/ma15093152
APA StyleChen, S., Liu, Z., Shen, Y., & Liu, S. (2022). High-Temperature Corrosion of Ni-Cr-Mo Cladding Layers with Different Si Contents in NaCl-KCl-Na2SO4-K2SO4 Mixed Salt Medium. Materials, 15(9), 3152. https://doi.org/10.3390/ma15093152