Study on Deterioration Law and Mechanism of Gray Brick Due to Salt Crystallization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Material
2.1.1. Gray Brick
2.1.2. Type and Concentration of Salt
- (1)
- Choice of soluble salt. The salt used in the test is classified as sodium chloride, calcium chloride and magnesium sulfate, according to the characteristic table of chemical composition of groundwater in Kaifeng City [26]. The cations in surface water are mainly Na+, Ca2+, Mg2+, accounting for 43.6%, 36.1% and 20.3% of the total content, respectively. The anions are dominated by HCO3−, accounting for 71.7% of the total, followed by SO42−, accounting for 16.8%, and the content of Cl− is the lowest, accounting for only 11.4% changes. The types of anions and cations in the groundwater are the same as those in the surface water, only their content varies.
- (2)
- Determination of salt solution concentration. According to the characteristics table of groundwater chemical components in Kaifeng City [26] and the XRF test results of salt frost collected from the surface of the north side of Kaifeng City Wall at different heights, 1%, 3%, and 5% were finally set as the test salt solution concentration values.
2.2. Cyclic Deterioration Test
2.3. Nominal Strength Test
2.4. Compressive Strength Test
3. Results and Discussion
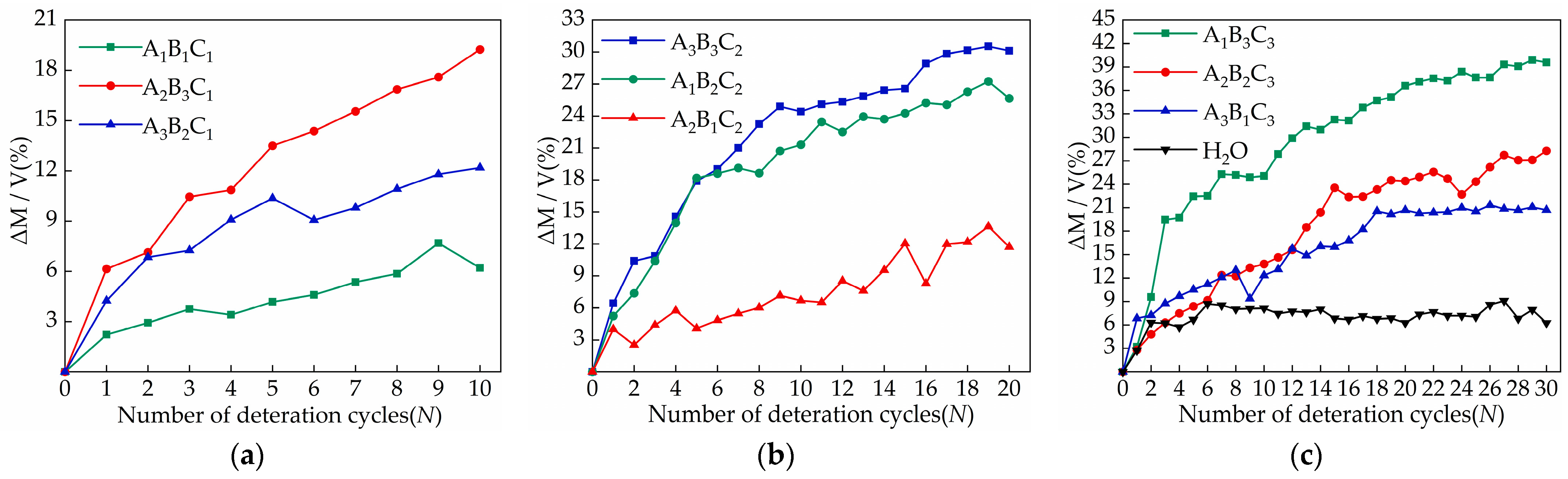
3.1. Changes in Sample Appearance and Quality Due to Salt Crystallization
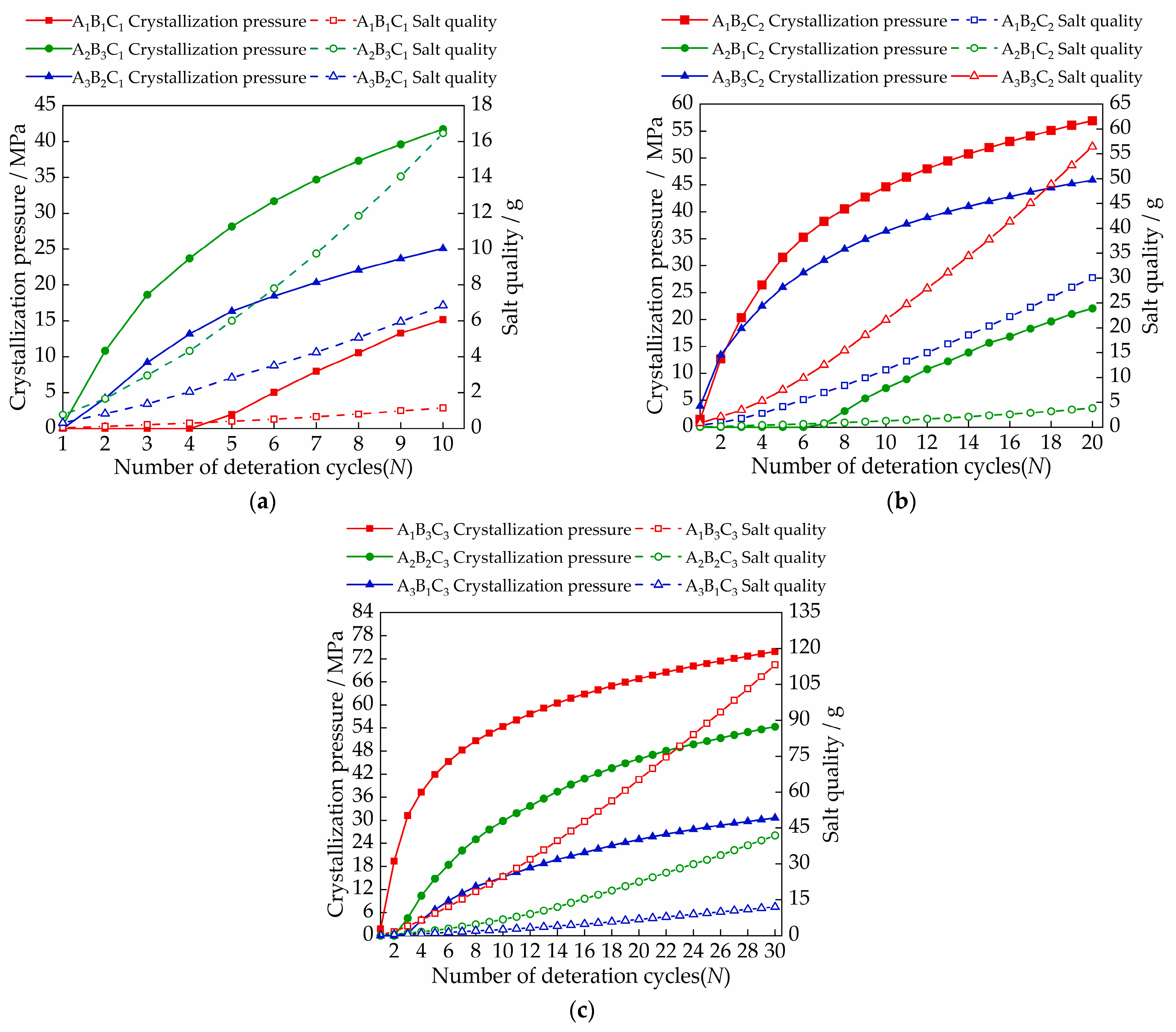
3.2. The Variation in the Uniaxial Compression Strength Values Due to Salt Crystallization
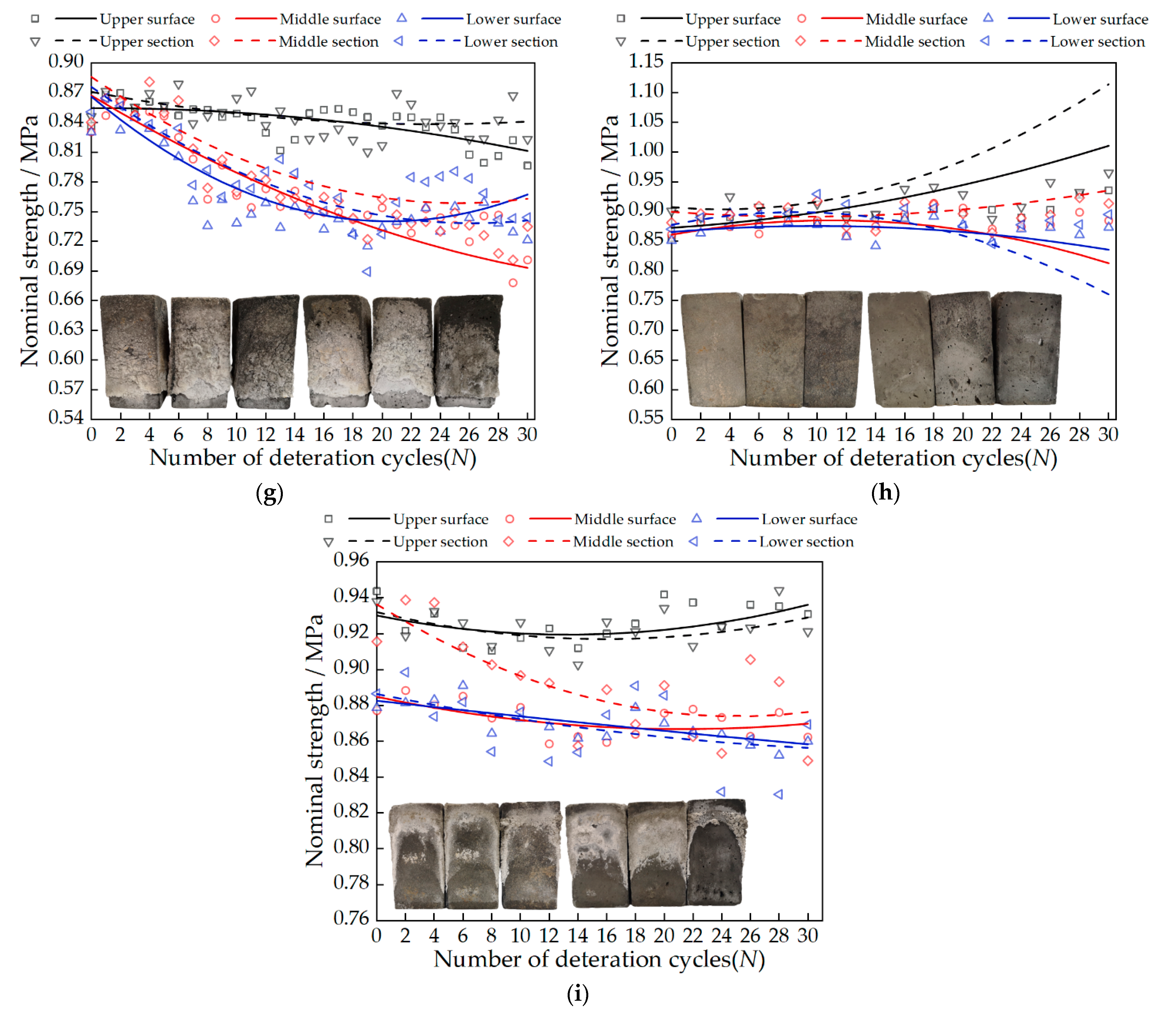
3.3. Change in Nominal Strength
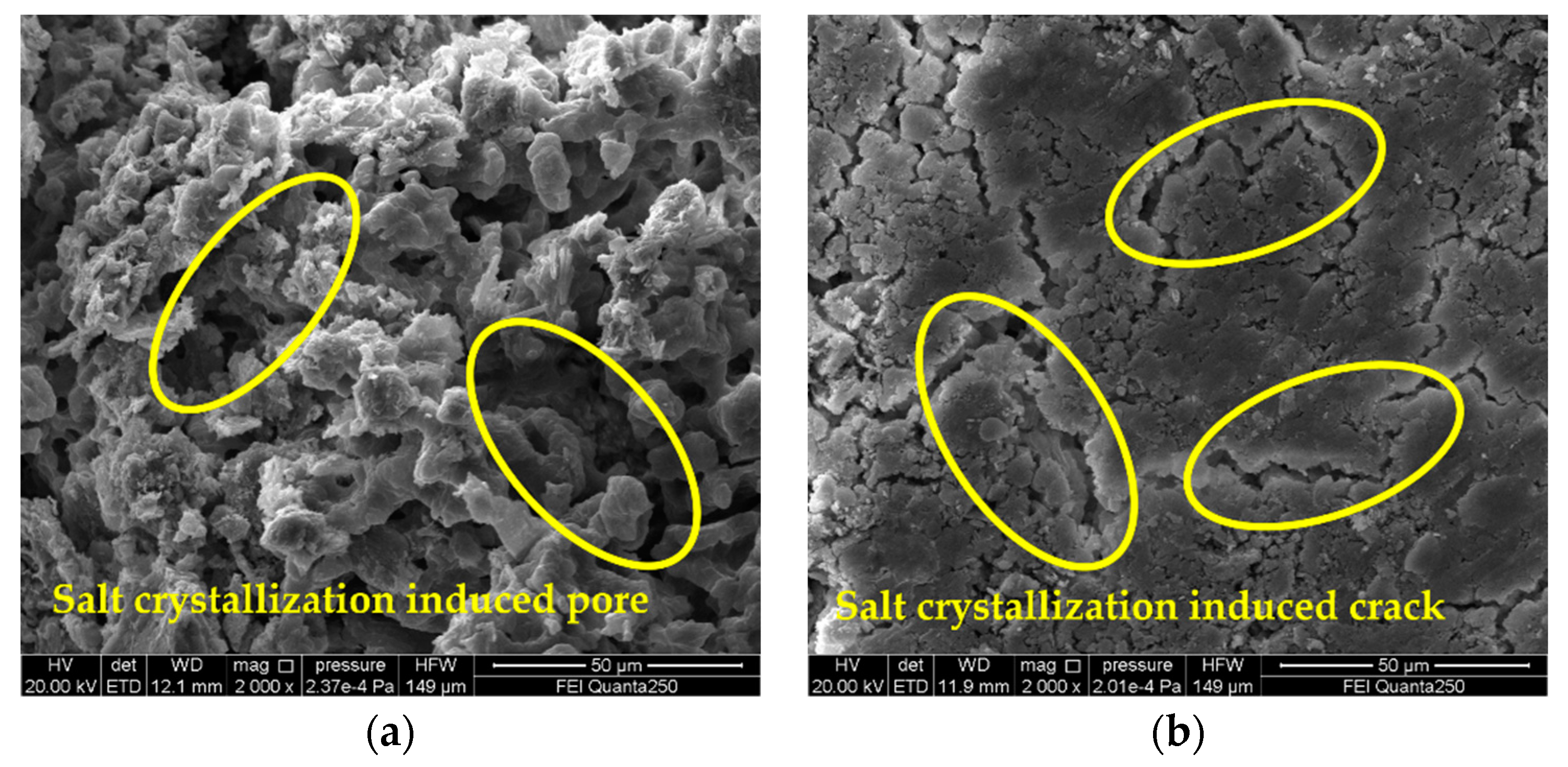
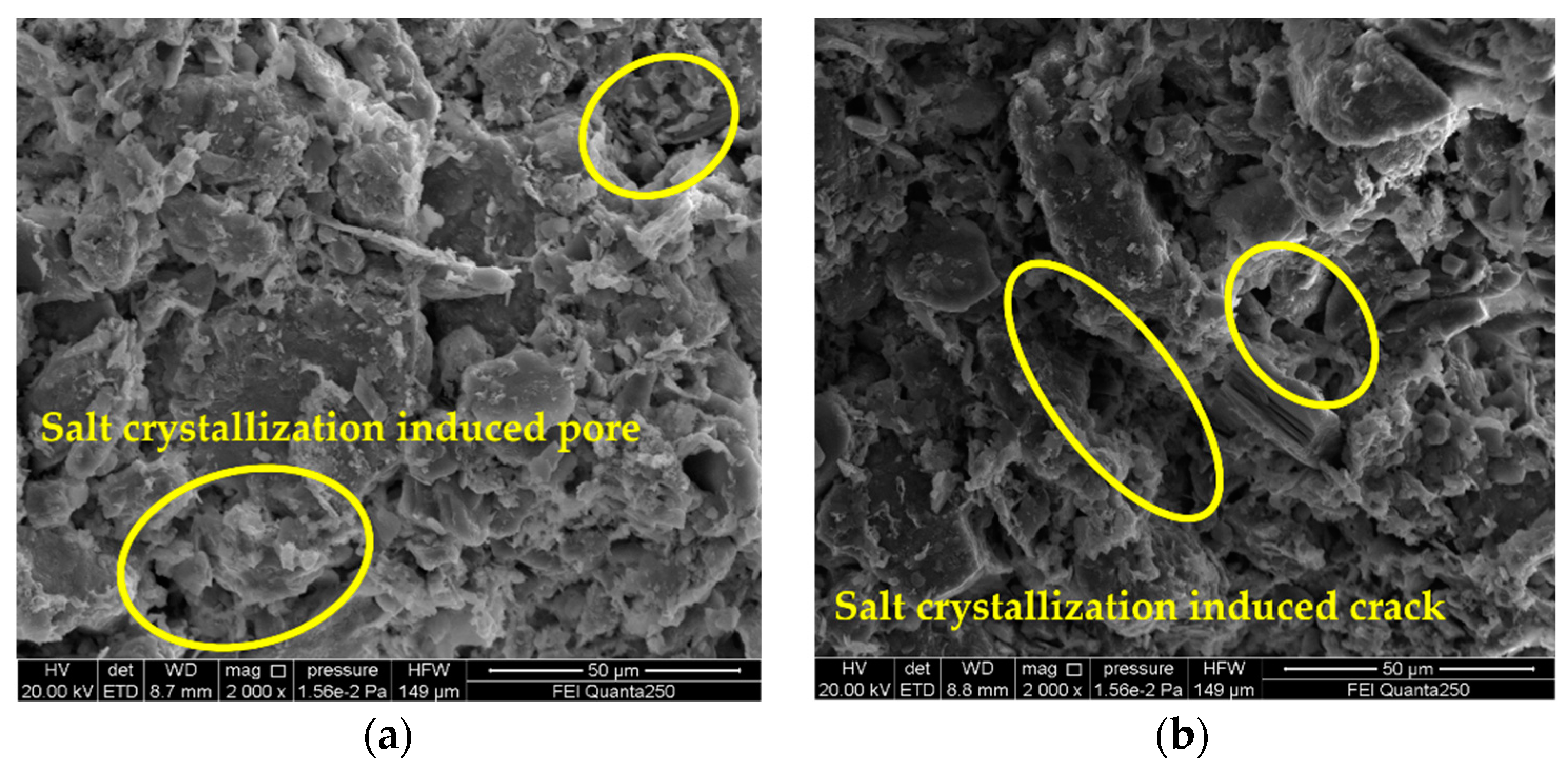
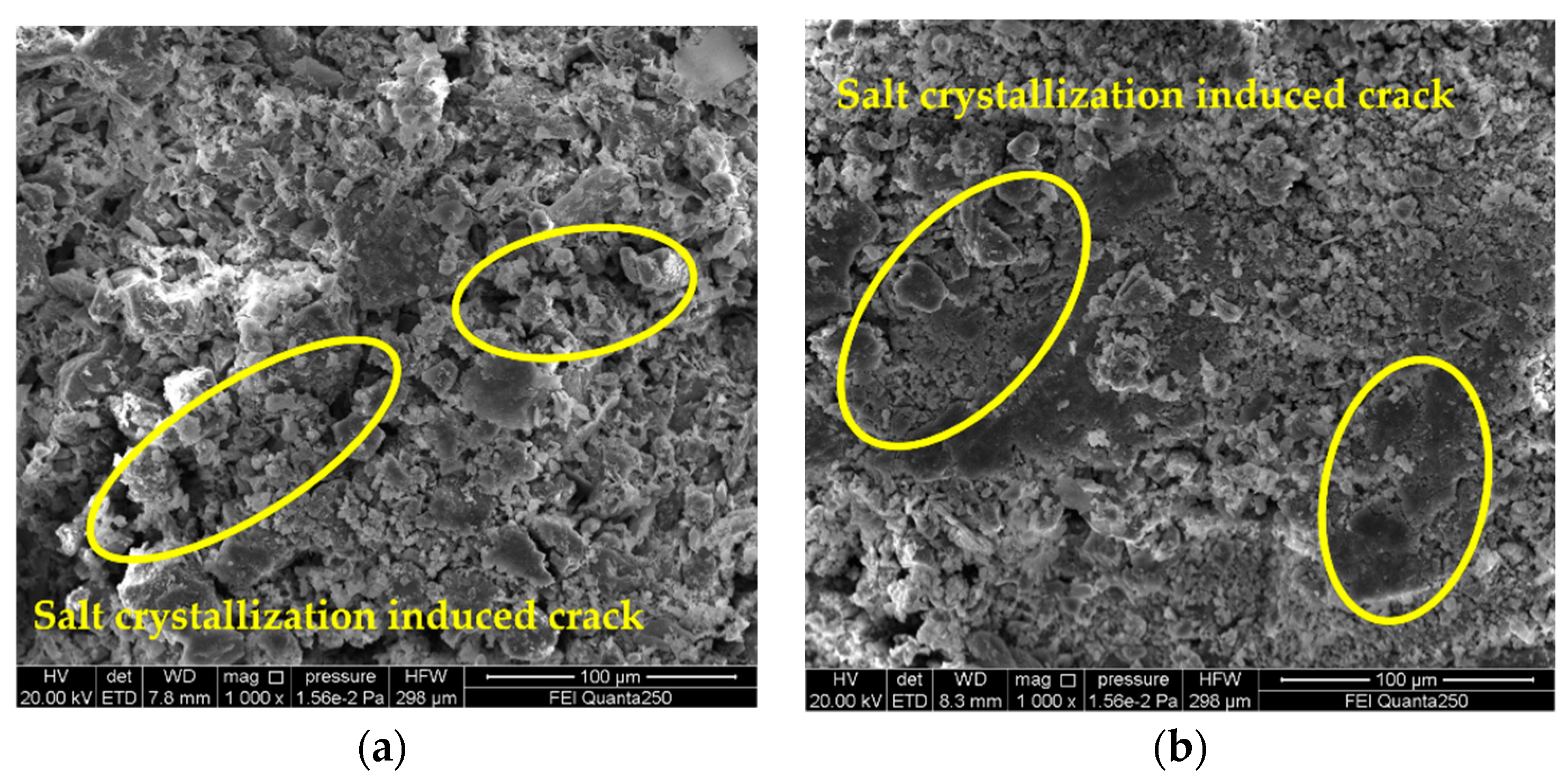
3.4. Analysis on Deterioration Mechanism of Gray Brick Caused by Salt Crystallization
4. Conclusions
- The concentration of salt solution is the main factor affecting the relative change in sample quality. Samples of the A2B3C1, A3B3C2, and A1B3C3 groups with a salt concentration of 5% increased the most, which were 19.25%, 30.12%, and 39.58%, respectively. In appearance, the damage to the middle and lower part of the sample is more serious when the sodium chloride solution acts on the sample. With the increase in the number of cycles, the surface will form crusts and fall off. The damage of the magnesium sulfate solution to the upper part of the sample is more significant, mainly including pulverization, cracks, and causing parts to fall off. The calcium chloride solution has no obvious damage characteristics on the surface of the sample;
- There are two factors that affect nominal strength: salt solution concentration and salt type. Nominal strength decreases with increasing salt solution concentration. Magnesium sulfate crystals have the most serious damage to the tiny pores of gray bricks, followed by sodium chloride and calcium chloride, which has the least. The nominal strength of the samples in the A3B3C2 group decreased the most, with an average decrease of 0.27 MPa. When the concentrations of sodium chloride solution, calcium chloride solution and magnesium sulfate solution are less than 13.73 mol/L, 11.47 mol/L and 17 mol/L, respectively, the nominal strength of the sample increases;
- The size of the crystallization pressure has a lot to do with the quality of the salt inside the brick. When the concentrations of sodium chloride solution, calcium chloride solution and magnesium sulfate solution are in the range of 8.17~8.93 mol/L, 6.98~9.9 mol/L, and 4.73~8.94 mol/L, respectively, the crystallization pressure begins to occur;
- By observing the SEM images, it is found that the degree of sample deterioration caused by salt crystallization not only depends on the size of the crystallization pressure, but is also related to the distribution of salt crystals in the pores of different sizes of the sample. In order to better understand the damage mechanism of brick masonry caused by salinization under actual conditions, the next research topics include conducting mixed-salt cyclic deterioration experiments and determining the pore size of the crystalline distribution of different types of salts.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Çelik, M.Y.; Sert, M. The role of different salt solutions and their concentration ratios in salt crystallization test on the durability of the Döğer tuff (Afyonkarahisar, Turkey) used as building stones of cultural heritages. Bull. Eng. Geol. Environ. 2020, 79, 5553–5568. [Google Scholar] [CrossRef]
- Zhang, F.; Hu, Z.; Wei, F.; Wen, X.; Li, X.; Dai, L.; Liu, L. Study on Concrete Deterioration in Different NaCl-Na2SO4 Solutions and the Mechanism of Cl− Diffusion. Matererials 2021, 14, 5054. [Google Scholar] [CrossRef] [PubMed]
- Kliukas, R.; Jaras, A.; Lukoševičienė, O. The Impact of Long-Term Physical Salt Attack and Multicycle Temperature Gradient on the Mechanical Properties of Spun Concrete. Materials 2021, 14, 4811. [Google Scholar] [CrossRef] [PubMed]
- Lubelli, B.; Cnudde, V.; Diaz-Goncalves, T.; Franzoni, E.; Van Hees, R.P.J.; Ioannou, I.; Menéndez, B.; Nunes, C.; Siedel, H.; Stefanidou, M.; et al. Towards a more effective and reliable salt crystallization test for porous building materials: State of the art. Mater. Struct. 2018, 51, 55. [Google Scholar] [CrossRef]
- Koniorczyk, M.; Gawin, D.; Schrefler, B.A. Multiphysics model for spalling prediction of brick due to in-pore salt crystallization. Comput. Struct. 2018, 196, 233–245. [Google Scholar] [CrossRef]
- Xiong, R.; Jiang, W.; Yang, F.; Li, K.; Guan, B.; Zhao, H. Investigation of Voids Characteristics in an Asphalt Mixture Exposed to Salt Erosion Based on CT Images. Materials 2019, 12, 3774. [Google Scholar] [CrossRef] [Green Version]
- Benavente, D.; De Jongh, M.; Cañaveras, J.C. Weathering Processes and Mechanisms Caused by Capillary Waters and Pigeon Droppings on Porous Limestones. Minerals 2020, 11, 18. [Google Scholar] [CrossRef]
- Chang, C.; An, L.; Zheng, W.; Wen, J.; Dong, J.; Yan, F.; Xiao, X. Research and Engineering Application of Salt Erosion Resistance of Magnesium Oxychloride Cement Concrete. Materrials 2021, 14, 7880. [Google Scholar] [CrossRef]
- Manohar, S.; Santhanam, M.; Chockalingam, N. Performance and microstructure of bricks with protective coatings subjected to salt weathering. Constr. Build. Mater. 2019, 226, 94–105. [Google Scholar] [CrossRef]
- Graziani, G.; Sassoni, E.; Franzoni, E. Experimental study on the salt weathering resistance of fired clay bricks consolidated by ethyl silicate. Mater. Struct. 2015, 49, 2525–2533. [Google Scholar] [CrossRef]
- Coussy, O. Deformation and stress from in-pore drying-induced crystallization of salt. J. Mech. Phys. Solids 2006, 54, 1517–1547. [Google Scholar] [CrossRef]
- Ghobadi, M.H.; Babazadeh, R. Experimental Studies on the Effects of Cyclic Freezing-Thawing, Salt Crystallization, and Thermal Shock on the Physical and Mechanical Characteristics of Selected Sandstones. Rock Mech. Rock Eng. 2015, 48, 1001–1016. [Google Scholar] [CrossRef]
- Wang, F.; Qin, X.; Pang, W.; Wang, W. Performance Deterioration of Asphalt Mixture under Chloride Salt Erosion. Materials 2021, 14, 3339. [Google Scholar] [CrossRef] [PubMed]
- Benavente, D.; Cueto, N.; Martínez-Martínez, J.; Del Cura, M.A.G.; Cañaveras, J.C. The influence of petrophysical properties on the salt weathering of porous building rocks. Environ. Earth Sci. 2006, 52, 215–224. [Google Scholar] [CrossRef]
- Manohar, S.; Santhanam, M. Correlation between Physical-mineralogical Properties and Weathering Resistance Using Characterisation Case Studies in Historic Indian Bricks. Int. J. Arch. Heritage 2020, 2, 1–14. [Google Scholar] [CrossRef]
- Liu, J.-B.; Zhang, Z.-J. Characteristics and weathering mechanisms of the traditional Chinese blue brick from the ancient city of Ping Yao. R. Soc. Open Sci. 2020, 7, 200058. [Google Scholar] [CrossRef]
- Stryszewska, T.; Dudek, M. Selection of Method of Chemical Analysis in Measuring the Salinity of Mineral Materials. Materials 2020, 13, 559. [Google Scholar] [CrossRef] [Green Version]
- Fragata, A.; Veiga, M.R.; Velosa, A. Substitution ventilated render systems for historic masonry: Salt crystallization tests evaluation. Constr. Build. Mater. 2016, 102, 592–600. [Google Scholar] [CrossRef]
- Lubelli, B.; de Rooij, M.R. NaCl crystallization in restoration plasters. Constr. Build. Mater. 2009, 23, 1736–1742. [Google Scholar] [CrossRef]
- Çelik, M.Y.; Sert, M. An assessment of capillary water absorption changes related to the different salt solutions and their concentrations ratios in the der tuff (Afyonkarahisar-Turkey) used as building stone of cultural heritages. J. Build. Eng. 2021, 35, 102102. [Google Scholar] [CrossRef]
- Menéndez, B.; Petráňová, V. Effect of mixed vs single brine composition on salt weathering in porous carbonate building stones for different environmental conditions. Eng. Geol. 2016, 210, 124–139. [Google Scholar] [CrossRef]
- Ludovico-Marques, M.; Chastre, C. Effect of salt crystallization ageing on the compressive behavior of sandstone blocks in historical buildings. Eng. Fail. Anal. 2012, 26, 247–257. [Google Scholar] [CrossRef]
- Schiro, M.; Ruiz-Agudo, E.; Rodriguez-Navarro, C. Damage Mechanisms of Porous Materials due to In-Pore Salt Crystallization. Phys. Rev. Lett. 2012, 109, 265503. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Feng, W.; Zhang, Y.; Zhao, H. Stress from crystallization in ideal pores. Chin. J. Rock Mech. Eng. 2016, 35, 8. (In Chinese) [Google Scholar]
- Test Methods for Wall Bricks. In Chinese Standard GB/T 2542-2012; Chinese Standard: Beijing, China, 2013.
- Du, Q.-H.; Qu, J.-H.; Song, X. An Analysis of Chemical Characteristics and Causes of Groundwater near the Yellow River in Kaifeng City. China Rural. Water Hydropower 2020, 172–176, 181. Available online: https://www.cnki.net (accessed on 21 December 2021). (In Chinese).
- Yu, S.; Oguchi, C.T. Role of pore size distribution in salt uptake, damage, and predicting salt susceptibility of eight types of Japanese building stones. Eng. Geol. 2010, 115, 226–236. [Google Scholar] [CrossRef]
- Lindström, N.; Talreja, T.; Linnow, K.; Stahlbuhk, A.; Steiger, M. Crystallization behavior of Na2SO4-MgSO4 salt mixtures in sandstone and comparison to single salt behavior. Appl. Geochem. 2016, 69, 50–70. [Google Scholar] [CrossRef]
- Steiger, M. Crystal growth in porous materials—I: The crystallization pressure of large crystals. J. Cryst. Growth 2005, 282, 455–469. [Google Scholar] [CrossRef]
- Steiger, M. Crystal growth in porous materials—II: Influence of crystal size on the crystallization pressure. J. Cryst. Growth 2005, 282, 470–481. [Google Scholar] [CrossRef]
- Yan, S.J.; Fang, Y.; Liu, J.H.; Tan, S.E. Deterioration experiment with soluble salt on sandstone of Yungang grottoes and its model creation. Rock Soil Mech. 2013, 34, 7. (In Chinese) [Google Scholar]
- Foraboschi, P.; Vanin, A. Experimental investigation on bricks from historical Venetian buildings subjected to moisture and salt crystallization. Eng. Fail. Anal. 2014, 45, 185–203. [Google Scholar] [CrossRef]
- Ju, X.D.; Feng, W.J.; Zhang, Y.J.; Zhao, H.B. Crystallization stresses in brittle porous media. Chin. J. Geotech. Eng. 2016, 38, 1246–1253. [Google Scholar] [CrossRef]
- Wang, F.-R.; Jiao, D.-D.; Liu, P. Experimental study on the effect of sulfate on glutenite weathering in Maijishan grottoes. Rock Soil Mech. 2020, 41, 8. (In Chinese) [Google Scholar] [CrossRef]
- Li, D.A.; Yxa, B.; Qha, B.; Xu, X.; Zh, A.; Gha, B. Evaporation-induced salt crystallization and feedback on hydrological functions in porous media with different grain morphologies. J. Hydrol. 2021, 598, 126427. [Google Scholar]
- Sun, Q.; Dong, Z.; Jia, H. Decay of sandstone subjected to a combined action of repeated freezing-thawing and salt crystallization. Bull. Eng. Geol. Environ. 2019, 78, 5951–5964. [Google Scholar] [CrossRef]
- Yu, S.; Oguchi, C.T. Complex relationships between salt type and rock properties in a durability experiment of multiple salt–rock treatments. Earth Surf. Process. Landf. 2009, 34, 2096–2110. [Google Scholar] [CrossRef]
- Derluyn, H.; Moonen, P.; Carmeliet, J. Deformation and damage due to drying-induced salt crystallization in porous limestone. J. Mech. Phys. Solids 2014, 63, 242–255. [Google Scholar] [CrossRef] [Green Version]
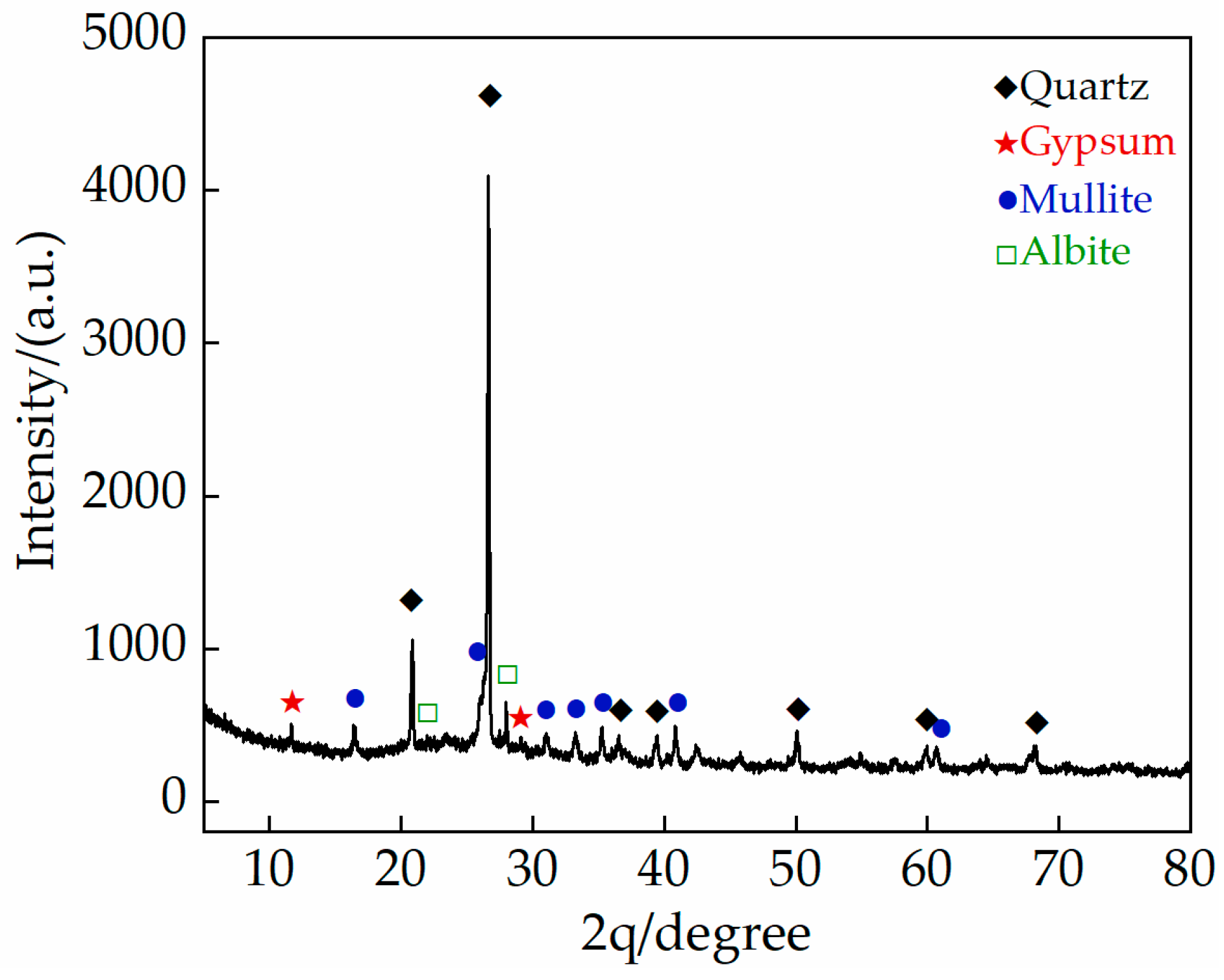
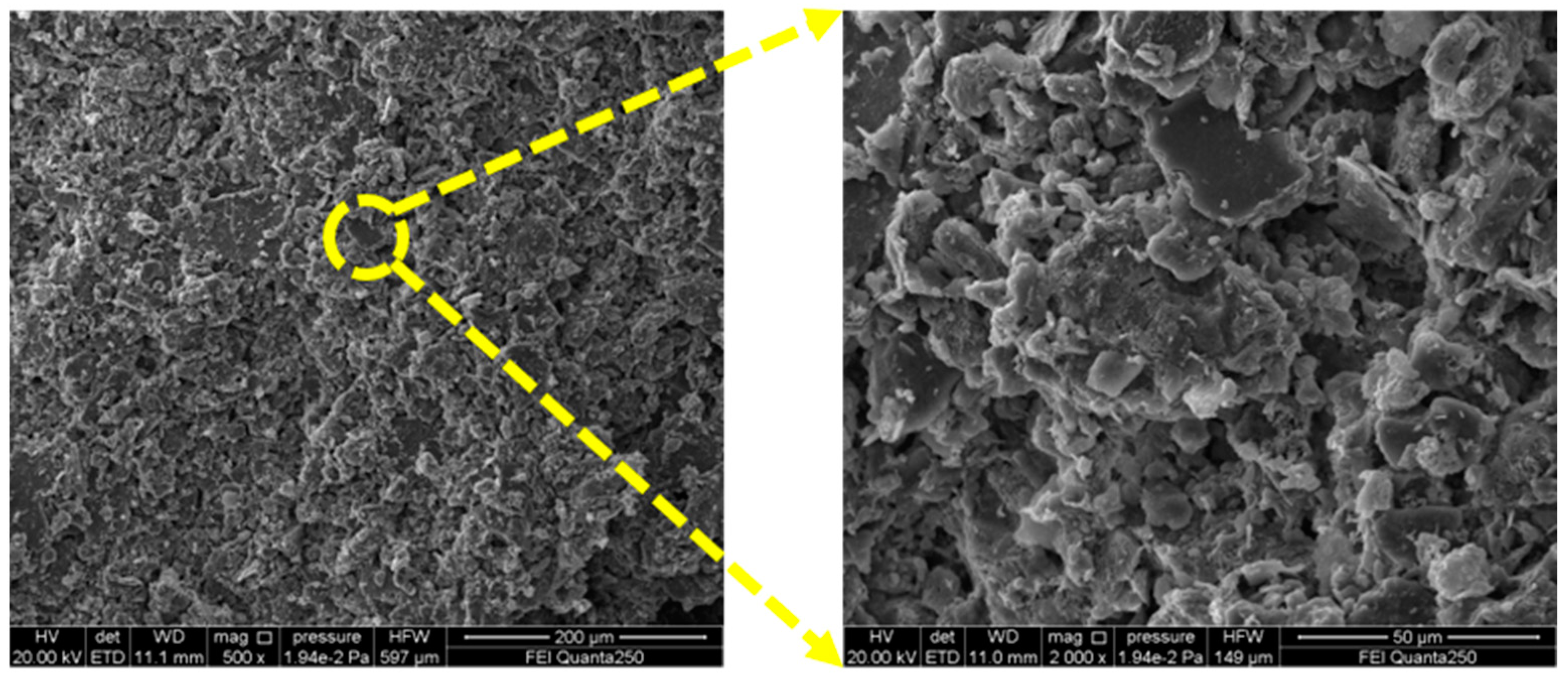


| Compound | SiO2 | Al2O3 | CaO | Fe2O3 | K2O | MgO | Na2O | TiO2 |
|---|---|---|---|---|---|---|---|---|
| Concentration (%) | 62.29 | 15.63 | 6.69 | 5.23 | 3.74 | 3.20 | 1.95 | 0.68 |
| Density/(kg/m3) | Poisson’s Ratio | Porosity /% | Compressive Strength/MPa | Elastic Modulus/GPa | Elastic Modulus (GPa) |
|---|---|---|---|---|---|
| 1800 | 0.15 | 15.63 | 19.27 | 3.74 | 3.34 |
| Test No. | Variable | ||
|---|---|---|---|
| Salt (A) | Salt Concentration (B) | Cycles (C) | |
| A1B1C1 | NaCl | 1% | 10 |
| A1B2C2 | NaCl | 3% | 20 |
| A1B3C3 | NaCl | 5% | 30 |
| A2B2C3 | CaCl2 | 3% | 30 |
| A2B3C1 | CaCl2 | 5% | 10 |
| A2B1C2 | CaCl2 | 1% | 20 |
| A3B3C2 | MgSO4 | 5% | 20 |
| A3B1C3 | MgSO4 | 1% | 30 |
| A3B2C1 | MgSO4 | 3% | 10 |
| Test No. | Salt | Salt Concentration/% | Cycle | Saturated Water Absorption/% | Compressive Strength/MPa |
|---|---|---|---|---|---|
| 0 | H2O | 0 | 30 | 20.4 | 18 |
| 1 | NaCl | 1% | 10 | 21.2 | 15.7 |
| 2 | NaCl | 3% | 20 | 22.6 | 15.3 |
| 3 | NaCl | 5% | 30 | 24.4 | 16.4 |
| 4 | CaCl2 | 3% | 30 | 23 | 16.7 |
| 5 | CaCl2 | 5% | 10 | 23.8 | 16 |
| 6 | CaCl2 | 1% | 20 | 22.7 | 17.4 |
| 7 | MgSO4 | 5% | 20 | 22.3 | 13.1 |
| 8 | MgSO4 | 1% | 30 | 21 | 15.5 |
| 9 | MgSO4 | 3% | 10 | 21.9 | 14 |
| Test No. | Salt | Salt Concentration/% | Cycle | Saturated Water Absorption/% | Compressive Strength/MPa |
|---|---|---|---|---|---|
| 0 | H2O | 0 | 30 | 20.4 | 18 |
| 1 | NaCl | 1% | 10 | 21.2 | 15.7 |
| 2 | NaCl | 3% | 20 | 22.6 | 15.3 |
| 3 | NaCl | 5% | 30 | 24.4 | 16.4 |
| 4 | CaCl2 | 3% | 30 | 23 | 16.7 |
| 5 | CaCl2 | 5% | 10 | 23.8 | 16 |
| 6 | CaCl2 | 1% | 20 | 22.7 | 17.4 |
| 7 | MgSO4 | 5% | 20 | 22.3 | 13.1 |
| 8 | MgSO4 | 1% | 30 | 21 | 15.5 |
| 9 | MgSO4 | 3% | 10 | 21.9 | 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, J.; Li, Y.; Luo, Z.; Huang, X.; Kong, Q.; Wang, Z. Study on Deterioration Law and Mechanism of Gray Brick Due to Salt Crystallization. Materials 2022, 15, 2936. https://doi.org/10.3390/ma15082936
Yue J, Li Y, Luo Z, Huang X, Kong Q, Wang Z. Study on Deterioration Law and Mechanism of Gray Brick Due to Salt Crystallization. Materials. 2022; 15(8):2936. https://doi.org/10.3390/ma15082936
Chicago/Turabian StyleYue, Jianwei, Yuan Li, Zhenxian Luo, Xuanjia Huang, Qingmei Kong, and Zifa Wang. 2022. "Study on Deterioration Law and Mechanism of Gray Brick Due to Salt Crystallization" Materials 15, no. 8: 2936. https://doi.org/10.3390/ma15082936
APA StyleYue, J., Li, Y., Luo, Z., Huang, X., Kong, Q., & Wang, Z. (2022). Study on Deterioration Law and Mechanism of Gray Brick Due to Salt Crystallization. Materials, 15(8), 2936. https://doi.org/10.3390/ma15082936






