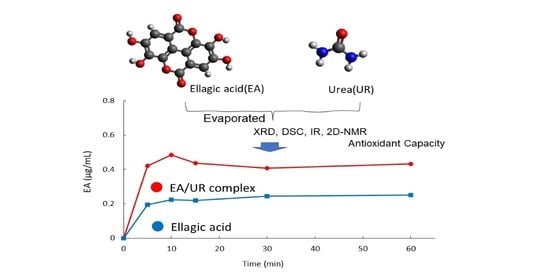
Preparation, Characterization, Solubility, and Antioxidant Capacity of Ellagic Acid-Urea Complex
Abstract
:1. Introduction
2. Materials and Methods
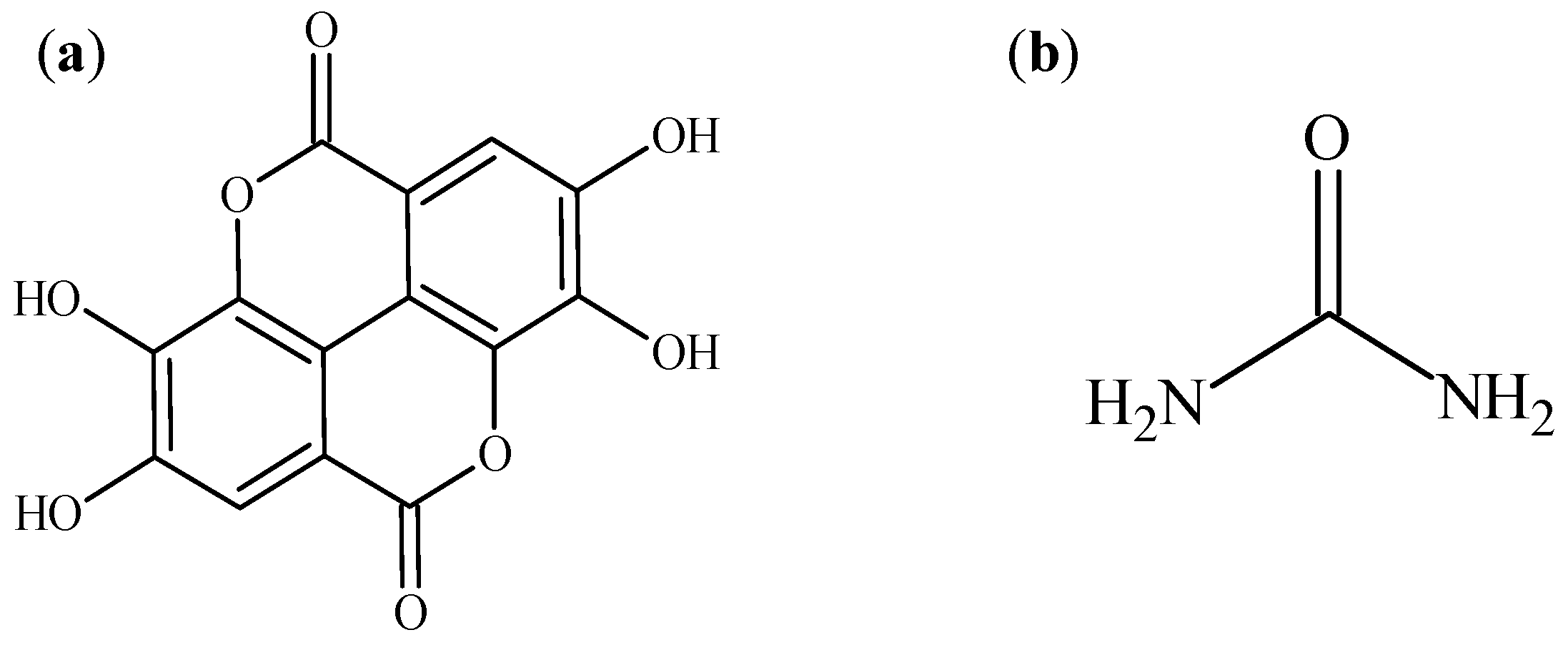
2.1. Materials
2.2. Methods
2.2.1. Preparation of Physical Mixture and Solvent Evaporated Solid Dispersion
2.2.2. Solubility Test
2.2.3. Dissolution Test
2.2.4. DPPH Radical Scavenging Test
2.2.5. Differential Scanning Calorimetry (DSC) Measurement
2.2.6. Powder X-ray Diffraction (PXRD) Measurement
2.2.7. Solid-State Fluorescence Measurement
2.2.8. Fourier Transform Infrared (FT-IR) Absorption Spectrum
2.2.9. Near-Infrared (NIR) Measurement
2.2.10. Scanning Electron Microscopy (SEM)
2.2.11. Nuclear Overhauser Effect Spectroscopy (NOESY) Measurement
2.2.12. Statistical Analysis
3. Results and Discussion
3.1. Solubility Test
3.2. Dissolution Test
3.3. DPPH Radical Scavenging Test
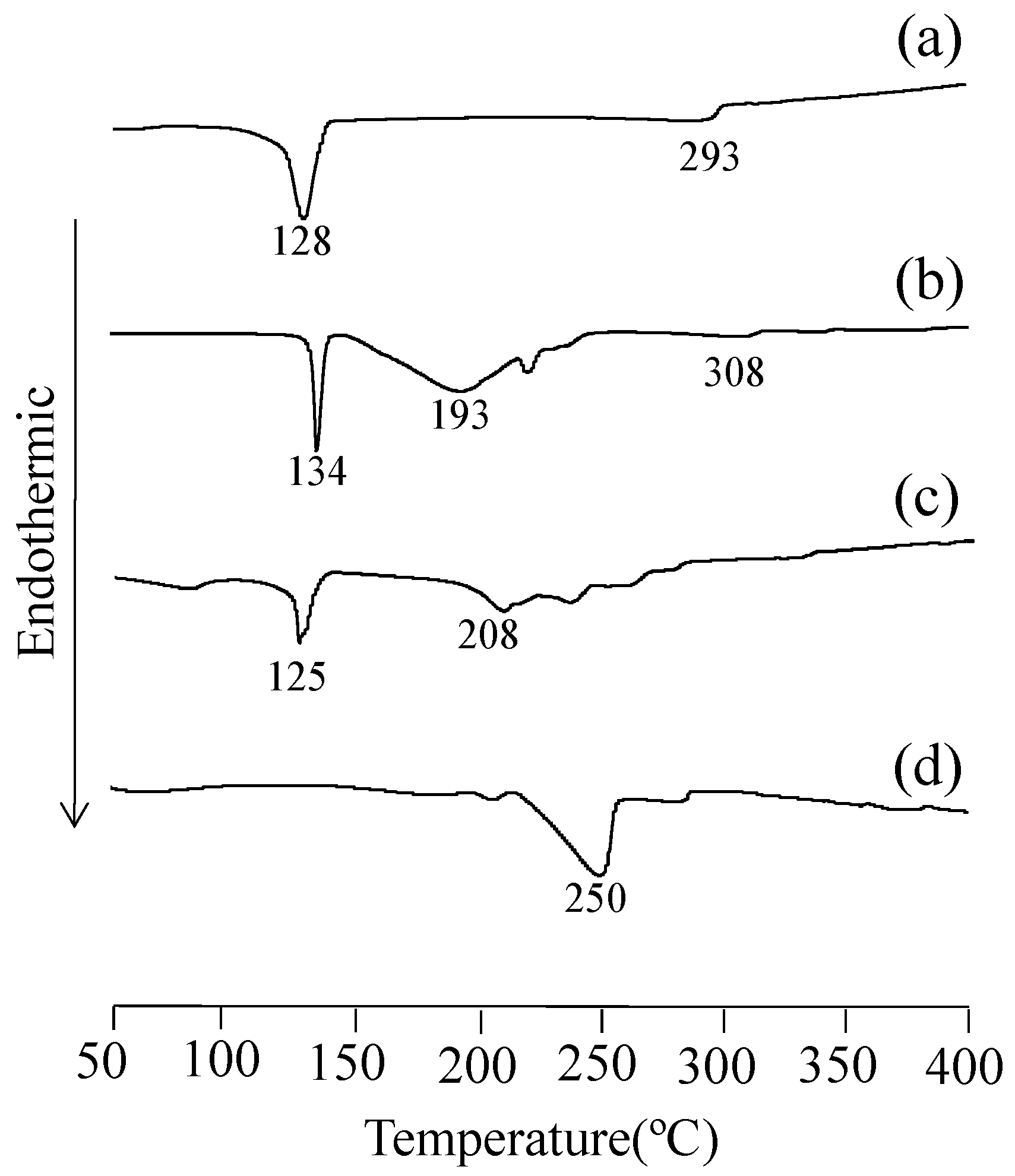
3.4. DSC Measurement
3.5. PXRD Measurement
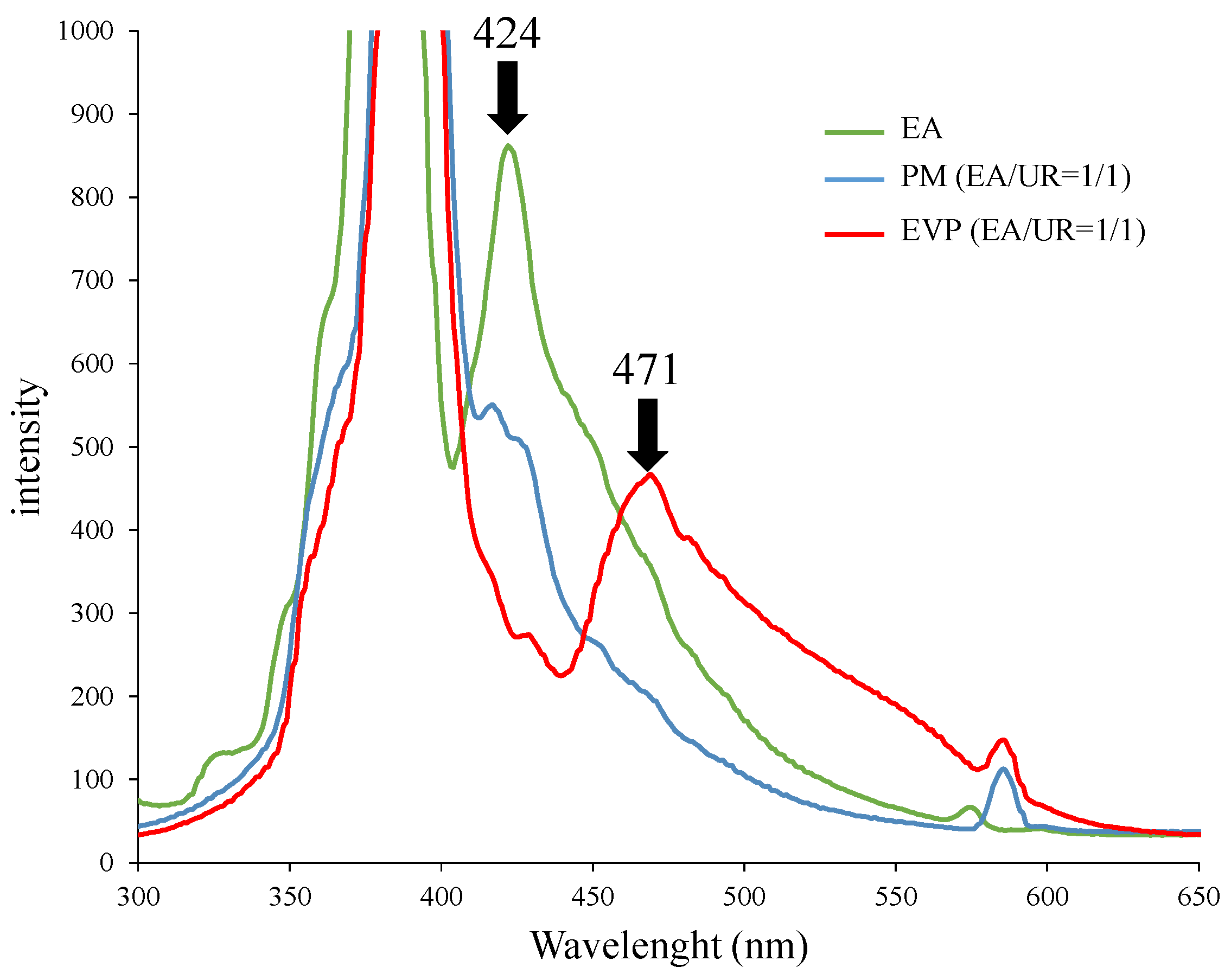
3.6. Solid Fluorescence Measurement
3.7. FT-IR Absorption Spectrum
3.8. NIR Measurement
3.9. SEM Analysis
3.10. NOESY NMR Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EA | Ellagic acid |
| UR | Urea |
| PM | Physical mixture |
| EVP | Evaporated |
| ASC | Ascorbic acid |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| PXRD | Powder X-ray diffraction |
| DSC | Differential scanning calorimetry |
| FT-IR | Fourier transform infrared spectroscopy |
| NIR | Near-infrared |
| SEM | Scanning electron microscopy |
| NOESY | Nuclear Overhauser effect spectroscopy |
References
- Ceci, C.; Lacal, P.M.; Tentori, L.; Gabriella De Martino, M.; Miano, R.; Graziani, G. Experimental evidence of the antitumor, antimetastatic and antiangiogenic activity of ellagic acid. Nutrients 2018, 10, 1756. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, G.; Andlauer, W. Innovative microwave-assisted hydrolysis of ellagitannins and quantification as ellagic acid equivalents. Food Chem. 2013, 138, 2430–2434. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Lee, R.; Heber, D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin. Chim. Acta 2004, 348, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Watanabe, Y.; Kasai, K.; Yamakoshi, J.; Koga, T. Inhibitory effect of an ellagic acid-rich pomegranate extract on tyrosinase activity and ultraviolet-induced pigmentation. Biosci. Biotechnol. Biochem. 2005, 69, 2368–2373. [Google Scholar] [CrossRef] [PubMed]
- Mertens-Talcott, S.U.; Talcott, S.T.; Percival, S.S. Low concentrations of quercetin and ellagic acid synergistically influence proliferation, cytotoxicity and apoptosis in MOLT-4 human leukemia cells. J. Nutr. 2003, 133, 2669–2674. [Google Scholar] [CrossRef]
- Marín, M.; Giner, R.M.; Ríos, J.-L.; Recio, M.C. Intestinal anti-inflammatory activity of ellagic acid in the acute and chronic dextrane sulfate sodium models of mice colitis. J. Ethnopharmacol. 2013, 150, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Castonguay, A.; Boukharta, M.; Teel, R. Biodistribution of, antimutagenic efficacies in Salmonella typhimurium OF, and inhibition of P450 activities by ellagic acid and one analogue. Chem. Res. Toxicol. 1998, 11, 1258–1264. [Google Scholar] [CrossRef]
- Nyamba, I.; Lechanteur, A.; Semdé, R.; Evrard, B. Physical formulation approaches for improving aqueous solubility and bioavailability of ellagic acid: A review. Eur. J. Pharm. Biopharm. 2021, 159, 198–210. [Google Scholar] [CrossRef]
- Mueller, J.; Oliveira, J.S.L.; Barker, R.; Trapp, M.; Schroeter, A.; Brezesinski, G.; Neubert, R.H.H. The effect of urea and taurine as hydrophilic penetration enhancers on stratum corneum lipid models. Biochim. Biophys. Acta 2016, 1858, 2006–2018. [Google Scholar] [CrossRef]
- Trommer, H.; Neubert, R.H.H. Overcoming the stratum corneum: The modulation of skin penetration. Skin Pharmacol. Physiol. 2006, 19, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Herbig, M.E.; Evers, D.-H. Correlation of hydrotropic solubilization by urea with logD of drug molecules and utilization of this effect for topical formulations. Eur. J. Pharm. Biopharm. 2013, 85, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Suzuki, T.; Sakano, H.; Mizuno, N. Effect of vehicles on diclofenac permeation across excised rat skin. Biol. Pharm. Bull. 1955, 18, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Niiyama, D.; Murata, I.; Kanamoto, I. Usefulness of urea as a means of improving the solubility of poorly water-soluble ascorbyl palmitate. Int. J. Med. Chem. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Beig, A.; Lindley, D.; Miller, J.M.; Agbaria, R.; Dahan, A. Hydrotropic solubilization of lipophilic drugs for oral delivery: The effects of urea and nicotinamide on carbamazepine solubility-permeability interplay. Front. Pharmacol. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Cysewski, P.; Przybylek, M.; Ziolkowska, D.; Mroczynska, K. Exploring the cocrystallization potential of urea and benzamide. J. Mol. Model. 2016, 22, 1–10. [Google Scholar] [CrossRef]
- Fukami, T. Current status and promising future of pharmaceutical cocrystals in development of oral dosage forms. Nippon Yakurigaku Zasshi 2017, 150, 36–40. [Google Scholar] [CrossRef]
- Yanez, M.; Jhanji, M.; Murphy, K.; Gower, R.M.; Sajish, M.; Jabbarzadeh, E. Nicotinamide augments the anti-inflammatory properties of resveratrol through PAPR1 activation. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Trask, A.V.; Motherwell, W.D.S.; Jones, W. Physical stability enhancement of theophylline via cocrystallization. Int. J. Pharm. 2006, 320, 114–123. [Google Scholar] [CrossRef]
- Chao, J.; Wang, H.; Zhao, W.; Zhang, M.; Zhang, L. Investigation of the inclusion behavior of chlorogenic acid with hydroxypropyl-β-cyclodextrin. Int. J. Biol. Macromol. 2012, 50, 277–282. [Google Scholar] [CrossRef]
- Inoue, Y.; Yoshida, M.; Ezawa, T.; Tanikawa, T.; Arce, F.; See, G.L.; Tomita, J.; Suzuki, M.; Oguchi, T. Inclusion complexes of daidzein with cyclodextrin-based metal-organic framework-1 enhance its solubility and antioxidant capacity. AAPS PharmSciTech. 2022, 23, 2. [Google Scholar] [CrossRef] [PubMed]
- Brittain, H.G.; Elder, B.J.; Isbester, P.K.; Salerno, A.H. Solid-state fluorescence studies of some polymorphs of diflunisal. Pharm. Res. 2005, 22, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Moribe, K.; Tsuchiya, M.; Tozuka, Y.; Yamaguchi, K.; Oguchi, T.; Yamamoto, K. Grinding-induced equimolar complex formation between thiourea and ethenzamide. Chem. Pharm. Bull. 2004, 52, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Robeyns, K.; Leyssens, T. Urea as a cocrystal former–Study of 3 urea based pharmaceutical cocrystals. Pharmaceutics 2021, 13, 671. [Google Scholar] [CrossRef] [PubMed]
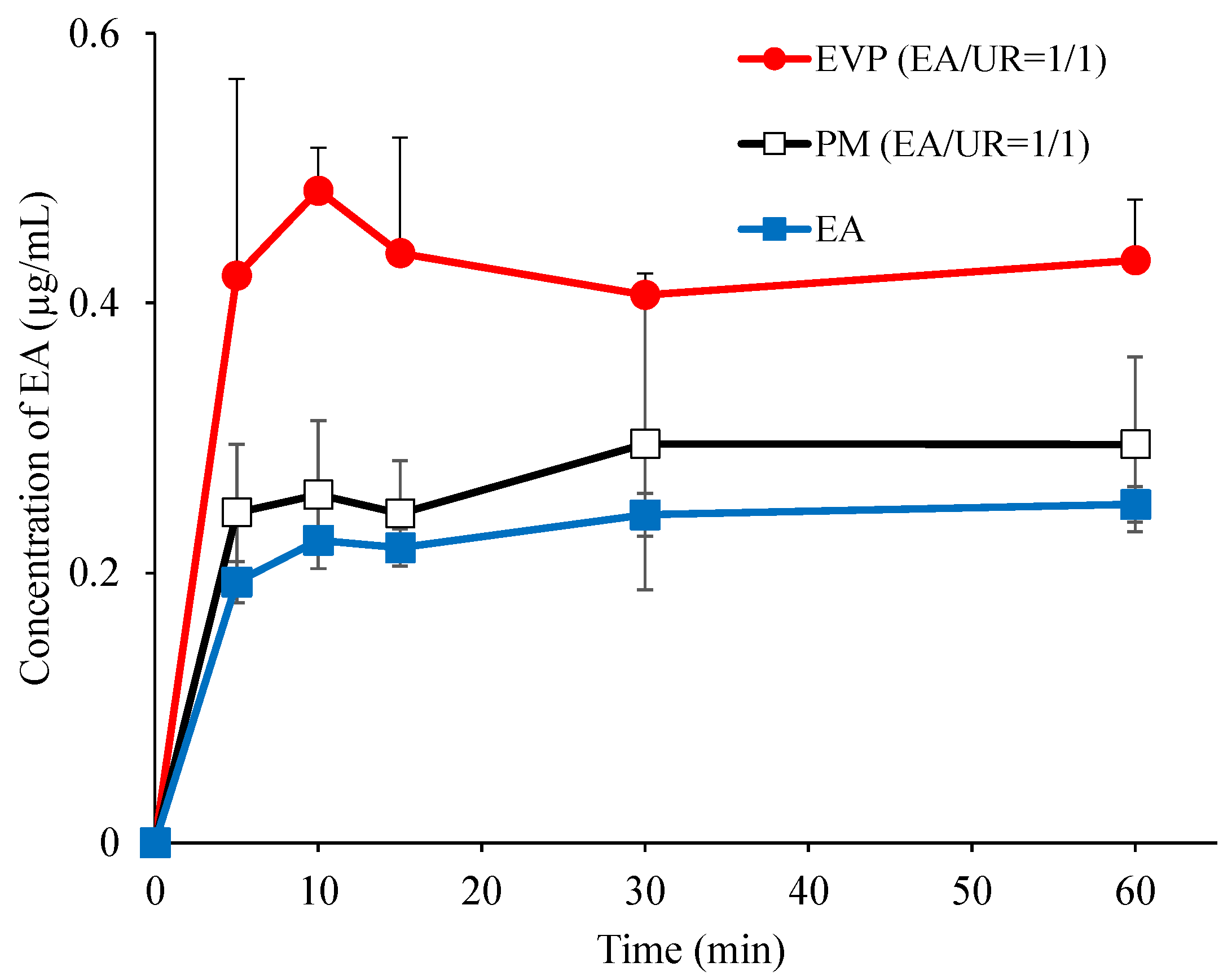
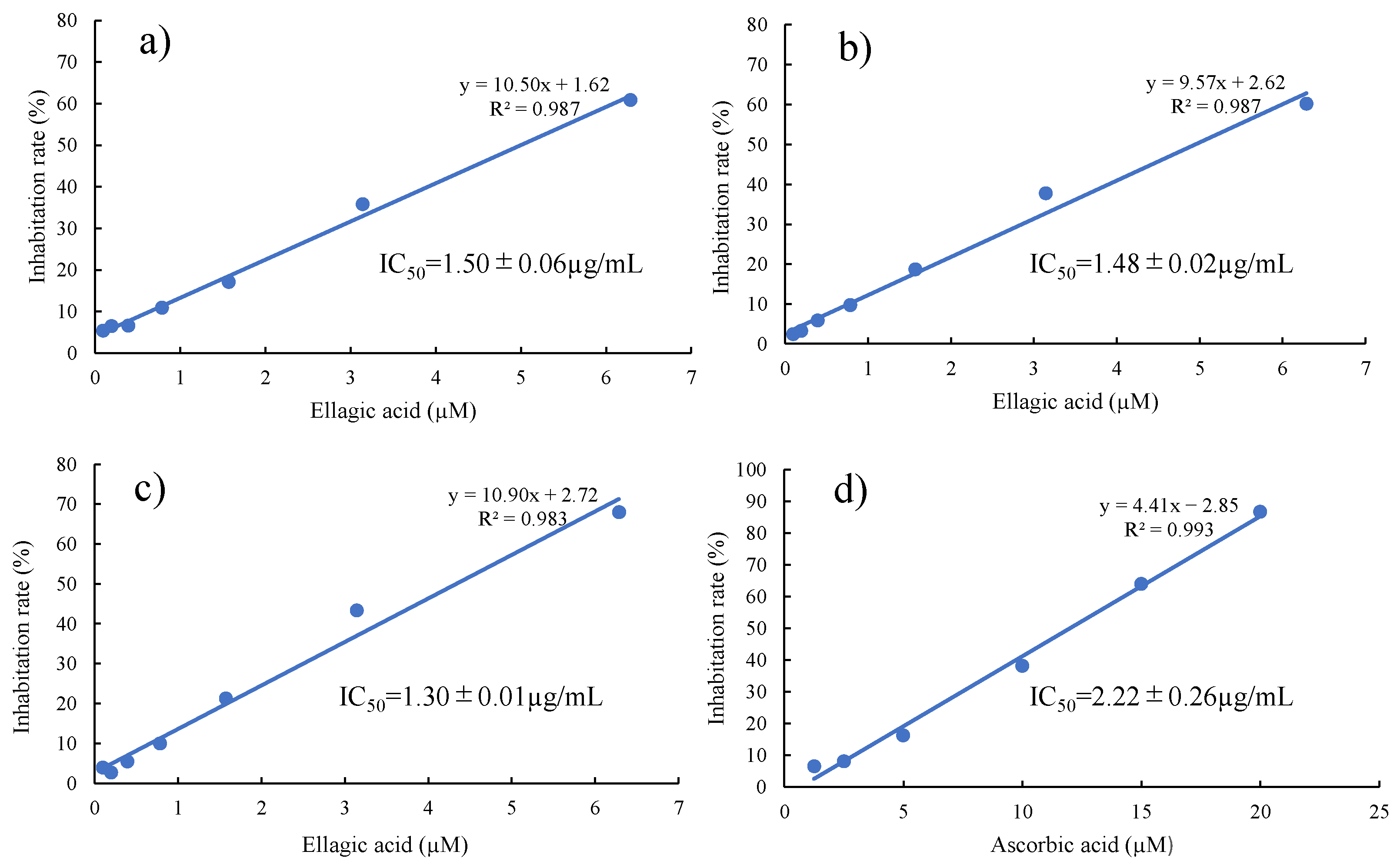






| Time | EA | PM | EVP |
|---|---|---|---|
| 3 | 1.36 ± 0.26 | 2.06 ± 0.69 | 5.40 ± 2.27 |
| 24 | 3.77 ± 0.36 | 5.18 ± 0.30 | 7.45 ± 1.65 |
| 72 | 3.99 ± 0.59 | 5.04 ± 1.33 | 7.13 ± 0.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakurai, H.; Suzuki, M.; Itakura, S.; Todo, H.; Arce, F.; See, G.L.; Tanikawa, T.; Inoue, Y. Preparation, Characterization, Solubility, and Antioxidant Capacity of Ellagic Acid-Urea Complex. Materials 2022, 15, 2836. https://doi.org/10.3390/ma15082836
Sakurai H, Suzuki M, Itakura S, Todo H, Arce F, See GL, Tanikawa T, Inoue Y. Preparation, Characterization, Solubility, and Antioxidant Capacity of Ellagic Acid-Urea Complex. Materials. 2022; 15(8):2836. https://doi.org/10.3390/ma15082836
Chicago/Turabian StyleSakurai, Hitomi, Mitsuaki Suzuki, Shoko Itakura, Hiroaki Todo, Florencio Arce, Gerard Lee See, Takashi Tanikawa, and Yutaka Inoue. 2022. "Preparation, Characterization, Solubility, and Antioxidant Capacity of Ellagic Acid-Urea Complex" Materials 15, no. 8: 2836. https://doi.org/10.3390/ma15082836
APA StyleSakurai, H., Suzuki, M., Itakura, S., Todo, H., Arce, F., See, G. L., Tanikawa, T., & Inoue, Y. (2022). Preparation, Characterization, Solubility, and Antioxidant Capacity of Ellagic Acid-Urea Complex. Materials, 15(8), 2836. https://doi.org/10.3390/ma15082836