Preparation and Adsorption Properties of MCM-41 with Novel Gemini Ionic Liquid Surfactants as Template
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
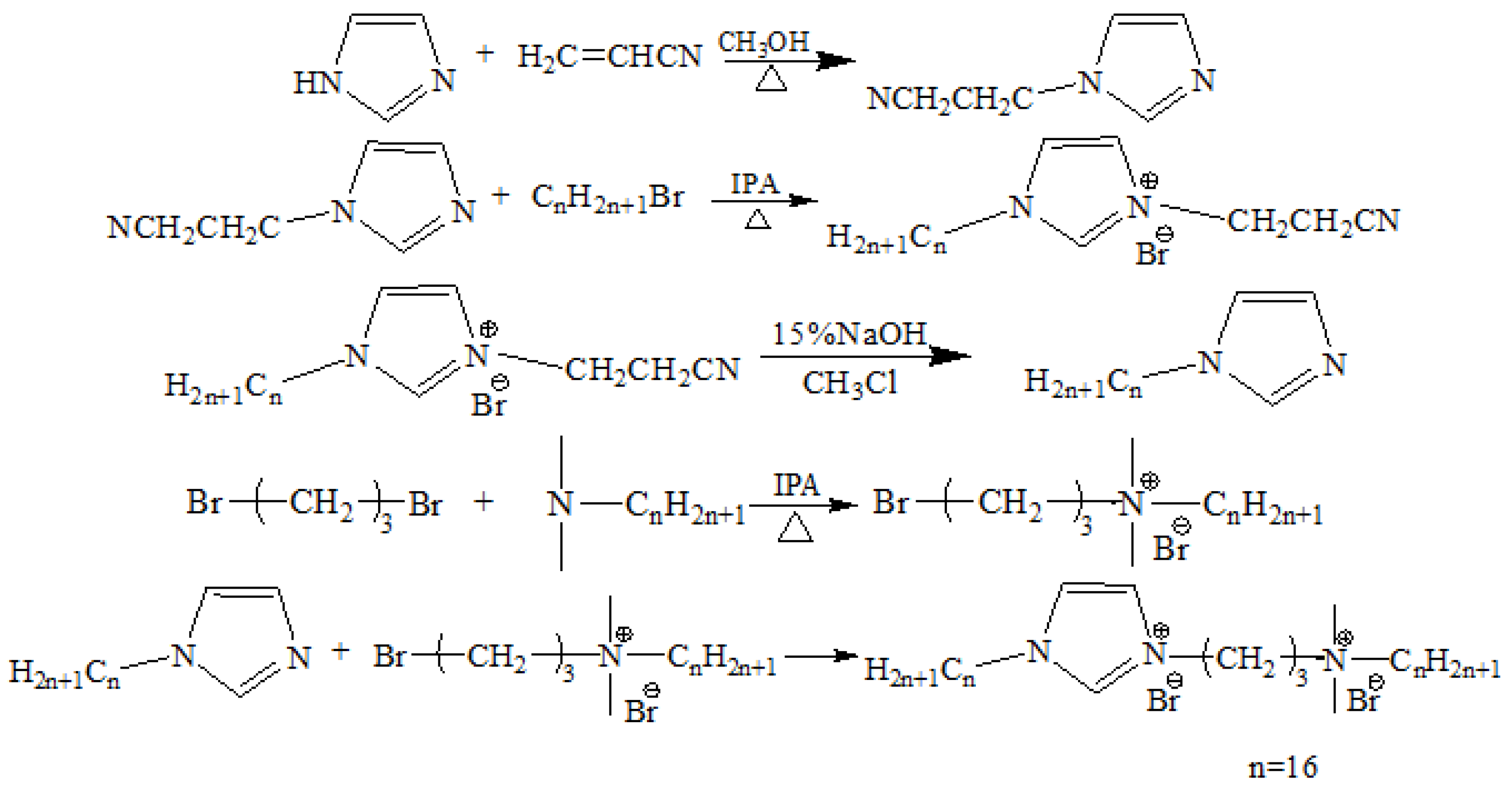
2.2. Synthesis and Characterization of Surfactants
2.3. Preparation and Characterization of Mesoporous Silica
2.4. Adsorption Experiments
3. Results and Discussion
3.1. The 1H-NMR and 13C-NMR Analysis of [N1116C3IMC16][Br]2
3.2. Structural Characterization of Mesoporous Molecular Sieves
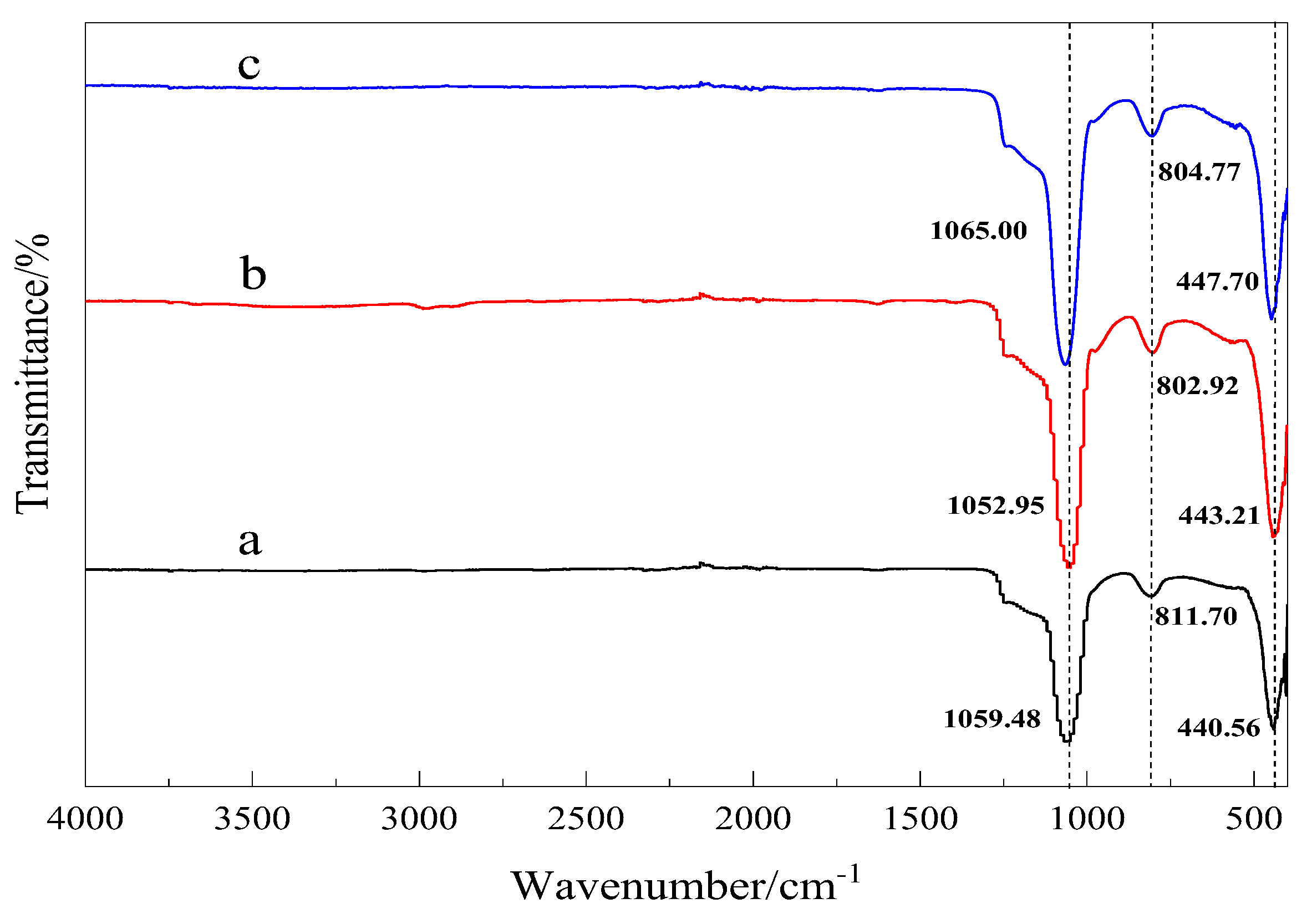
3.2.1. FTIR Spectroscopy Analysis
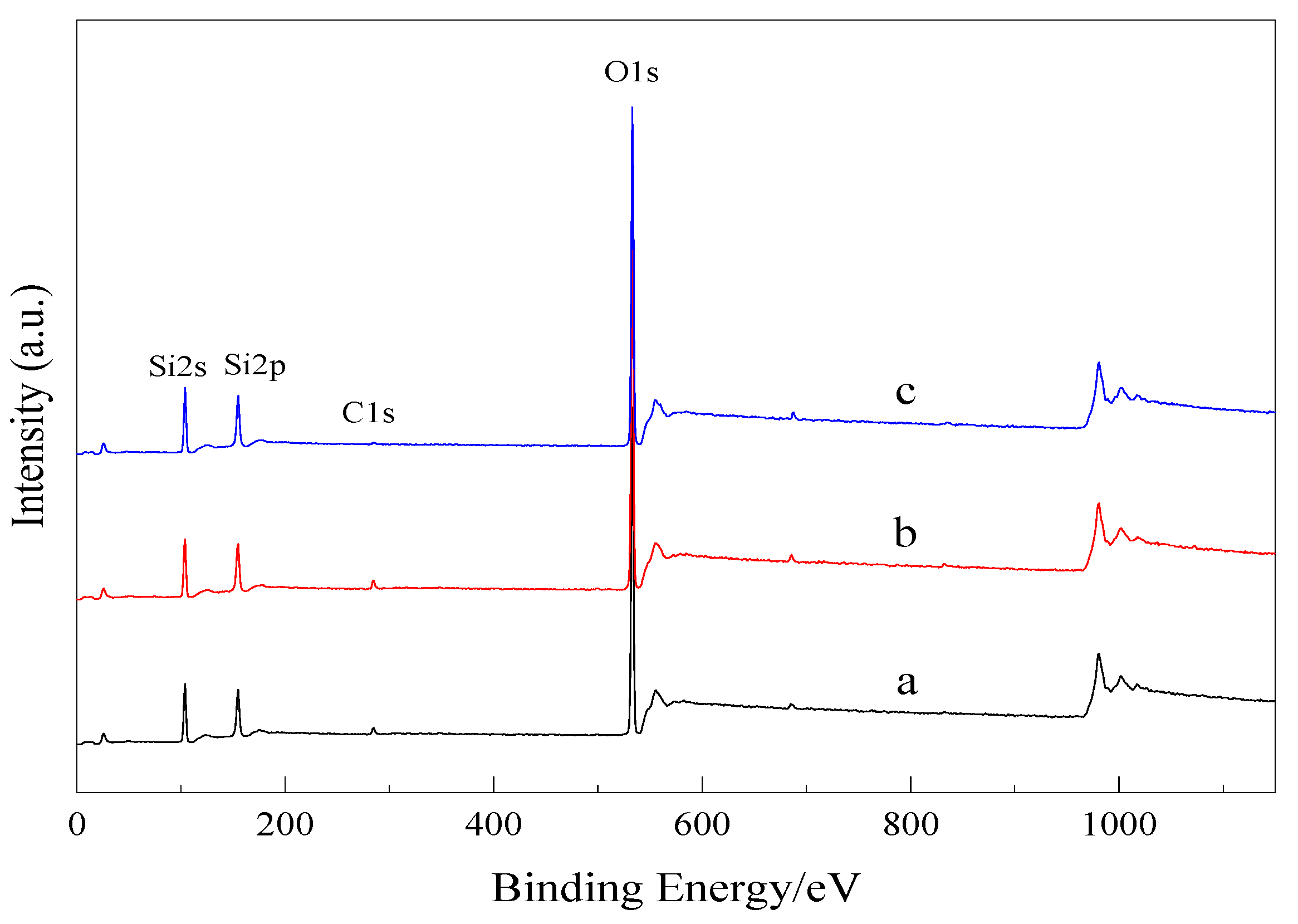
3.2.2. XPS Analysis
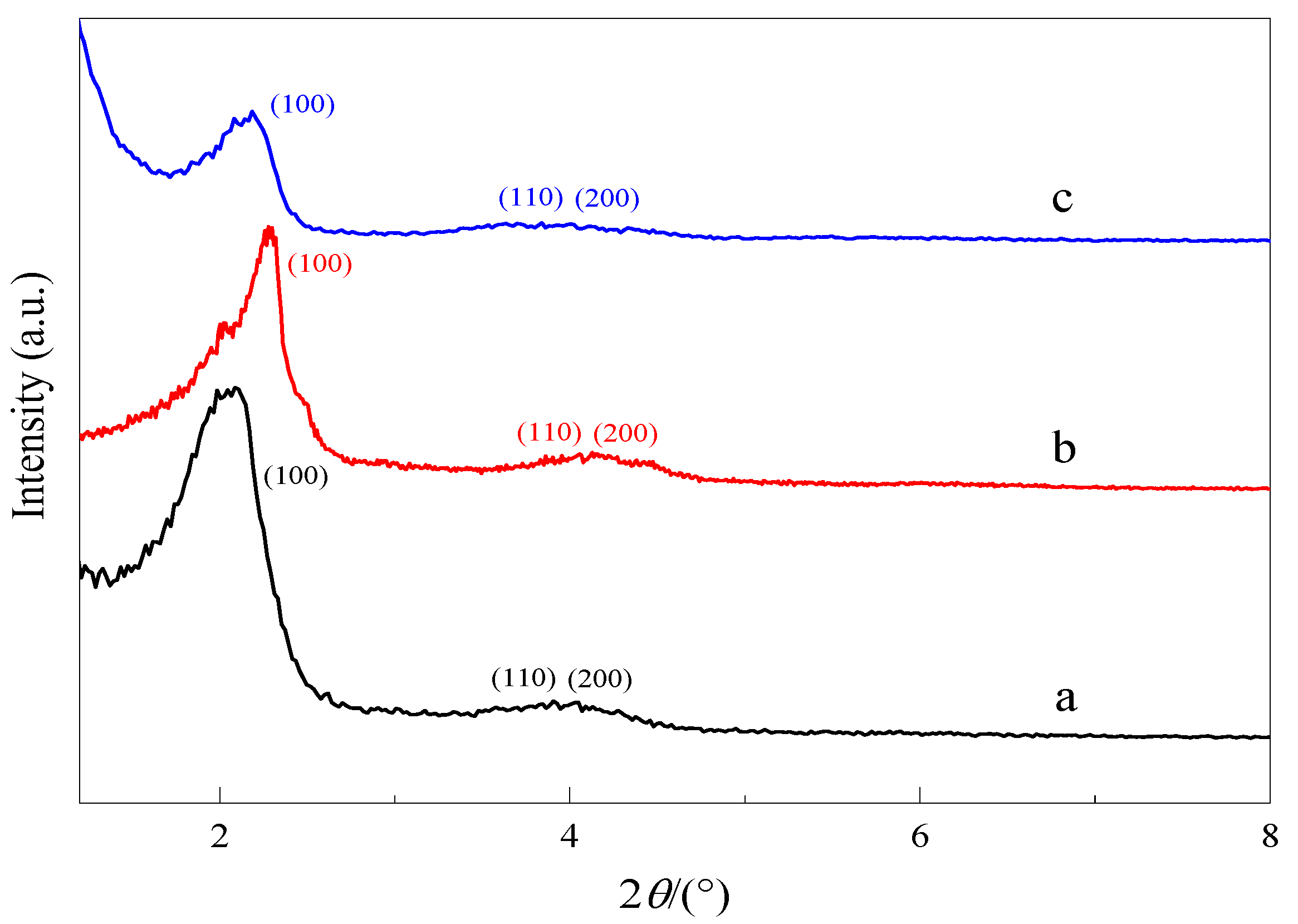
3.2.3. X-ray Diffraction
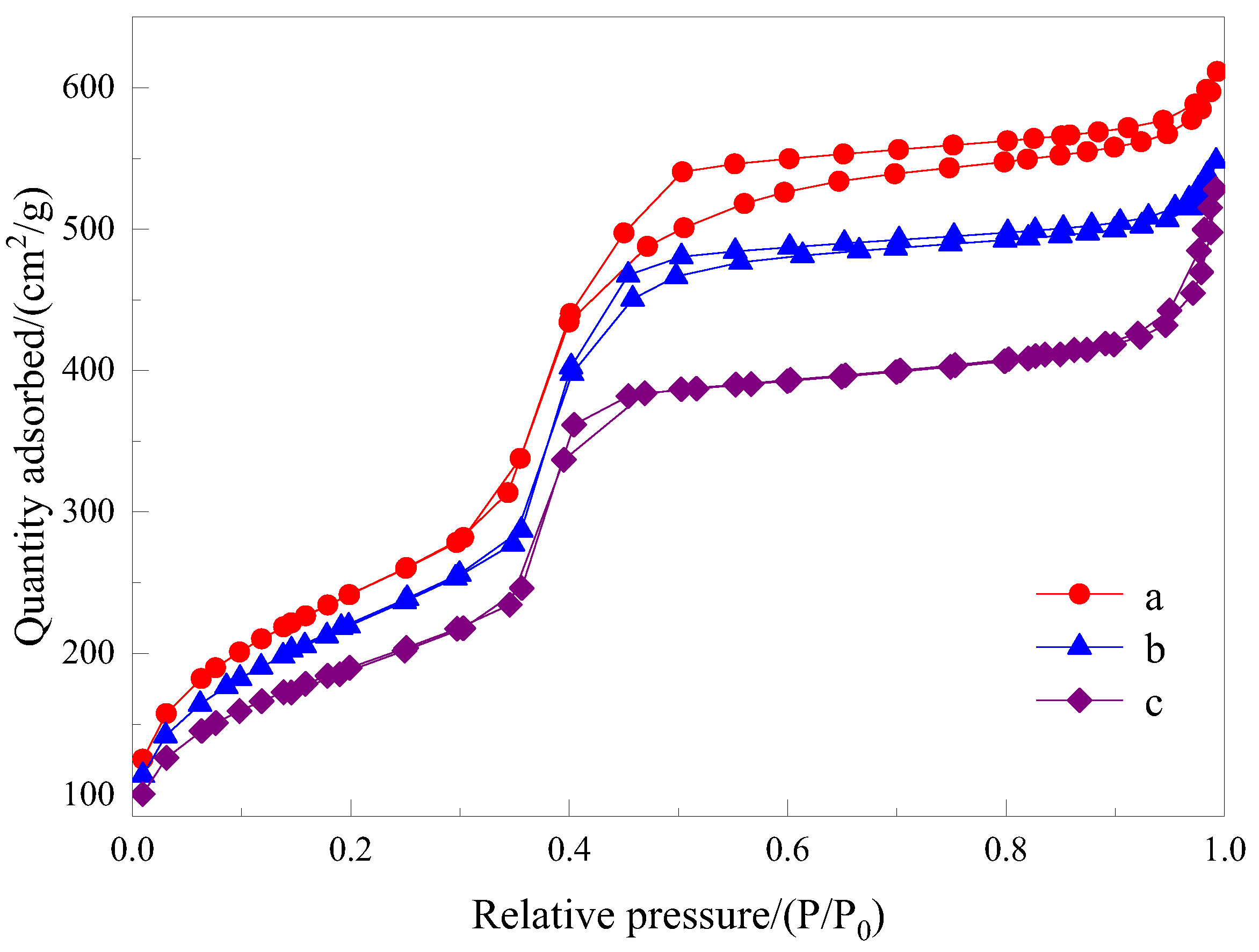
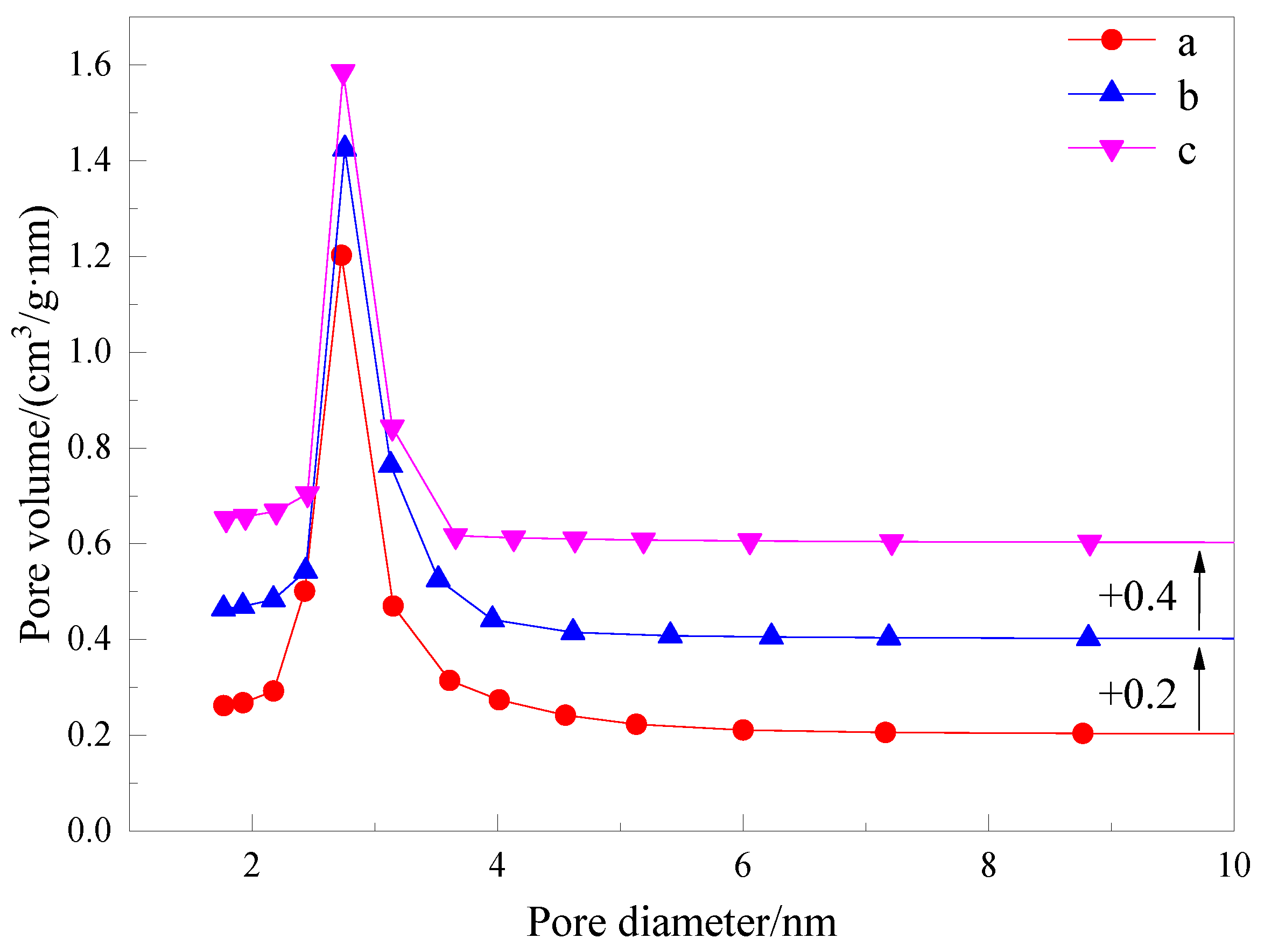
3.2.4. Nitrogen Adsorption–Desorption Isotherms
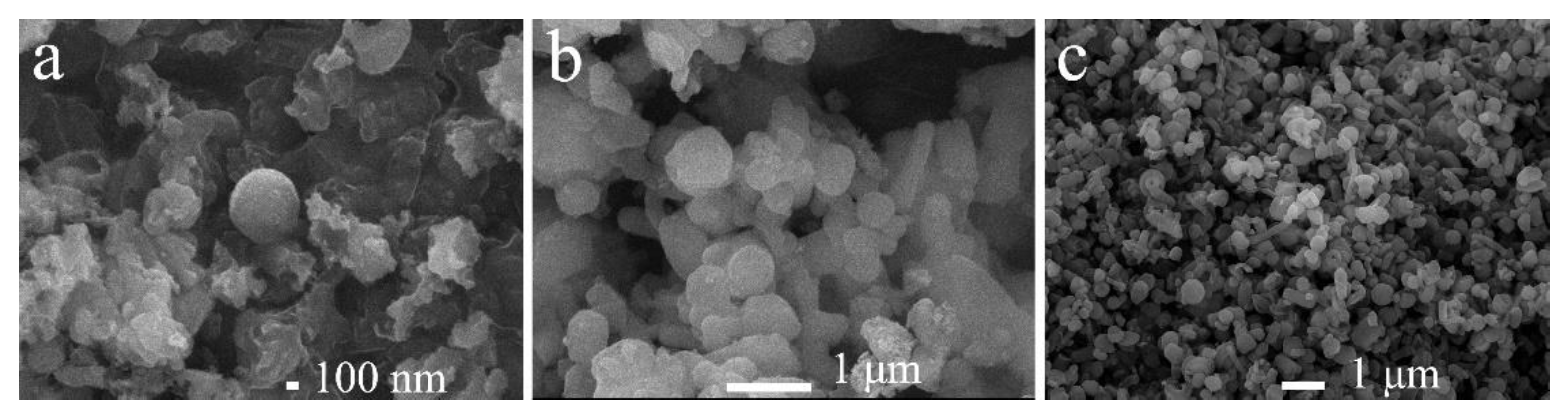
3.2.5. Scanning and Transmission Electron Microscopy
3.3. Formation Mechanism of Mesoporous Molecular Sieves
3.4. Adsorption Properties of Mesoporous Material
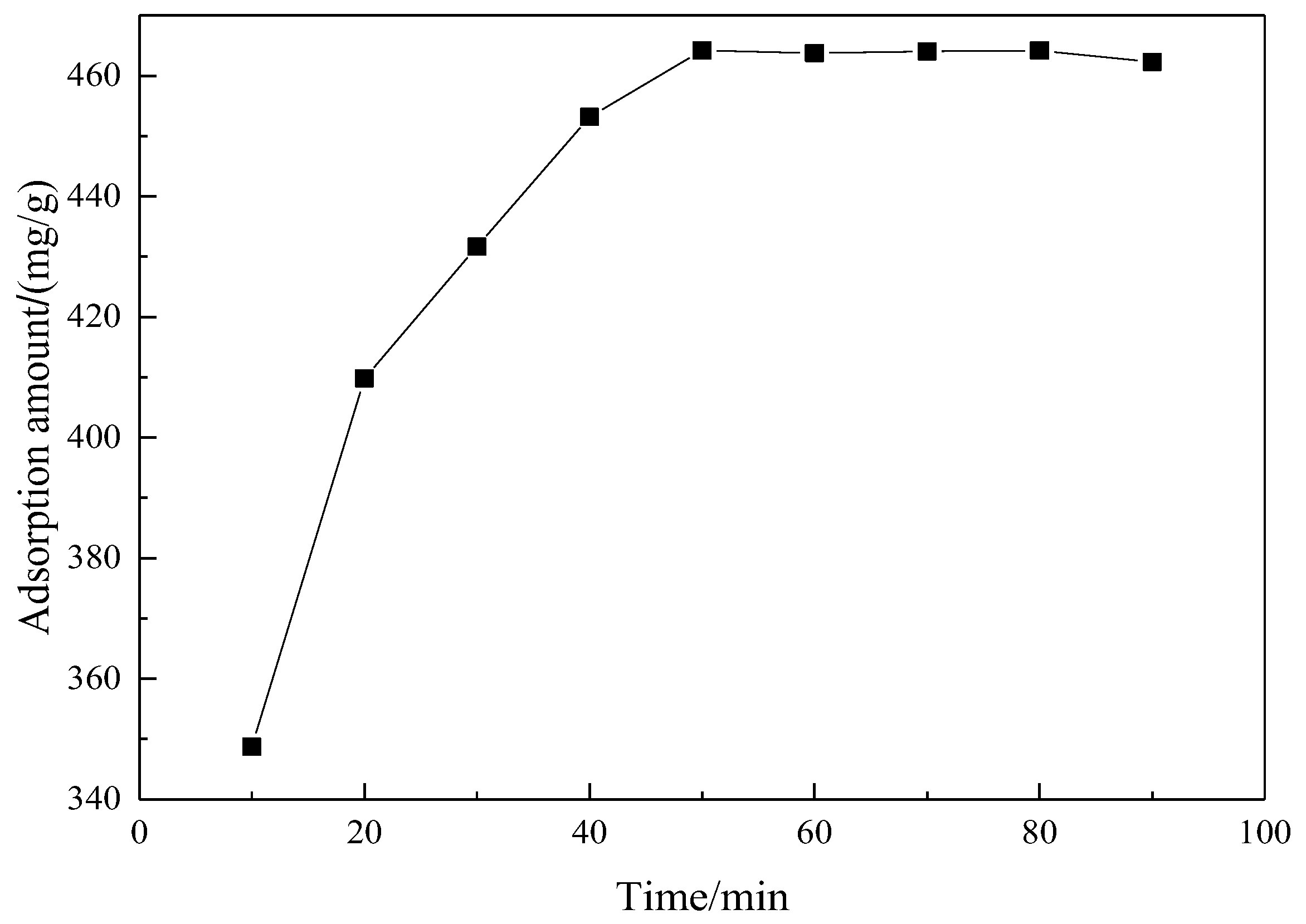
3.4.1. The Effect of Adsorption Time on the Adsorption Performance of Mesoporous Material
3.4.2. The Effect of pH of Solution on the Adsorption Performance of Mesoporous Material
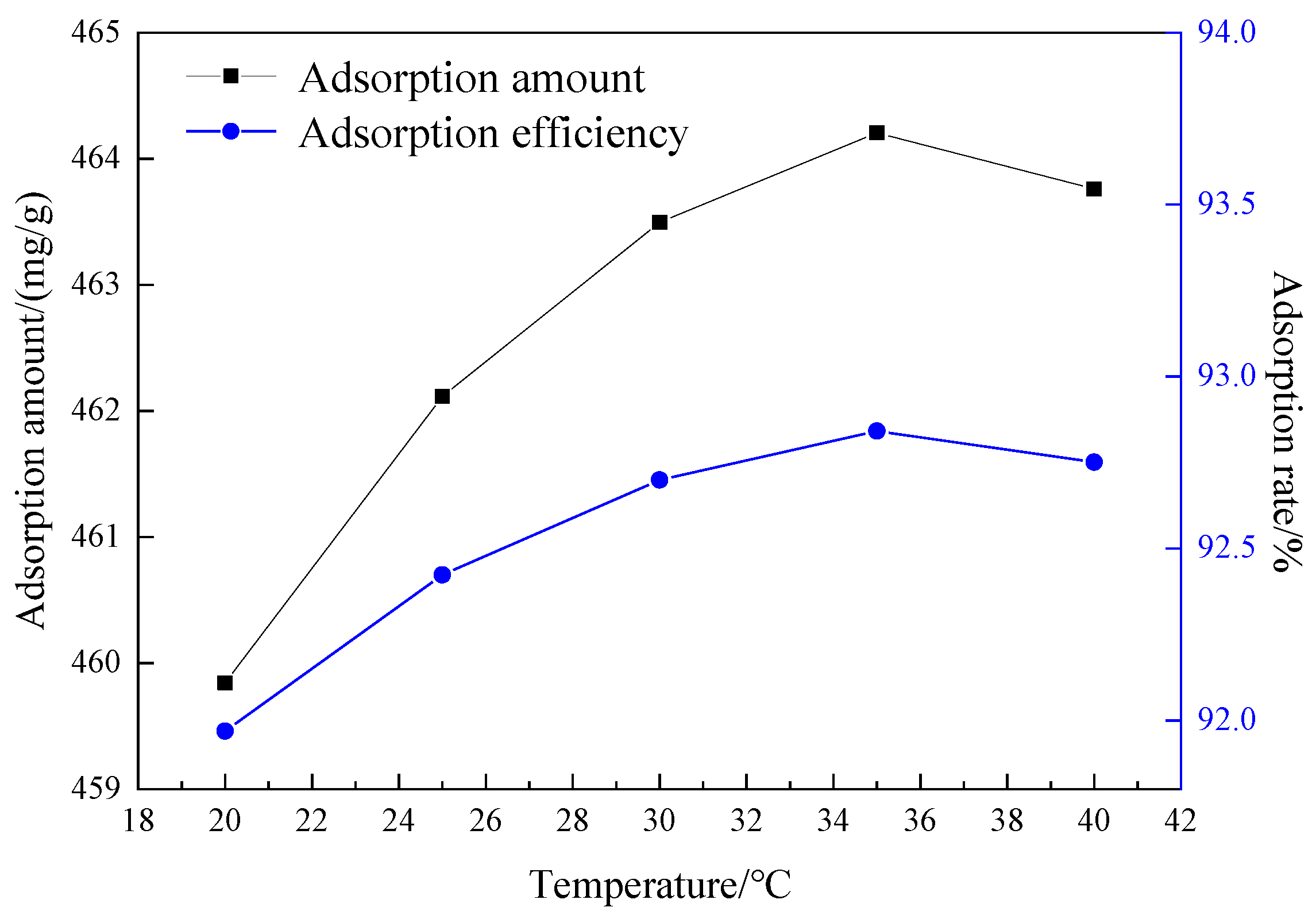
3.4.3. The Effect of Adsorption Temperature on the Adsorption Performance of Mesoporous Material
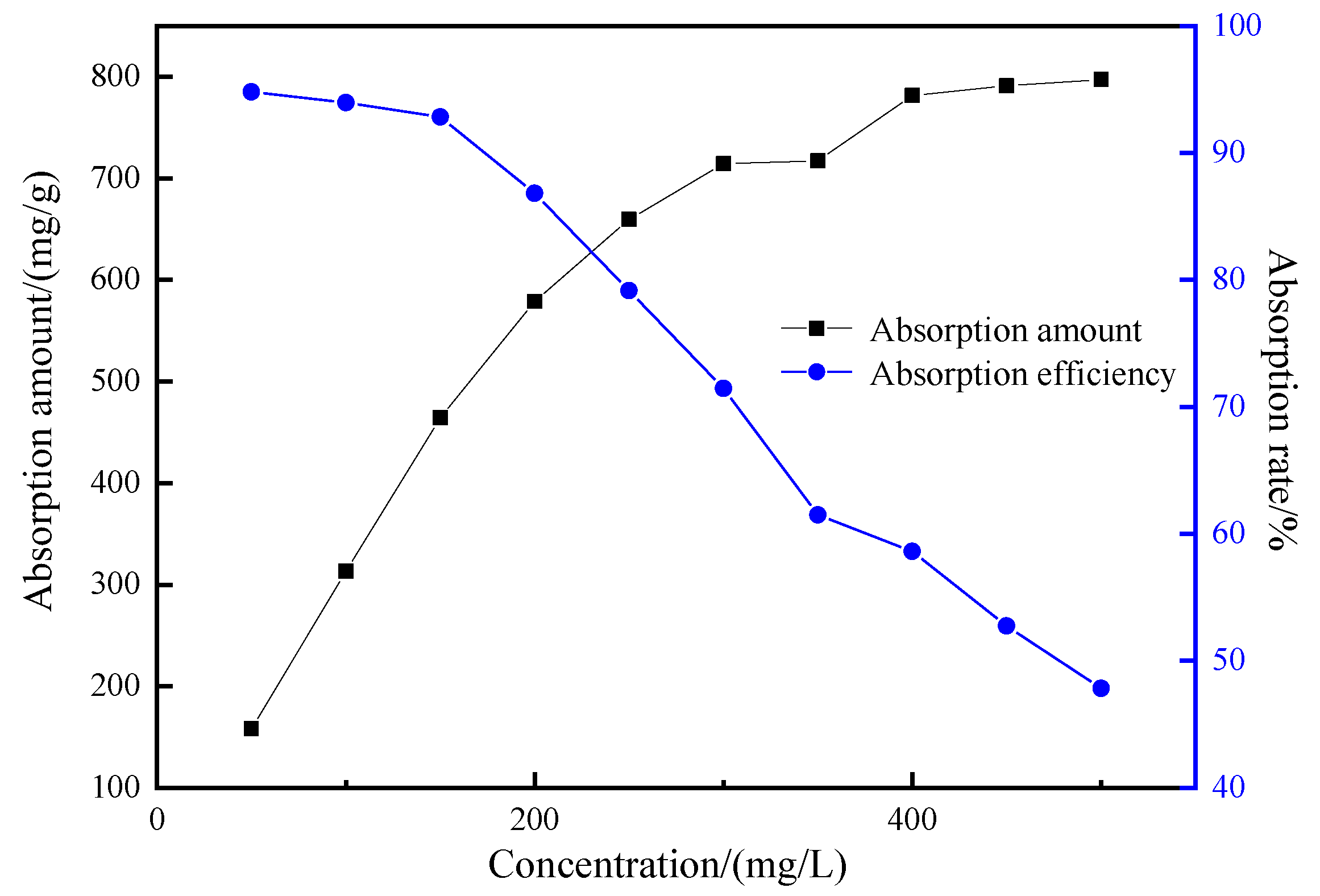
3.4.4. The Effect of Initial Concentration of Adsorbate on the Adsorption Performance of Mesoporous Material
3.5. Analysis of Adsorption Kinetics and Adsorption Isotherm Regression Analysis of Mesoporous Materials
3.5.1. Analysis of Adsorption Kinetics of Mesoporous Materials
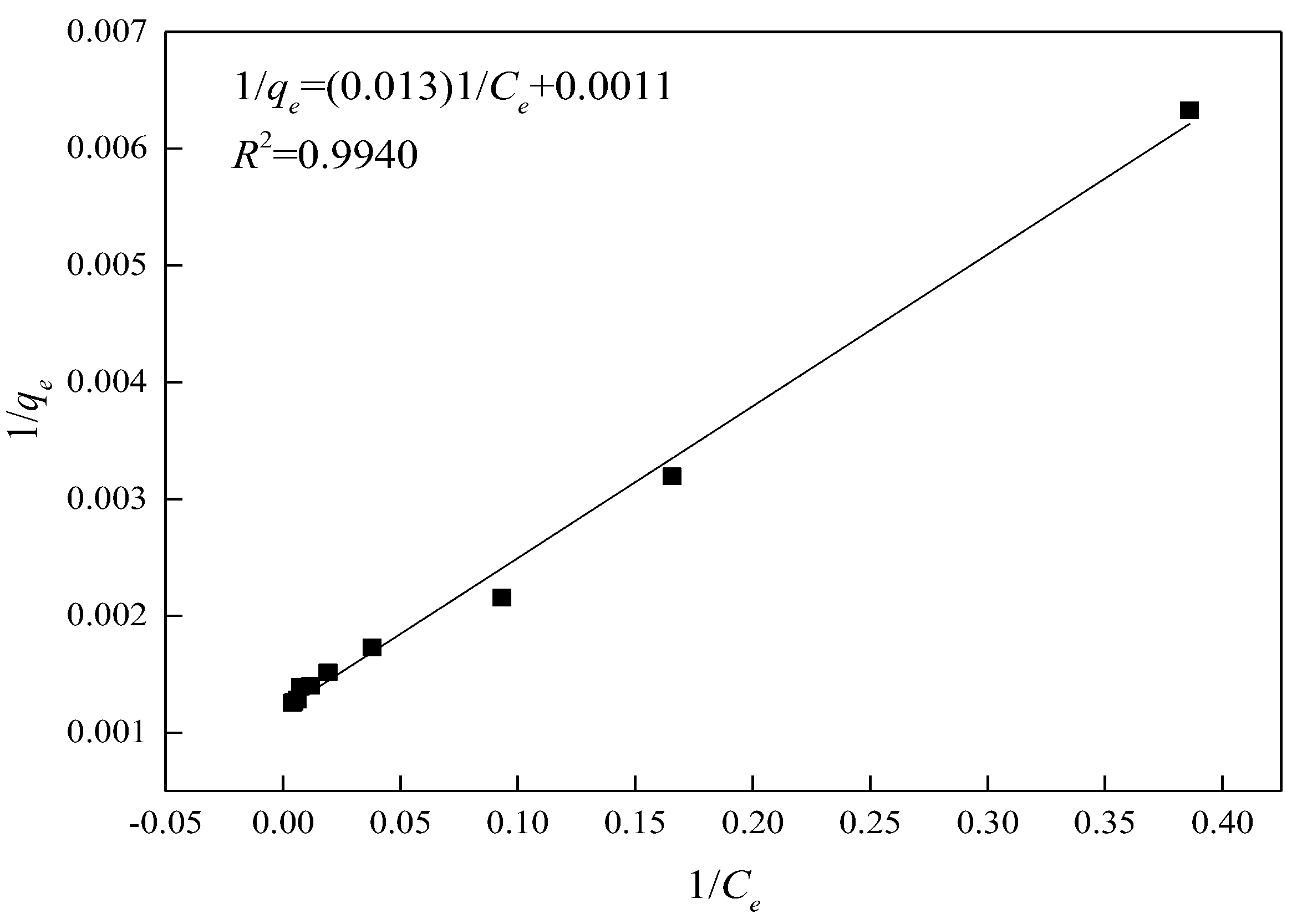
3.5.2. Analysis of the Adsorption Isotherm Regression
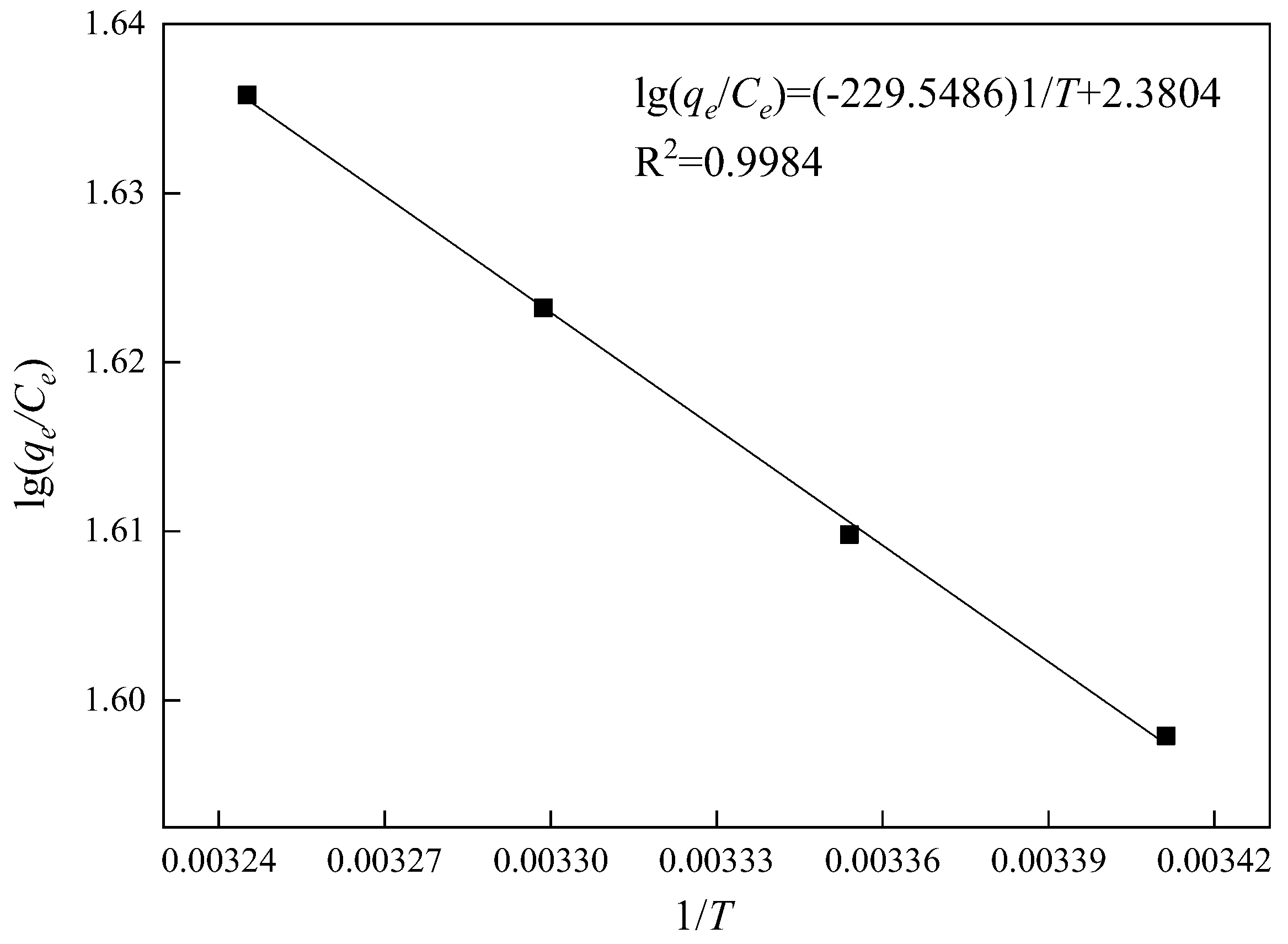
3.5.3. Adsorption Thermodynamic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Du, X.; Liu, Z.; Shi, S.; Lv, H. Dendritic fibrous nano-particles (DFNPs): Rising stars of mesoporous materials. J. Mater. Chem. A 2019, 7, 5111–5152. [Google Scholar] [CrossRef]
- Liu, J.; Wei, X.; Xue, J.; Su, H. Preparation and adsorption properties of mesoporous material PS-MCM-41 with low-silicon content peanut shell ash as silicon source. Mater. Chem. Phys. 2020, 241, 122355. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Zhao, X.H. Synthesis and Characterization of Ordered Porous Silica, Using New Surfac-Tants as Templates. Master’s Thesis, Shandong University, Shandong, China, April 2008. [Google Scholar]
- Voort, P.; Mathieu, M.; Mees, F.; Vansant, E.F.J. Synthesis of high-quality MCM-48 and MCM-41 by means of the GEMINI surfactant method. J. Phys. Chem. B 1998, 102, 8847–8851. [Google Scholar] [CrossRef]
- Gianotti, E.; Berlier, G.; Costabello, K.; Coluccia, S.; Meneau, F. In situ synchrotron small-angle X-ray scattering study of MCM-41 crystallisation using Gemini surfactants. Catal. Today 2007, 126, 203–210. [Google Scholar] [CrossRef]
- Wang, S.; Han, S.; Cui, X.; Qiu, X. Effects of the spacer length of gemini surfactants on the ordered pore of silica. J. Porous Mater. 2012, 19, 243–249. [Google Scholar] [CrossRef]
- Li, M.; Zhang, C.; Yang, X.-L.; Xu, H.-B. Controllable synthesis of hollow mesoporous silica nanoparticles templated by kinetic self-assembly using a gemini surfactant. RSC Adv. 2013, 3, 16304–16307. [Google Scholar] [CrossRef]
- Ji, J.W. Synthesis of Novel Amphiphiles and their Application in Preparation of Mesoporous Materials. Master’s Thesis, Tsinghua University, Beijing, China, April 2017. [Google Scholar]
- Lee, C.K.; Liu, S.S.; Juang, L.C.; Wang, C.C.; Lin, K.S.; Lyu, M. Application of MCM-41 for dyes removal from wastewater. J. Hazard Mater. 2007, 147, 997–1005. [Google Scholar] [CrossRef]
- Shao, Y.M.; Sun, Q.Q.; Zhang, Q.Y.; Li, L.S.; Liu, P.H. Application of nickel modi-fied MCM-41 as an adsorbent to remove methyl blue from aqueous solution. Acta. Sci. Circum. 2014, 34, 3011–3016. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.L.; Ran, G.; Cui, Z.; Chen, S.H.; Xie, K. Application of Amino-Functionalized MCM-41 as an adsorbent to remove methylene blue from water. Fine Chem. 2016, 33, 188–194. [Google Scholar]
- Sun, Z.B.; Li, B.; Guo, C.L.; Fan, J.X. Research progress on adsorption proper-ties of mesoporous silica zeolite. N. Chem. Mater. 2021, 3, 1–11. [Google Scholar]
- Guo, W.; Yu, C.; Li, S.F.; Wang, Z.; Yu, J.H.; Huang, H.W.; Qiu, J.S. Strategies and insights towards the intrinsic capacitive properties of MnO2 for supercapacitors: Challenges and perspectives. Nano Energy 2019, 57, 459–472. [Google Scholar] [CrossRef]
- Li, Q.C.; Deng, Y.L.; Huang, J.P. Low-cost synthesis of MCM-41 mesoporous molecular sieves and its adsporption property of crystal violet. B Chin. Ceram. Soc. 2019, 38, 3807–3813. [Google Scholar]
- An, Q.L. Studies on the Synthesis and Properties of Mesoporous Molecular sieve MCM-41. Master’s Thesis, Xi’an University of Science and Technology, Xi’an, China, April 2005. [Google Scholar]
- Zana, R.; Benrraou, M.; Rueff, R. Alkanediyl-alpha, omega-bis(dimethylalkylammonium bromide) surfactants. 1. Effect of the spacer chain length on the critical micelle concentration and micelle ionization degree. Langmuir 1991, 7, 1072–1075. [Google Scholar] [CrossRef]
- Li, J.J.; Gao, G. The thermodynamic and kinetic analysis of adsorption properties of MCM-41 mesoporous molecular sieves. Contemp. Chem. Ind. 2017, 46, 841–845. [Google Scholar]
- Sanaeishoar, H.; Sabbaghan, M.; Mohave, F. Synthesis and characterization of micro-mesoporous MCM-41 using various ionic liquids as co-templates. Micropor. Mesopor. Mater. 2015, 217, 219–224. [Google Scholar] [CrossRef]
- Rameli, N.; Jumbri, K.; Wahab, R.A.; Ramli, A.; Huyop, F. Synthesis and characterization of mesoporous silica nanoparticles using ionic liquids as a template. J. Phys. Conf. Ser. 2018, 1123, 012068. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.L.; Bai, Y.R.; Li, Q.; Yang, X.Z. Synthesis of mesoporous silica with ionic liquid surfactant as template. Mater. Lett. 2021, 291, 129556. [Google Scholar] [CrossRef]
- Zhai, Q.Z. Studies of adsorption of crystal violet from aqueous solution by nano mesocellular foam silica: Process equilibrium, kinetic, isotherm, and thermodynamic studies. Water Sci. Technol. 2020, 81, 2092–2108. [Google Scholar] [CrossRef]
- Li, Y. Research of Reduction of Acrylic Fiber Wastewater by Fenton Process. Master’s Thesis, Hebei University of Engineering, Handan, China, May 2010. [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Jiang, S.; Lu, L.L.; Guo, H.W. Adsorption of methylene blue by hazelnut shell powder. Nat. Prod. Res. Dev. 2017, 29, 110–113. [Google Scholar]

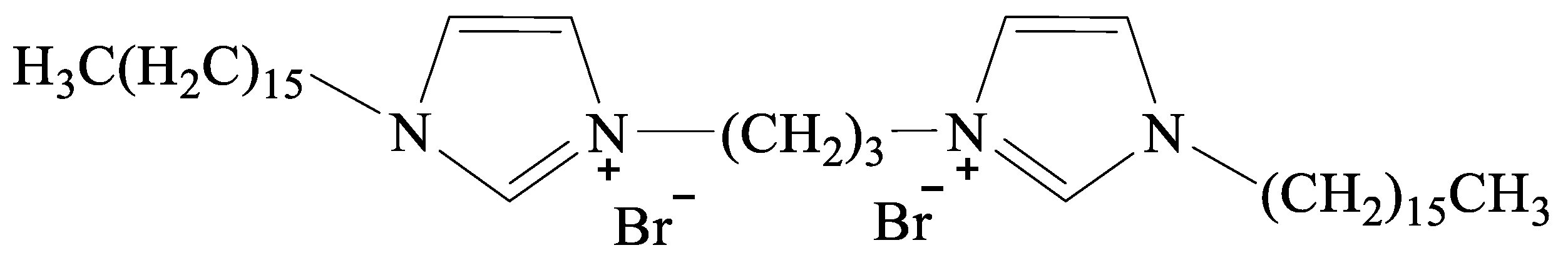
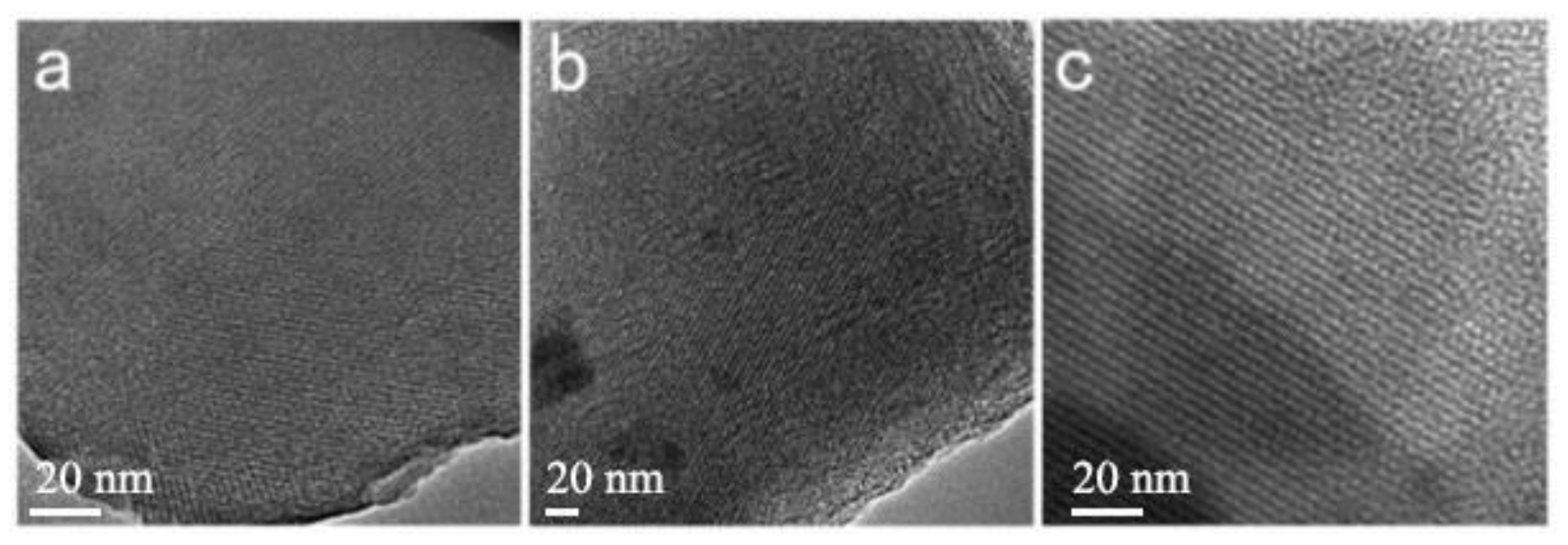



| Sample | Reactants | Molar Ratio | Mass Ratio |
|---|---|---|---|
| a | TEOS/[N1116C3IMC16][Br]2/H2O | 1.0:0.1:100.0 | 2.08/0.79/17.97 |
| b | TEOS/[N1116C3N1116][Br]2/H2O | 1.0:0.1:100.0 | 2.08/0.74/17.97 |
| c | TEOS/[C16IMC3C16IM][Br]2/H2O | 1.0:0.1:100.0 | 2.08/0.79/17.97 |
| Sample | Name | Peak Position/eV | Full Width at Half Maximum /(FWHM) | Atomic Concentration/% |
|---|---|---|---|---|
| a | Si2p | 103.96 | 1.54 | 12.79 |
| O1s | 533.31 | 1.49 | 84.97 | |
| C1s | 284.88 | 1.53 | 2.24 | |
| b | Si2p | 103.84 | 1.52 | 12.60 |
| O1s | 533.22 | 1.44 | 84.92 | |
| C1s | 284.82 | 1.42 | 2.48 | |
| c | Si2p | 103.93 | 2.75 | 31.42 |
| O1s | 533.14 | 2.73 | 64.52 | |
| C1s | 285.32 | 4.19 | 2.34 |
| Sample | SBET/(m2/g) | VBJH/(cm3/g) | a0/nm | Dp/nm | Wt/nm |
|---|---|---|---|---|---|
| a | 879.37 | 0.85 | 3.88 | 3.28 | 0.90 |
| b | 803.01 | 0.95 | 4.23 | 3.32 | 0.59 |
| c | 684.08 | 0.82 | 4.78 | 3.86 | 0.92 |
| t/min | Ce/(mg/L) | qt/(mg/g) | t/qt |
|---|---|---|---|
| 10 | 45.38 | 348.73 | 0.0287 |
| 20 | 27.05 | 409.83 | 0.0488 |
| 30 | 20.51 | 431.63 | 0.0695 |
| 40 | 14.05 | 453.16 | 0.0883 |
| 50 | 10.74 | 464.21 | |
| 60 | 10.87 | 463.76 | |
| 70 | 10.79 | 464.02 | |
| 80 | 10.74 | 464.21 | |
| 90 | 11.32 | 462.26 |
| C0/(mg/L) | Ce/(mg/L) | qe/(mg/g) | 1/Ce | 1/qe |
|---|---|---|---|---|
| 50 | 2.59 | 158.03 | 0.3861 | 0.0063 |
| 100 | 6.04 | 313.21 | 0.1657 | 0.0032 |
| 150 | 10.74 | 464.21 | 0.0931 | 0.0022 |
| 200 | 26.34 | 578.88 | 0.0380 | 0.0017 |
| 250 | 52.12 | 659.61 | 0.0192 | 0.0015 |
| 300 | 85.63 | 714.57 | 0.0117 | 0.0014 |
| 350 | 134.77 | 717.43 | 0.0074 | 0.0014 |
| 400 | 165.51 | 781.62 | 0.0060 | 0.0013 |
| 450 | 212.64 | 791.18 | 0.0047 | 0.0013 |
| 500 | 260.83 | 797.24 | 0.0038 | 0.0013 |
| T/K | Ce (mg/L) | qe (mg/g) | qe/Ce | lg(qe/Ce) |
|---|---|---|---|---|
| 293.15 | 11.65 | 461.17 | 39.59 | 1.60 |
| 298.15 | 11.35 | 462.17 | 40.72 | 1.61 |
| 303.15 | 11.03 | 463.23 | 41.99 | 1.62 |
| 308.15 | 10.74 | 464.21 | 43.23 | 1.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Bai, Y.; Li, Q.; Wang, J. Preparation and Adsorption Properties of MCM-41 with Novel Gemini Ionic Liquid Surfactants as Template. Materials 2022, 15, 2780. https://doi.org/10.3390/ma15082780
Yang X, Bai Y, Li Q, Wang J. Preparation and Adsorption Properties of MCM-41 with Novel Gemini Ionic Liquid Surfactants as Template. Materials. 2022; 15(8):2780. https://doi.org/10.3390/ma15082780
Chicago/Turabian StyleYang, Xuzhao, Yarong Bai, Qing Li, and Jun Wang. 2022. "Preparation and Adsorption Properties of MCM-41 with Novel Gemini Ionic Liquid Surfactants as Template" Materials 15, no. 8: 2780. https://doi.org/10.3390/ma15082780
APA StyleYang, X., Bai, Y., Li, Q., & Wang, J. (2022). Preparation and Adsorption Properties of MCM-41 with Novel Gemini Ionic Liquid Surfactants as Template. Materials, 15(8), 2780. https://doi.org/10.3390/ma15082780






