Thermal Properties of Geopolymer Based on Fayalite Waste from Copper Production and Metakaolin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Geopolymer Precursors
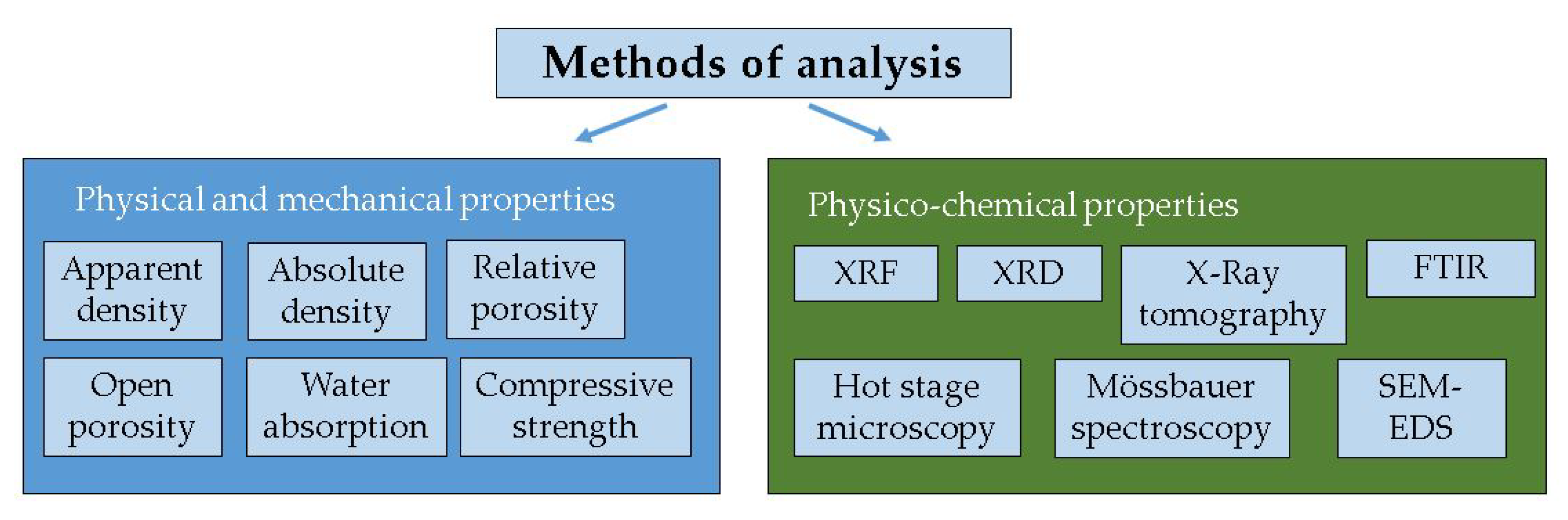
2.2. Methods of Analysis
2.3. Geopolymer Synthesis
3. Results
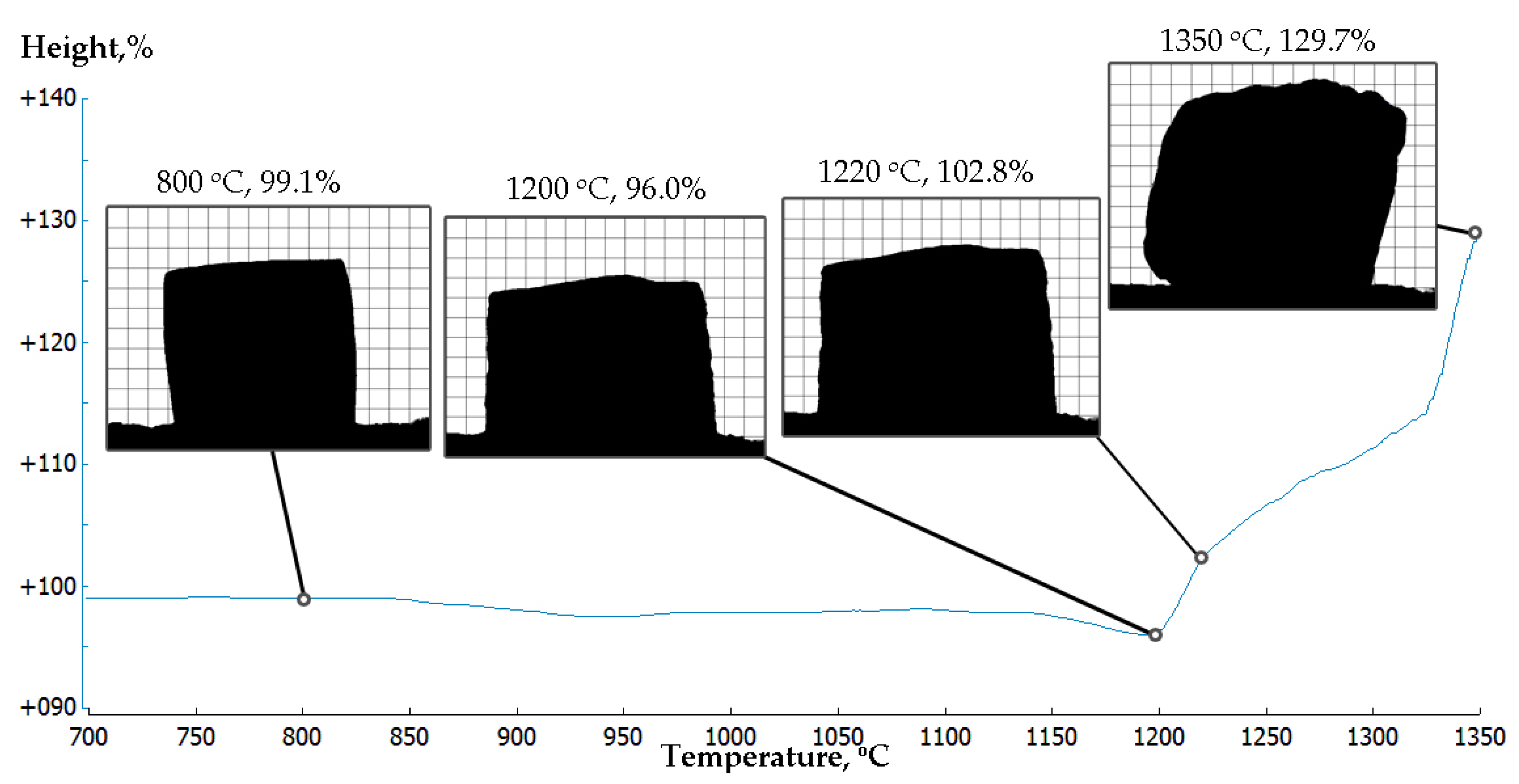
3.1. Hot Stage Experiments
3.2. Thermal Treatment of the Prepared Geopolymer Specimens
3.2.1. Physical and Mechanical Properties
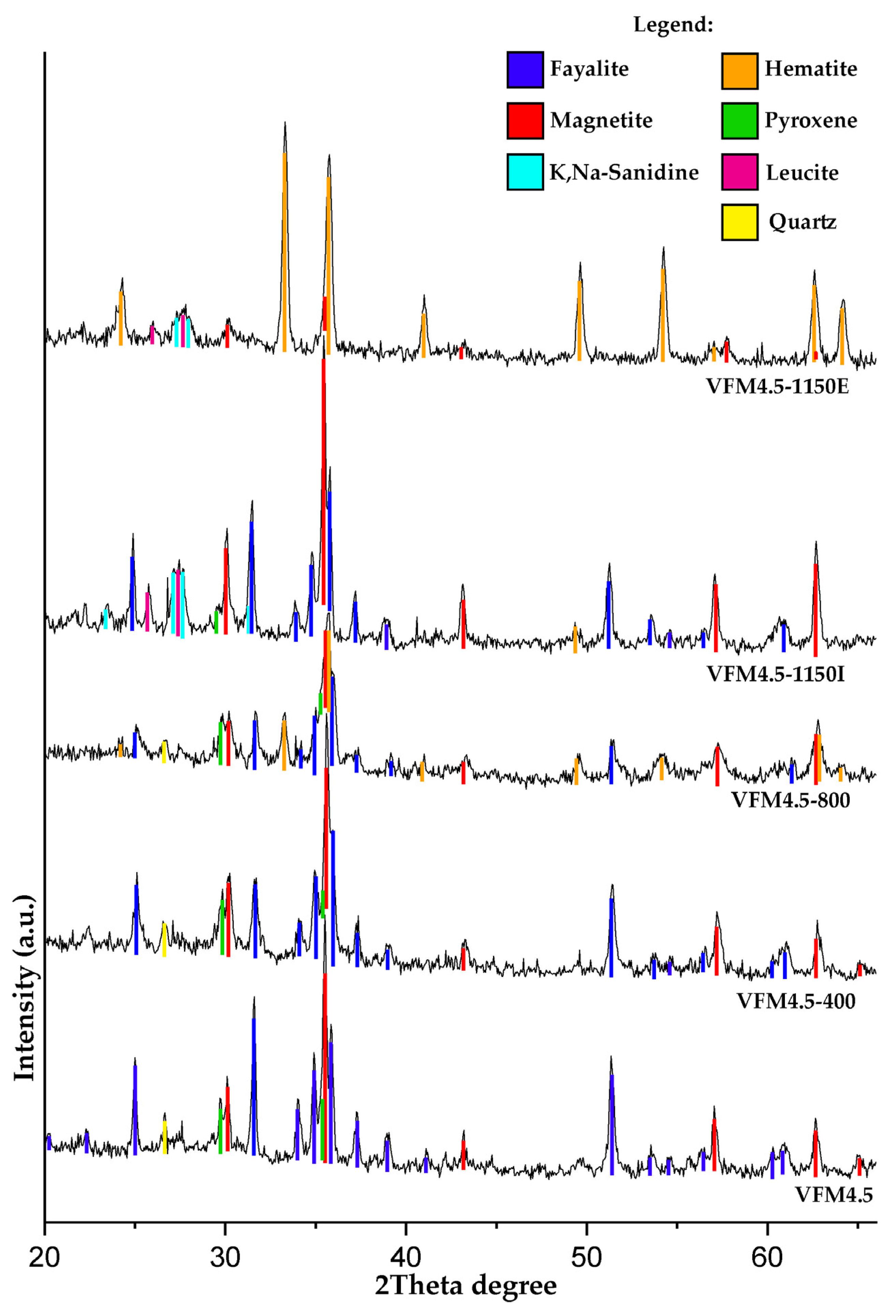
3.2.2. Powder XRD
3.2.3. Mössbauer Spectroscopy
3.2.4. FTIR
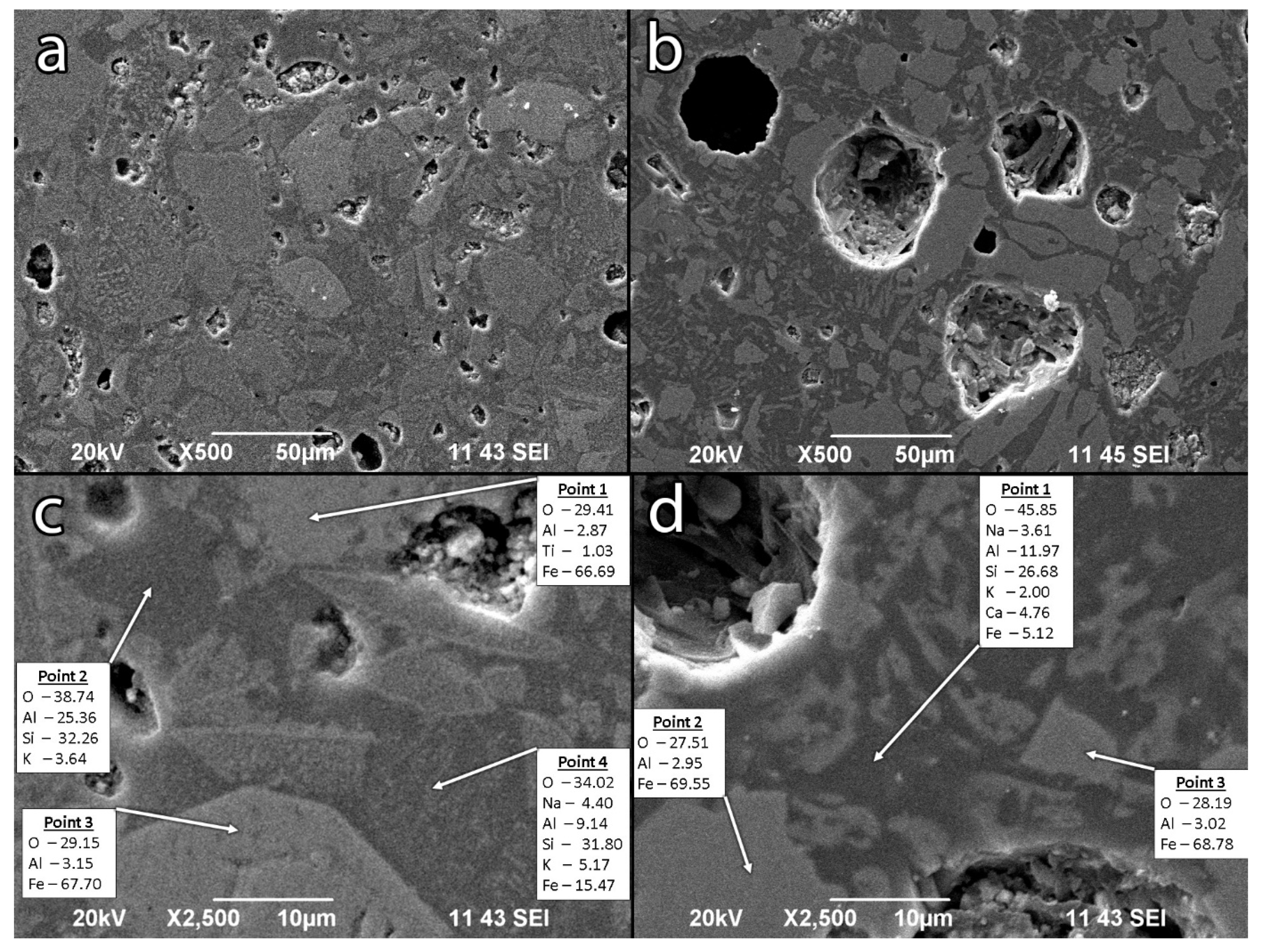
3.2.5. SEM-EDS
4. Conclusions
- Geopolymers based on fayalite slag, a waste from a copper producing plant, showed fire-resistance up to 1150 °C.
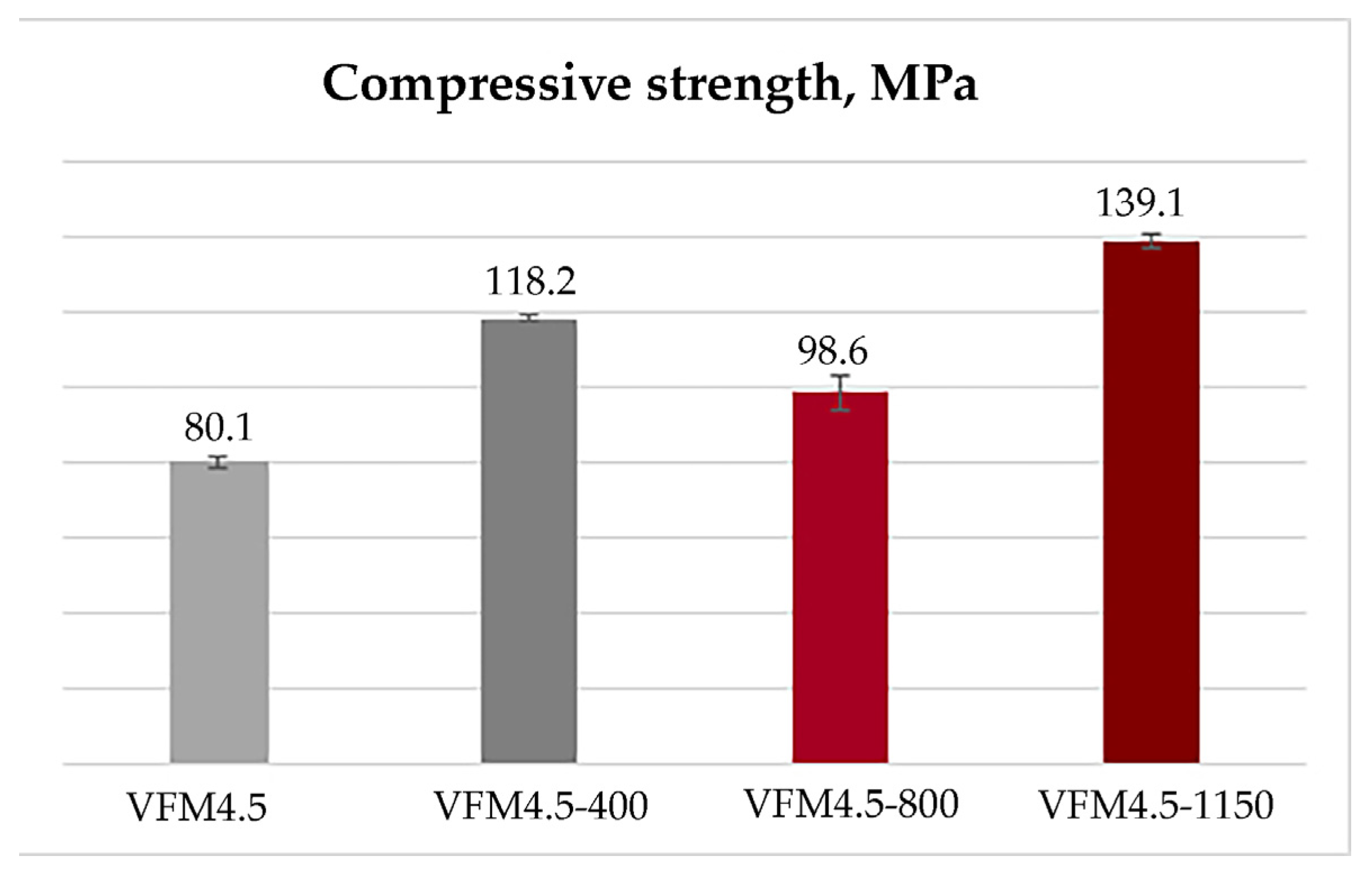
- The thermal exposure of the obtained geopolymers led to significant changes in their physical properties and microstructure. Contrary to the ordinary Portland cement materials, the compressive strength of the obtained geopolymers increased from 80 MPa at room temperature to 139 MPa after heating to 1150 °C, and water absorption and open porosity decreased significantly to only 1.21% and 3%, respectively.
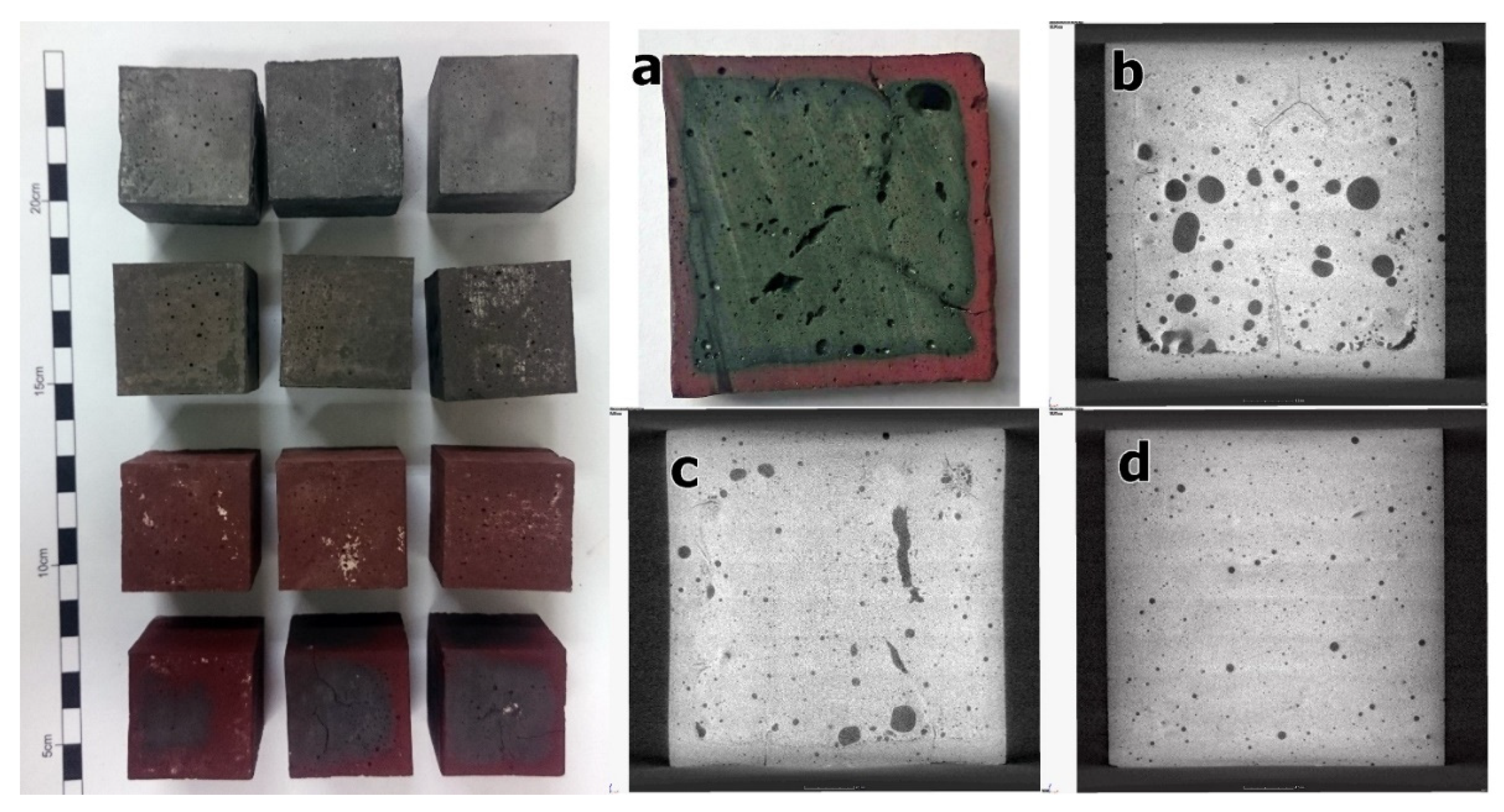
- Changes between the outer and inner layers of the specimens were observed above 800 °C. At 1150 °C, the structure of the outer layer was characterized by a color change to reddish due to the oxidation of the iron phases to hematite, while the inner core remained black due to the presence of magnetite. In both layers, there was partial crystallization of the geopolymer gel into leucite and K,Na-sanidine.
- At 1150 °C, the outer layer was more rigid, while the inner core was characterized by a lower viscosity due to the presence of Fe2+. As a result, coalescence processes in the inner core were observed.
- Partial substitutions of Al and Fe were detected in the geopolymer gel and magnetite/hematite phases after exposure to 1150 °C.
- At temperatures above 1200 °C, the geopolymers based on fayalite waste started to melt and expand vigorously.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davidovits, J. Geopolymer Chemistry and Applications, 4th ed.; J. Davidovits: Saint-Quentin, France, 2015; p. 11. [Google Scholar]
- Provis, J.L.; Van Deventer, J.S.J. Geopolymers: Structures, Processing, Properties and Industrial Applications; Woodhead Publishing Limited: Cambridge, UK, 2009; p. 78. [Google Scholar]
- Ren, B.; Zhao, Y.; Bai, H.; Kang, S.; Zhang, T.; Song, S. Eco-friendly geopolymer prepared from solid wastes: A critical review. Chemosphere 2021, 267, 128900. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, S.; Yilmaz, E.; Liu, Y. Compressive fatigue behavior and failure evolution of additive fiber-reinforced cemented tailings composites. Int. J. Miner. Metall. Mater. 2022, 29, 345–355. [Google Scholar] [CrossRef]
- De Azevedo, A.R.; Marvila, M.T.; Ali, M.; Khan, M.I.; Masood, F.; Vieira, C.M.F. Effect of the addition and processing of glass polishing waste on the durability of geopolymeric mortars. Case Stud. Constr. Mater. 2021, 15, e00662. [Google Scholar] [CrossRef]
- Alvee, A.R.; Malinda, R.; Akbar, A.M.; Ashar, R.D.; Rahmawati, C.; Alomayri, T.; Raza, A.; Shaikh, F.U.A. Experimental study of the mechanical properties and microstructure of geopolymer paste containing nano-silica from agricultural waste and crystalline admixtures. Case Stud. Constr. Mater. 2022, 16, e00792. [Google Scholar] [CrossRef]
- Vickers, L.; Van Riessen, A.; Rickard, W.D. Fire-Resistant Geopolymers: Role of Fibres and Fillers to Enhance Thermal Properties; Springer: Berlin/Heidelberg, Germany, 2015; pp. 10–14. [Google Scholar]
- Xiao, J.; König, G. Study on concrete at high temperature in China—An overview. Fire Saf. J. 2004, 39, 89–103. [Google Scholar] [CrossRef]
- Lahoti, M.; Wong, K.K.; Yang, E.H.; Tan, K.H. Effects of Si/Al molar ratio on strength endurance and volume stability of metakaolin geopolymers subject to elevated temperature. Ceram. Int. 2018, 44, 5726–5734. [Google Scholar] [CrossRef]
- Bakharev, T. Thermal behaviour of geopolymers prepared using class F fly ash and elevated temperature curing. Cem. Concr. Res. 2006, 36, 1134–1147. [Google Scholar] [CrossRef]
- Luhar, S.; Nicolaides, D.; Luhar, I. Fire Resistance Behaviour of Geopolymer Concrete: An Overview. Buildings 2021, 11, 82. [Google Scholar] [CrossRef]
- Gambo, S.; Ibrahim, K.; Aliyu, A.; Ibrahim, A.G.; Abdulsalam, H. Performance of metakaolin based geopolymer concrete at elevated temperature. Niger. J. Technol. 2020, 39, 732–737. [Google Scholar] [CrossRef]
- Peng, X.; Li, H.; Shuai, Q.; Wang, L. Fire Resistance of Alkali Activated Geopolymer Foams Produced from Metakaolin and Na2O2. Materials 2020, 13, 535. [Google Scholar] [CrossRef] [Green Version]
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Ariffin, M.A.M.; Bhutta, M.A.R.; Hussin, M.W.; Tahir, M.M.; Aziah, N. Sulfuric acid resistance of blended ash geopolymer concrete. Constr. Build. Mater. 2013, 43, 80–86. [Google Scholar] [CrossRef]
- Mehta, A.; Siddique, R. Sulfuric acid resistance of fly ash based geopolymer concrete. Constr. Build. Mater. 2017, 146, 136–143. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, L.; San Nicolas, R. Degradation process of alkali-activated slag/fly ash and Portland cement-based pastes exposed to phosphoric acid. Constr. Build. Mater. 2020, 232, 117209. [Google Scholar] [CrossRef]
- Wong, L.S. Durability Performance of Geopolymer Concrete: A Review. Polymers 2022, 14, 868. [Google Scholar] [CrossRef]
- Valencia Saavedra, W.G.; Angulo, D.E.; de Gutiérrez, R. Fly ash slag geopolymer concrete: Resistance to sodium and magnesium sulfate attack. J. Mater. Civ. Eng. 2016, 28, 4016148. [Google Scholar] [CrossRef]
- Thokchom, S.; Ghosh, P.; Ghosh, S. Durability of fly ash geopolymer mortars in nitric acid—Effect of alkali (Na2O) content. J. Civ. Eng. Manag. 2011, 17, 393–399. [Google Scholar] [CrossRef]
- Nugteren, H.W.; Butselaar-Orthlieb, V.C.L.; Izquierdo, M. High strength geopolymers produced from coal combustion fly ash. Glob. NEST J. 2009, 11, 155–161. [Google Scholar]
- Mahmoodi, O.; Siad, H.; Lachemi, M.; Dadsetan, S.; Sahmaran, M. Development of normal and very high strength geopolymer binders based on concrete waste at ambient environment. J. Clean. Prod. 2021, 279, 123436. [Google Scholar] [CrossRef]
- Nikolov, A.; Titorenkova, R.; Velinov, N.; Delcheva, Z. Characterization of a novel geopolymer based on acid-activated fayalite slag from local copper industry. Bulg. Chem. Commun. 2018, 50, 54–61. [Google Scholar]
- Wijaya, S.W.; Satria, J.; Sugiarto, A.; Hardjito, D. The use of borax in deterring flash setting of high calcium fly ash based geopolymer. Mater. Sci. Forum 2016, 857, 416–420. [Google Scholar]
- Nikolov, A.; Rostovsky, I.; Nugteren, H. Geopolymer materials based on natural zeolite. Case Stud. Constr. Mater. 2017, 6, 198–205. [Google Scholar] [CrossRef]
- Latella, B.A.; Perera, D.S.; Escott, T.R.; Cassidy, D.J. Adhesion of glass to steel using a geopolymer. J. Mater. Sci. 2006, 41, 1261–1264. [Google Scholar] [CrossRef]
- Van Deventer, J.S.J.; Provis, J.L.; Duxson, P. Technical and commercial progress in the adoption of geopolymer cement. Miner. Eng. 2012, 29, 89–104. [Google Scholar] [CrossRef]
- Müller, N.; Harnisch, J. A Blueprint for a Climate Friendly Cement Industry; Gland WWF Lafarge Conservation Partnership: Nürnberg, Germany, 2008; p. 7. [Google Scholar]
- Kovalchuk, G.; Krienko, P.V. Producing Fire-and Heat-Resistant Geopolymers; Woodhead Publishing: Cambridge, UK, 2009; pp. 227–266. [Google Scholar]
- Zhao, R.; Sanjayan, J.G. Geopolymer and Portland cement concretes in simulated fire. Mag. Concr. Res. 2011, 63, 163–173. [Google Scholar] [CrossRef]
- Lahoti, M.; Tan, K.H.; Yang, E.-H. A critical review of geopolymer properties for structural fire-resistance applications. Constr. Build. Mater. 2019, 221, 514–526. [Google Scholar] [CrossRef]
- Barbosa, V.F.F.; MacKenzie, K.J.D. Synthesis and thermal behaviour of potassium sialate geopolymers. Mater. Lett. 2003, 57, 1477–1482. [Google Scholar] [CrossRef]
- Uddin Ahmed Shaikh, F.; Haque, S.; Sanjayan, J. Behavior of fly ash geopolymer as fire resistant coating for timber. J. Sustain. Cem. Mater. 2019, 8, 259–274. [Google Scholar] [CrossRef]
- Rickard, W.D.A.; Temuujin, J.; van Riessen, A. Thermal analysis of geopolymer pastes synthesised from five fly ashes of variable composition. J. Non-Cryst. Solids 2012, 358, 1830–1839. [Google Scholar] [CrossRef]
- Ali, M.; Vijayalakshmi, M.M. Thermal properties of geopolymer composites–A Review. Solid State Technol. 2020, 63, 7215–7233. [Google Scholar] [CrossRef]
- He, R.; Dai, N.; Wang, Z. Thermal and mechanical properties of geopolymers exposed to high temperature: A literature review. Adv. Civ. Eng. Mater. 2020, 2020, 17. [Google Scholar] [CrossRef] [Green Version]
- Haddaji, Y.; Majdoubi, H.; Mansouri, S.; Alomayri, T.S.; Allaoui, D.; Manoun, B.; Oumam, M.; Hannache, H. Microstructure and flexural performances of glass fibers reinforced phosphate sludge based geopolymers at elevated temperatures. Case Stud. Constr. Mater. 2022, 16, e00928. [Google Scholar] [CrossRef]
- Duxson PFernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.C.; van Deventer, J.S.J. The thermal evolution of metakaolin geopolymers: Part 2—Phase stability and structural development. J. Non-Cryst. Solids 2007, 353, 2186–2200. [Google Scholar] [CrossRef]
- Barbosa, V.F.; MacKenzie, K.J. Thermal behaviour of inorganic geopolymers and composites derived from sodium polysialate. Mater. Res. bull. 2003, 38, 319–331. [Google Scholar] [CrossRef]
- Rickard, W.D.A.; Williams, R.; Temuujin, J.; Van Riessen, A. Assessing the suitability of three Australian fly ashes as an aluminosilicate source for geopolymers in high temperature applications. Mater. Sci. Eng. 2011, 528, 3390–3397. [Google Scholar] [CrossRef]
- Rickard, W.D.; Riessen, A.V.; Walls, P. Thermal character of geopolymers synthesized from class F fly ash containing high concentrations of iron and $α$-quartz. Int. J. Appl. Ceram. Technol. 2010, 7, 81–88. [Google Scholar] [CrossRef]
- Davidovits, J.; Davidovits, R. Ferro-Sialate Geopolymers (-Fe-O-Si-O-Al-O-); Geopolymer Institute Library: Saint-Quentin, France, 2020. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Bartzas, G. Effect of sulphate and nitrate anions on heavy metal immobilisation in ferronickel slag geopolymers. Appl. Clay Sci. 2013, 73, 103–109. [Google Scholar] [CrossRef]
- Komnitsas KBartzas, G.; Karmali, V.; Petrakis, E.; Kurylak, W.; Pietek, G.; Kanasiewicz, J. Assessment of alkali activation potential of a Polish ferronickel slag. Sustainability 2019, 11, 1863. [Google Scholar] [CrossRef] [Green Version]
- Komnitsas, K.; Zaharaki, D.; Perdikatsis, V. Effect of synthesis parameters on the compressive strength of low-calcium ferronickel slag inorganic polymers. J. Hazard. Mater. 2009, 161, 760–768. [Google Scholar] [CrossRef]
- Onisei, S.; Douvalis, A.P.; Malfliet, A.; Peys, A.; Pontikes, Y. Inorganic polymers made of fayalite slag: On the microstructure and behavior of Fe. J. Am. Ceram. Soc. 2018, 101, 2245–2257. [Google Scholar] [CrossRef]
- Nikolov, A. Alkali-activated geopolymers based on iron-rich slag from copper industry. IOP Conf. Ser. Mater. Sci. Eng. 2020, 951, 012006. [Google Scholar] [CrossRef]
- Nikolov, A. Alkali and acid activated geopolymers based on iron-silicate fines—By-product from copper industry. Int. Sci. J. Mach. Technol. Mater. 2020, 14, 37–39. [Google Scholar]
- Nikolov, A. Characterization of geopolymer based on fayalite and metakaolin with standard consistence. Comptes Rendus L’académie Bulg. Sci. 2021, 74, 1461–1468. [Google Scholar] [CrossRef]
- Žák, T.; Jirásková, Y. CONFIT: Mössbauer spectra fitting program. Surf. Interface Anal. 2006, 38, 710–714. [Google Scholar] [CrossRef]
- EN 196-3:2016; Methods of Testing Cement—Part 3: Determination of Setting Times and Soundness. iTeh, Inc.: Newark, DE, USA, 2016.
- Karamanov, A.; Aloisi, M.; Pelino, M. Vitrification of copper flotation waste. J. Hazard. Mater. 2007, 140, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Rahier, H.; Van Mele, B.; Biesemans, M.; Wastiels, J.; Wu, X. Low-temperature synthesized aluminosilicate glasses. J. Mater. Sci. 1996, 31, 71–79. [Google Scholar] [CrossRef]
- Pan, Z.; Sanjayan, J.G. Stress-strain behaviour and abrupt loss of stiffness of geopolymer at elevated temperatures. Cem. Concr. Compos. 2010, 32, 657–664. [Google Scholar] [CrossRef]
- Nikolov, A. Physical properties and powder XRD characterization of fly ash-based geopolymers heated up to 1150 oC. Rev. Bulg. Geol. Soc. 2019, 80, 36–38. [Google Scholar]
- Jiang, X.; Zhang, Y.; Xiao, R.; Polaczyk, P.; Zhang, M.; Hu, W.; Bai, Y.; Huang, B. A comparative study on geopolymers synthesized by different classes of fly ash after exposure to elevated temperatures. J. Clean. Prod. 2020, 270, 122500. [Google Scholar] [CrossRef]
- Peys ADouvalis, A.P.; Hallet, V.; Rahier, H.; Blanpain, B.; Pontikes, Y. Inorganic polymers from CaO-FeOx-SiO2 slag: The start of oxidation of Fe and the formation of a mixed valence binder. Front. Mater. 2019, 6, 212. [Google Scholar] [CrossRef]
- Mysen, B.O. The structural behavior of ferric and ferrous iron in aluminosilicate glass near meta-aluminosilicate joins. Geochim. Cosmochim. Acta 2006, 70, 2337–2353. [Google Scholar] [CrossRef]
- Perera, D.S.; Cashion, J.D.; Blackford, M.G.; Zhang, Z.; Vance, E.R. Fe speciation in geopolymers with Si/Al molar ratio of ∼2. J. Eur. Ceram. Soc. 2007, 27, 2697–2703. [Google Scholar] [CrossRef]
- Mihailova, I.; Mehandjiev, D. Characterization of fayalite from copper slags. J. Univ. Chem. Technol. Metall. 2010, 45, 317–326. [Google Scholar]
- Pisciella, P.; Pelino, M. FTIR spectroscopy investigation of the crystallisation process in an iron rich glass. J. Eur. Ceram. Soc. 2005, 25, 1855–1861. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Zhang, W.; Li, Z.; Zhang, Y.; Li, Y.; Ren, Y. Effects of Si/Al ratio on the efflorescence and properties of fly ash based geopolymer. J. Clean. Prod. 2020, 244, 118852. [Google Scholar] [CrossRef]


| Fe2O3 | SiO2 | Al2O3 | CaO | ZnO | MgO | K2O | Na2O | CuO | PbO | TiO2 | MoO3 | SO3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fayalite | 58.42 | 29.34 | 4.40 | 2.66 | 1.32 | 0.89 | 0.71 | 0.58 | 0.49 | 0.37 | 0.30 | 0.27 | 0.26 |
| Metakaolin | 1.14 | 53.94 | 43.20 | 0.15 | - | 0.09 | 0.62 | 0.11 | - | - | 0.74 | - | 0.01 |
| Series | Apparent Density, g/cm3 | Absolute Density, g/cm3 | Relative Porosity, % | Water Absorption, % | Open Porosity, % |
|---|---|---|---|---|---|
| VFM4.5 | 2.38 | 3.59 | 34 | 10.7 ± 0.11 | 25 |
| VFM4.5-400 | 2.49 | 3.58 | 30 | 10.6 ± 0.04 | 26 |
| VFM4.5-800 | 2.61 | 3.44 | 24 | 7.12 ± 0.49 | 19 |
| VFM4.5-1150 | 2.73 | 3.41 | 20 | 1.21 ± 0.44 | 3 |
| Sample | Components | IS, mm/s | QS, mm/s | B, T | Γexp, mm/s | G, % |
|---|---|---|---|---|---|---|
| RAW-F | Sx1-Fe3O4, Fe3+ tetra | 0.3 | 0 | 48 | 0.36 | 13 |
| Sx2-Fe3O4, Fe2.5+ octa | 0.62 | −0.05 | 45.1 | 0.54 | 15 | |
| Sx3-Fe3O4, Fe2.5+ octa | 0.72 | −0.05 | 42.3 | 0.78 | 13 | |
| Db1-Fe2SiO4, Fe2+—M1 | 1.14 | 2.68 | - | 0.3 | 21 | |
| Db2-Fe2SiO4, Fe2+—M2 | 1.17 | 2.88 | - | 0.3 | 27 | |
| Db3—Fe2+ | 1.24 | 2.19 | - | 0.65 | 11 | |
| VFM4.5 | Sx1-Fe3O4, Fe3+tetra | 0.3 | 0 | 48.8 | 0.37 | 13 |
| Sx2-Fe3O4, Fe2.5+octa | 0.6 | −0.02 | 45.8 | 0.62 | 17 | |
| Sx3-Fe3O4, Fe2.5+octa | 0.74 | −0.05 | 42.5 | 0.91 | 14 | |
| Db1-Fe2SiO4, Fe2+—M1 | 1.12 | 2.77 | - | 0.3 | 18 | |
| Db2-Fe2SiO4, Fe2+—M2 | 1.21 | 2.87 | - | 0.3 | 28 | |
| Db3—Fe2+ | 1.18 | 1.96 | - | 0.77 | 9 | |
| Db4—Fe3+ | 0.47 | 0.76 | - | 0.3 | 1 | |
| VFM4.5-1150I | Sx1-Fe3O4, Fe3+ tetra | 0.31 | 0 | 48.4 | 0.41 | 16 |
| Sx2-Fe3O4, Fe2.5+ octa | 0.54 | −0.04 | 45.8 | 0.61 | 14 | |
| Sx3-Fe3O4, Fe2.5+ octa | 0.7 | −0.01 | 42.8 | 1.04 | 27 | |
| Db1-Fe2SiO4, Fe2+—M1 | 1.15 | 2.71 | - | 0.3 | 14 | |
| Db2-Fe2SiO4, Fe2+—M2 | 1.18 | 2.92 | - | 0.3 | 23 | |
| Db3—Fe2+ | 1.07 | 1.76 | - | 0.71 | 6 | |
| VFM4.5-1150E | Sx1-α-Fe2O3, Fe3+ octa | 0.38 | −0.2 | 51.9 | 0.28 | 67 |
| Sx2-α-Fe2O3, Fe3+ octa | 0.36 | −0.2 | 50.4 | 0.56 | 28 | |
| Db—Fe3+ | 0.39 | 1 | - | 0.76 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolov, A.; Karamanov, A. Thermal Properties of Geopolymer Based on Fayalite Waste from Copper Production and Metakaolin. Materials 2022, 15, 2666. https://doi.org/10.3390/ma15072666
Nikolov A, Karamanov A. Thermal Properties of Geopolymer Based on Fayalite Waste from Copper Production and Metakaolin. Materials. 2022; 15(7):2666. https://doi.org/10.3390/ma15072666
Chicago/Turabian StyleNikolov, Aleksandar, and Alexandar Karamanov. 2022. "Thermal Properties of Geopolymer Based on Fayalite Waste from Copper Production and Metakaolin" Materials 15, no. 7: 2666. https://doi.org/10.3390/ma15072666
APA StyleNikolov, A., & Karamanov, A. (2022). Thermal Properties of Geopolymer Based on Fayalite Waste from Copper Production and Metakaolin. Materials, 15(7), 2666. https://doi.org/10.3390/ma15072666








