Solvent-Free and Efficient One-Pot Strategy for Synthesis of the Triazine-Heterocycle Azacyanines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
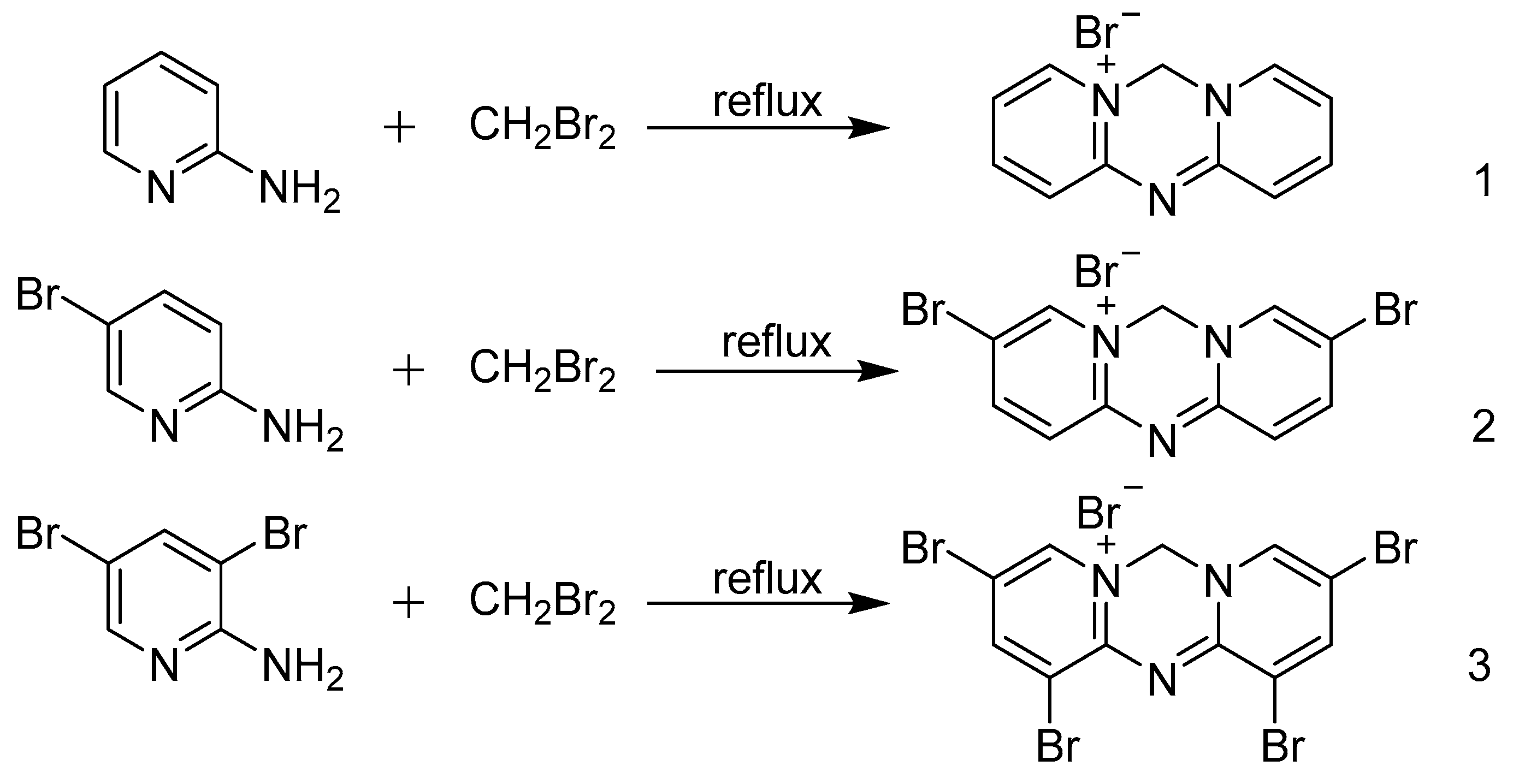
2.2. Preparation of Azacyanine 1
2.3. Preparation of Azacyanine 2
2.4. Preparation of Azacyanine 3
3. Characterization Methods
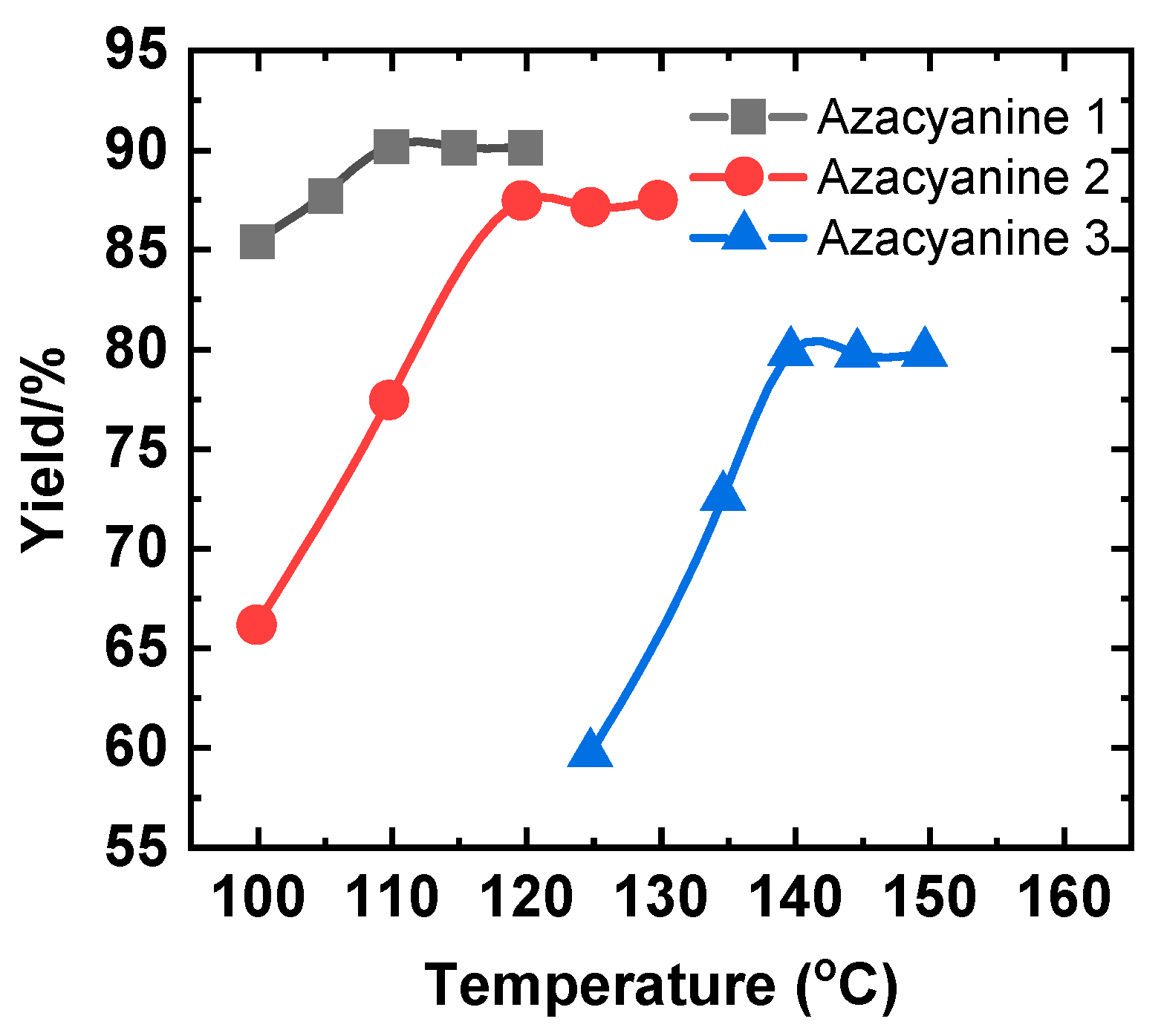
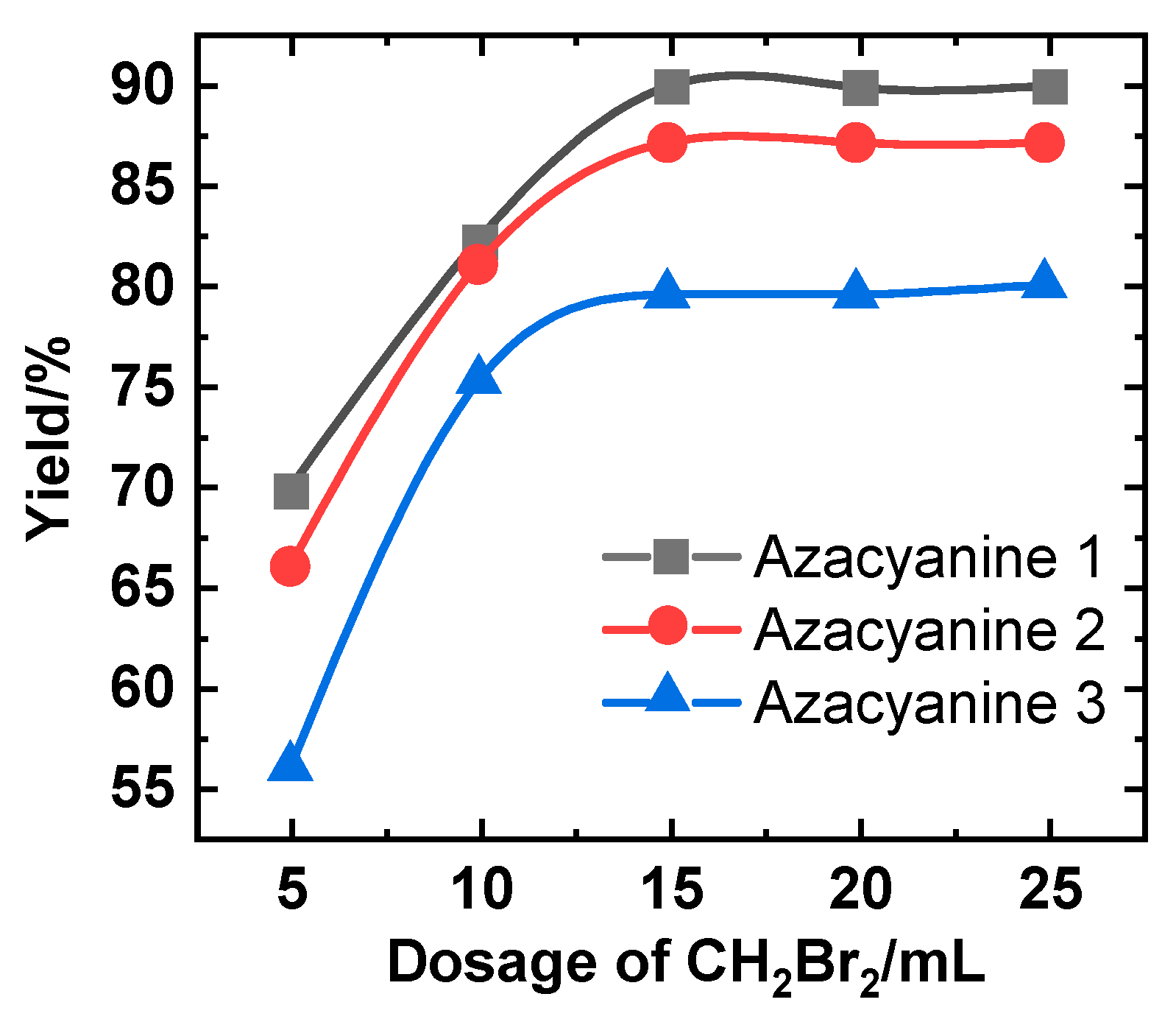
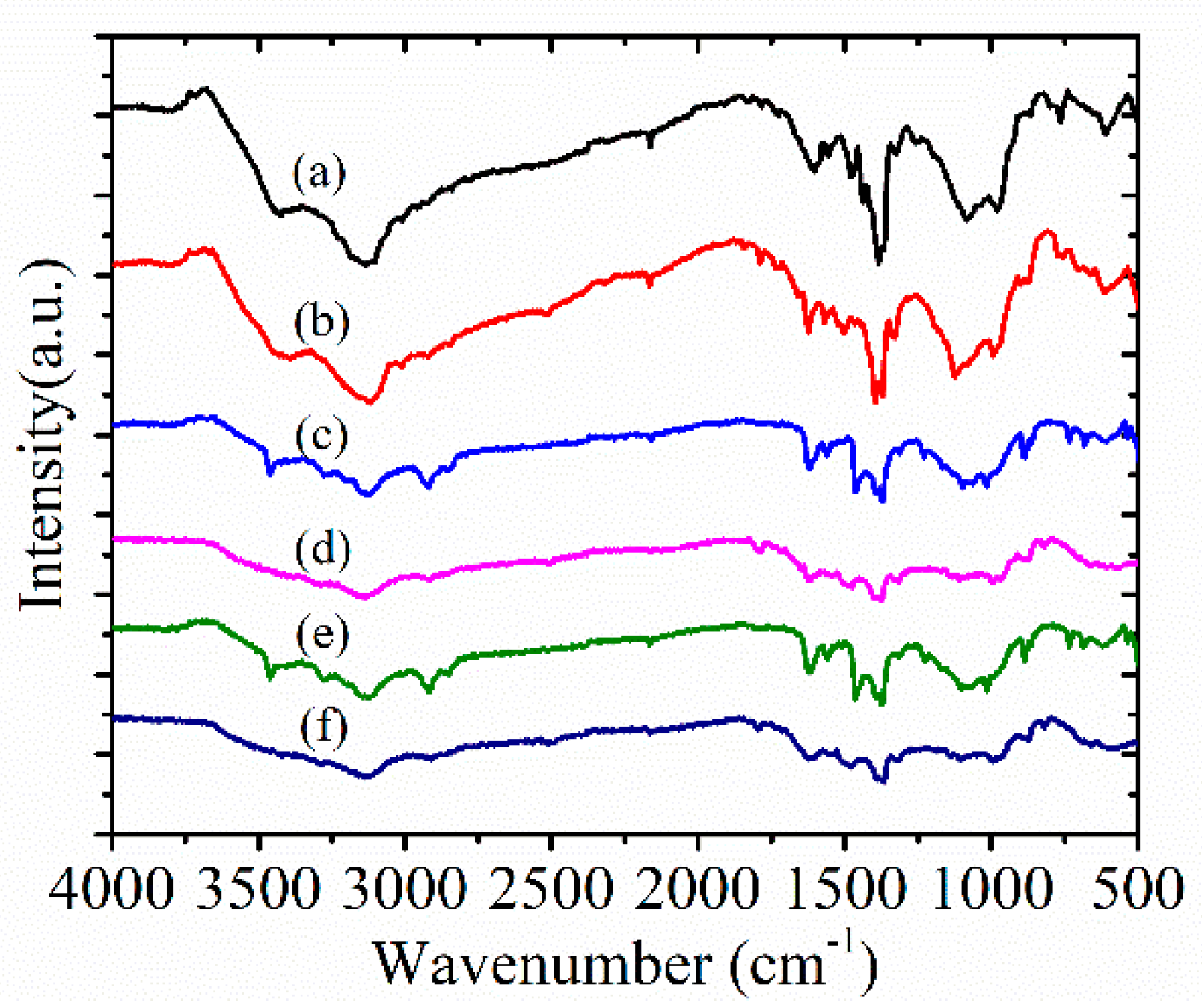
4. Results and Discussion
Structure Analysis and Thermo-Response Properties of HBPS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahapatra, S.S.; Karak, N. s-Triazine containing flame retardant hyperbranched polyamines: Synthesis, characterization and properties evaluation. Polym. Degrad. Stab. 2007, 92, 947–955. [Google Scholar] [CrossRef]
- Borah, J.; Karak, N. Blends of triazine-based hyperbranched polyether with LDPE and plasticized PVC. J. Appl. Polym. Sci. 2007, 104, 648–654. [Google Scholar] [CrossRef]
- Oettmeier, W.; Hilp, U.; Draber, W.; Fedtke, C.; Schmidt, R.R. Structure-activity relationships of triazinone herbicides on resistant weeds and resistant Chlamydomonas reinhardtii. J. Pestic. Sci. 1991, 33, 399–409. [Google Scholar] [CrossRef]
- Liu, K.C.; Shih, B.J.; Chuen-Hsiang, L.E.E. Cyclocondensation of 3-amino-2-iminonaphtho [1, 2-d]-thiazole with α-ketocarboxylic acid derivatives: Synthesis of 2-substituted 3-Oxo-3H-naphtho [1′, 2′: 4, 5] thiazolo-[3, 2-b][1, 2, 4] triazines as potential anti-HIV agents. J. Heterocycl. Chem. 1992, 29, 97–101. [Google Scholar] [CrossRef]
- Sztanke, K.; Pasternak, K.; Sztanke, M.; Kandefer-Szerszeń, M.; Kozioł, A.E.; Dybała, I. Crystal structure, antitumour and antimetastatic activities of disubstituted fused 1, 2, 4-triazinones. Bioorg. Med. Chem. Lett. 2009, 19, 5095–5100. [Google Scholar] [CrossRef]
- Ayça, K.; Doğu, K.; Çetinkol, Ö.P. Targeting human telomeric DNA with azacyanines. Turk. J. Chem. 2019, 43, 1040–1051. [Google Scholar]
- Hu, X.P.; Li, W.Y.; Wang, Y.Z. Synthesis and characterization of a novel nitrogen-containing flame retardant. J. Appl. Polym. Sci. 2004, 94, 1556–1561. [Google Scholar] [CrossRef]
- Zeynalov, E.B.; Allen, N.S. Modelling light stabilizers as thermal antioxidants. Polym. Degrad. Stab. 2006, 91, 3390–3396. [Google Scholar] [CrossRef]
- Singh, M.K. Time-resolved single molecule fluorescence spectroscopy of Cy5-dCTP: Influence of the immobilization strategy. Phys. Chem. Chem. Phys. 2009, 11, 7225–7230. [Google Scholar] [CrossRef]
- Patra, D. Application and new developments in fluorescence spectroscopic techniques in studying individual molecules. Appl. Spectrosc. Rev. 2008, 43, 389–415. [Google Scholar] [CrossRef]
- Patra, D. Single molecule studies in chemical biology and nanosciences. Curr. Chem. Biol. 2008, 2, 267–277. [Google Scholar]
- Leubner, I.H. Synthesis and properties of pyrido-and azapyridocyanines. J. Org. Chem. 1973, 38, 1098–1102. [Google Scholar] [CrossRef]
- Haddadin, M.J.; Kurth, M.J.; Olmstead, M.M. One-step synthesis of new heterocyclic azacyanines. Tetrahedron Lett. 2000, 41, 5613–5616. [Google Scholar] [CrossRef]
- Huang, K.S.; Haddadin, M.J.; Olmstead, M.M.; Kurth, M.J. Synthesis and Reactions of Some Heterocyclic Azacyanines1. J. Org. Chem. 2001, 66, 1310–1315. [Google Scholar] [CrossRef]
- Munavalli, S.; Hsu, F.L.; Poziomek, E.J. Synthesis of novel azapyridocyanines. Heterocycles 1986, 24, 1893–1898. [Google Scholar]
- Kaye, G.W.C.; Laby, T.H. Tables of Physical and Chemical Constants; Longman: Harlow, UK, 1995; p. 241. [Google Scholar]
- Attanasi, O.A.; Baccolini, G.; Boga, C.; De Crescentini, L.; Filippone, P.; Mantellini, F. Solvent-Free Reaction of Some 1,2-Diaza-1,3-butadienes with Phosphites: Environmentally Friendly Access to New Diazaphospholes and E-Hydrazonophosphonates. J. Org. Chem. 2005, 70, 4033–4037. [Google Scholar] [CrossRef]
- Loh, T.-P.; Huang, J.-M.; Goh, S.-H.; Vittal, J.J. Aldol Reaction under Solvent-Free Conditions: Highly Stereoselective Synthesis of 1,3-Amino Alcohols. Org. Lett. 2000, 2, 1291–1294. [Google Scholar] [CrossRef]
- Sato, I.; Saito, T.; Soai, K. Solvent-Free Catalytic Enantioselective Addition of Diethylzinc to Aldehydes. Chem. Commun. 2000, 24, 2471–2472. [Google Scholar] [CrossRef]
- Shan, Y.K.; Sun, Z.D.; Zhang, H.Q.; Shen, H.H. Method for Synthesizing Hydrated Dipyridino Hexahydro Triazine Bromide Salt. CN Patent 102532145A, 4 July 2012. [Google Scholar]
- Xiaohui Jiang, Chunmei Wang, Xuewei Hou, Determination of bromine content in brominated polystyrene. Petrochem. Technol. Appl. 2002, 20, 277–280.
- Jose, S.P.; Mohan, S. Vibrational spectra and normal co-ordinate analysis of 2-aminopyridine and 2-amino picoline. Spectrochim. Acta Part A 2006, 64, 240–245. [Google Scholar] [CrossRef]
- Waiblinger, F.; Keck, J.; Stein, M.; Fluegge, A.P.; Kramer, H.E.; Leppard, D. Light-induced opening of the intramolecular hydrogen bond of UV absorbers of the 2-(2-hydroxyphenyl)-1, 3, 5-triazine and the 2-(2-hydroxyphenyl) benzotriazole type. J. Phys. Chem. 2000, 104, 1100–1106. [Google Scholar] [CrossRef]
- Liepins, R.; Walker, C. Azo, Cyanine, and Merocyanine Dyes with 1, 2-Dinitrile Groups. Ind. Eng. Chem. Prod. Res. Develop. 1971, 10, 401–405. [Google Scholar] [CrossRef]
- Patra, D.; Malaeb, N.N.; Haddadin, M.J.; Kurth, M.J. Influence of substituent and solvent on the radiative process of singlet excited states of novel cyclic azacyanine derivatives. J. Fluoresc. 2012, 22, 707–717. [Google Scholar] [CrossRef]

| Sample a | C (wt%) | H (wt%) | N (wt%) | Br (wt%) |
|---|---|---|---|---|
| Azacyanine 1 | 50.10% (50.00%) | 3.79% (3.82%) | 15.71% (15.91%) | 30.37% (30.20%) |
| Azacyanine 2 | 31.22% (31.31%) | 2.08% (1.91%) | 9.90% (9.95%) | 57.03% (56.87%) |
| Azacyanine 3 | 22.81% (22.76%) | 1.10% (1.03%) | 7.39% (7.24%) | 68.82% (68.97%) |
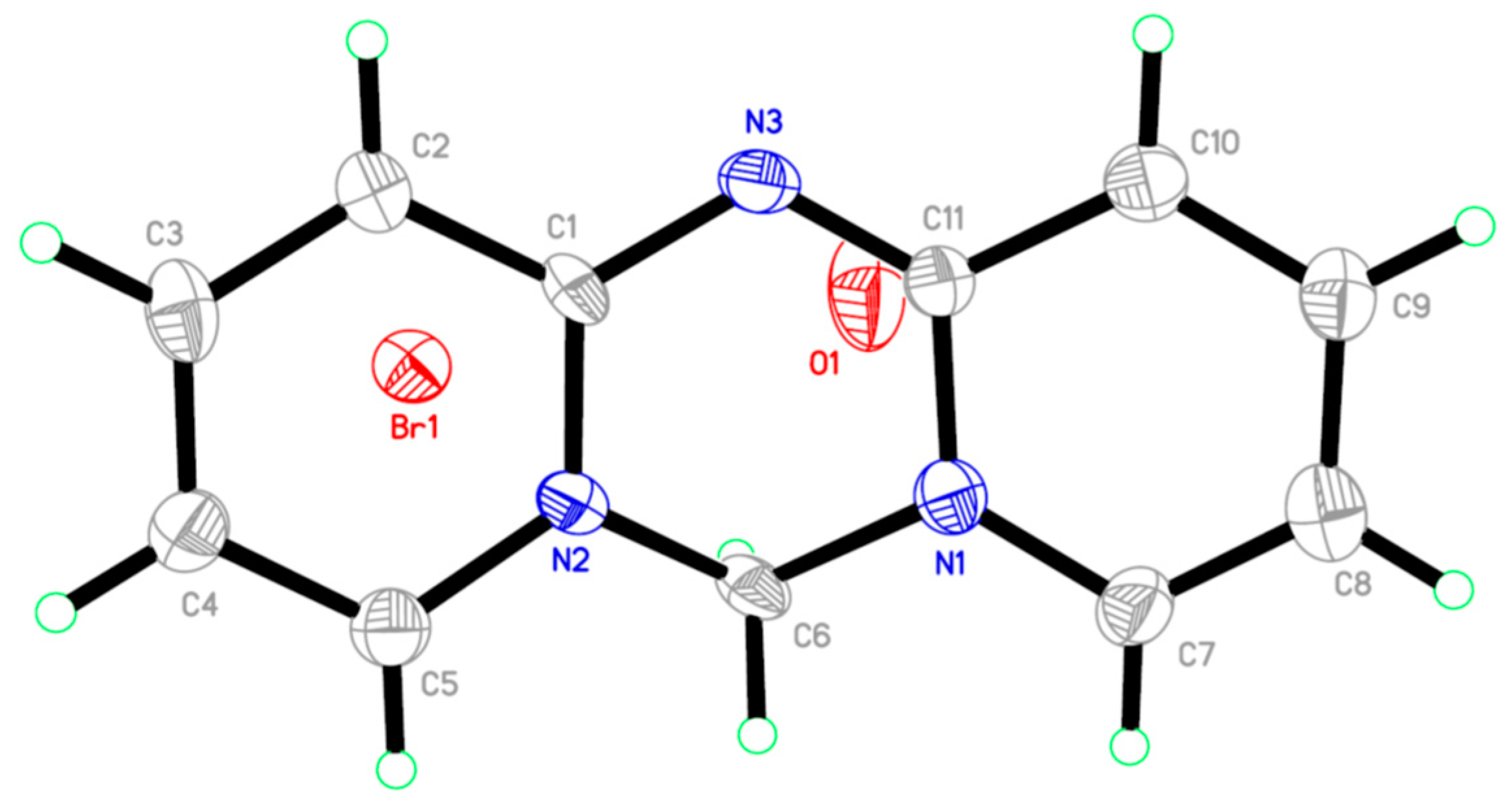
| Bond | Bond-Length/Å | Bond | Bond-Length/Å |
|---|---|---|---|
| N(1)-C(7) | 1.357(18) | C(5)-H(5) | 0.9300 |
| N(1)-C(11) | 1.399(18) | C(6)-H(6A) | 0.9700 |
| N(1)-C(6) | 1.454(17) | C(6)-H(6B) | 0.9700 |
| N(2)-C(1) | 1.369(15) | C(7)-C(8) | 1.34(2) |
| N(2)-C(5) | 1.374(19) | C(7)-H(7) | 0.9300 |
| N(2)-C(6) | 1.451(17) | C(8)-C(9) | 1.40(2) |
| N(3)-C(11) | 1.291(18) | C(8)-H(8) | 0.9300 |
| N(3)-C(1) | 1.433(17) | C(9)-C(10) | 1.38(2) |
| C(1)-C(2) | 1.406(18) | C(9)-H(9) | 0.9300 |
| C(2)-C(3) | 1.37(2) | C(10)-C(11) | 1.42(2) |
| C(2)-H(2) | 0.9300 | C(10)-H(10) | 0.9300 |
| C(4)-C(5) | 1.40(2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Sun, Z.; Wang, Y. Solvent-Free and Efficient One-Pot Strategy for Synthesis of the Triazine-Heterocycle Azacyanines. Materials 2022, 15, 2619. https://doi.org/10.3390/ma15072619
Jiang X, Sun Z, Wang Y. Solvent-Free and Efficient One-Pot Strategy for Synthesis of the Triazine-Heterocycle Azacyanines. Materials. 2022; 15(7):2619. https://doi.org/10.3390/ma15072619
Chicago/Turabian StyleJiang, Xianwu, Zhuodong Sun, and Yu Wang. 2022. "Solvent-Free and Efficient One-Pot Strategy for Synthesis of the Triazine-Heterocycle Azacyanines" Materials 15, no. 7: 2619. https://doi.org/10.3390/ma15072619
APA StyleJiang, X., Sun, Z., & Wang, Y. (2022). Solvent-Free and Efficient One-Pot Strategy for Synthesis of the Triazine-Heterocycle Azacyanines. Materials, 15(7), 2619. https://doi.org/10.3390/ma15072619





