Corrosion Behavior in Magnesium-Based Alloys for Biomedical Applications
Abstract
1. Introduction
2. Corrosion Behavior
2.1. Galvanic Corrosion
2.2. Pitting Corrosion
2.3. Stress Corrosion
2.4. Hydrogen Evolution (HE)
2.5. Corrosion Behavior in Different Corrosive Environments
2.5.1. Corrosive Electrolytes
2.5.2. In Vitro and In Vivo Environments
3. Influential Factors for Corrosion Behavior in Magnesium
3.1. Alloy Composition
3.1.1. Non-Rare Earth (RE) Elements
3.1.2. Rare Earth (RE) Elements
3.2. Organizational Structure
3.2.1. Crystal Structure
3.2.2. Amorphous Structures
4. A Methodology for Improving Corrosion Resistance
4.1. Composition Design
4.2. Heat Treatment
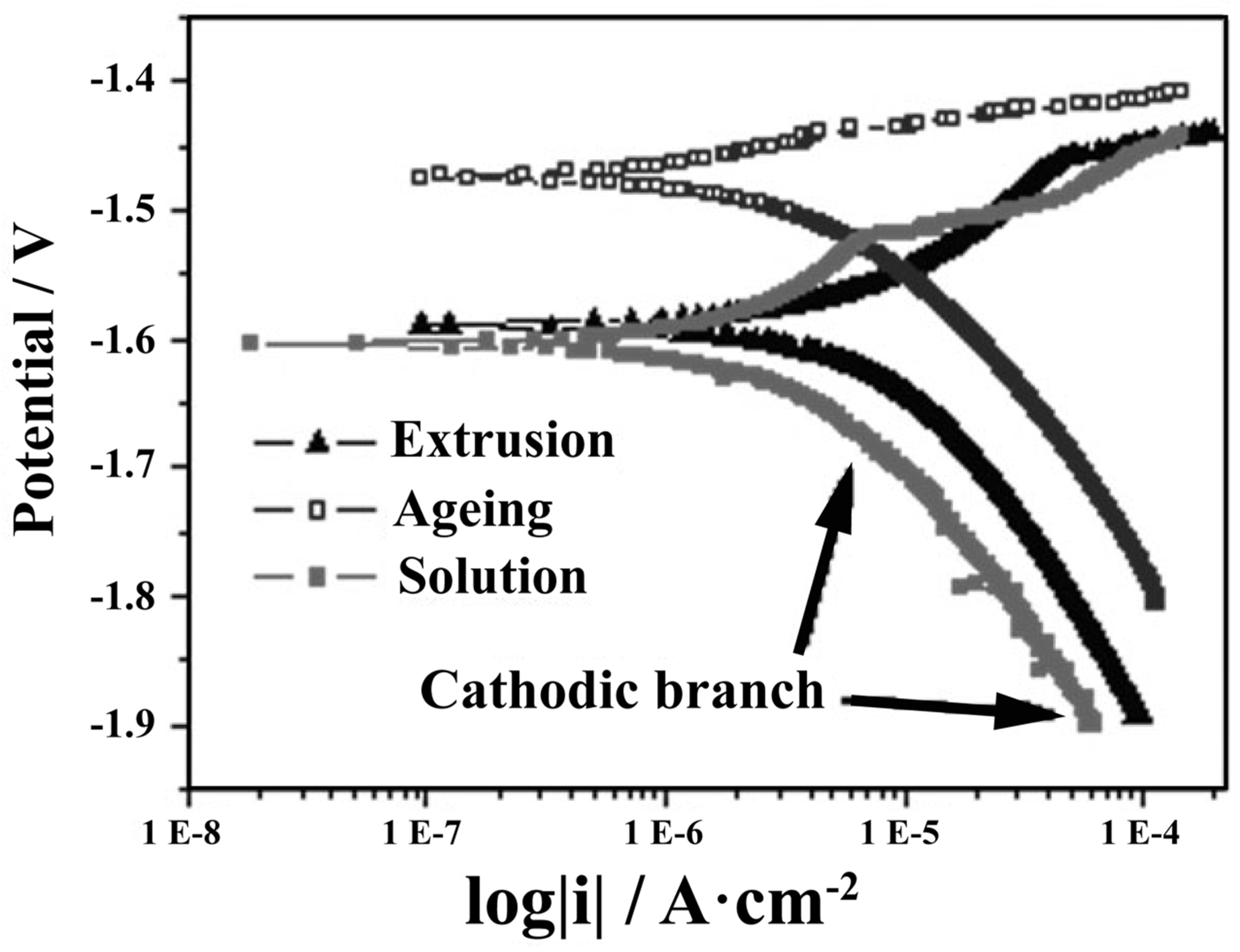
4.3. Severe Plastic Deformation (SPD)
4.4. Surface Modifications
4.4.1. Surface Machining Processes
4.4.2. Ion Implantation
4.4.3. Coating Technology
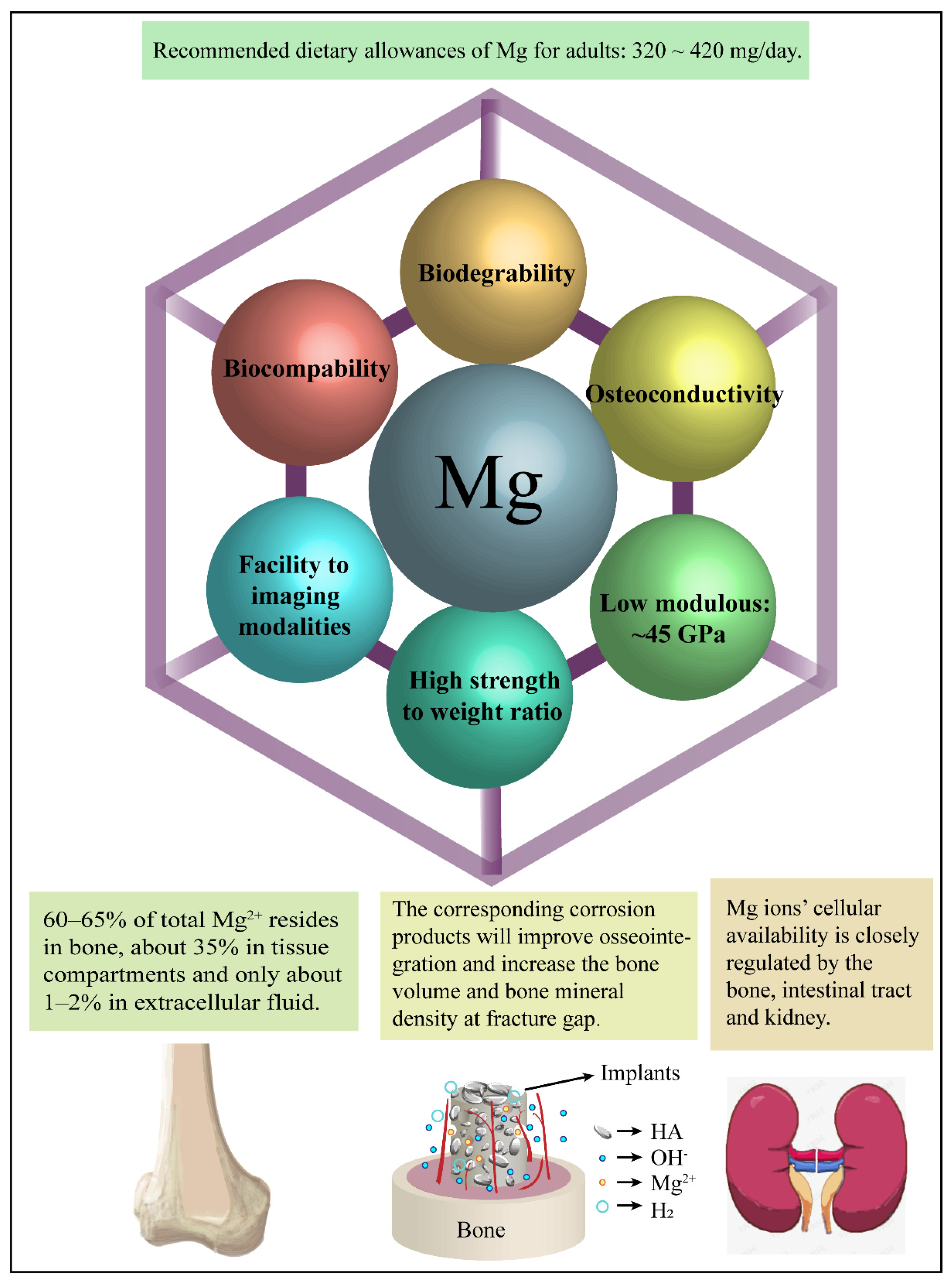
5. The Positive Biological Effects of Mg Alloys
5.1. Magnesium Ions
5.2. Hydroxyl Ions
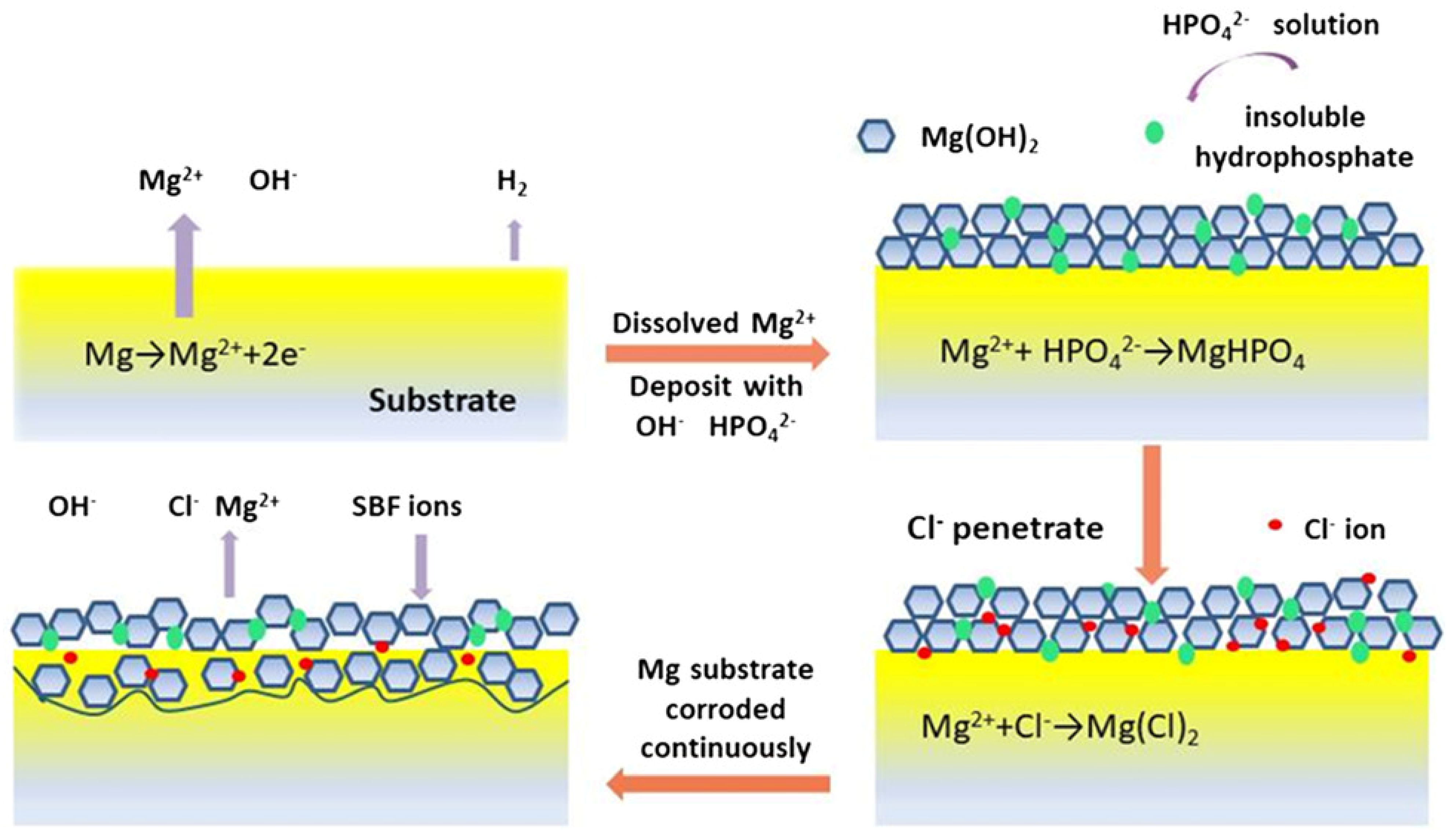
5.3. Corrosion Layers
6. Outlook
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Glossary
| CGRP | Calcitonin gene-related polypeptide-a |
| CREB1 | CAMP-responsive element-binding protein 1 |
| CT | Computed tomography |
| CF | Corrosion fatigue |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| H2 | Hydrogen |
| HE | Hydrogen evolution |
| HERs | Hydrogen evolution reactions |
| HA | Hydroxyapatite |
| MEM | Minimum essential medium |
| NFATc1 | Osteoclastogenesis-related gene |
| PBR | Pilling–Bedworth ratio |
| PLA | Polylactic acid |
| T6 treatment | Precipitation heat treatment |
| RE | Rare earth |
| REEs | Rare earth elements |
| ROS | Reactive oxygen species |
| SPD | Severe plastic deformation |
| SBF | Simulated body fluid |
| Ksp | Solubility product constant |
| T4 treatment | Solution treatment |
| SCC | Stress corrosion cracking |
| TM | Transition metal |
| Tris | Tris (hydroxymethyl) aminomethane |
References
- Nagels, J.; Stokdijk, M.; Rozing, P.M. Stress shielding and bone resorption in shoulder arthroplasty. J. Shoulder Elb. Surg. 2003, 12, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.R.; McCreary, D.L.; Chau, M.; Cunningham, B.P.; Pena, F.; Swiontkowski, M.F. Functional outcomes of symptomatic implant removal following ankle fracture open reduction and internal fixation. Foot Ankle Int. 2018, 39, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Nesti, L.J.; Tuli, R.; Lazatin, J.; Danielson, K.G.; Sharkey, P.F.; Tuan, R.S. Titanium particles suppress expression of osteoblastic phenotype in human mesenchymal stem cells. J. Orthop. Res. 2002, 20, 1175–1184. [Google Scholar] [CrossRef]
- Puleo, D.A.; Huh, W.W. Acute toxicity of metal ions in cultures of osteogenic cells derived from bone marrow stromal cells. J. Appl. Biomater. 1995, 6, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Wicklund, B.H.; Gustilo, R.B.; Tsukayarna, D.T. Titanium, chromium and cobalt ions modulate the release of bone-associated cytokines by human monocytes/macrophages in vitro. Biomoterials 1996, 17, 2233–2240. [Google Scholar] [CrossRef]
- Haynes, D.R.; Boyle, S.J.; Rogers, S.D.; Howie, D.W.; Vernon-Roberts, B. Variation in cytokines induced by particles from different prosthetic materials. Clin. Orthop. 1998, 352, 223–230. [Google Scholar] [CrossRef]
- Lhotka, C.; Szekeres, T.; Steffan, I.; Zhuber, K.; Zweymiiller, K. Four-year study of cobalt and chromium blood levels in patients managed with two different metal-on-metal total hip replacements. J. Orthop. Res. 2003, 21, 189–195. [Google Scholar] [CrossRef]
- Nikia, Y.; Matsumotoa, H.; Sudaa, Y.; Otania, T.; Fujikawab, K.; Toyamaa, Y.; Hisamoric, N.; Nozuec, A. Metal ions induce bone-resorbing cytokine production through the redox pathway in synoviocytes and bone marrow macrophages. Biomaterials 2003, 24, 1447–1457. [Google Scholar] [CrossRef]
- Filli, L.; Luechinger, R.; Frauenfelder, T.; Beck, S.; Guggenberger, R.; Farshad-Amacker, N.; Andreisek, G. Metal-induced artifacts in computed tomography and magnetic resonance imaging: Comparison of a biodegradable magnesium alloy versus titanium and stainless steel controls. Skelet. Radiol. 2015, 44, 849–856. [Google Scholar] [CrossRef]
- Sonnow, L.; Konneker, S.; Vogt, P.M.; Wacker, F.; von Falck, C. Biodegradable magnesium Herbert screw—Image quality and artifacts with radiography, CT and MRI. BMC Med. Imaging 2017, 17, 16. [Google Scholar] [CrossRef]
- Shils, M.E. Modern Nutrition in Health and Disease, 8th ed.; Lea & Febiger: Philadelphia, PA, USA, 1994; pp. 164–184. [Google Scholar] [CrossRef]
- Vormann, J. Magnesium: Nutrition and metabolism. Mol. Aspects Med. 2003, 24, 27–37. [Google Scholar] [CrossRef]
- Christine, B.; Darlene, G.S.; Chen, J.J.; Susan, M. What is next for the dietary reference intakes for bone metabolism related nutrients beyond calcium: Phosphorus, magnesium, vitamin d, and fluoride? Crit. Rev. Food Sci. Nutr. 2009, 49, 136–144. [Google Scholar]
- Quamme, G.A.; Rouffignac, C.D. Epithelial magnesium transport and regulation by the kidney. Front. Biosci. 2000, 5, 694–711. [Google Scholar] [CrossRef][Green Version]
- Zhang, E.; Xu, L.; Yu, G.; Pan, F.; Yang, K. In vivo evaluation of biodegradable magnesium alloy bone implant in the first 6 months implantation. J. Biomed. Mater. Res. A 2009, 90, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Slottow, P.T.L.; Pakala, R.; Waksman, R. Serial imaging and histology illustrating the degradation of a bioabsorbable magnesium stent in a porcine coronary artery. Eur. Heart J. 2008, 29, 314. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; He, X.; Ding, P.; Liu, D.; Chen, M. Fabrication, microstructure, and properties of a biodegradable Mg-Zn-Ca clip. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, L.; Qi, H.; Zhao, Q.; Liu, Y.; Zhang, Y. Dual function of magnesium in bone biomineralization. Adv. Healthc. Mater. 2019, 8, e1901030. [Google Scholar] [CrossRef]
- Berglund, I.S.; Dirr, E.W.; Ramaswamy, V.; Allen, J.B.; Allen, K.D.; Manuel, M.V. The effect of Mg-Ca-Sr alloy degradation products on human mesenchymal stem cells. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 697–704. [Google Scholar] [CrossRef]
- Penga, Q.; Meng, J.; Li, Y.; Huang, Y.; Horta, N. Effect of yttrium addition on lattice parameter, Young’s modulus and vacancy of magnesium. Mater. Sci. Eng. A 2011, 528, 2106–2109. [Google Scholar] [CrossRef]
- Xu, Y.L.; Wang, L.; Huang, M.; Gensch, F.; Kainer, K.U.; Hort, N. The effect of solid solute and precipitate phase on Young’s modulus of binary Mg-RE alloys. Adv. Eng. Mater. 2018, 20, 1800271. [Google Scholar] [CrossRef]
- Deng, C.Z.; Radhakrishnan, R.; Larsen, S.R.; Boismer, D.A.; Stinson, J.S.; Hotchkiss, A.K.; Petersen, E.M.; Weber, J.; Scheuermann, T. Magnesium alloys for bioabsorbable stents: A feasibility assessment. In Magnesium Technology 2011; Springer: Cham, Switzerland, 2011; pp. 413–418. [Google Scholar]
- Parande, G.; Manakari, V.; Kopparthy, S.D.S.; Gupta, M. Utilizing low-cost eggshell particles to enhance the mechanical response of Mg-2.5Zn magnesium alloy matrix. Adv. Eng. Mater. 2018, 20, 1700919. [Google Scholar] [CrossRef]
- Quamme, G.A.; Dirks, J.H. The physiology of renal magnesium handling. Ren. Physiol. 1986, 9, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Berglund, I.S.; Brar, H.S.; Dolgova, N.; Acharya, A.P.; Keselowsky, B.G.; Sarntinoranont, M.; Manuel, M.V. Synthesis and characterization of Mg-Ca-Sr alloys for biodegradable orthopedic implant applications. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1524–1534. [Google Scholar] [CrossRef]
- Qi, Z.R.; Zhang, Q.; Tan, L.L.; Lin, X.; Yin, Y.; Wang, X.L.; Yang, K.; Wang, Y. Comparison of degradation behavior and the associated bone response of ZK60 and PLLA in vivo. J. Biomed. Mater. Res. A 2014, 102, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.K.; Byun, S.H.; Lee, J.Y.; Lee, J.W.; Kim, S.M.; Lee, S.M.; Kim, H.E.; Lee, J.H. Radiological, histological, and hematological evaluation of hydroxyapatite-coated resorbable magnesium alloy screws placed in rabbit tibia. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Luffy, S.A.; Wu, J.; Kumta, P.N.; Gilbert, T.W. Evaluation of magnesium alloys for use as an intraluminal tracheal for pediatric applications in a rat tracheal bypass model. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1844–1853. [Google Scholar] [CrossRef]
- Di Virgilio, A.L.; Reigosa, M.; de Mele, M.F. Biocompatibility of magnesium particles evaluated by in vitro cytotoxicity and genotoxicity assays. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 99, 111–119. [Google Scholar] [CrossRef]
- Elkaiam, L.; Hakimi, O.; Yosafovich-Doitch, G.; Ovadia, S.; Aghion, E. In vivo evaluation of Mg-5%Zn-2%Nd alloy as an innovative biodegradable implant material. Ann. Biomed. Eng. 2020, 48, 380–392. [Google Scholar] [CrossRef]
- Heublein, B.; Rohde, R.; Kaese, V.; Niemeyer, M.; Hartung, W.; Haverich, W. Biocorrosion of magnesium alloys: A new principle in cardiovascular implant technology? Heart 2003, 89, 651–656. [Google Scholar] [CrossRef]
- Li, Z.; Gu, X.; Lou, S.; Zheng, Y. The development of binary Mg-Ca alloys for use as biodegradable materials within bone. Biomaterials 2008, 29, 1329–1344. [Google Scholar] [CrossRef]
- Kraus, T.; Fischerauer, S.F.; Hanzi, A.C.; Uggowitzer, P.J.; Loffler, J.F.; Weinberg, A.M. Magnesium alloys for temporary implants in osteosynthesis: In vivo studies of their degradation and interaction with bone. Acta Biomater. 2012, 8, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Hanzi, A.C.; Gerber, I.; Schinhammer, M.; Loffler, J.F.; Uggowitzer, P.J. On the in vitro and in vivo degradation performance and biological response of new biodegradable Mg-Y-Zn alloys. Acta Biomater. 2010, 6, 1824–1833. [Google Scholar] [CrossRef] [PubMed]
- Noviana, D.; Paramitha, D.; Ulum, M.F.; Hermawan, H. The effect of hydrogen gas evolution of magnesium implant on the postimplantation mortality of rats. J. Orthop. Transl. 2016, 5, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Lee, K.B.; Kim, S.Y.; Bode, K.; Jang, Y.S.; Kwon, T.Y.; Jeon, M.H.; Lee, M.H. Gas formation and biological effects of biodegradable magnesium in a preclinical and clinical observation. Sci. Technol. Adv. Mater. 2018, 19, 324–335. [Google Scholar] [CrossRef]
- Rahim, M.I.; Szafranski, S.P.; Ingendoh-Tsakmakidis, A.; Stiesch, M.; Mueller, P.P. Evidence for inoculum size and gas interfaces as critical factors in bacterial biofilm formation on magnesium implants in an animal model. Colloids Surf. B Biointerfaces 2020, 186, 110684. [Google Scholar] [CrossRef]
- Song, G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Miao, H.; Zhang, D.; Chen, C.; Zhang, L.; Pei, J.; Su, Y.; Huang, H.; Wang, Z.; Kang, B.; Ding, W.; et al. Research on biodegradable Mg–Zn–Gd alloys for potential orthopedic implants: In vitro and in vivo evaluations. ACS Biomater. Sci. Eng. 2019, 5, 1623–1634. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, J.; Dong, Q.; Ba, Z.; Wu, Y. Corrosion behavior and mechanical degradation of as-extruded Mg-Gd-Zn-Zr alloys for orthopedic application. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 698–708. [Google Scholar] [CrossRef]
- Luffy, S.A.; Chou, D.T.; Waterman, J.; Wearden, P.D.; Kumta, P.N.; Gilbert, T.W. Evaluation of magnesium-yttrium alloy as an extraluminal tracheal stent. J. Biomed. Mater. Res. A 2014, 102, 611–620. [Google Scholar] [CrossRef]
- Rouffignac, C.D.; Gary, Q. Renal magnesium handling and its hormonal control. Physiol. Rev. 1994, 74, 305–322. [Google Scholar] [CrossRef]
- Gupta, M.; Sharon, N.M.L. Magnesium, Magnesium Alloys, and Magnesium Composites; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics. J. Am. Ceram. Soc. 2005, 81, 1705–1728. [Google Scholar] [CrossRef]
- Ducheyne, P.; Hench, L.L.; Kagan, A.I.; Martens, M.; Bursens, A.; Mulier, J.C. Effect of hydroxyapatite impregnation on skeletal bonding of porous coated implants. J. Biomed. Mater. Res. 1980, 14, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.I.; Weizbauer, A.; Evertz, F.; Hoffmann, A.; Rohde, M.; Glasmacher, B.; Windhagen, H.; Gross, G.; Seitz, J.M.; Mueller, P.P. Differential magnesium implant corrosion coat formation and contribution to bone bonding. J. Biomed. Mater. Res. A 2017, 105, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Lamaka, S.V.; Karavai, O.V.; Bastos, A.C.; Zheludkevich, M.L.; Ferreira, M.G.S. Monitoring local spatial distribution of Mg2+, pH and ionic currents. Electrochem. Commun. 2008, 10, 259–262. [Google Scholar] [CrossRef]
- Soltan, A.; Dargusch, M.S.; Shi, Z.; Gerrard, D.; Atrens, A. Understanding the corrosion behaviour of the magnesium alloys EV31A, WE43B, and ZE41A. Mater. Corros. 2019, 70, 1527–1552. [Google Scholar] [CrossRef]
- Vaughan, M.W.; Karayan, A.I.; Srivastava, A.; Mansoor, B.; Seitz, J.M.; Eifler, R.; Karaman, I.; Castaneda, H.; Maier, H.J. The effects of severe plastic deformation on the mechanical and corrosion characteristics of a bioresorbable Mg-ZKQX6000 alloy. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 115, 111130. [Google Scholar] [CrossRef]
- Li, T.; Zhang, H.; He, Y.; Wang, X. Comparison of corrosion behavior of Mg-1.5Zn-0.6Zr and AZ91D alloys in a NaCl solution. Mater. Corros. 2015, 66, 7–15. [Google Scholar] [CrossRef]
- Liu, X.; Shan, D.; Song, Y.; Han, E.-h. Influence of yttrium element on the corrosion behaviors of Mg–Y binary magnesium alloy. J. Magnes. Alloy. 2017, 5, 26–34. [Google Scholar] [CrossRef]
- Cain, T.; Bland, L.G.; Birbilis, N.; Scully, J.R. A compilation of corrosion potentials for magnesium alloys. Corrosion 2014, 70, 1043–1051. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; National Association of Corrosion Engineers: Houston, TX, USA, 1974. [Google Scholar] [CrossRef]
- McCafferty, E. Standard electrode potentials of the elements as a fundamental periodic property of atomic number. Electrochim. Acta 2007, 52, 5884–5890. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Mei, D.; Lamaka, S.V.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Vyaliy, I.E.; Zheludkevich, M.L.; Gnedenkov, S.V. Localized currents and pH distribution studied during corrosion of MA8 Mg alloy in the cell culture medium. Corros. Sci. 2020, 170, 108689. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Gonzalez, J.; Mei, D.; Feyerabend, F.; Willumeit-Römer, R.; Zheludkevich, M.L. Local PH and its evolution near Mg alloy surfaces exposed to simulated body fluids. Adv. Mater. Interfaces 2018, 5, 1800169. [Google Scholar] [CrossRef]
- Nordlien, J.H.; One, S.; Masuko, N. Morphology and structure of oxide films formed on magnesium by exposure to air and water. J. Electrochem. Soc. 1995, 142, 3320–3322. [Google Scholar] [CrossRef]
- Ascencio, M.; Pekguleryuz, M.; Omanovic, S. An investigation of the corrosion mechanisms of WE43 Mg alloy in a modified simulated body fluid solution: The influence of immersion time. Corros. Sci. 2014, 87, 489–503. [Google Scholar] [CrossRef]
- Zeng, R.C.; Sun, L.; Zheng, Y.F.; Cui, H.Z.; Han, E.H. Corrosion and characterisation of dual phase Mg–Li–Ca alloy in Hank’s solution: The influence of microstructural features. Corros. Sci. 2014, 79, 69–82. [Google Scholar] [CrossRef]
- Liu, M.; Zanna, S.; Ardelean, H.; Frateur, I.; Schmutz, P.; Song, G.; Atrens, A.; Marcus, P. A first quantitative XPS study of the surface films formed, by exposure to water, on Mg and on the Mg–Al intermetallics: Al3Mg2 and Mg17Al12. Corros. Sci. 2009, 51, 1115–1127. [Google Scholar] [CrossRef]
- Curioni, M. The behaviour of magnesium during free corrosion and potentiodynamic polarization investigated by real-time hydrogen measurement and optical imaging. Electrochim. Acta 2014, 120, 284–292. [Google Scholar] [CrossRef]
- Yang, J.; Yim, C.D.; You, B.S. Effects of Solute Zn on Corrosion Film of Mg–Sn–Zn Alloy Formed in NaCl Solution. J. Electrochem. Soc. 2016, 163, C839–C844. [Google Scholar] [CrossRef]
- Ardelean, H.; Seyeux, A.; Zanna, S.; Prima, F.; Frateur, I.; Marcus, P. Corrosion processes of Mg–Y–Nd–Zr alloys in Na2SO4 electrolyte. Corros. Sci. 2013, 73, 196–207. [Google Scholar] [CrossRef]
- Marco, I.; Feyerabend, F.; Willumeit-Römer, R.; Van der Biest, O.; The Minerals, Metals & Materials Society. Influence of testing environment on the degradation behavior of magnesium alloys for bioabsorbable implants. In TMS 2015 144th Annual Meeting & Exhibition; Springer: Cham, Switzerland, 2015; pp. 497–506. [Google Scholar] [CrossRef]
- Tie, D.; Feyerabend, F.; Hort, N.; Hoeche, D.; Kainer, K.U.; Willumeit, R.; Mueller, W.D. In vitro mechanical and corrosion properties of biodegradable Mg-Ag alloys. Mater. Corros. 2014, 65, 569–576. [Google Scholar] [CrossRef]
- Rettig, R.; Virtanen, S. Composition of corrosion layers on a magnesium rare-earth alloy in simulated body fluids. J. Biomed. Mater. Res. A 2009, 88, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, X.; Zhang, Z.; Farrell, N.; Chen, D.; Zheng, Y. Comparative, real-time in situ monitoring of galvanic corrosion in Mg-Mg2Ca and Mg-MgZn2 couples in Hank’s solution. Corros. Sci. 2019, 161, 108185. [Google Scholar] [CrossRef]
- Li, H.; Pang, S.; Liu, Y.; Sun, L.; Liaw, P.K.; Zhang, T. Biodegradable Mg–Zn–Ca–Sr bulk metallic glasses with enhanced corrosion performance for biomedical applications. Mater. Des. 2015, 67, 9–19. [Google Scholar] [CrossRef]
- Xin, Y.; Hu, T.; Chu, P.K. Degradation behaviour of pure magnesium in simulated body fluids with different concentrations of HCO3−. Corros. Sci. 2011, 53, 1522–1528. [Google Scholar] [CrossRef]
- Cao, X.; Jia, Q.; Xu, C.; Zhang, Z.; Ren, C.; Yang, W.; Zhang, J. Research on dynamic corrosion behavior and the microstructure of biomedical Mg–Y–Zn–Zr–Sr in simulated body fluid solution after processing by solution treatment. Adv. Eng. Mater. 2020, 22, 1901146. [Google Scholar] [CrossRef]
- Gonzalez, J.; Lamaka, S.V.; Mei, D.; Scharnagl, N.; Feyerabend, F.; Zheludkevich, M.L.; Willumeit-Romer, R. Mg biodegradation mechanism deduced from the local surface environment under simulated physiological conditions. Adv. Healthc. Mater. 2021, 10, e2100053. [Google Scholar] [CrossRef]
- Rossrucker, L.; Samaniego, A.; Grote, J.P.; Mingers, A.M.; Laska, C.A.; Birbilis, N.; Frankel, G.S.; Mayrhofer, K.J.J. The pH dependence of magnesium dissolution and hydrogen evolution during anodic polarization. J. Electrochem. Soc. 2015, 162, C333–C339. [Google Scholar] [CrossRef]
- Danaie, M.; Asmussen, R.M.; Jakupi, P.; Shoesmith, D.W.; Botton, G.A. The role of aluminum distribution on the local corrosion resistance of the microstructure in a sand-cast AM50 alloy. Corros. Sci. 2013, 77, 151–163. [Google Scholar] [CrossRef]
- Chu, P.-W.; Le Mire, E.; Marquis, E.A. Microstructure of localized corrosion front on Mg alloys and the relationship with hydrogen evolution. Corros. Sci. 2017, 128, 253–264. [Google Scholar] [CrossRef]
- Gray-Munro, J.E.; Strong, M. A study on the interfacial chemistry of magnesium hydroxide surfaces in aqueous phosphate solutions: Influence of Ca2+, Cl− and protein. J. Colloid Interface Sci. 2013, 393, 421–428. [Google Scholar] [CrossRef]
- Yao, H.B.; Li, Y.; Wee, A.T.S. An XPS investigation of the oxidationrcorrosion of melt-spun Mg. Appl. Surf. Sci. 2000, 158, 112–119. [Google Scholar] [CrossRef]
- McCafferty, E. Introduction to Corrosion Science; Springer: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Phillips, R.C.; Kish, J.R. On the self-passivation tendency of Mg-Al-Zn (AZ) alloys in aqueous solutions. ECS Trans. 2012, 41, 167. [Google Scholar] [CrossRef]
- Taheri, M.; Phillips, R.C.; Kish, J.R.; Botton, G.A. Analysis of the surface film formed on Mg by exposure to water using a FIB cross-section and STEM–EDS. Corros. Sci. 2012, 59, 222–228. [Google Scholar] [CrossRef]
- Brady, M.P.; Rother, G.; Anovitz, L.M.; Littrell, K.C.; Unocic, K.A.; Elsentriecy, H.H.; Song, G.L.; Thomson, J.K.; Gallego, N.C.; Davis, B. Film breakdown and nano-porous Mg(OH)2 formation from corrosion of magnesium alloys in salt solutions. J. Electrochem. Soc. 2015, 162, C140–C149. [Google Scholar] [CrossRef]
- Zhang, X.G. Galvanic corrosion. In Uhlig’s Corrosion Handbook, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 123–143. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, C.; Xu, S.; Gao, Y.; Jiang, S. Accelerated degradation rate of AZ31 magnesium alloy by copper additions. Mater. Corros. 2018, 69, 760–769. [Google Scholar] [CrossRef]
- Deshpande, K.B. Experimental investigation of galvanic corrosion: Comparison between SVET and immersion techniques. Corros. Sci. 2010, 52, 2819–2826. [Google Scholar] [CrossRef]
- Chen, Y.F.; Liu, M.; Zeng, X.Q.; Ding, W.J. Investigation of effect of geometry design on the galvanic corrosion of Mg component. Mater. Corros. 2015, 66, 949–962. [Google Scholar] [CrossRef]
- Jia, J.X.; Song, G.; Atrens, A. Experimental measurement and computer simulation of galvanic corrosion of magnesium coupled to steel. Adv. Eng. Mater. 2007, 9, 65–74. [Google Scholar] [CrossRef]
- Silva, E.L.; Höche, D.; Bouali, A.C.; Serdechnova, M.; Sesenes, R.L.; Scholz, C.S.; Zheludkevich, M.L. Digital modelling of the galvanic corrosion behaviour of a self-piercing riveted AZ31–AA5083 hybrid joint. Mater. Werkst 2017, 48, 529–545. [Google Scholar] [CrossRef]
- Gusieva, K.; Davies, C.H.J.; Scully, J.R.; Birbilis, N. Corrosion of magnesium alloys: The role of alloying. Int. Mater. Rev. 2015, 60, 169–194. [Google Scholar] [CrossRef]
- Ghali, E. Performance and corrosion forms of magnesium and its alloys. In Corrosion Resistance of Aluminum and Magnesium Alloys: Understanding, Performance, and Testing; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 320–347. [Google Scholar] [CrossRef]
- Ghali, E. Magnesium and magnesium alloys. In Uhlig’s Corrosion Handbook, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 809–835. [Google Scholar] [CrossRef]
- Tang, R.Z.; Tian, R.Z. Binary Alloy Phase Diagrams and Crystal Structure of Intermediate Phase; Central South University Press: Changsha, China, 2009. [Google Scholar]
- Südholz, A.D. Electrochemical properties of intermetallic phases and common impurity elements in magnesium alloys. Electrochem. Solid State Lett. 2011, 14, C5. [Google Scholar] [CrossRef]
- Song, G.L.; Xu, Z.q. Effect of microstructure evolution on corrosion of different crystal surfaces of AZ31 Mg alloy in a chloride containing solution. Corros. Sci. 2012, 54, 97–105. [Google Scholar] [CrossRef]
- Song, G.l.; Atrens, A.; Dargusch, M. Influence of microstructure on the corrosion of diecast AZ91D. Corros. Sci. 1999, 41, 249–273. [Google Scholar] [CrossRef]
- Jia, Q.g.; Han, S.; Sun, Y.; Zhang, W.; Xu, C.; Zhang, J. Effect of yttrium addition on the microstructure and corrosion resistance of Mg−Zn−Zr alloys. Mater. Werkst 2019, 50, 1391–1398. [Google Scholar] [CrossRef]
- Ding, Y.; Li, Y.; Wen, C. Effects of Mg17Sr2 phase on the bio-corrosion behavior of Mg-Zr-Sr alloys. Adv. Eng. Mater. 2016, 18, 259–268. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Z.; Xiong, X.; Zhu, C.; Yan, P. Microstructures and corrosion behaviors of squeeze-cast Mg-4Al-2RE and Mg-4Al-0.5RE-xCa (x = 0.3, 0.8, and 1.5) alloys. Mater. Corros. 2018, 69, 1300–1309. [Google Scholar] [CrossRef]
- Ben-Hamu, G.; Eliezer, D.; Shin, K.S. The role of Mg2Si on the corrosion behavior of wrought Mg–Zn–Mn alloy. Intermetallics 2008, 16, 860–867. [Google Scholar] [CrossRef]
- Ben-Hamu, G.; Eliezer, D. Corrosion behavior of wrought Mg-6%Zn-1%Mn-XSi-YCa alloy. Mater. Corros. 2013, 64, 516–521. [Google Scholar] [CrossRef]
- Song, Y.; Shan, D.; Chen, R.; Han, E.-H. Effect of second phases on the corrosion behaviour of wrought Mg–Zn–Y–Zr alloy. Corros. Sci. 2010, 52, 1830–1837. [Google Scholar] [CrossRef]
- Tunold, R.; Holtan, H.; Berg, M.B.H.; Lasson, A.; Steen-hansen, R. The corrosion of magnesium in aqueous solution containing chloride ions. Corros. Sci. 1977, 17, 353–365. [Google Scholar] [CrossRef]
- Gholami-Kermanshahi, M.; Neubert, V.-D.; Tavakoli, M.; Pastorek, F.; Smola, B.; Neubert, V. Effect of ECAP processing on corrosion behavior and mechanical properties of the ZFW MP magnesium alloy as a biodegradable implant material. Adv. Eng. Mater. 2018, 20, 1800121. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, L.; Li, L.; Gu, X.; Zhang, K.; Xia, J.; Fan, Y. Effect of stress on corrosion of high-purity magnesium in vitro and in vivo. Acta Biomater. 2019, 83, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Peron, M.; Skaret, P.C.; Fabrizi, A.; Varone, A.; Montanari, R.; Roven, H.J.; Ferro, P.; Berto, F.; Torgersen, J. The effect of equal channel angular pressing on the stress corrosion cracking susceptibility of AZ31 alloy in simulated body fluid. J. Mech. Behav. Biomed. Mater. 2020, 106, 103724. [Google Scholar] [CrossRef] [PubMed]
- Elkaiam, L.; Hakimi, O.; Aghion, E. Stress corrosion and corrosion fatigue of biodegradable Mg-Zn-Nd-Y-Zr alloy in in-vitro conditions. Metals 2020, 10, 791. [Google Scholar] [CrossRef]
- Jiang, J.; Xie, Q.; Qiang, M.; Ma, A.; Taylor, E.-K.; Li, Y.; Song, D.; Chen, J. Stress corrosion cracking behaviors of RE-containing ME21 magnesium alloy processed by equal-channel angular pressing. J. Rare Earths 2019, 37, 88–94. [Google Scholar] [CrossRef]
- Wang, B.J.; Xu, D.K.; Sun, J.; Han, E.-H. Effect of grain structure on the stress corrosion cracking (SCC) behavior of an as-extruded Mg-Zn-Zr alloy. Corros. Sci. 2019, 157, 347–356. [Google Scholar] [CrossRef]
- Sozanska, M.; Moscicki, A.; Czujko, T. The characterization of stress corrosion cracking in the AE44 magnesium casting alloy using quantitative fractography methods. Materials 2019, 12, 4125. [Google Scholar] [CrossRef]
- Choudhary, L.; Singh Raman, R.K.; Hofstetter, J.; Uggowitzer, P.J. In-vitro characterization of stress corrosion cracking of aluminium-free magnesium alloys for temporary bio-implant applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 42, 629–636. [Google Scholar] [CrossRef]
- Prabhu, D.B.; Nampoothiri, J.; Elakkiya, V.; Narmadha, R.; Selvakumar, R.; Sivasubramanian, R.; Gopalakrishnan, P.; Ravi, K.R. Elucidating the role of microstructural modification on stress corrosion cracking of biodegradable Mg4Zn alloy in simulated body fluid. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 106, 110164. [Google Scholar] [CrossRef]
- Argade, G.R.; Panigrahi, S.K.; Mishra, R.S. Aging response on the stress corrosion cracking behavior of wrought precipitation-hardened magnesium alloy. J. Mater. Sci. 2019, 55, 1216–1230. [Google Scholar] [CrossRef]
- Koo, Y.; Jang, Y.; Yun, Y. A study of long-term static load on degradation and mechanical integrity of Mg alloys-based biodegradable metals. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2017, 219, 45–54. [Google Scholar] [CrossRef]
- Song, Y.; Liu, Q.; Wang, H.; Zhu, X. Effect of Gd on microstructure and stress corrosion cracking of the AZ91-extruded magnesium alloy. Mater. Corros. 2021, 72, 1189–1200. [Google Scholar] [CrossRef]
- Chen, K.; Lu, Y.; Tang, H.; Gao, Y.; Zhao, F.; Gu, X.; Fan, Y. Effect of strain on degradation behaviors of WE43, Fe and Zn wires. Acta Biomater. 2020, 113, 627–645. [Google Scholar] [CrossRef] [PubMed]
- Dubey, D.; Kadali, K.; Panda, S.S.; Kumar, A.; Jain, J.; Mondal, K.; Singh, S.S. Comparative study on the stress corrosion cracking susceptibility of AZ80 and AZ31 magnesium alloys. Mater. Sci. Eng. A Struct. 2020, 792, 139793. [Google Scholar] [CrossRef]
- Merson, E.; Poluyanov, V.; Myagkikh, P.; Merson, D.; Vinogradov, A. Inhibiting stress corrosion cracking by removing corrosion products from the Mg-Zn-Zr alloy pre-exposed to corrosion solutions. SSRN Electron. J. 2021, 205, 116570. [Google Scholar] [CrossRef]
- Merson, E.; Poluyanov, V.; Myagkikh, P.; Merson, D.; Vinogradov, A. On the role of pre-exposure time and corrosion products in stress-corrosion cracking of ZK60 and AZ31 magnesium alloys. Mater. Sci. Eng. A Struct. 2021, 806, 140876. [Google Scholar] [CrossRef]
- Merson, E.; Myagkikh, P.; Poluyanov, V.; Merson, D.; Vinogradov, A. On the role of hydrogen in stress corrosion cracking of magnesium and its alloys: Gas-analysis study. Mater. Sci. Eng. A Struct. 2019, 748, 337–346. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, L.L.; Gao, H.; Yuan, X.; Chen, X. Biodegradable behaviour and fatigue life of ZEK100 magnesium alloy in simulated physiological environment. Fatigue Fract. Eng. Mater. Struct. 2015, 38, 904–913. [Google Scholar] [CrossRef]
- Wang, B.J.; Xu, D.K.; Wang, S.D.; Sheng, L.Y.; Zeng, R.-C.; Han, E.-h. Influence of solution treatment on the corrosion fatigue behavior of an as-forged Mg-Zn-Y-Zr alloy. Int. J. Fatigue 2019, 120, 46–55. [Google Scholar] [CrossRef]
- Nan, Z.; Ishihara, S.; Goshima, T. Corrosion fatigue behavior of extruded magnesium alloy AZ31 in sodium chloride solution. Int. J. Fatigue 2008, 30, 1181–1188. [Google Scholar] [CrossRef]
- Bian, D.; Zhou, W.; Liu, Y.; Li, N.; Zheng, Y.; Sun, Z. Fatigue behaviors of HP-Mg, Mg-Ca and Mg-Zn-Ca biodegradable metals in air and simulated body fluid. Acta Biomater. 2016, 41, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Harandi, S.E.; Singh Raman, R.K. Corrosion fatigue of a magnesium alloy under appropriate human physiological conditions for bio-implant applications. Eng. Fract. Mech. 2017, 186, 134–142. [Google Scholar] [CrossRef]
- Bobby Kannan, M.; Singh Raman, R.K.; Witte, F.; Blawert, C.; Dietzel, W. Influence of circumferential notch and fatigue crack on the mechanical integrity of biodegradable magnesium-based alloy in simulated body fluid. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 96, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; Singh Raman, R.K.; Davies, C.H.J. Corrosion fatigue of a magnesium alloy in modified simulated body fluid. Eng. Fract. Mech. 2015, 137, 2–11. [Google Scholar] [CrossRef]
- Wittke, P.; Klein, M.; Dieringa, H.; Walther, F. Corrosion fatigue assessment of creep-resistant magnesium alloy Mg–4Al–2Ba–2Ca in aqueous sodium chloride solution. Int. J. Fatigue 2016, 83, 59–65. [Google Scholar] [CrossRef]
- Williams, G.; Birbilis, N.; McMurray, H.N. Controlling factors in localised corrosion morphologies observed for magnesium immersed in chloride containing electrolyte. Faraday Discuss. 2015, 180, 313–330. [Google Scholar] [CrossRef]
- Thomas, S.; Medhekar, N.V.; Frankel, G.S.; Birbilis, N. Corrosion mechanism and hydrogen evolution on Mg. Curr. Opin. Solid State Mater. Sci. 2015, 19, 85–94. [Google Scholar] [CrossRef]
- Lysne, D.; Thomas, S.; Hurley, M.F.; Birbilis, N. On the Fe enrichment during anodic polarization of Mg and its impact on hydrogen evolution. J. Electrochem. Soc. 2015, 162, C396–C402. [Google Scholar] [CrossRef]
- Wang, L.; Shinohara, T.; Zhang, B.-P.; Iwai, H. Characterization of surface products on AZ31 magnesium alloy in dilute NaCl solution. J. Alloys Compd. 2009, 485, 747–752. [Google Scholar] [CrossRef]
- Wang, L.; Shinohara, T.; Zhang, B.P. XPS study of the surface chemistry on AZ31 and AZ91 magnesium alloys in dilute NaCl solution. Appl. Surf. Sci. 2010, 256, 5807–5812. [Google Scholar] [CrossRef]
- Filotás, D.; Fernández-Pérez, B.M.; Nagy, L.; Nagy, G.; Souto, R.M. A novel scanning electrochemical microscopy strategy for the investigation of anomalous hydrogen evolution from AZ63 magnesium alloy. Sens. Actuators B Chem. 2020, 308, 127691. [Google Scholar] [CrossRef]
- Senf, J.; Broszeit, E.; Gugau, M.; Berger, C. Corrosion and galvanic corrosion of die casted magnesium alloys. In Magnesium Technology 2000; The Minerals, Metals & Materials Society; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000; pp. 136–142. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, Q.; Zheng, M.; Hou, B.; Li, Y. Corrosion behavior of Mg–8Li–3Zn–Al alloy in neutral 3.5% NaCl solution. J. Magnes. Alloy. 2016, 4, 22–26. [Google Scholar] [CrossRef]
- Xin, Y.; Huo, K.; Tao, H.; Tang, G.; Chu, P.K. Influence of aggressive ions on the degradation behavior of biomedical magnesium alloy in physiological environment. Acta Biomater. 2008, 4, 2008–2015. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, J.; Nirmalan, M. Acid–base balance: The traditional approach. Curr. Anaesth. Crit. Care 2005, 16, 137–142. [Google Scholar] [CrossRef]
- Bailey, J.E.; Pablo, L.S. Practical approach to acid–base disorders. Vet. Clin. N. Am. Small Anim. Pract. 1998, 28, 645–662. [Google Scholar] [CrossRef]
- Torne, K.; Ornberg, A.; Weissenrieder, J. The influence of buffer system and biological fluids on the degradation of magnesium. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1490–1502. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Waterman, J.; Birbilis, N.; Dias, G.; Woodfield, T.B.; Hartshorn, R.M.; Staiger, M.P. Buffer-regulated biocorrosion of pure magnesium. J. Mater. Sci. Mater. Med. 2012, 23, 283–291. [Google Scholar] [CrossRef]
- Walker, J.; Shadanbaz, S.; Kirkland, N.T.; Stace, E.; Woodfield, T.; Staiger, M.P.; Dias, G.J. Magnesium alloys: Predicting in vivo corrosion with in vitro immersion testing. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1134–1141. [Google Scholar] [CrossRef]
- Schinhammer, M.; Hofstetter, J.; Wegmann, C.; Moszner, F.; Löffler, J.F.; Uggowitzer, P.J. On the immersion testing of degradable implant materials in simulated body fluid: Active pH regulation using CO2. Adv. Eng. Mater. 2013, 15, 434–441. [Google Scholar] [CrossRef]
- Xin, Y.; Chu, P.K. Influence of Tris in simulated body fluid on degradation behavior of pure magnesium. Mater. Chem. Phys. 2010, 124, 33–35. [Google Scholar] [CrossRef]
- Crimu, C.; Bolat, G.; Munteanu, C.; Mareci, D. Degradation characteristics of Mg0.8Ca in saline solution with and without albumin protein investigated by electrochemical impedance spectroscopy. Mater. Corros. 2015, 66, 649–655. [Google Scholar] [CrossRef]
- Katz, S.; Klotz, I.M. Interactions of calcium with serum albumin. Arch. Biochem. Biophys. 1953, 23, 351–361. [Google Scholar] [CrossRef]
- Frye, R.M.; Lees, H. Magnesium-albumin binding measurements using ion-selective membrane electrodes. Clin. Biochem. 1974, 7, 258–270. [Google Scholar] [CrossRef]
- Willumeit, R.; Fischer, J.; Feyerabend, F.; Hort, N.; Bismayer, U.; Heidrich, S.; Mihailova, B. Chemical surface alteration of biodegradable magnesium exposed to corrosion media. Acta Biomater. 2011, 7, 2704–2715. [Google Scholar] [CrossRef]
- Seuss, F.; Seuss, S.; Turhan, M.C.; Fabry, B.; Virtanen, S. Corrosion of Mg alloy AZ91D in the presence of living cells. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 99, 276–281. [Google Scholar] [CrossRef]
- Kannan, M.B.; Yamamoto, A.; Khakbaz, H. Influence of living cells (L929) on the biodegradation of magnesium-calcium alloy. Colloids Surf. B Biointerfaces 2015, 126, 603–606. [Google Scholar] [CrossRef]
- Zhang, J.; Hiromoto, S.; Yamazaki, T.; Niu, J.; Huang, H.; Jia, G.; Li, H.; Ding, W.; Yuan, G. Effect of macrophages on in vitro corrosion behavior of magnesium alloy. J. Biomed. Mater. Res. A 2016, 104, 2476–2487. [Google Scholar] [CrossRef]
- Xu, L.; Yu, G.; Zhang, E.; Pan, F.; Yang, K. In vivo corrosion behavior of Mg-Mn-Zn alloy for bone implant application. J. Biomed. Mater. Res. A 2007, 83, 703–711. [Google Scholar] [CrossRef]
- Han, P.; Tan, M.; Zhang, S.; Ji, W.; Li, J.; Zhang, X.; Zhao, C.; Zheng, Y.; Chai, Y. Shape and site dependent in vivo degradation of Mg-Zn pins in rabbit femoral condyle. Int. J. Mol. Sci. 2014, 15, 2959–2970. [Google Scholar] [CrossRef]
- Willbold, E.; Kaya, A.A.; Kaya, R.A.; Beckmann, F.; Witte, F. Corrosion of magnesium alloy AZ31 screws is dependent on the implantation site. Mater. Sci. Eng. B Adv. 2011, 176, 1835–1840. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, C.; Lou, T.; Chen, B.; Yi, R.; Wang, W.; Zhang, R.; Zuo, M.; Xu, H.; Han, P.; et al. Crevice corrosion—A newly observed mechanism of degradation in biomedical magnesium. Acta Biomater. 2019, 98, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, L.; Shi, T.; Li, B.; Yu, W.; Zhang, C.; Yan, P.; Zhang, Q.; Tian, H.; Lv, Y. Corrosion decomposition and mechanical behaviors of as-cast Mg–xZn–Zr alloys. Mater. Corros. 2020, 71, 1453–1461. [Google Scholar] [CrossRef]
- Jiang, Q.T.; Li, J.R.; Ma, X.M.; Li, Y.T.; Hou, B.R. The relationship between microstructure and corrosion behaviors of Mg-3Y-xNd alloys (x = 0.5, 1.0, 1.5 wt%). Mater. Corros. 2016, 67, 876–881. [Google Scholar] [CrossRef]
- Yang, J.; Yim, C.D.; You, B.S. Characteristics of surface films formed on Mg–Sn alloys in NaCl solution. J. Electrochem. Soc. 2016, 163, C395–C401. [Google Scholar] [CrossRef]
- Cano, Z.P.; McDermid, J.R.; Kish, J.R. Cathodic activity of corrosion filaments formed on mg alloy AM30. J. Electrochem. Soc. 2015, 162, C732–C740. [Google Scholar] [CrossRef]
- Atrens, A.; Shi, Z.; Mehreen, S.U.; Johnston, S.; Song, G.-L.; Chen, X.; Pan, F. Review of Mg alloy corrosion rates. J. Magnesium. Alloy. 2020, 8, 989–998. [Google Scholar] [CrossRef]
- Howard, J.E. Metabolism of calcium and phosphorus in bone. Bull. N. Y. Acad. Med. 1951, 27, 24–41. [Google Scholar]
- Neubert, V.; Bakkar, A. Effect of small Ca-additions on the corrosion resistance of AS41-Mg alloy. In Magnesium: Proceedings of the 6th International Conference Magnesium Alloys and Their Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003; pp. 638–645. [Google Scholar] [CrossRef]
- Rao, K.P.; Prasad, Y.V.R.K.; Dharmendra, C.; Suresh, K.; Hort, N.; Dieringa, H. Review on hot working behavior and strength of calcium-containing magnesium alloys. Adv. Eng. Mater. 2018, 20, 1701102. [Google Scholar] [CrossRef]
- Cha, P.R.; Han, H.S.; Yang, G.F.; Kim, Y.C.; Hong, K.H.; Lee, S.C.; Jung, J.Y.; Ahn, J.P.; Kim, Y.Y.; Cho, S.Y.; et al. Biodegradability engineering of biodegradable Mg alloys: Tailoring the electrochemical properties and microstructure of constituent phases. Sci. Rep. 2013, 3, 2367. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Birbilis, N.; Walker, J.; Woodfield, T.; Dias, G.J.; Staiger, M.P. In-vitro dissolution of magnesium-calcium binary alloys: Clarifying the unique role of calcium additions in bioresorbable magnesium implant alloys. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 95, 91–100. [Google Scholar] [CrossRef]
- Li, C.Q.; Xu, D.K.; Chen, X.B.; Wang, B.J.; Wu, R.Z.; Han, E.H.; Birbilis, N. Composition and microstructure dependent corrosion behaviour of Mg-Li alloys. Electrochim. Acta 2018, 260, 55–64. [Google Scholar] [CrossRef]
- Bobby Kannan, M.; Singh Raman, R.K. In vitro degradation and mechanical integrity of calcium-containing magnesium alloys in modified-simulated body fluid. Biomaterials 2008, 29, 2306–2314. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Liu, K.; Ma, K.; Wang, Z.; Li, S. Effects of trace Ca/Sn addition on corrosion behaviors of biodegradable Mg–4Zn–0.2Mn alloy. J. Magnes. Alloy. 2018, 6, 1–14. [Google Scholar] [CrossRef]
- Bowen, P.K.; Drelich, J.; Goldman, J. Zinc exhibits ideal physiological corrosion behavior for bioabsorbable stents. Adv. Mater. 2013, 25, 2577–2582. [Google Scholar] [CrossRef]
- Bahmani, A.; Arthanari, S.; Shin, K.S. Corrosion behavior of Mg–Mn–Ca alloy: Influences of Al, Sn and Zn. J. Magnes. Alloy. 2019, 7, 38–46. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Fereidouni-Lotfabadi, A.; Daroonparvar, M.; Yajid, M.A.M.; Mezbahul-Islam, M.; Kasiri-Asgarani, M.; Medraj, M. Microstructure and bio-corrosion behavior of Mg-Zn and Mg-Zn-Ca alloys for biomedical applications. Mater. Corros. 2014, 65, 1178–1187. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Song, Y.; Zhao, C.; Xie, C.; Zhang, X. Influence of heat treatments on in vitro degradation behavior of Mg-6Zn alloy studied by electrochemical measurements. Adv. Eng. Mater. 2010, 12, B170–B174. [Google Scholar] [CrossRef]
- Sikder, P.; Bhaduri, S.B.; Ong, J.L.; Guda, T. Silver (Ag) doped magnesium phosphate microplatelets as next-generation antibacterial orthopedic biomaterials. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 976–989. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Ismail, A.F.; Aziz, M.; Kasiri-Asgarani, M.; Ghayour, H.; Razzaghi, M.; Hadisi, Z. In vitro corrosion behavior, bioactivity, and antibacterial performance of the silver-doped zinc oxide coating on magnesium alloy. Mater. Corros. 2017, 68, 1228–1236. [Google Scholar] [CrossRef]
- Ben-Hamu, G.; Eliezer, A. The influence of Ag and Si additions on the electrochemical behavior in extruded Mg–Zn alloys. Isr. J. Chem. 2007, 47, 309–317. [Google Scholar] [CrossRef]
- Gusieva, K.; Sato, T.; Sha, G.; Ringer, S.P.; Birbilis, N. Influence of low level Ag additions on Mg-alloy AZ91. Adv. Eng. Mater. 2013, 15, 485–490. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, X.; Yang, H. Sr microalloying for refining grain size of AZ91D Magnesium alloy. J. Wuhan Univ. Technol. 2007, 22, 74–76. [Google Scholar] [CrossRef]
- Du, J.; Yang, J.; Kuwabara, M.; Li, W.; Peng, J. Effect of strontium on the grain refining efficiency of Mg–3Al alloy refined by carbon inoculation. J. Alloys Compd. 2009, 470, 228–232. [Google Scholar] [CrossRef]
- Gu, X.N.; Xie, X.H.; Li, N.; Zheng, Y.F.; Qin, L. In vitro and in vivo studies on a Mg-Sr binary alloy system developed as a new kind of biodegradable metal. Acta Biomater. 2012, 8, 2360–2374. [Google Scholar] [CrossRef]
- Bornapour, M.; Muja, N.; Shum-Tim, D.; Cerruti, M.; Pekguleryuz, M. Biocompatibility and biodegradability of Mg-Sr alloys: The formation of Sr-substituted hydroxyapatite. Acta Biomater. 2013, 9, 5319–5330. [Google Scholar] [CrossRef]
- Kang, D.H.; Park, S.S.; Kim, N.J. Development of creep resistant die cast Mg–Sn–Al–Si alloy. Mater. Sci. Eng. A Struct. 2005, 413–414, 555–560. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Huang, S.; Zhou, X. Study of the corrosion behavior and the corrosion films formed on the surfaces of Mg–xSn alloys in 3.5wt.% NaCl solution. Appl. Surf. Sci. 2014, 317, 1143–1150. [Google Scholar] [CrossRef]
- Cain, T.W.; Glover, C.F.; Scully, J.R. The corrosion of solid solution Mg-Sn binary alloys in NaCl solutions. Electrochim. Acta 2019, 297, 564–575. [Google Scholar] [CrossRef]
- Yang, J.; Yim, C.D.; You, B.S. Effects of Sn in α-Mg matrix on properties of surface films of Mg-xSn (x = 0, 2, 5 wt%) alloys. Mater. Corros. 2016, 67, 531–541. [Google Scholar] [CrossRef]
- Hu, Y. Electrochemical Polarization behaviour of Mg-Al alloys in near-neutral solutions. Master’s Thesis, Materials Science & Engineering, McMaster University, Hamilton, ON, Canada, 2012. [Google Scholar]
- Gandel, D.S.; Easton, M.A.; Gibson, M.A.; Birbilis, N. Influence of Mn and Zr on the corrosion of Al-Free Mg alloys: Part 1—electrochemical behavior of Mn and Zr. Corrosion 2013, 69, 666–671. [Google Scholar] [CrossRef]
- Lun Sin, S.; Dubé, D.; Tremblay, R. Characterization of Al–Mn particles in AZ91D investment castings. Mater. Charact. 2007, 58, 989–996. [Google Scholar] [CrossRef]
- Simanjuntak, S.; Cavanaugh, M.K.; Gandel, D.S.; Easton, M.A.; Gibson, M.A.; Birbilis, N. The influence of iron, manganese, and zirconium on the corrosion of magnesium: An artificial neural network approach. Corrosion 2015, 71, 199–208. [Google Scholar] [CrossRef]
- Gandel, D.S.; Easton, M.A.; Gibson, M.A.; Birbilis, N. Influence of Mn and Zr on the corrosion of Al-free Mg alloys: Part 2—impact of Mn and Zr on Mg alloy electrochemistry and corrosion. Corrosion 2013, 69, 744–751. [Google Scholar] [CrossRef]
- Yang, L.; He, S.; Yang, C.; Zhou, X.; Lu, X.; Huang, Y.; Qin, G.; Zhang, E. Mechanism of Mn on inhibiting Fe-caused magnesium corrosion. J. Magnes. Alloy. 2021, 9, 676–685. [Google Scholar] [CrossRef]
- Gu, D.D.; Peng, J.; Wang, J.W.; Liu, Z.T.; Pan, F.S. Effect of Mn modification on the corrosion susceptibility of Mg–Mn alloys by magnesium scrap. Acta Metall. Sin. (Engl. Lett.) 2020, 34, 1–11. [Google Scholar] [CrossRef]
- Yang, L.; Shi, Y.; Shen, L.; Zhang, E.; Qin, G.; Lu, X.; Zhou, X. Effect of Ag on cathodic activation and corrosion behaviour of Mg-Mn-Ag alloys. Corros. Sci. 2021, 185. [Google Scholar] [CrossRef]
- Dong, Q.S.; Jia, Y.Q.; Ba, Z.X.; Tao, X.W.; Wang, Z.Z.; Xue, F.; Bai, J. Exploring the corrosion behavior of Mn-implanted biomedical Mg. J. Alloys Compd. 2021, 873, 159739. [Google Scholar] [CrossRef]
- Dai, J.H.; Jiang, B.; Peng, C.; Pan, F.S. Effect of Mn additions on diffusion behavior of Fe in molten magnesium alloys by solid-liquid diffusion couples. J. Alloys Compd. 2017, 710, 260–266. [Google Scholar] [CrossRef]
- Yang, W.; Yoon, Y.O.; Kim, S.K.; Lim, H.K.; Kim, D. Effects of heat treatment on bio-corrosion properties of Mg-Zn-xMn (x = 0.5, 1.0, and 1.5 wt.%) alloys as biodegradable materials. Magnes. Technol. 2015, 2015, 407–411. [Google Scholar] [CrossRef]
- Song, G.; StJohn, D. The effect of zirconium grain refinement on the corrosion behaviour of magnesium-rare earth alloy MEZ. J. Light Met. 2002, 2, 1–16. [Google Scholar] [CrossRef]
- Wu, G.; Sun, M.; Dai, J.; Ding, W. Study on the grain refinement behavior of Mg-Zr master alloy and Zr containing compounds in Mg-10Gd-3Y magnesium alloy. In Magnesium Technology 2011; The Minerals, Metals & Materials Society; Springer: Cham, Switzerland, 2011. [Google Scholar] [CrossRef]
- Gandel, D.S.; Easton, M.A.; Gibson, M.A.; Abbott, T.; Birbilis, N. The influence of zirconium additions on the corrosion of magnesium. Corros. Sci. 2014, 81, 27–35. [Google Scholar] [CrossRef]
- Gandel, D.S.; Easton, M.A.; Gibson, M.A.; Abbott, T.; Birbilis, N. The influence of Mg-Zr master alloy microstructure on the corrosion of Mg. In Magnesium Technology 2013; The Minerals, Metals & Materials Society; Springer: Cham, Switzerland, 2013; pp. 157–162. [Google Scholar] [CrossRef]
- Ding, Y.; Li, Y.; Lin, J.; Wen, C. Effects of zirconium and strontium on the biocorrosion of Mg-Zr-Sr alloys for biodegradable implant applications. J. Mater. Chem. B 2015, 3, 3714–3729. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Wu, G.; Wang, W.; Ding, W. Effect of Zr on the microstructure, mechanical properties and corrosion resistance of Mg–10Gd–3Y magnesium alloy. Mater. Sci. Eng. A Struct. 2009, 523, 145–151. [Google Scholar] [CrossRef]
- Ben-Hamu, G.; Eliezer, D.; Shin, K.S.; Cohen, S. The relation between microstructure and corrosion behavior of Mg–Y–RE–Zr alloys. J. Alloys Compd. 2007, 431, 269–276. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, Y.; Wei, Z.; Chen, Q.; Gan, F. Improvement of corrosion resistance of AZ91D magnesium alloy by holmium addition. Corros. Sci. 2006, 48, 4223–4233. [Google Scholar] [CrossRef]
- Mert, F.; Blawert, C.; Kainer, K.U.; Hort, N. Influence of cerium additions on the corrosion behaviour of high pressure die cast AM50 alloy. Corros. Sci. 2012, 65, 145–151. [Google Scholar] [CrossRef]
- Song, Y.L.; Liu, Y.H.; Wang, S.H.; Yu, S.R.; Zhu, X.Y. Effect of cerium addition on microstructure and corrosion resistance of die cast AZ91 magnesium alloy. Mater. Corros. 2007, 58, 189–192. [Google Scholar] [CrossRef]
- Campos, R.S.; Höche, D.; Blawert, C.; Kainer, K.U. Influence of lanthanum concentration on the corrosion behaviour of binary Mg-La alloys. In Magnesium Technology 2011; The Minerals, Metals & Materials Society; Springer: Cham, Switzerland, 2011; pp. 507–511. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Z.; Liu, Y.; Yang, M.; Qu, Q. Influence of erbium, cerium on the stress corrosion cracking behavior of AZ91 alloy in humid atmosphere. Adv. Eng. Mater. 2017, 19, 1700021. [Google Scholar] [CrossRef]
- Das, T.; Sharma, A.; Talukder, G. Effects of lanthanum in cellular systems. Biol. Trace Elem. Res. 1988, 18, 201–228. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tsumura, Y.; Tonogai, Y.; Shibata, T.; Ito, Y. Differences in behavior among the chlorides of seven rare earth elements administered intravenously to rats. Fund. Appl. Limnol. 1997, 37, 106–116. [Google Scholar] [CrossRef]
- Schranz, D.; Zartner, P.; Michel-Behnke, I.; Akintürk, H. Bioabsorbable metal stents for percutaneous treatment of critical recoarctation of the aorta in a newborn. Catheter. Cardiovasc. Int. 2006, 67, 671–673. [Google Scholar] [CrossRef] [PubMed]
- Drueke, T.B. Lanthanum carbonate as a first-line phosphate binder: The “cons”. Semin. Dial. 2007, 20, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.H.; Gou, B.D.; Zhang, T.L.; Wang, K. Lanthanum chloride bidirectionally influences calcification in bovine vascular smooth muscle cells. J. Cell. Biochem. 2012, 113, 1776–1786. [Google Scholar] [CrossRef]
- Liu, M.; Schmutz, P.; Uggowitzer, P.J.; Song, G.; Atrens, A. The influence of yttrium (Y) on the corrosion of Mg–Y binary alloys. Corros. Sci. 2010, 52, 3687–3701. [Google Scholar] [CrossRef]
- Johnson, I.; Perchy, D.; Liu, H. In vitro evaluation of the surface effects on magnesium-yttrium alloy degradation and mesenchymal stem cell adhesion. J. Biomed. Mater. Res. A 2012, 100, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Zheng, D.; Song, G.-L.; Shi, Z.; Atrens, A. The corrosion behavior of Mg5Y in nominally distilled water. Adv. Eng. Mater. 2018, 20, 1700986. [Google Scholar] [CrossRef]
- Dai, Y.; Tang, Y.; Xu, X.; Luo, Z.; Zhang, Y.; Li, Z.; Lin, Z.; Zhao, S.; Zeng, M.; Sun, B.; et al. Evaluation of the mechanisms and effects of Mg-Ag-Y alloy on the tumor growth and metastasis of the MG63 osteosarcoma cell line. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2537–2548. [Google Scholar] [CrossRef]
- Li, H.; Wen, J.; He, J.; Shi, H.; Liu, Y. Effects of Dy addition on the mechanical and degradation properties of Mg–2Zn–0.5Zr alloy. Adv. Eng. Mater. 2020, 22, 1901360. [Google Scholar] [CrossRef]
- Ding, Y.; Lin, J.; Wen, C.; Zhang, D.; Li, Y. Mechanical properties, corrosion, and biocompatibility of Mg-Zr-Sr-Dy alloys for biodegradable implant applications. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2425–2434. [Google Scholar] [CrossRef]
- Kutniy, K.V.; Papirov, I.I.; Tikhonovsky, M.A.; Pikalov, A.I.; Sivtzov, S.V.; Pirozhenko, L.A.; Shokurov, V.S.; Shkuropatenko, V.A. Influence of grain size on mechanical and corrosion properties of magnesium alloy for medical implants. Mater. Werkst 2009, 40, 242–246. [Google Scholar] [CrossRef]
- Zheng, T.; Hu, Y.; Yang, S. Effect of grain size on the electrochemical behavior of pure magnesium anode. J. Magnes. Alloy. 2017, 5, 404–411. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N.; Davies, C.H.J. Revealing the relationship between grain size and corrosion rate of metals. Scr. Mater. 2010, 63, 1201–1204. [Google Scholar] [CrossRef]
- Liao, J.; Hotta, M.; Yamamoto, N. Corrosion behavior of fine-grained AZ31B magnesium alloy. Corros. Sci. 2012, 61, 208–214. [Google Scholar] [CrossRef]
- Huang, L.; Wang, K.; Wang, W.; Peng, P.; Qiao, K.; Liu, Q. Microstructural evolution and corrosion behavior of friction stir processed fine-grained AZ80 Mg alloy. Mater. Corros. 2019, 71, 93–108. [Google Scholar] [CrossRef]
- Ralston, K.D.; Williams, G.; Birbilis, N. Effect of pH on the grain size dependence of magnesium corrosion. Corrosion 2012, 68, 507–517. [Google Scholar] [CrossRef]
- Argade, G.R.; Panigrahi, S.K.; Mishra, R.S. Effects of grain size on the corrosion resistance of wrought magnesium alloys containing neodymium. Corros. Sci. 2012, 58, 145–151. [Google Scholar] [CrossRef]
- Xin, R.; Luo, Y.; Zuo, A.; Gao, J.; Liu, Q. Texture effect on corrosion behavior of AZ31 Mg alloy in simulated physiological environment. Mater. Lett. 2012, 72, 1–4. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, Z.; Long, C.; Chen, H.; Wu, Y.; Huang, J.a.; Zhou, W.; Wu, Y. Effect of crystal orientation and {101-2} twins on the corrosion behaviour of AZ31 magnesium alloy. J. Alloys Compd. 2020, 827, 154096. [Google Scholar] [CrossRef]
- Song, G.L.; Mishra, R.; Xu, Z.Q. Crystallographic orientation and electrochemical activity of AZ31 Mg alloy. Electrochem. Commun. 2010, 12, 1009–1012. [Google Scholar] [CrossRef]
- Fu, B.Q.; Liu, W.; Li, Z.L. Calculation of the surface energy of hcp-metals with the empirical electron theory. Appl. Surf. Sci. 2009, 255, 9348–9357. [Google Scholar] [CrossRef]
- Xin, R.; Li, B.; Li, L.; Liu, Q. Influence of texture on corrosion rate of AZ31 Mg alloy in 3.5wt.% NaCl. Mater. Des. 2011, 32, 4548–4552. [Google Scholar] [CrossRef]
- Liu, M.; Qiu, D.; Zhao, M.-C.; Song, G.; Atrens, A. The effect of crystallographic orientation on the active corrosion of pure magnesium. Scr. Mater. 2008, 58, 421–424. [Google Scholar] [CrossRef]
- Orlov, D.; Ralston, K.D.; Birbilis, N.; Estrin, Y. Enhanced corrosion resistance of Mg alloy ZK60 after processing by integrated extrusion and equal channel angular pressing. Acta Mater. 2011, 59, 6176–6186. [Google Scholar] [CrossRef]
- Gebert, A.; Subba Rao, R.V.; Wolff, U.; Baunack, S.; Eckert, J.; Schultz, L. Corrosion behaviour of the Mg65Y10Cu15Ag10 bulk metallic glass. Mater. Sci. Eng. A Struct. 2004, 375–377, 280–284. [Google Scholar] [CrossRef]
- Xu, Y.K.; Xu, J. Ceramics particulate reinforced Mg65Cu20Zn5Y10 bulk metallic glass composites. Scr. Mater. 2003, 49, 843–848. [Google Scholar] [CrossRef]
- Wong, C.C.; Wong, P.C.; Tsai, P.H.; Jang, J.S.; Cheng, C.K.; Chen, H.H.; Chen, C.H. Biocompatibility and osteogenic capacity of Mg-Zn-Ca bulk metallic glass for rabbit tendon-bone interference fixation. Int. J. Mol. Sci. 2019, 20, 2191. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Y.; Guo, S.; Jiang, W.; Liu, Q. Effect of Sr on the microstructure and biodegradable behavior of Mg–Zn–Ca-Mn alloys for implant application. Mater. Des. 2018, 153, 308–316. [Google Scholar] [CrossRef]
- Wang, C.; Fang, H.; Qi, X.; Hang, C.; Sun, Y.; Peng, Z.; Wei, W.; Wang, Y. Silk fibroin film-coated MgZnCa alloy with enhanced in vitro and in vivo performance prepared using surface activation. Acta Biomater. 2019, 91, 99–111. [Google Scholar] [CrossRef]
- Zheng, Q.; Cheng, S.; Strader, J.H.; Ma, E.; Xu, J. Critical size and strength of the best bulk metallic glass former in the Mg–Cu–Gd ternary system. Scr. Mater. 2007, 56, 161–164. [Google Scholar] [CrossRef]
- Wei, Y.X.; Xi, X.K.; Zhao, D.Q.; Pan, M.X.; Wang, W.H. Formation of MgNiPr bulk metallic glasses in air. Mater. Lett. 2005, 59, 945–947. [Google Scholar] [CrossRef]
- Zberg, B.; Uggowitzer, P.J.; Loffler, J.F. MgZnCa glasses without clinically observable hydrogen evolution for biodegradable implants. Nat. Mater. 2009, 8, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, N.T.; Lespagnol, J.; Birbilis, N.; Staiger, M.P. A survey of bio-corrosion rates of magnesium alloys. Corros. Sci. 2010, 52, 287–291. [Google Scholar] [CrossRef]
- Limmer, K.R.; Williams, K.S.; Labukas, J.P.; Andzelm, J.W. First principles modeling of cathodic reaction thermodynamics in dilute magnesium alloys. Corrosion 2017, 73, 506–517. [Google Scholar] [CrossRef]
- Li, Y.m.; Wu, Y.; Cheng, Y.m. Inorganic Chemistry; HuaZhong University of Science & Technology Press: Wuhan, China, 2010. [Google Scholar]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 97th ed.; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2017. [Google Scholar]
- Zheng, Y.; Xu, X.; Xu, Z.; Wang, J.; Cai, H. Development of Mg-based degradable metallic biomaterials. In Metallic Biomaterials: New Directions and Technologies; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; pp. 59–112. [Google Scholar]
- Yang, L.; Huang, Y.; Peng, Q.; Feyerabend, F.; Kainer, K.U.; Willumeit, R.; Hort, N. Mechanical and corrosion properties of binary Mg–Dy alloys for medical applications. Mater. Sci. Eng. B Adv. 2011, 176, 1827–1834. [Google Scholar] [CrossRef]
- Zhang, C.; Guan, S.; Wang, L.; Zhu, S.; Wang, J.; Guo, R. Effect of solution pretreatment on homogeneity and corrosion resistance of biomedical Mg-Zn-Ca alloy processed by high pressure torsion. Adv. Eng. Mater. 2017, 19, 1600236. [Google Scholar] [CrossRef]
- Chelliah, N.M.; Padaikathan, P.; Kumar, R. Evaluation of electrochemical impedance and biocorrosion characteristics of as-cast and T4 heat treated AZ91 Mg-alloys in Ringer’s solution. J. Magnes. Alloy. 2019, 7, 134–143. [Google Scholar] [CrossRef]
- Li, L.; Cao, H.; Qi, F.; Wang, Q.; Zhao, N.; Liu, Y.; Ye, X.; Ouyang, X. Effect of heat treatment on microstructure and mechanical properties of Mg-5Zn-1Mn alloy tube. Metals 2020, 10, 301. [Google Scholar] [CrossRef]
- Li, L.; Wang, T.; Wang, Y.; Zhang, C.-C.; Lv, H.; Lin, H.; Yu, W.-b.; Huang, C.-j. Effects of ytterbium addition and heat treatment on the mechanical properties and biocorrosion behaviors of Mg–Zn–Zr alloy. J. Magnes. Alloy. 2020, 8, 499–509. [Google Scholar] [CrossRef]
- Lin, D.J.; Hung, F.Y.; Liu, H.J.; Yeh, M.L. Dynamic corrosion and material characteristics of Mg–Zn–Zr mini-tubes: The influence of microstructures and extrusion parameters. Adv. Eng. Mater. 2017, 19, 1700159. [Google Scholar] [CrossRef]
- Lopes, D.R.; Silva, C.L.P.; Soares, R.B.; Pereira, P.H.R.; Oliveira, A.C.; Figueiredo, R.B.; Langdon, T.G.; Lins, V.F.C. Cytotoxicity and corrosion behavior of magnesium and magnesium alloys in Hank’s solution after processing by high-pressure torsion. Adv. Eng. Mater. 2019, 21, 1900391. [Google Scholar] [CrossRef]
- Birbilis, N.; Ralston, K.D.; Virtanen, S.; Fraser, H.L.; Davies, C.H.J. Grain character influences on corrosion of ECAPed pure magnesium. Corros. Eng. Sci. Technol. 2013, 45, 224–230. [Google Scholar] [CrossRef]
- Zhang, T.; Ji, Z.; Wu, S. Effect of extrusion ratio on mechanical and corrosion properties of AZ31B alloys prepared by a solid recycling process. Mater. Des. 2011, 32, 2742–2748. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Chen, J.; Zou, Z. Effects of tensile and compressive deformation on corrosion behaviour of a Mg–Zn alloy. Corros. Sci. 2015, 90, 445–450. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N. Effect of grain size on corrosion: A review. Corrosion 2010, 66, 7500501–7500513. [Google Scholar] [CrossRef]
- Li, S.; Yi, L.; Liu, T.; Deng, H.; Ji, B.; Zhang, K.; Zhou, L. Formation of a protective layer against corrosion on Mg alloy via alkali pretreatment followed by vanillic acid treatment. Mater. Corros. 2020, 71, 1330–1338. [Google Scholar] [CrossRef]
- Xiong, P.; Jia, Z.; Zhou, W.; Yan, J.; Wang, P.; Yuan, W.; Li, Y.; Cheng, Y.; Guan, Z.; Zheng, Y. Osteogenic and pH stimuli-responsive self-healing coating on biomedical Mg-1Ca alloy. Acta Biomater. 2019, 92, 336–350. [Google Scholar] [CrossRef]
- Xin, Y.; Jiang, J.; Huo, K.; Tang, G.; Tian, X.; Chu, P.K. Corrosion resistance and cytocompatibility of biodegradable surgical magnesium alloy coated with hydrogenated amorphous silicon. J. Biomed. Mater. Res. A 2009, 89, 717–726. [Google Scholar] [CrossRef]
- Shen, N.; Ding, H. Microstructure prediction for cryogenic cutting using a physics-based material model. Procedia CIRP 2016, 45, 107–110. [Google Scholar] [CrossRef][Green Version]
- Outeiro, J.C.; Batista, A.C.; Marques, M.J. Residual stresses induced by dry and cryogenic cooling during machining of AZ31B magnesium alloy. Adv. Mater. Res. 2014, 996, 658–663. [Google Scholar] [CrossRef]
- Pu, Z.; Dillon, O.W.; Jawahir, J.; Jawahir, I.S.; Puleo, D.A. Microstructural changes of AZ31 magnesium alloys induced by cryogenic machining and its influence on corrosion resistance in simulated body fluid for biomedical applications. In Proceedings of the ASME 2010 International Manufacturing Science and Engineering Conference, Erie, PA, USA, 12–15 October 2010. [Google Scholar]
- Peron, M.; Bertolini, R.; Ghiotti, A.; Torgersen, J.; Bruschi, S.; Berto, F. Enhancement of stress corrosion cracking of AZ31 magnesium alloy in simulated body fluid thanks to cryogenic machining. J. Mech. Behav. Biomed. Mater. 2020, 101, 103429. [Google Scholar] [CrossRef]
- Ghiotti, A.; Bertolini, R.; Pezzato, L.; Savio, E.; Terzini, M.; Bruschi, S. Surface texturing to enhance sol-gel coating performances for biomedical applications. CIRP Ann. 2021, 70, 459–462. [Google Scholar] [CrossRef]
- Ramesh, S.; Aditya Kudva, S.; Anne, G.; Manne, B.; Arya, S. Optimization of ball-burnishing process parameters on surface roughness, micro hardness of Mg–Zn–Ca alloy and investigation of corrosion behavior. Mater. Res. Express 2019, 6, 1065e8. [Google Scholar] [CrossRef]
- Pu, Z.; Song, G.L.; Yang, S.; Outeiro, J.C.; Dillon, O.W.; Puleo, D.A.; Jawahir, I.S. Grain refined and basal textured surface produced by burnishing for improved corrosion performance of AZ31B Mg alloy. Corros. Sci. 2012, 57, 192–201. [Google Scholar] [CrossRef]
- Pu, Z.; Yang, S.; Song, G.L.; Dillon, O.W.; Puleo, D.A.; Jawahir, I.S. Ultrafine-grained surface layer on Mg–Al–Zn alloy produced by cryogenic burnishing for enhanced corrosion resistance. Scr. Mater. 2011, 65, 520–523. [Google Scholar] [CrossRef]
- Cao, C.Y.; Zhu, J.; Tanaka, T. Influence of burnishing process on microstructure and corrosion properties of mg alloy AZ31. In Advanced Surface Enhancement; Springer Nature Singapore Pte Ltd.: Singapore, 2020; pp. 97–107. [Google Scholar]
- Zhao, Y.; Jamesh, M.I.; Li, W.K.; Wu, G.; Wang, C.; Zheng, Y.; Yeung, K.W.; Chu, P.K. Enhanced antimicrobial properties, cytocompatibility, and corrosion resistance of plasma-modified biodegradable magnesium alloys. Acta Biomater. 2014, 10, 544–556. [Google Scholar] [CrossRef]
- Liang, T.; Zeng, L.; Shi, Y.; Pan, H.; Chu, P.K.; Yeung, K.W.K.; Zhao, Y. In vitro and in vivo antibacterial performance of Zr & O PIII magnesium alloys with high concentration of oxygen vacancies. Bioact Mater. 2021, 6, 3049–3061. [Google Scholar] [CrossRef]
- Liu, Y.; Bian, D.; Wu, Y.; Li, N.; Qiu, K.; Zheng, Y.; Han, Y. Influence of biocompatible metal ions (Ag, Fe, Y) on the surface chemistry, corrosion behavior and cytocompatibility of Mg-1Ca alloy treated with MEVVA. Colloids Surf. B Biointerfaces 2015, 133, 99–107. [Google Scholar] [CrossRef]
- Fan, J.M.; Li, Q.; Kang, W.; Zhang, S.Y.; Chen, B. Composite cerium oxide/titanium oxide thin films for corrosion protection of AZ91D magnesium alloy via sol-gel process. Mater. Corros. 2009, 60, 438–443. [Google Scholar] [CrossRef]
- Hou, S.; Mi, L.; Wang, L.; Zhu, S.; Hu, J.; Ding, Q.; Guan, S. Corrosion protection of Mg-Zn-Y-Nd alloy by flower-like nanostructured TiO2 film for vascular stent application. J. Chem. Technol. Biot. 2013, 88, 2062–2066. [Google Scholar] [CrossRef]
- Peron, M.; Berto, F.; Torgersen, J. Stress corrosion cracking behavior of zirconia ALD–coated AZ31 alloy in simulated body fluid. Mater. Des. Process. Commun. 2020, 2, e126. [Google Scholar] [CrossRef]
- Samiee, M.; Hanachi, M.; Seyedraoufi, Z.S.; Eshraghi, M.J.; Shajari, Y. Biodegradable magnesium alloy coated with TiO2/MgO two-layer composite via magnetic sputtering for orthopedic applications: A study on the surface characterization, corrosion, and biocompatibility. Ceram. Int. 2021, 47, 6179–6186. [Google Scholar] [CrossRef]
- Kim, S.M.; Jo, J.H.; Lee, S.M.; Kang, M.H.; Kim, H.E.; Estrin, Y.; Lee, J.H.; Lee, J.W.; Koh4, Y.H. Hydroxyapatite-coated magnesium implants with improved in vitro and in vivo biocorrosion, biocompatibility, and bone response. J. Biomed. Mater. Res. A 2013, 102, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Revell, P.A.; Damien, E.; Zhang, X.S.; Evans, P.; Howlett, C.R. The effect of magnesium ions on bone bonding to hydroxyapatite coating on titanium alloy implants. Key Eng. Mat. 2003, 254–256, 447–450. [Google Scholar] [CrossRef]
- Gu, X.N.; Zheng, Y.F.; Lan, Q.X.; Cheng, Y.; Zhang, Z.X.; Xi, T.F.; Zhang, D.Y. Surface modification of an Mg-1Ca alloy to slow down its biocorrosion by chitosan. Biomed. Mater. 2009, 4, 044109. [Google Scholar] [CrossRef] [PubMed]
- Córdoba, L.C.; Marques, A.; Taryba, M.; Coradin, T.; Montemor, F. Hybrid coatings with collagen and chitosan for improved bioactivity of Mg alloys. Surf. Coat. Technol. 2018, 341, 103–113. [Google Scholar] [CrossRef]
- Gray-Munro, J.E.; Seguin, C.; Strong, M. Influence of surface modification on the in vitro corrosion rate of magnesium alloy AZ31. J. Biomed. Mater. Res. A 2009, 91, 221–230. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Y.; Zhang, S.; Li, J.; Zhao, C.; Zhang, X. Interaction between a high purity magnesium surface and PCL and PLA coatings during dynamic degradation. Biomed. Mater. 2011, 6, 025005. [Google Scholar] [CrossRef]
- Wong, H.M.; Yeung, K.W.; Lam, K.O.; Tam, V.; Chu, P.K.; Luk, K.D.; Cheung, K.M. A biodegradable polymer-based coating to control the performance of magnesium alloy orthopaedic implants. Biomaterials 2010, 31, 2084–2096. [Google Scholar] [CrossRef]
- Wong, H.M.; Zhao, Y.; Leung, F.K.L.; Xi, T.; Zhang, Z.; Zheng, Y.; Wu, S.; Luk, K.D.K.; Cheung, K.M.C.; Chu, P.K.; et al. Functionalized polymeric membrane with enhanced mechanical and biological properties to control the degradation of magnesium alloy. Adv. Healthc. Mater. 2017, 6, 1601269. [Google Scholar] [CrossRef]
- Toorani, M.; Aliofkhazraei, M.; Naderi, R. Ceria-embedded MAO process as pretreatment for corrosion protection of epoxy films applied on AZ31-magnesium alloy. J. Alloys Compd. 2019, 785, 669–683. [Google Scholar] [CrossRef]
- Asadi, H.; Suganthan, B.; Ghalei, S.; Handa, H.; Ramasamy, R.P. A multifunctional polymeric coating incorporating lawsone with corrosion resistance and antibacterial activity for biomedical Mg alloys. Prog. Org. Coat. 2021, 153, 106157. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, W.; Zhang, X.; Nune, K.C.; Zhao, Y.; Liu, N.; Misra, R.D.K.; Yang, K.; Tan, L.; Yan, J. The effect of different coatings on bone response and degradation behavior of porous magnesium-strontium devices in segmental defect regeneration. Bioact. Mater. 2021, 6, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Kou, F.; Liu, C.S.; Wang, L.G.; Yasin, A.; Li, J.A.; Guan, S.K. Fabrication of citric acid/RGD multilayers on Mg-Zn-Y-Nd alloy via layer-by-layer self-assembly for promoting surface biocompatibility. Adv. Mater. Interfaces 2021, 8, 2170074. [Google Scholar] [CrossRef]
- Durán, K.S.; Hernández, N.; Rueda, L.M.; Hernández-Barrios, C.A.; Coy, A.E.; Viejo, F. Design of multilayer hybrid sol-gel coatings with bifunctional barrier-bioactive response on the Elektron 21 magnesium alloy for biomedical applications. J. Magnes. Alloy. 2021, 9, 2097–2112. [Google Scholar] [CrossRef]
- Peng, F.; Cheng, S.; Zhang, R.; Li, M.; Zhou, J.; Wang, D.; Zhang, Y. Zn-contained mussel-inspired film on Mg alloy for inhibiting bacterial infection and promoting bone regeneration. Regen. Biomater. 2021, 8, rbaa044. [Google Scholar] [CrossRef]
- Chen, Y.; Li, W.; Wang, W.; Zhao, Y.; Chen, M. Microstructure, corrosion resistance, and antibacterial properties of an Ag/Mg-Al layered double hydroxide coating synthesized in situ on biomedical Mg-Zn-Ca alloy. Ceram. Int. 2022, 48, 4172–4187. [Google Scholar] [CrossRef]
- Singh, S.; Singh, G.; Bala, N. Synthesis and characterization of iron oxide-hydroxyapatite-chitosan composite coating and its biological assessment for biomedical applications. Prog. Org. Coat. 2021, 150, 106011. [Google Scholar] [CrossRef]
- Negrescu, A.M.; Necula, M.G.; Gebaur, A.; Golgovici, F.; Nica, C.; Curti, F.; Iovu, H.; Costache, M.; Cimpean, A. In vitro macrophage immunomodulation by poly(epsilon-caprolactone) based-coated AZ31 Mg alloy. Int. J. Mol. Sci. 2021, 22, 909. [Google Scholar] [CrossRef]
- Ma, X.; Huang, W.; Niu, Z.; Li, W.; Mei, D.; Zhu, S.; Guan, S. A drug-loaded bio-functional anticorrosion coating on Mg alloy for orthopedic applications. Mater. Lett. 2022, 311, 131581. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, Y.; Zhao, Y.; Gao, F.; Guo, X.; Yang, M.; Hong, Q.; Yang, Z.; Dai, J.; Pan, C. Incorporation of heparin/BMP2 complex on GOCS-modified magnesium alloy to synergistically improve corrosion resistance, anticoagulation, and osteogenesis. J. Mater. Sci. Mater. Med. 2021, 32, 24. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Ruan, Y.C.; Yu, M.K.; O’Laughlin, M.; Wise, H.; Chen, D.; Tian, L.; Shi, D.; Wang, J.; et al. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat. Med. 2016, 22, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Qu, X.; Li, H.; Yang, K.; Wan, P.; Tan, L.; Ouyang, Z.; Liu, X.; Tian, B.; Xiao, F.; et al. The effect of metallic magnesium degradation products on osteoclast-induced osteolysis and attenuation of NF-κB and NFATc1 signaling. Biomaterials 2014, 35, 6299–6310. [Google Scholar] [CrossRef] [PubMed]
- Hamushan, M.; Cai, W.; Zhang, Y.; Ren, Z.; Du, J.; Zhang, S.; Zhao, C.; Cheng, P.; Zhang, X.; Shen, H.; et al. High-purity magnesium pin enhances bone consolidation in distraction osteogenesis via regulating Ptch protein activating Hedgehog-alternative Wnt signaling. Bioact. Mater. 2021, 6, 1563–1574. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Geng, Z.; Huang, Y.; Jia, Z.; Cui, Z.; Li, Z.; Wu, S.; Liang, Y.; Zhu, S.; Yang, X.; et al. Unraveling the osteogenesis of magnesium by the activity of osteoblasts in vitro. J. Mater. Chem. B 2018, 6, 6615–6621. [Google Scholar] [CrossRef]
- Peng, H.; Fan, K.; Zan, R.; Gong, Z.J.; Sun, W.; Sun, Y.; Wang, W.; Jiang, H.; Lou, J.; Ni, J.; et al. Degradable magnesium implants inhibit gallbladder cancer. Acta Biomater. 2021, 128, 514–522. [Google Scholar] [CrossRef]
- Silver, I.A.; Deas, J.; Erecinska, M. Interactions of bioactive glasses with osteoblasts in vitro: Effects of 45S5 Bioglass, and 58S and 77S bioactive glasses on metabolism, intracellular ion concentrations and cell viability. Biomaterials 2001, 22, 175–185. [Google Scholar] [CrossRef]
- Rahim, M.I.; Eifler, R.; Rais, B.; Mueller, P.P. Alkalization is responsible for antibacterial effects of corroding magnesium. J. Biomed. Mater. Res. A 2015, 103, 3526–3532. [Google Scholar] [CrossRef]
- Brooks, E.K.; Ahn, R.; Tobias, M.E.; Hansen, L.A.; Luke-Marshall, N.R.; Wild, L.; Campagnari, A.A.; Ehrensberger, M.T. Magnesium alloy AZ91 exhibits antimicrobial properties in vitro but not in vivo. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 221–227. [Google Scholar] [CrossRef]
- Zeng, J.; Ren, L.; Yuan, Y.; Wang, Y.; Zhao, J.; Zeng, R.; Yang, K.; Mei, X. Short-term effect of magnesium implantation on the osteomyelitis modeled animals induced by Staphylococcus aureus. J. Mater. Sci. Mater. Med. 2013, 24, 2405–2416. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, G.; Wei, M. Controlling the biodegradation rate of magnesium using biomimetic apatite coating. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 89, 408–414. [Google Scholar] [CrossRef]
- Jonasova, L.; Muller, F.A.; Helebrant, A.; Strnad, J.; Greil, P. Biomimetic apatite formation on chemically treated titanium. Biomaterials 2004, 25, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, Y.; Huang, Y.; Song, R.; Chen, M. Effects of solidification cooling rate on the corrosion resistance of Mg–Zn–Ca alloy. Prog. Nat. Sci. 2014, 24, 452–457. [Google Scholar] [CrossRef]
- Ghafarzadeh, M.; Kharaziha, M.; Atapour, M. Bilayer micro-arc oxidation-poly (glycerol sebacate) coating on AZ91 for improved corrosion resistance and biological activity. Prog. Org. Coat. 2021, 161, 106495. [Google Scholar] [CrossRef]
- Dong, X.; Wei, B.; Legut, D.; Zhang, H.; Zhang, R. Electrochemical Pourbaix diagrams of Mg-Zn alloys from first-principles calculations and experimental thermodynamic data. Phys. Chem. Chem. Phys. 2021, 23, 19602–19610. [Google Scholar] [CrossRef] [PubMed]





| Element. | Atomic% | Weight% | System | Ref. |
|---|---|---|---|---|
| Lithium | 17 | 5.5 | Eutectic | [88,89] |
| Aluminum | 11.8 | 12.7 | Eutectic | [88,89] |
| Silicon | ~0.00 | ~0.00 | Eutectic | [90] |
| Calcium | 0.82 | 1.34 | Eutectic | [89,90] |
| Titanium | 0.1 | 0.2 | Peritectic | [88,89] |
| Manganese | 1 | 2.2 | Peritectic | [88,89] |
| Iron | ~0.00 | ~0.00 | Eutectic | [90] |
| Copper | 0.02 | 0.04 | Eutectic | [90] |
| Zinc | 2.4 | 6.2 | Eutectic | [88,89] |
| Strontium | 0.03 | 0.11 | Eutectic | [90] |
| Zirconium | 1 | 3.8 | Peritectic | [88,89] |
| Silver | 3.8 | 15 | Eutectic | [88,89] |
| Cadmium | 100 | 100 | Complete SS | [88,89] |
| Indium | 19.4 | 53.2 | Peritectic | [90] |
| Tin | 3.35 | 14.48 | Eutectic | [88,89] |
| Gold | 0.1 | 0.8 | Eutectic | [88,89] |
| Yttrium | 3.75 | 12.47 | Eutectic | [90] |
| Cerium | 0.09 | 0.52 | Eutectic | [90] |
| Neodymium | 0.52 | ~3.00 | Eutectic | [90] |
| Samarium | 0.9 | 5.3 | Eutectic | [90] |
| Gadolinium | 4.53 | 23.49 | Eutectic | [90] |
| Dysprosium | 4.76 | 25.34 | Eutectic | [90] |
| Erbium | 6.91 | 33.8 | Eutectic | [90] |
| Formula | Ksp | Formula | Ksp | Formula | Ksp |
|---|---|---|---|---|---|
| Ag3PO4 | 8.89 × 10−17 | Ag2CO3 | 8.46 × 10−12 | Ag(OH)2 | 2.00 × 10−8 |
| AlPO4 | 9.84 × 10−21 | BaCO3 | 2.58 × 10−9 | Al(OH)3 | 1.30 × 10−33 |
| Ba3(PO4)2 | 3.40 × 10−23 | CaCO3 | 3.36 × 10−9 | Au(OH)3 | 5.50 × 10−46 |
| Ca3(PO4)2 | 2.07 × 10−33 | CdCO3 | 1.00 × 10−12 | Bi(OH)3 | 6.00 × 10−31 |
| Cd3(PO4)2 | 2.53 × 10−33 | CuCO3 | 1.40 × 10−10 | Ca(OH)2 | 5.02 × 10−6 |
| Cu3(PO4)2 | 1.40 × 10−37 | CoCO3 | 1.40 × 10−13 | Cd(OH)2 | 7.20·10−15 |
| Li3PO4 | 2.37 × 10−11 | FeCO3 | 3.13 × 10−11 | Ce(OH)3 | 1.60 × 10−20 |
| Mg3(PO4)2 | 1.04 × 10−24 | Li2CO3 | 8.15 × 10−4 | Ce(OH)4 | 2.00 × 10−28 |
| Ni3(PO4)2 | 4.74 × 10−32 | MgCO3 | 6.82 × 10−6 | Cu(OH) | 1.00 × 10−14 |
| AgCl | 1.77 × 10−10 | MnCO3 | 2.24 × 10−11 | Ca(OH)2 | 2.2 × 10−20 |
| AuCl | 2.00 × 10−13 | Nd2(CO3)3 | 1.08 × 10−33 | Co(OH)2 | 5.92 × 10−15 |
| AuCl3 | 3.20 × 10−46 | NiCO3 | 1.42 × 10−7 | Co(OH)3 | 1.60 × 10−44 |
| CuCl | 1.72 × 10−7 | SrCO3 | 5.60 × 10−10 | Cr(OH)3 | 6.30 × 10−31 |
| Y2(CO3)3 | 1.03 × 10−31 | Cu(OH)2 | 2.20 × 10−20 | ||
| ZnCO3 | 1.46 × 10−10 | Eu(OH)3 | 9.38 × 10−27 | ||
| Ga(OH)3 | 7.28 × 10−36 | ||||
| Fe(OH)2 | 4.87 × 10−17 | ||||
| Fe(OH)3 | 2.79 × 10−39 | ||||
| Pb(OH)2 | 1.43 × 10−20 | ||||
| La(OH)3 | 2.00 × 10−19 | ||||
| Mg(OH)2 | 5.61 × 10−12 | ||||
| Mn(OH)2 | 1.90 × 10−13 | ||||
| Ni(OH)2 | 5.48 × 10−16 | ||||
| Pr(OH)3 | 3.39 × 10−24 | ||||
| Tl(OH)3 | 1.68 × 10−44 | ||||
| Sn(OH)2 | 5.45 × 10−27 | ||||
| Sn(OH)4 | 1.00 × 10−56 | ||||
| Y(OH)3 | 1.00 × 10−22 | ||||
| Zn(OH)2 | 3.00 × 10−17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Liu, X.; Sun, K.; Fu, R.; Wang, G. Corrosion Behavior in Magnesium-Based Alloys for Biomedical Applications. Materials 2022, 15, 2613. https://doi.org/10.3390/ma15072613
Xu L, Liu X, Sun K, Fu R, Wang G. Corrosion Behavior in Magnesium-Based Alloys for Biomedical Applications. Materials. 2022; 15(7):2613. https://doi.org/10.3390/ma15072613
Chicago/Turabian StyleXu, Liming, Xingwang Liu, Kang Sun, Rao Fu, and Gang Wang. 2022. "Corrosion Behavior in Magnesium-Based Alloys for Biomedical Applications" Materials 15, no. 7: 2613. https://doi.org/10.3390/ma15072613
APA StyleXu, L., Liu, X., Sun, K., Fu, R., & Wang, G. (2022). Corrosion Behavior in Magnesium-Based Alloys for Biomedical Applications. Materials, 15(7), 2613. https://doi.org/10.3390/ma15072613






