Nanoengineering of N-doped Mesoporous Carbon Nanoparticles with Adjustable Internal Cavities via Emulsion-Induced Assembly
Abstract
:1. Introduction
2. Experimental Sections
2.1. Chemicals and Materials
2.2. Synthesis of NMMCNs with Cherimoya-Shaped Rough Surfaces
2.3. Cytotoxicity Assays of NMMCNs-2 with Cherimoya-Shaped Rough Surfaces
2.4. Hemolysis Experiment
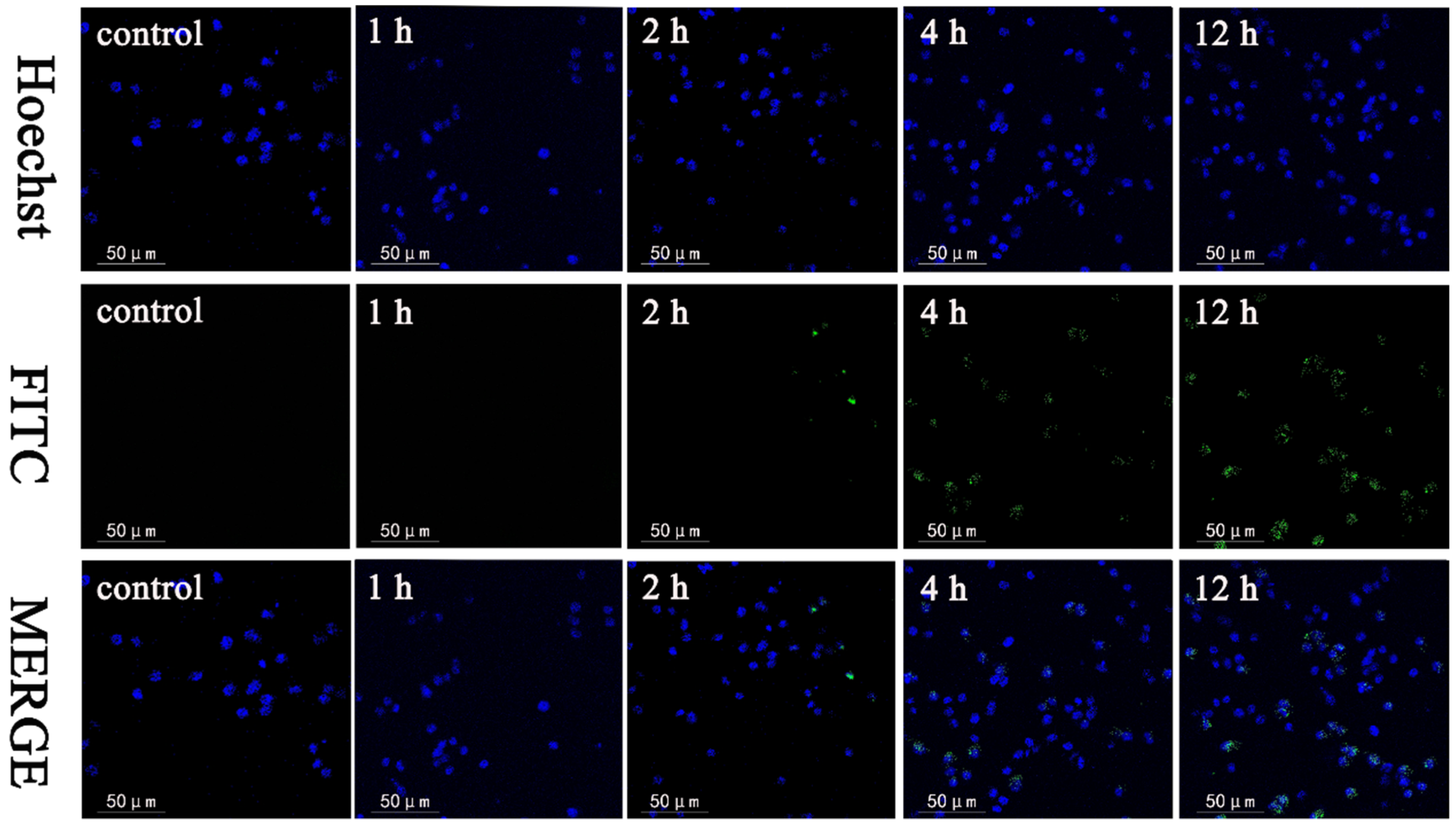
2.5. Cell Uptake Assay
2.6. Construction of the Res-Loaded Delivery System and In Vitro Release Profile Study
2.7. Determination of DPPH• Radical Scavenging Activity of the Res–NMMCNs-2 Delivery System
2.8. In Vitro Antitumor Activity Test of the Res–NMMCNs-2 Delivery System
2.9. Characterization
3. Results and Discussions
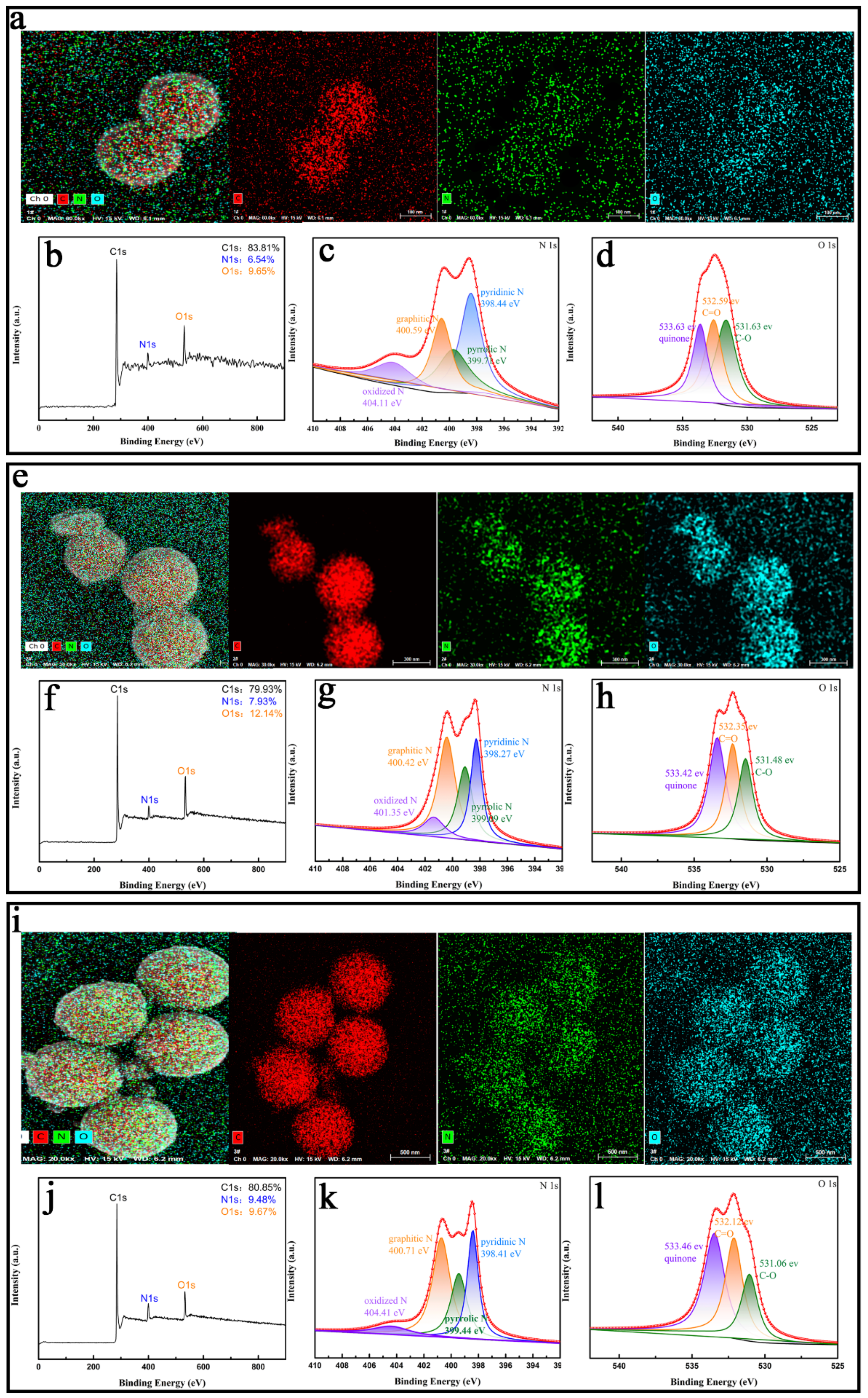
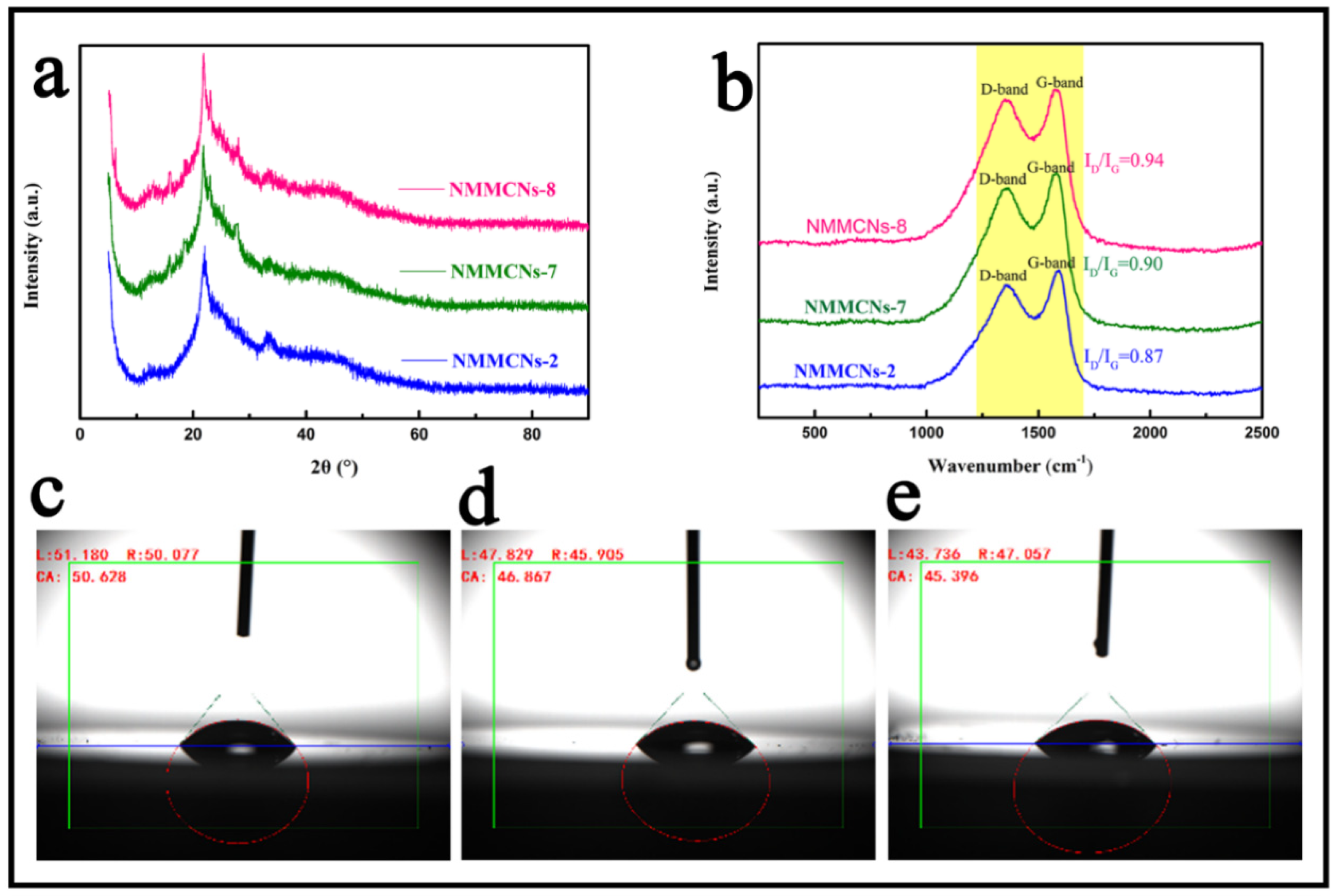
3.1. Structural Characterization
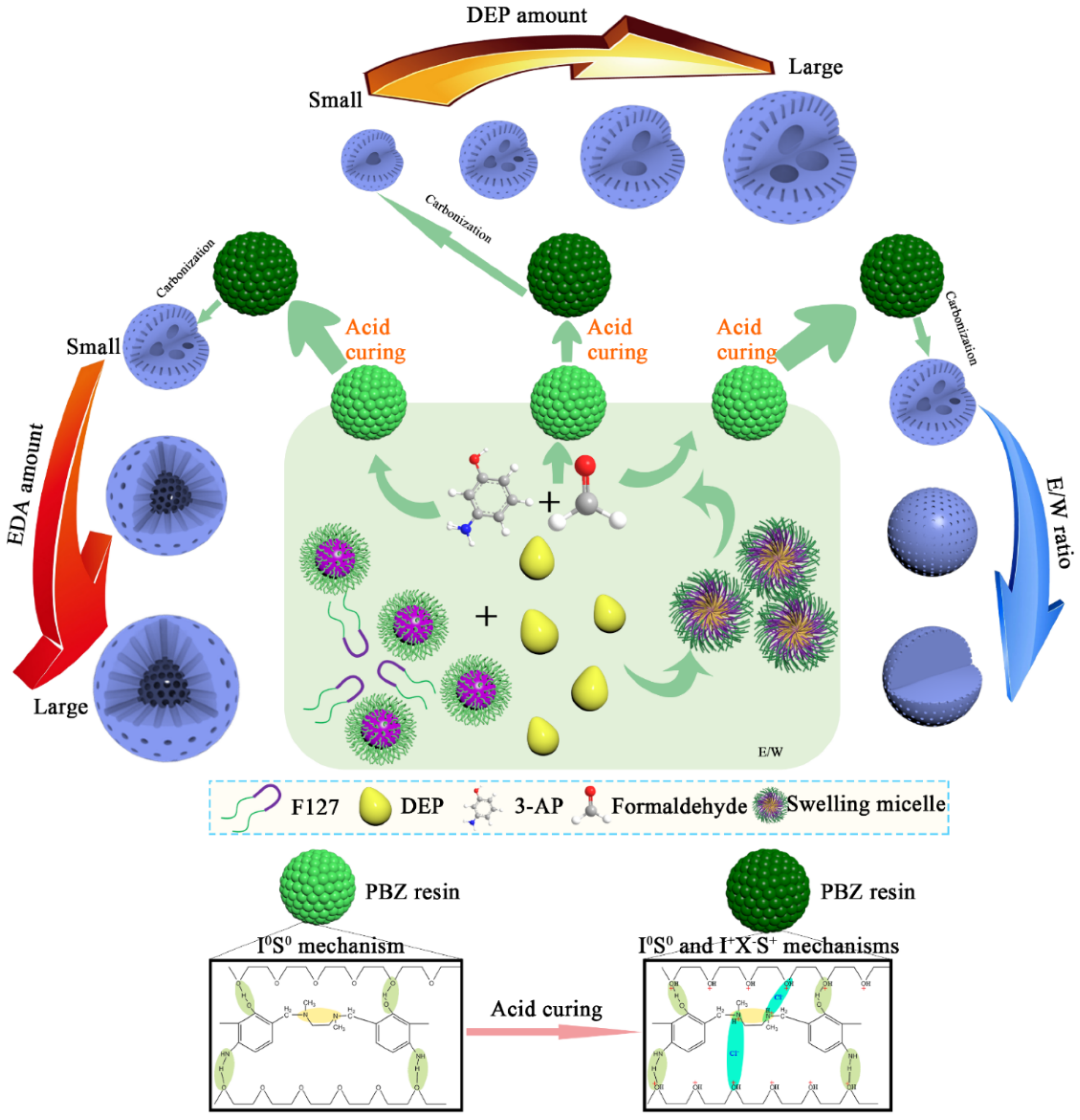
3.2. Synthesis Mechanism
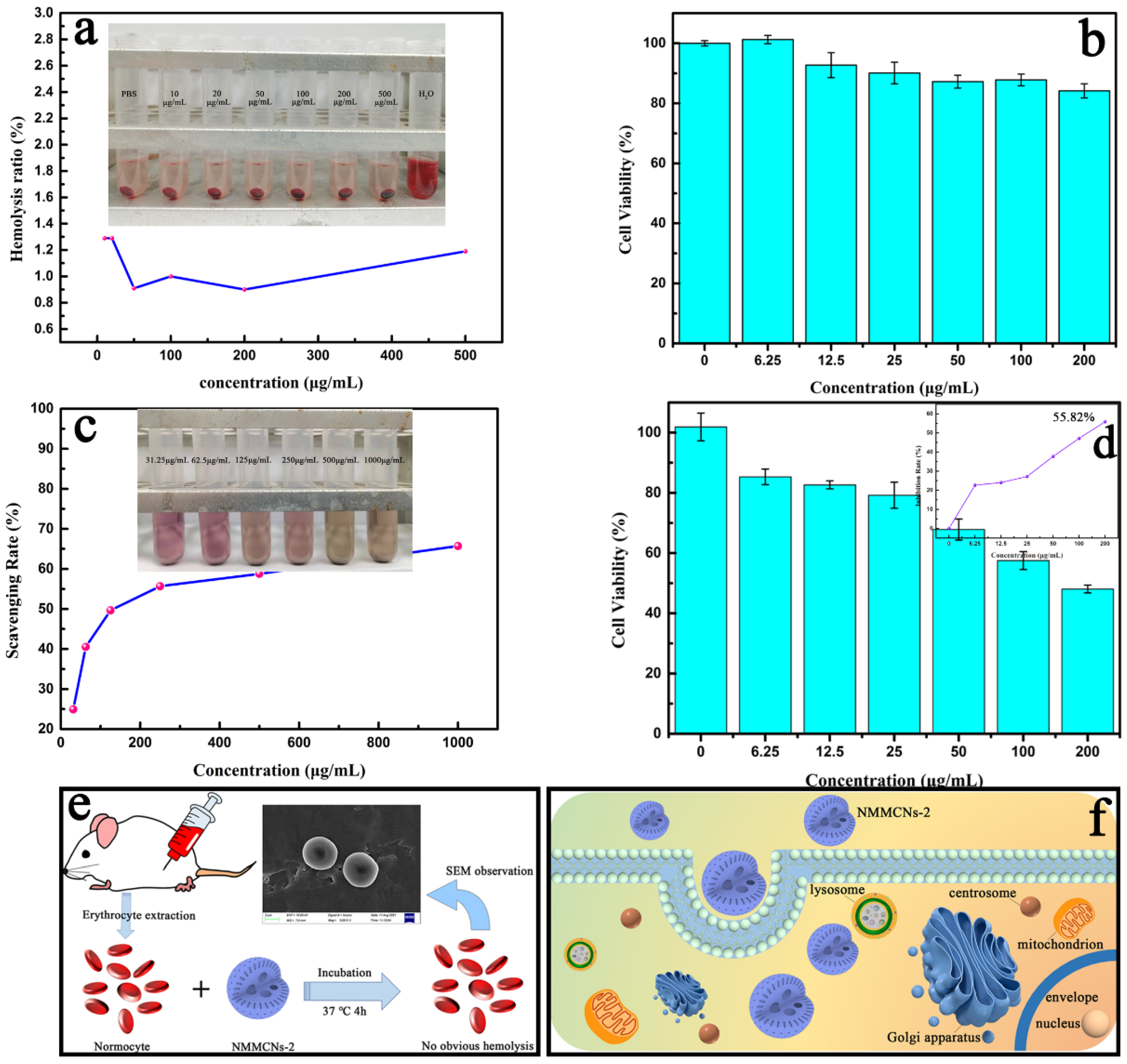
3.3. Biological Effects
3.4. Drug Carrier
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, R.; Huang, X.; Liu, Y.; Kong, Y.; Gu, Z.; Yang, Y.; Wang, Y.; Ban, W.; Song, H.; Yu, C. Shaping Nanoparticles for Interface Catalysis: Concave Hollow Spheres via Deflation-Inflation Asymmetric Growth. Adv. Sci. 2020, 7, 2000393. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Zhu, Z.; Ye, S.; Lin, G.; Chen, H.; Tu, Y.; Bai, G.; Zhang, L.; Yang, X. Surface Polymerization and Controlled Pyrolysis: Tailorable Synthesis of Bumpy Hollow Carbon Spheres for Energy Storage. Langmuir 2021, 37, 4007–4015. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xia, Y.; Zhang, X.; Al-Khalaf, A.A.; Zhao, T.; Xu, J.; Peng, L.; Hozzein, W.N.; Li, W.; Zhao, D. Synthesis of carbon nanotubes@mesoporous carbon core–shell structured electrocatalysts via a molecule-mediated interfacial co-assembly strategy. J. Mater. Chem. A 2019, 7, 8975–8983. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, Y.; Tian, Y.; Wang, X.; Yan, M. Tailored synthesis from rhombic dodecahedron to spherical ordered mesoporous carbon nanoparticles via one-step strategy. Carbon 2019, 152, 295–304. [Google Scholar] [CrossRef]
- Tian, Z.; Jiang, H.; Huang, M.; Wang, G.H. Facile Synthesis of Size-Controlled Nitrogen-Doped Mesoporous Carbon Nanosphere Supported Ultrafine Ru Nanoparticles for Selective Hydrogenation of Quinolines. Chemistry 2020, 26, 17000–17004. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.F.; Long, Y.; Yu, H.J.; Yavitt, B.M.; Fan, W.; Ribbe, A.; Watkins, J.J. Bimodal Mesoporous Carbon Spheres with Small and Ultra-Large Pores Fabricated Using Amphiphilic Brush Block Copolymer Micelle Templates. ACS Appl. Mater. Interfaces 2020, 12, 57322–57329. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Xie, H.; Tian, Y.; Wang, X. Controlled Growth of N-Doped and Large Mesoporous Carbon Spheres with Adjustable Litchi-Like Surface and Particle Size as a Giant Guest Molecule Carrier. ACS Appl. Mater. Interfaces 2018, 10, 20073–20084. [Google Scholar] [CrossRef]
- Liu, J.; Xie, L.; Deng, J.; Gong, Y.; Tang, G.; Bai, H.; Wang, Y. Annular Mesoporous Carbonaceous Nanospheres from Biomass-Derived Building Units with Enhanced Biological Interactions. Chem. Mater. 2019, 31, 7186–7191. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, W.; Yu, P.; Pang, H.; Liang, Y. Improved ion-diffusion performance by engineering an ordered mesoporous shell in hollow carbon nanospheres. Chem. Comm. 2020, 56, 2467–2470. [Google Scholar] [CrossRef]
- Lu, A.H.; Sun, T.; Li, W.C.; Sun, Q.; Han, F.; Liu, D.H.; Guo, Y. Synthesis of discrete and dispersible hollow carbon nanospheres with high uniformity by using confined nanospace pyrolysis. Angew. Chem. Int. Engl. 2011, 50, 11765–11768. [Google Scholar] [CrossRef]
- Song, Y.; Li, Z.; Guo, K.; Shao, T. Hierarchically ordered mesoporous carbon/graphene composites as supercapacitor electrode materials. Nanoscale 2016, 8, 15671–15680. [Google Scholar] [CrossRef]
- Wang, M.; Wang, X.; Yue, Q.; Zhang, Y.; Wang, C.; Chen, J.; Cai, H.; Lu, H.; Elzatahry, A.A.; Zhao, D.; et al. Templated Fabrication of Core–Shell Magnetic Mesoporous Carbon Microspheres in 3-Dimensional Ordered Macroporous Silicas. Chem. Mater. 2014, 26, 3316–3321. [Google Scholar] [CrossRef]
- Shen, G.; Sun, X.; Zhang, H.; Liu, Y.; Zhang, J.; Meka, A.; Zhou, L.; Yu, C. Nitrogen-doped ordered mesoporous carbon single crystals: Aqueous organic–organic self-assembly and superior supercapacitor performance. J. Mater. Chem. A 2015, 3, 24041–24048. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, S.; Huang, W.; Tian, Y.; Wang, X. Controllable synthesis of mesoporous carbon nanospheres with uniform size by a facile one-pot aqueous strategy under highly acidic conditions. Carbon 2016, 105, 521–528. [Google Scholar] [CrossRef]
- Shao, W.; Hu, F.; Liu, S.; Zhang, T.; Song, C.; Weng, Z.; Wang, J.; Jian, X. Carbon spheres with rational designed surface and secondary particle-piled structures for fast and stable sodium storage. J. Energy Chem. 2021, 54, 368–376. [Google Scholar] [CrossRef]
- Zhang, F.; Zong, S.; Zhang, Y.; Lv, H.; Liu, X.; Du, J.; Chen, A. Preparation of hollow mesoporous carbon spheres by pyrolysis-deposition using surfactant as carbon precursor. J. Power Sources 2021, 484, 229274. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Xu, L.; Wang, X.; Tian, Y. Dual soft-templated congenerous assembly to raspberry-shaped mesoporous carbon nanoparticles with hierarchical mesopores. Microporous Mesoporous Mater. 2020, 293, 109803. [Google Scholar] [CrossRef]
- Zhang, L.-H.; He, B.; Li, W.-C.; Lu, A.-H. Surface Free Energy-Induced Assembly to the Synthesis of Grid-Like Multicavity Carbon Spheres with High Level In-Cavity Encapsulation for Lithium-Sulfur Cathode. Adv. Energy Mater. 2017, 7, 1071518. [Google Scholar] [CrossRef]
- Chen, C.; Wang, H.; Han, C.; Deng, J.; Wang, J.; Li, M.; Tang, M.; Jin, H.; Wang, Y. Asymmetric Flasklike Hollow Carbonaceous Nanoparticles Fabricated by the Synergistic Interaction between Soft Template and Biomass. J. Am. Chem. Soc. 2017, 139, 2657–2663. [Google Scholar] [CrossRef]
- Yang, X.; Lu, P.; Yu, L.; Pan, P.; Elzatahry, A.A.; Alghamdi, A.; Luo, W.; Cheng, X.; Deng, Y. An Efficient Emulsion-Induced Interface Assembly Approach for Rational Synthesis of Mesoporous Carbon Spheres with Versatile Architectures. Adv. Funct. Mater. 2020, 30, 2002488. [Google Scholar] [CrossRef]
- Wang, T.; Sun, Y.; Zhang, L.; Li, K.; Yi, Y.; Song, S.; Li, M.; Qiao, Z.A.; Dai, S. Space-Confined Polymerization: Controlled Fabrication of Nitrogen-Doped Polymer and Carbon Microspheres with Refined Hierarchical Architectures. Adv. Mater. 2019, 31, 1807876. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-F.; Li, W.-C.; Hu, Y.-R.; Ye, C.-Y.; Lu, A.-H. Sculpturing solid polymer spheres into internal gridded hollow carbon spheres under controlled pyrolysis micro-environment. Nano Res. 2021, 14, 1565–1573. [Google Scholar] [CrossRef]
- Zhang, Z.; Jia, B.; Liu, L.; Zhao, Y.; Wu, H.; Qin, M.; Han, K.; Wang, W.A.; Xi, K.; Zhang, L.; et al. Hollow Multihole Carbon Bowls: A Stress-Release Structure Design for High-Stability and High-Volumetric-Capacity Potassium-Ion Batteries. ACS Nano 2019, 13, 11363–11371. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, W.C.; Hao, G.P.; Hao, Y.; Sun, Q.; Zhang, X.Q.; Lu, A.H. Temperature-programmed precise control over the sizes of carbon nanospheres based on benzoxazine chemistry. J. Am. Chem. Soc. 2011, 133, 15304–15307. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.-P.; Li, W.-C.; Qian, D.; Wang, G.-H.; Zhang, W.-P.; Zhang, T.; Wang, A.-Q.; Schüth, F.; Bongard, H.-J.; Lu, A.-H. Structurally Designed Synthesis of Mechanically Stable Poly(benzoxazine-co-resol)-Based Porous Carbon Monoliths and Their Application as High-Performance CO2Capture Sorbents. J. Am. Chem. Soc. 2011, 133, 11378–11388. [Google Scholar] [CrossRef] [PubMed]
- Konnola, R.; Anirudhan, T.S. Efficient carbon dioxide capture by nitrogen and sulfur dual-doped mesoporous carbon spheres from polybenzoxazines synthesized by a simple strategy. J. Environ. Chem. Eng. 2020, 8, 103614. [Google Scholar] [CrossRef]
- Manmuanpom, N.; Thubsuang, U.; Dubas, S.T.; Wongkasemjit, S.; Chaisuwan, T. Enhanced CO2 capturing over ultra-microporous carbon with nitrogen-active species prepared using one-step carbonization of polybenzoxazine for a sustainable environment. J. Environ. Manag. 2018, 223, 779–786. [Google Scholar] [CrossRef]
- Li, Y.; Qi, J.; Li, J.; Shen, J.; Liu, Y.; Sun, X.; Shen, J.; Han, W.; Wang, L. Nitrogen-Doped Hollow Mesoporous Carbon Spheres for Efficient Water Desalination by Capacitive Deionization. ACS Sustain. Chem. Eng. 2017, 5, 6635–6644. [Google Scholar] [CrossRef]
- Zhao, Y.; Lyu, H.; Liu, Y.; Liu, W.; Tian, Y.; Wang, X. Rational design and synthesis of multimorphology mesoporous carbon@silica nanoparticles with tailored structure. Carbon 2021, 183, 912–928. [Google Scholar] [CrossRef]
- Yao, Y.; Ye, H.; Qi, Z.; Mo, L.; Yue, Q.; Baral, A.; Hoon, D.S.B.; Vera, J.C.; Heiss, J.D.; Chen, C.C.; et al. B7-H4(B7x)-Mediated Cross-talk between Glioma-Initiating Cells and Macrophages via the IL6/JAK/STAT3 Pathway Lead to Poor Prognosis in Glioma Patients. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 2778–2790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Wang, Y.; Yang, H.; Liu, X.; Lu, Z. Preparation and biological activity studies of resveratrol loaded ionically cross-linked chitosan-TPP nanoparticles. Carbohydr. Polym. 2017, 175, 170–177. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, L.; Liu, G. Synthesis of Benzoxazine-Based N-Doped Mesoporous Carbons as High-Performance Electrode Materials. Appl. Sci. 2020, 10, 422. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Gilani, M.R.H.S.; Lai, J.; Nsabimana, A.; Liu, Z.; Luque, R.; Xu, G. Autocatalysis Synthesis of Poly(benzoxazine-co-resol)-Based Polymer and Carbon Spheres. Macromolecules 2018, 51, 5494–5500. [Google Scholar] [CrossRef]
- Wan, L.; Wang, J.; Xie, L.; Sun, Y.; Li, K. Nitrogen-enriched hierarchically porous carbons prepared from polybenzoxazine for high-performance supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 15583–15596. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Sun, X.; Xu, L.; Zhang, H.; Liu, G. Synthesis and electrochemical properties of benzoxazine-based heteroatom-doped carbon materials. J. Mater. 2019, 34, 1219–1228. [Google Scholar] [CrossRef]
- Wan, L.; Wang, J.; Feng, C.; Sun, Y.; Li, K. Synthesis of polybenzoxazine based nitrogen-rich porous carbons for carbon dioxide capture. Nanoscale 2015, 7, 6534–6544. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, L.; Lv, P. Facile synthesis of ordered mesoporous carbon monolith with close-packed microspheres structure through emulsion templating. Microporous Mesoporous 2020, 297, 110056. [Google Scholar] [CrossRef]
- Yue, F.; Gao, G.; Li, F.; Zheng, Y.; Hou, S. Size-controlled synthesis of urchin-like reduced graphene oxide microspheres with highly packed density by emulsion-assisted in-situ assembly and their supercapacitor performance. Carbon 2018, 134, 112–122. [Google Scholar] [CrossRef]
- Hu, T.; Li, Y.; Gao, W.; Wang, X.; Tian, Y. Engineering of rich nitrogen-doped and magnetic mesoporous carbon nanospheres with predictable size uniformity for acid dye molecules adsorption. Microporous Mesoporous 2019, 279, 234–244. [Google Scholar] [CrossRef]
- Chong, J.; Zhu, X.; Huang, W.; Wang, X.; Tian, Y. The fabrication of size-tunable nitrogen-doped dual-mesoporous carbon nanospheres with excellent thermal stability via colloidal silica driving co-assembly strategy. Carbon 2018, 126, 156–164. [Google Scholar] [CrossRef]
- Liu, J.; Yang, T.; Wang, D.W.; Lu, G.Q.; Zhao, D.; Qiao, S.Z. A facile soft-template synthesis of mesoporous polymeric and carbonaceous nanospheres. Nat. Commun. 2013, 4, 1432. [Google Scholar] [CrossRef]
- Du, J.; Chen, A.; Liu, L.; Li, B.; Zhang, Y. N-doped hollow mesoporous carbon spheres prepared by polybenzoxazines precursor for energy storage. Carbon 2020, 160, 265–272. [Google Scholar] [CrossRef]
- Zhao, R.; Jin, Z.; Wang, J.; Zhang, G.; Zhang, D.; Sun, Y.; Guan, T.; Zhao, J.; Li, K. Adsorptive desulfurization of model fuel by S, N-codoped porous carbons based on polybenzoxazine. Fuel 2018, 218, 258–265. [Google Scholar] [CrossRef]
- Li, W.; Li, B.; Shen, M.; Gao, Q.; Hou, J. Use of Gemini surfactant as emulsion interface microreactor for the synthesis of nitrogen-doped hollow carbon spheres for high-performance supercapacitors. Chem. Eng. J. 2020, 384, 123309. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Teng, W.; Zhu, H.; Wang, J.; Elzatahry, A.A.; Al-Dahyan, D.; Li, W.; Deng, Y.; Zhao, D. A template-catalyzed in situ polymerization and co-assembly strategy for rich nitrogen-doped mesoporous carbon. J. Mater. Chem. A 2018, 6, 3162–3170. [Google Scholar] [CrossRef]
- Peng, L.; Hung, C.T.; Wang, S.; Zhang, X.; Zhu, X.; Zhao, Z.; Wang, C.; Tang, Y.; Li, W.; Zhao, D. Versatile Nanoemulsion Assembly Approach to Synthesize Functional Mesoporous Carbon Nanospheres with Tunable Pore Sizes and Architectures. J Am. Chem. Soc. 2019, 141, 7073–7080. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yao, Z.; Ma, R.; Zhou, Z.; Liu, G.; Qian, L.; Zhu, Y.; Wang, J. In situ formation of nitrogen-doped carbon nanoparticles on hollow carbon spheres as efficient oxygen reduction electrocatalysts. Nanoscale 2016, 8, 18134–18142. [Google Scholar] [CrossRef]
- Taylor, P. Ostwald ripening in emulsions: Estimation of solution thermodynamics of the disperse phase. Adv. Colloid Interface Sci. 2003, 106, 261–285. [Google Scholar] [CrossRef]
- Long, D.; Lu, F.; Zhang, R.; Qiao, W.; Zhan, L.; Liang, X.; Ling, L. Suspension assisted synthesis of triblock copolymer-templated ordered mesoporous carbon spheres with controlled particle size. Chem. Comm. 2008, 23, 2647–2649. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Song, P.; Hou, J.; Wang, B.; Xu, F.; Zhang, X. Revealing the Dynamic Formation Process and Mechanism of Hollow Carbon Spheres: From Bowl to Sphere. ACS Sustain. Chem. Eng. 2018, 6, 2797–2805. [Google Scholar] [CrossRef]
- Carter, L.G.; D’Orazio, J.A.; Pearson, K.J. Resveratrol and cancer: Focus on in vivo evidence. Endocr.-Relat. Cancer 2014, 21, 209–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]

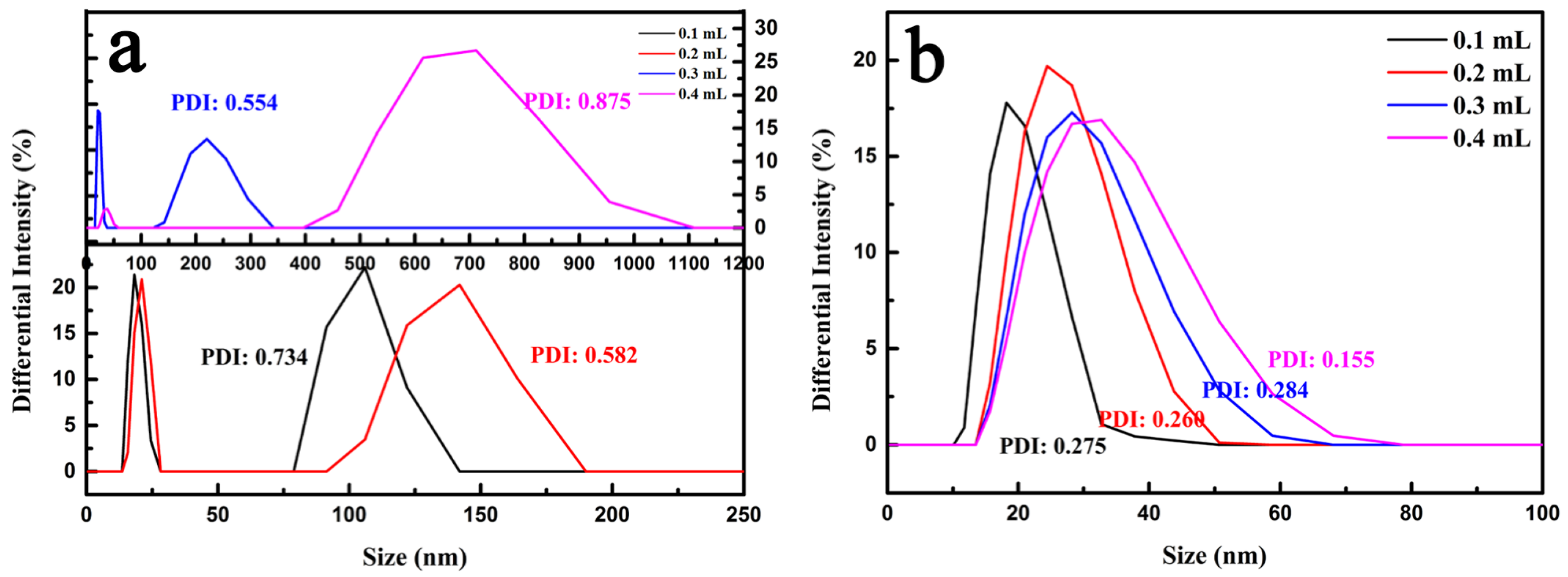
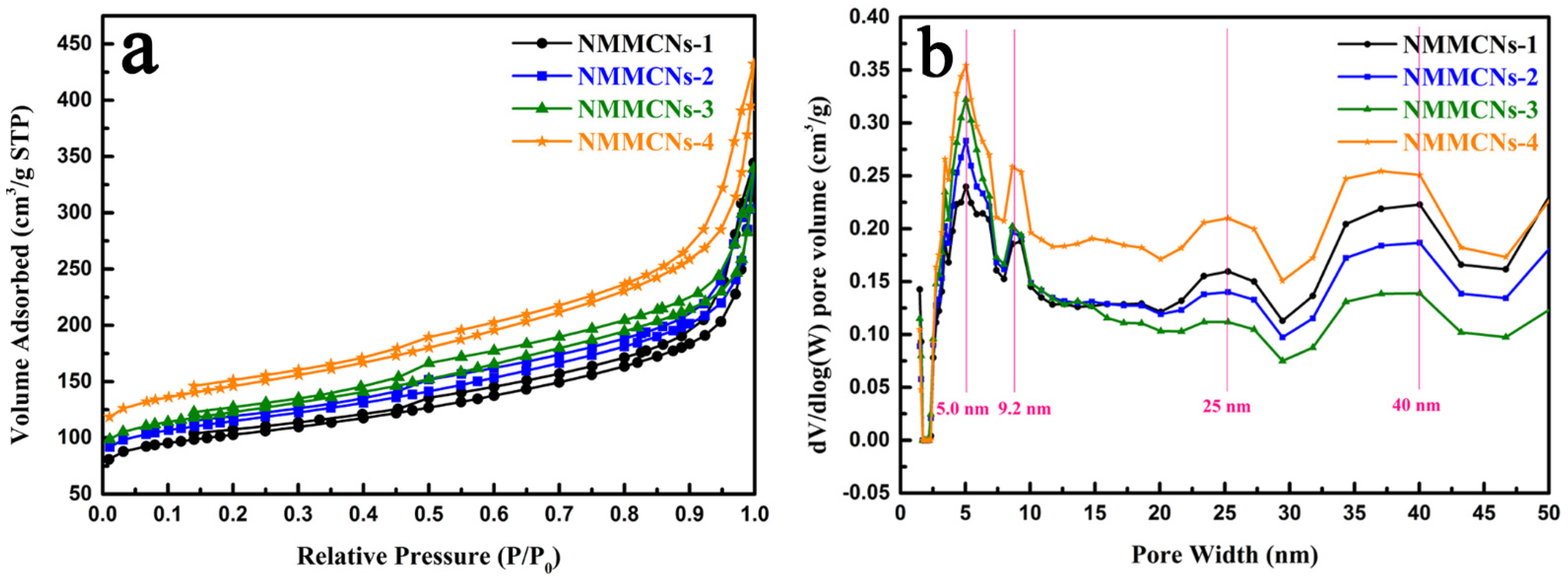
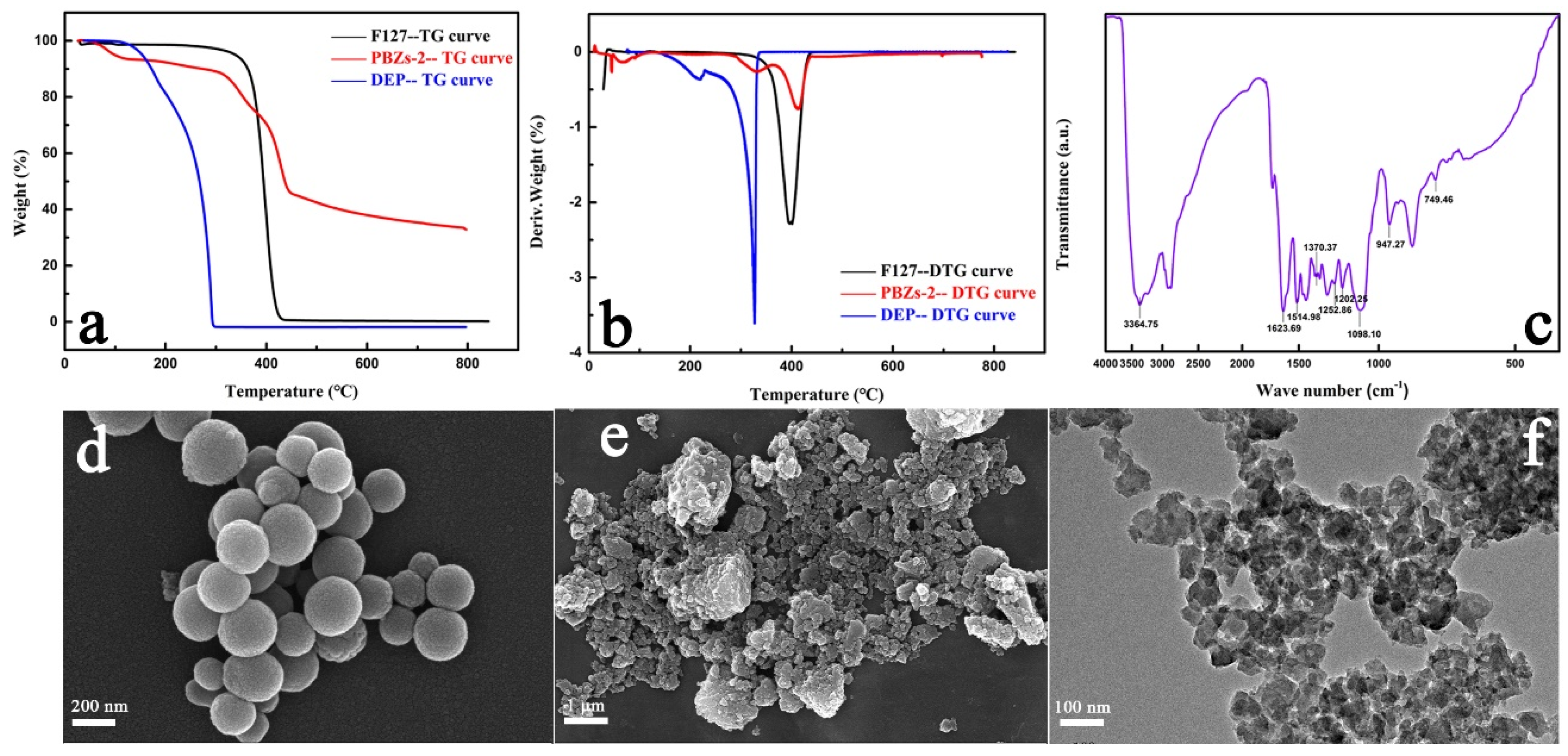
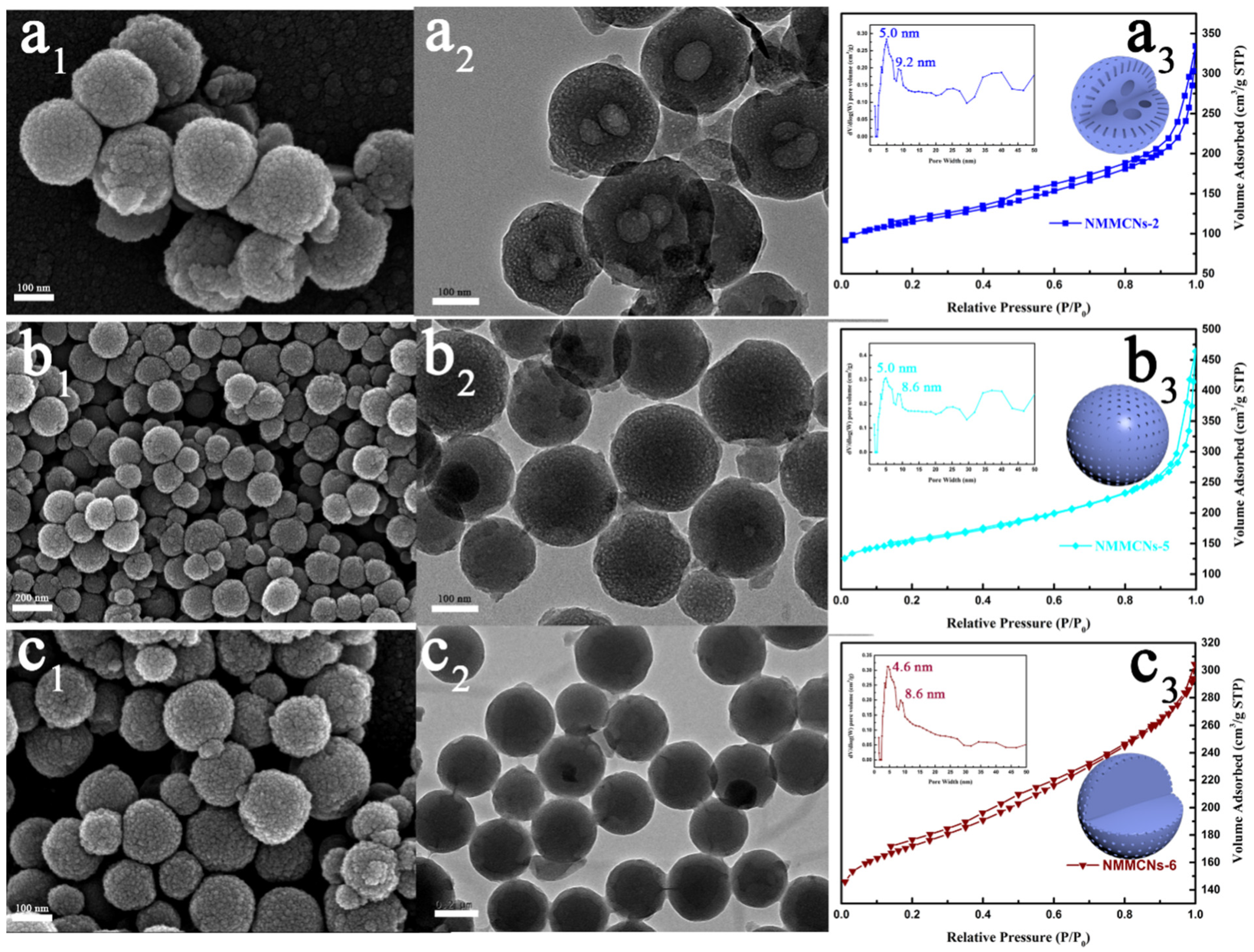


| Samples | Synthesis Parameters | ||||
|---|---|---|---|---|---|
| Before Carbonization | After Carbonization | F127 (g) | DEP (mL) | Ethanol/Water E/W (v/v) | EDA (mL) |
| PBZs-1 | NMMCNs-1 | 0.4 | 0.1 | 5/35 | 0.1 |
| PBZs-2 | NMMCNs-2 | 0.4 | 0.2 | 5/35 | 0.1 |
| PBZs-3 | NMMCNs-3 | 0.4 | 0.3 | 5/35 | 0.1 |
| PBZs-4 | NMMCNs-4 | 0.4 | 0.4 | 5/35 | 0.1 |
| PBZs-5 | NMMCNs-5 | 0.4 | 0.3 | 10/30 | 0.1 |
| PBZs-6 | NMMCNs-6 | 0.4 | 0.3 | 15/25 | 0.1 |
| PBZs-7 | NMMCNs-7 | 0.4 | 0.3 | 5/35 | 0.2 |
| PBZs-8 | NMMCNs-8 | 0.4 | 0.3 | 5/35 | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Zhao, X.; Wang, X.; Tian, Y. Nanoengineering of N-doped Mesoporous Carbon Nanoparticles with Adjustable Internal Cavities via Emulsion-Induced Assembly. Materials 2022, 15, 2591. https://doi.org/10.3390/ma15072591
Wang C, Zhao X, Wang X, Tian Y. Nanoengineering of N-doped Mesoporous Carbon Nanoparticles with Adjustable Internal Cavities via Emulsion-Induced Assembly. Materials. 2022; 15(7):2591. https://doi.org/10.3390/ma15072591
Chicago/Turabian StyleWang, Cong, Xiaoxi Zhao, Xiufang Wang, and Yong Tian. 2022. "Nanoengineering of N-doped Mesoporous Carbon Nanoparticles with Adjustable Internal Cavities via Emulsion-Induced Assembly" Materials 15, no. 7: 2591. https://doi.org/10.3390/ma15072591
APA StyleWang, C., Zhao, X., Wang, X., & Tian, Y. (2022). Nanoengineering of N-doped Mesoporous Carbon Nanoparticles with Adjustable Internal Cavities via Emulsion-Induced Assembly. Materials, 15(7), 2591. https://doi.org/10.3390/ma15072591







