Investigation of the Initial Corrosion Destruction of a Metal Matrix around Different Non-Metallic Inclusions on Surfaces of Pipeline Steels
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials and Procedures
2.2. Extractions and Investigations of Inclusions
3. Results and Discussions
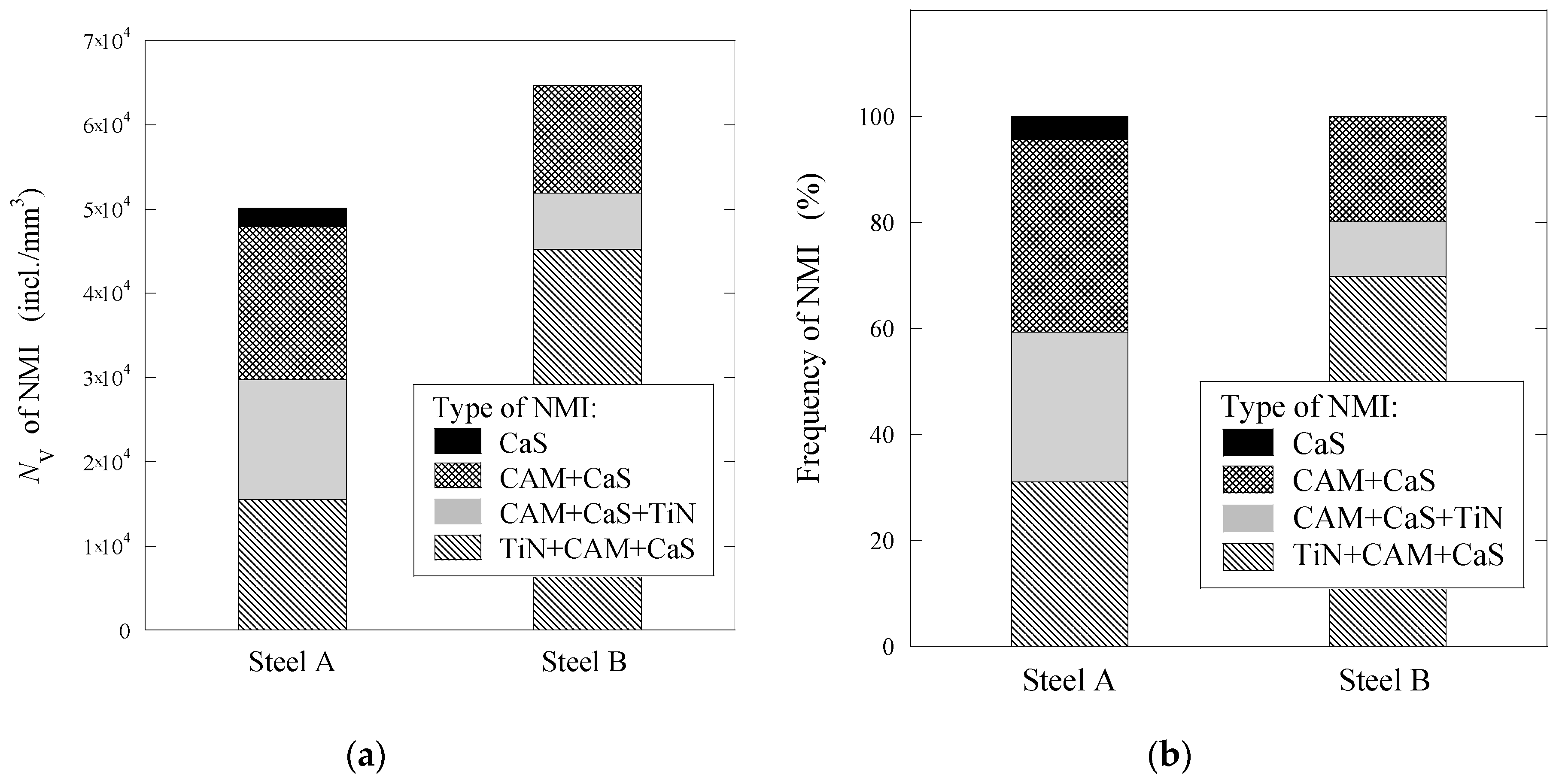
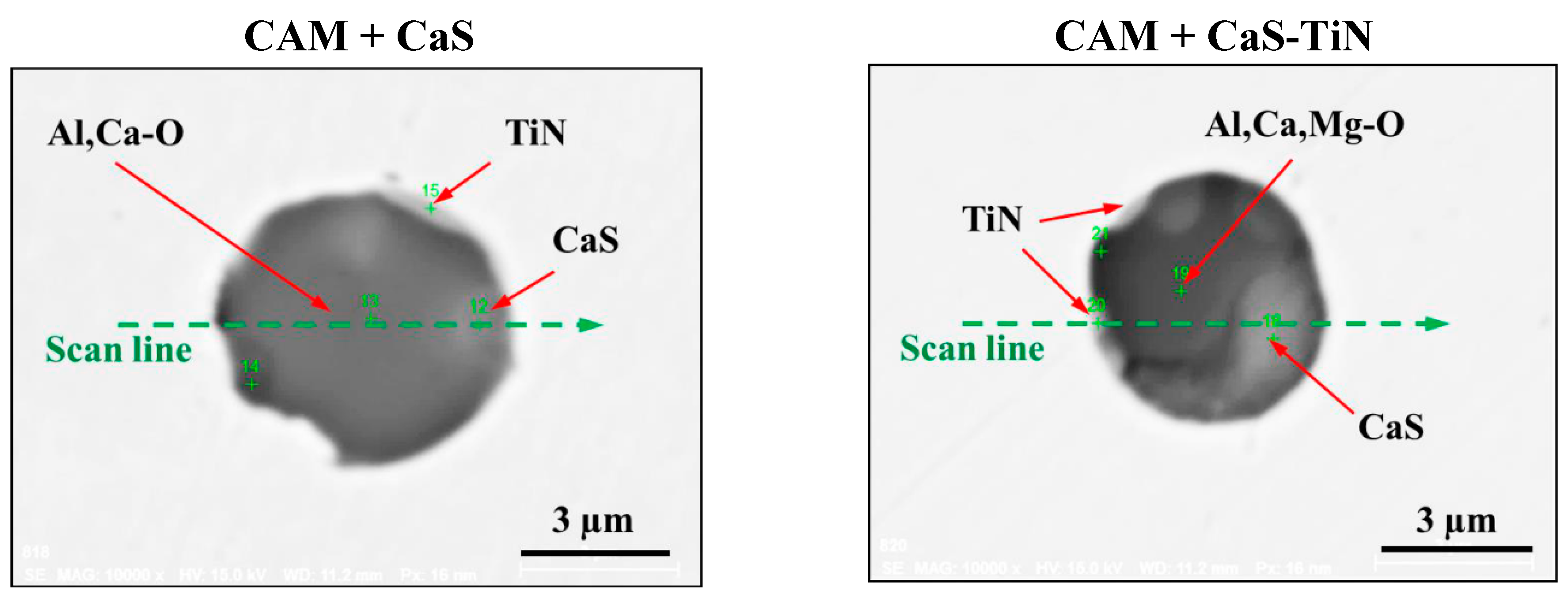
3.1. Characterization of Typical Non-Metallic Inclusions after Electrolytic Extraction of Steel Samples
3.2. Evaluation of Depletion Zone of Steel Matrix around Different NMIs
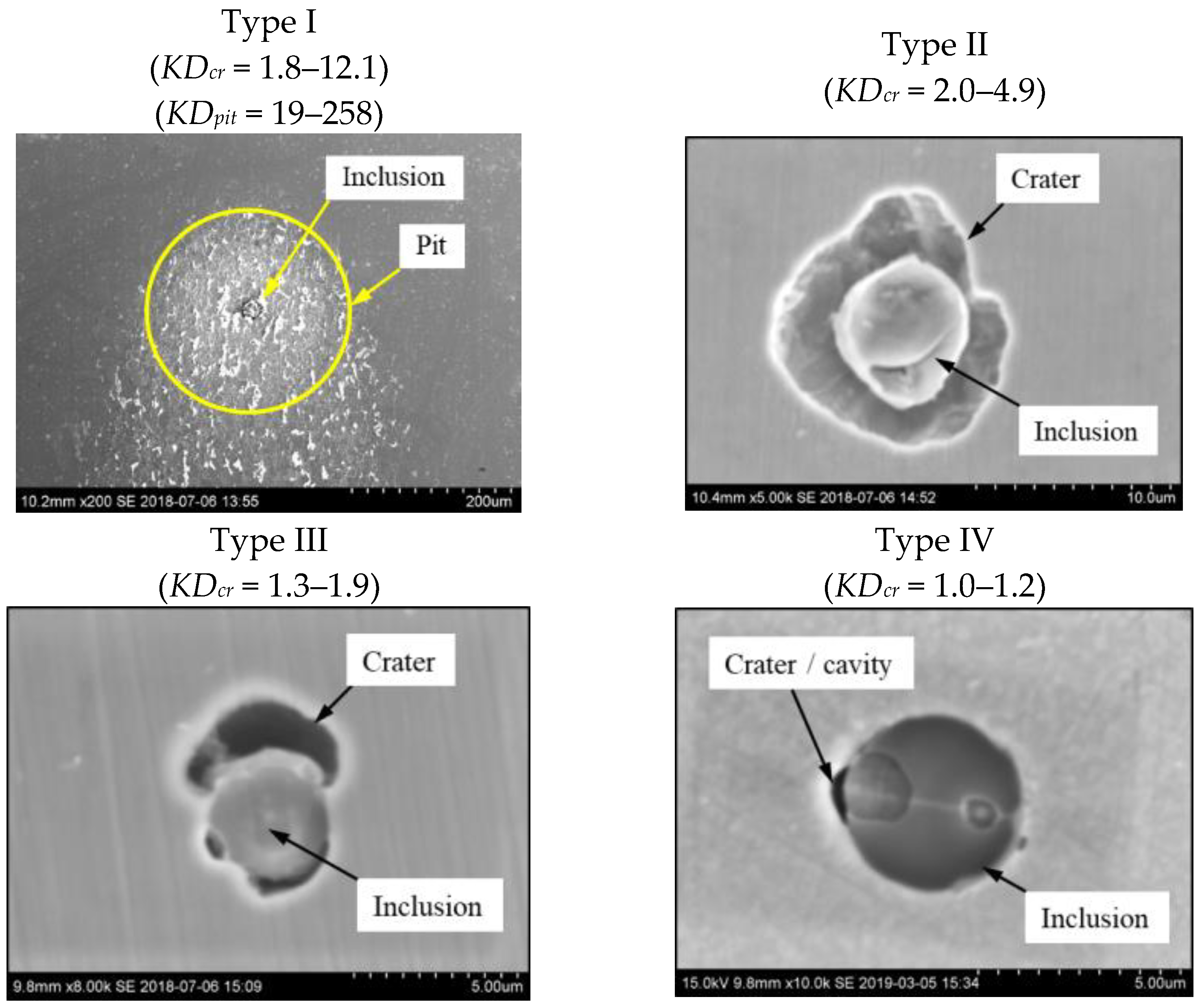
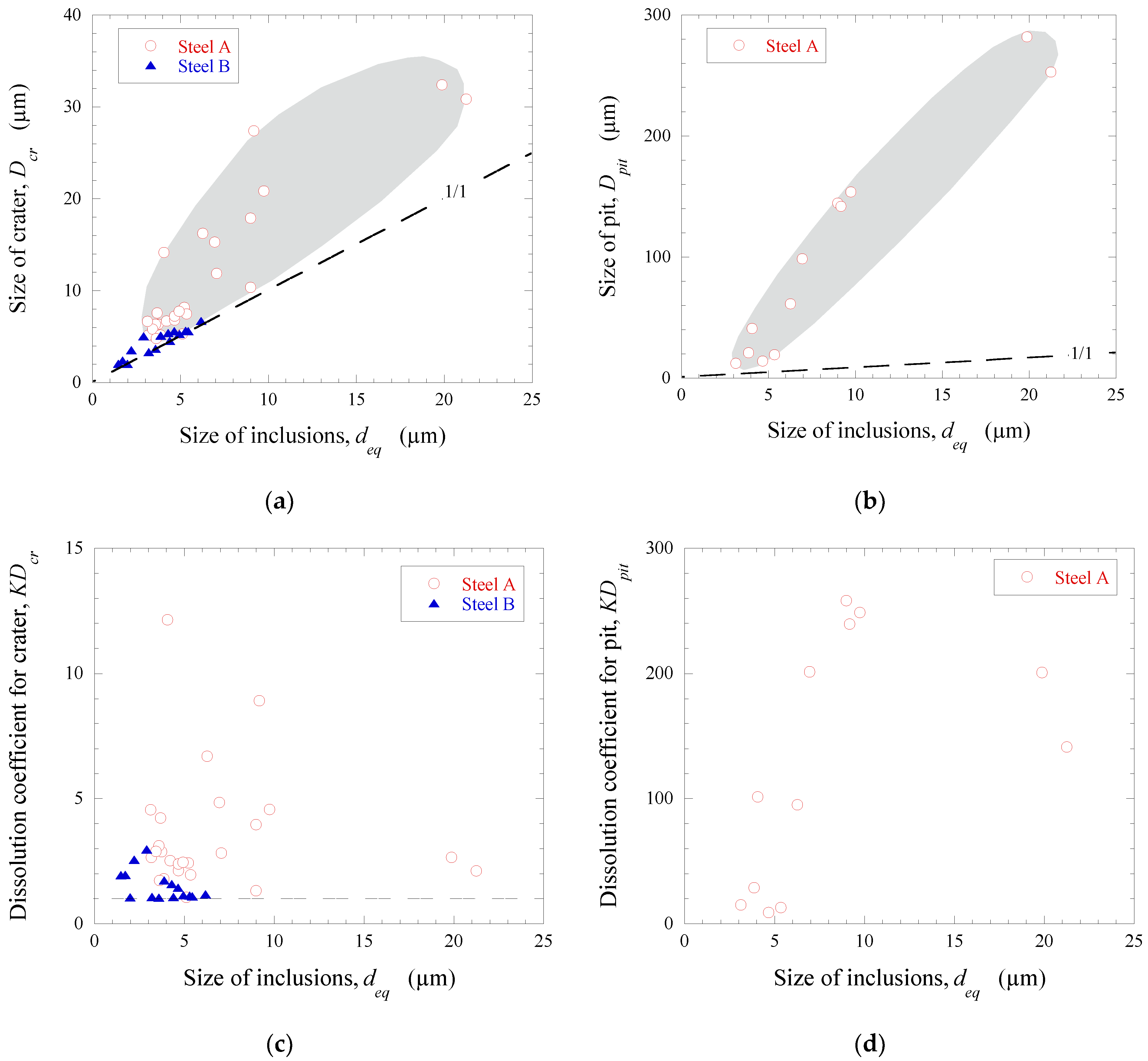
3.3. Evaluation of Corrosion Destruction of a Steel Matrix around Different NMI
3.4. Physical Properties of the Matrix/Inclusion System
- (1)
- dissolution of the metal matrix due to electrochemical processes taking place between different inclusions (or phases) and the metal;
- (2)
- chemical dissolution of a metal matrix due to chemical reactions between inclusions with the present solution (water, oil, gas, etc.), and air;
- (3)
- dissolution of the weakened metal matrix due to formation of depletion zones of anticorrosion alloying elements in the steel matrix around inclusions containing these alloying elements;
- (4)
- dissolution of the metal matrix around some inclusions containing some voids (or micro-crevices), which are formed during the solidification of steel; and,
- (5)
- dissolution of a stressed metal matrix around some inclusions due to differences in physical properties (such as thermal expansion) and mechanical properties of the metal and inclusions;
4. Conclusions
- 1.
- Four groups of typical inclusions were observed in the industrial steel samples: (i) pure CaS inclusions or oxide cores completely covered by a CaS layer (97–100% of the CaS being present in the outer inclusion layer); (ii) CaO-Al2O3-MgO inclusions partially covered by a CaS layer; (iii) oxide inclusions containing small precipitations of CaS and TiN inclusions; and (iv) oxide inclusions completely covered by TiN or pure TiN inclusions.
- 2.
- The developed and tested method of short chemical extraction using a soft 10%AA electrolyte can successfully be applied for the evaluation of initial corrosion processes of the steel matrix around various non-metallic inclusions present in industrial pipeline steel samples. Quantitative parameters (such as the diameters of corrosion craters (Dcr) and pits (Dpit) and the relative coefficients of metal matrix dissolution (KD) around different inclusions) can be used for the determination and comparison of “corrosion active” inclusions and their effect on the initial corrosion of the surrounding layer of the steel matrix.
- 3.
- During cooling of steels after hot rolling, significant tensile stresses appear in the steel layer surrounding CaS inclusions due to the larger thermal expansion coefficient (αi) of CaS compared to the value of steel. This promotes faster dissolution of the steel matrix around these inclusions, which corresponds to the initial corrosion stage. However, the compressive stresses in the steel layer, which appear around CaO-Al2O3 and TiN inclusions having smaller αi values, do not contribute to the initiation of corrosion of the matrix surrounding these inclusions.
Author Contributions
Funding
Conflicts of Interest
References
- Shibaeva, T.V.; Laurinavichyute, V.K.; Tsirlina, G.A.; Arsenkin, A.M.; Grigorovich, K.V. The effect of microstructure and non-metallic inclusions on corrosion behavior of low carbon steel in chloride containing solutions. Corros. Sci. 2014, 80, 299–308. [Google Scholar] [CrossRef]
- Weber, R.A.; Somers, B.R.; Kaufmann, E.J. Low-Carbon, Age-Hardenable Steels for Use in Construction: A Review; Materials Science; United States Army Corps of Engineers: Washington, DC, USA, 1996. [Google Scholar]
- Edmonds, D.V.; Cochrane, R.C. The effect of alloying on the resistance of carbon steel for oilfield applications to CO2 corrosion. Materials Research-ibero-american. J. Mater. 2005, 8, 377–385. [Google Scholar]
- Wu, W.; Liu, Z.; Wang, Q.; Li, X. Improving the resistance of high-strength steel to SCC in a SO2-polluted marine atmosphere through Nb and Sb microalloying. Corros. Sci. 2020, 170, 108693. [Google Scholar] [CrossRef]
- Dong, J.; Li, C.; Liu, C.; Huang, Y.; Yu, L.; Li, H.; Liu, Y. Microstructural and mechanical properties development during quenching-partitioning-tempering process of Nb-V-Ti microalloyed ultra-high strength steel. Mater. Sci. Eng. A 2017, 705, 249–256. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, L.; Gao, C.; Chang, W.; Lu, M. Corrosion behavior of novel 3% Cr pipeline steel in CO2 Top-of-Line Corrosion environment. Mater. Des. 2012, 36, 54–57. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Deng, Y.; Han, X.; Hu, W.; Zhong, C. Ex situ characterization of metallurgical inclusions in X100 pipeline steel before and after immersion in a neutral pH bicarbonate solution. J. Alloys Compd. 2016, 673, 28–37. [Google Scholar] [CrossRef]
- Williams, D.E.; Kilburn, M.R.; Cliff, J.; Waterhouse, G.I.N. Composition changes around sulphide inclusions in stainless steels, and implications for the initiation of pitting corrosion. Corros. Sci. 2010, 52, 3702–3716. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, G.; Li, Y. Observation of the pitting corrosion and uniform corrosion for X80 steel in 3.5 wt.% NaCl solutions using in-situ and 3-D measuring microscope. Corros. Sci. 2016, 111, 508–517. [Google Scholar] [CrossRef]
- Liu, C.; Revilla, R.I.; Liu, Z.; Zhang, D.; Li, X.; Terryn, H. Effect of inclusions modified by rare earth elements (Ce, La) on localized marine corrosion in Q460NH weathering steel. Corros. Sci. 2017, 129, 82–90. [Google Scholar] [CrossRef]
- Li, J.; Gao, X.; Du, L.; Liu, Z. Relationship between microstructure and hydrogen induced cracking behavior in a low alloy pipeline steel. J. Mater. Sci. Technol. 2017, 33, 1504–1512. [Google Scholar] [CrossRef]
- Torkkeli, J.; Saukkonen, T.; Hänninen, H. Effect of MnS inclusion dissolution on carbon steel stress corrosion cracking in fuel-grade ethanol. Corros. Sci. 2015, 96, 14–22. [Google Scholar] [CrossRef]
- Wranglen, G. Pitting and sulphide inclusions in steel. Corros. Sci. 1974, 14, 331–349. [Google Scholar] [CrossRef]
- Wei, J.; Dong, J.; Ke, W.; He, X. Influence of inclusions on early corrosion development of ultra-low carbon bainitic steel in NaCl solution. Corrosion 2015, 71, 1467–1480. [Google Scholar] [CrossRef]
- Anijdan, S.; Mousavi, H.; Arab, G.; Sabzi, M.; Sadeghi, M.; Eivani, A.R.; Jafarian, H.R. Sensitivity to hydrogen induced cracking, and corrosion performance of an API X65 pipeline steel in H2S containing environment: Influence of heat treatment and its subsequent microstructural changes. J. Mater. Res. Technol. 2021, 15, 1–16. [Google Scholar] [CrossRef]
- Wang, L.; Xin, J.; Cheng, L.; Zhao, K.; Sun, B.; Li, J.; Wang, X.; Cui, Z. Influence of inclusions on initiation of pitting corrosion and stress corrosion cracking of X70 steel in near-neutral pH environment. Corros. Sci. 2019, 147, 108–127. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Revilla, R.I.; Sun, T.; Zhao, J.; Zhang, D.; Yang, S.; Liu, Z.; Cheng, X.; Terryn, H.; et al. Towards a better understanding of localised corrosion induced by typical non-metallic inclusions in low-alloy steels. Corros. Sci. 2021, 179, 109150. [Google Scholar] [CrossRef]
- Liu, C.; Revilla, R.I.; Zhang, D.; Liu, Z.; Lutz, A.; Zhang, F.; Zhao, T.; Ma, H.; Li, X.; Terryn, H. Role of Al2O3 inclusions on the localized corrosion of Q460NH weathering steel in marine environment. Corros. Sci. 2018, 138, 96–104. [Google Scholar] [CrossRef]
- Jin, T.Y.; Liu, Z.Y.; Cheng, Y.F. Effect of non-metallic inclusions on hydrogen-induced cracking of API5L X100 steel. Int. J. Hydrog. Energy 2010, 35, 8014–8021. [Google Scholar] [CrossRef]
- Szklarska-śmialowska, Z.; Lunarska, E. The effect of sulfide inclusions on the susceptibility of steels to pitting, stress corrosion cracking and hydrogen embrittlement. Mater. Corros. 2004, 32, 478–485. [Google Scholar] [CrossRef]
- Reformatskaya, I.I.; Podobaev, A.N.; Florianovich, G.M.; Ashcheulova, I.I. Evaluation of the corrosion resistance of low-carbon pipe steels under conditions of hot-water supply. Prot. Met. 1999, 35, 4–9. [Google Scholar]
- Wang, Y.; Zhang, X.; Cheng, L.; Liu, J.; Hou, T.; Wu, K. Correlation between active/inactive (Ca, Mg, Al)-Ox-Sy inclusions and localised marine corrosion of EH36 steels. J. Mater. Res. Technol. 2021, 13, 2419–2432. [Google Scholar] [CrossRef]
- Serov, G.V.; Komissarov, A.; Tikhonov, S.; Sidorova, E.P.; Kushnerev, V.; Mishnev, P.A.; Kuznetsov, D.V. Effect of Deoxidation on Low-Alloy Steel Nonmetallic Inclusion Composition. Refract. Ind. Ceram. 2019, 59, 573–578. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Q.; Li, X.; Hu, J.; Cao, F. Insight into the triggering effect of (Al, Mg, Ca, Mn)-oxy-sulfide inclusions on localized corrosion of weathering steel. J. Mater. Sci. Technol. 2021, 64, 99–113. [Google Scholar] [CrossRef]
- Hou, Y.; Li, T.; Li, G.; Cheng, C. Mechanism of Yttrium composite inclusions on the localized corrosion of pipeline steels in NaCl solution. Micron 2020, 130, 102820. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Jiang, Z.; Zhao, J.; Cheng, X.; Liu, Z.; Zhang, D.; Li, X. Influence of rare earth metals on mechanisms of localised corrosion induced by inclusions in Zr-Ti deoxidised low alloy steel. Corros. Sci. 2020, 166, 108463. [Google Scholar] [CrossRef]
- Sidorova, E.; Karasev, A.V.; Kuznetsov, D.; Jönsson, P.G. Modification of non-metallic inclusions in oil-pipeline steels by ca-treatment. Metals 2019, 9, 391. [Google Scholar] [CrossRef] [Green Version]
- Karasev, A.V.; Suito, H. Analysis of Size Distributions of Primary Oxide Inclusions in Fe-10 mass pct Ni-M (M = Si, Ti, Al, Zr, and Ce) Alloy. Metall. Mater. Trans. B 1999, 30, 259–270. [Google Scholar] [CrossRef]
- Inoue, R.; Kiyokawa, K.; Tomoda, K.; Ueda, S.; Ariyama, T. Three-Dimensional Estimation of Multi-Component Inclusion Particle in Steel. In Proceedings of the 8th International Workshop on Progress in Analytical Chemistry and Materials Characterization in the Steel and Metal Industries (CETAS-2011), Luxembourg, 17–19 May 2011; Volume OC17, pp. 118–125. [Google Scholar]
- Brooksbank, D.; Andrews, K. Thermal expansion of some inclusions found in steels and relation to tessellated stresses. J. Iron Steel Inst. 1968, 206, 595–599. [Google Scholar]
- Brooksbank, D.; Andrews, K. Stress fields around inclusions and their relation to mechanical properties. Int. Conf. Prod. Appl. Clean Steels 1972, 1972, 186–198. [Google Scholar]
- Brooksbank, D.; Andrews, K. Tessellated stresses associated with some inclusions in steel. J. Iron. Steel Inst. 1969, 207, 474–483. [Google Scholar]


| Steel | Corrosion Test | Investigated Parameters of NMI | Pitting Formation | Ref. |
|---|---|---|---|---|
| X100 steel | NS4 solution (g/L): 0.483 NaHCO3, 0.122 KCl, 0.181 CaCl2·2H2O and 0.131 MgSO4·7H2O, near-neutral pH | Morphologies, compositions and sizes of complex inclusions, such as Al–Mg–Ca–O, Al–Si–Ca–O and Al–Si–Ca–S–O mixtures | Formation of micropits around or at an inclusion involves the dissolution at the inclusion/matrix interface and the “drop-off” of the inclusion | [7] |
| X80 steel | 3.5% NaCl solution in H2O. Temperatures: 18, 35 and 65 °C | Size and chemical composition of non-metallic inclusions | Pits are mostly initiated by mechanical defects, but some pits are initiated by non-metallic inclusions | [9] |
| X70 steel | Near-neutral pH NS4 solution (pH = 6.8) | Morphologyand composition of complex oxide inclusions with/without CaS | The dissolution of CaS in complex oxide inclusions induces formation of pits at the inclusions | [16] |
| Low-alloy steel (0.03% C, 0.25% Si, 0.1% Mn, 1.2% Cr, 0.3% Ni, 0.4% Cu, 0.002% S) | Xisha atmospheric simulated solution (0.1% NaCl, 0.05% Na2SO4 and 0.05% CaCl2 in H2O) | Morphology and size of typical non-metallic inclusions: Al2O3, ZrO2-Ti2O3-Al2O3 and (RE)AlO3-(RE)2O2S-(RE)xSy | Local corrosion by the dissolution of adjacent matrix with high energy and electrochemical activity around NMIs (Al2O3, ZrO2-Ti2O3-Al2O3) | [17] |
| Q460NH steel | 0.1% NaCl, 0.05% Na2SO4 and 0.05% CaCl2 in H2O. Times: 5, 30 min and 72 h | Morphology of Al2O3 inclusions after being immersed for different times | Localized corrosion was induced at the interface between Al2O3 inclusions and the steel matrix | [18] |
| EH36 steel | 0.5% NaCl solution in H2O. Times: 1, 5, 15, 60 min and 24 h | Morphology of (Ca, Mg, Al)-Ox-Sy complex inclusions | Dissolution of the steel matrix leads to the formation of a micro-crevice | [22] |
| X80 steel | 3.5% NaCl solution in H2O. Times: 10, 30 and 60 min | Morphologies and element distributions of typical Mg-YS composite inclusions | Steel matrix dissolves prior to Mg-YS inclusion during the initial immersion stage | [25] |
| Low-alloy steel (0.045%C, 0.24%Si, 0.12% Mn, 1.22% Cr, 0.32% Ni, 0.43% Cu, 0.002% S, 0.036% RE) | 0.1% NaCl, 0.05% Na2SO4 and 0.05% CaCl2 in H2O (pH = 4.9) | Morphology and composition of (RE)2O2S-(RE)xSy inclusions | Localized corrosion was initiated by the dissolution of (RE)2O2S-(RE)xSy | [26] |
| Steel | C | Si | Mn | Cu | Cr | Ni | Ti | Al | Ca | S | N |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 0.06 | 0.24 | 0.63 | 0.33 | 0.43 | 0.17 | 0.019 | 0.025 | 0.0020 | 0.002 | 0.005 |
| B | 0.05 | 0.23 | 0.67 | 0.35 | 0.43 | 0.18 | 0.021 | 0.022 | 0.0014 | 0.001 | 0.007 |
| NMI * | SEM Image on Film Filter | SEM Image on Metal Surface | Composition (mass.%) | Size (µm) |
|---|---|---|---|---|
| CaS |  | 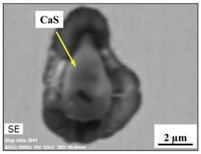 | CaS—97–100%, Al2O3—0–2%, MgO—0–1%, CaO—0–2% | 2.5–8.7 |
| CAM + CaS |  |  | CaO—9–69%, Al2O3—2–54%, MgO—0–22%, SiO2—0–7%, CaS—9–62% | 0.9–21.3 |
| CAM + CaS + TiN |  |  | CaO—4–45%, Al2O3—4–45%, MgO—0–19%, SiO2—1–5%, CaS—0–50%, TiN—1–53% | 1.1–5.5 |
| TiN + CAM + CaS | 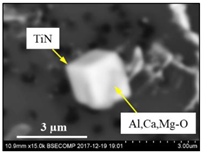 | 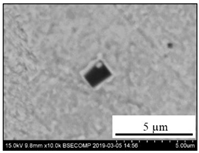 | TiN—78–100%, Al2O3—0–5%, MgO—0–5%, CaO—0–5%, CaS—0–13% | 0.7–4.3 |
| Steel | Type of NMI | deq (µm) | Dcr (µm) | KDcr | Dpit (µm) | KDpit |
|---|---|---|---|---|---|---|
| A | I | 8.6 ± 6.0 (3.1~21.3) | 16.8 ± 9.5 (5.2~32.4) | 4.7 ± 3.2 (1.8~12.1) | 103.5 ± 93.3 (12.1~281.8) | 129.3 ± 98.4 (19.1~258.1) |
| II | 4.4 ± 1.2 (3.2~7.1) | 7.3 ± 1.9 (5.2~11.9) | 2.8 ± 0.6 (2.1~4.2) | |||
| III | 6.3 ± 3.8 (3.6~9.0) | 7.6 ± 4.0 (4.8~10.4) | 1.5 ± 0.3 (1.3~1.7) | |||
| IV | 5.1 | 5.3 | 1.1 | |||
| B | I | no | ||||
| II | 2.3 ± 0.6 (1.7~2.9) | 3.7 ± 1.3 (2.4~5.0) | 2.5 ± 0.5 (2.0~3.0) | |||
| III | 3.6 ± 1.4 (1.5~4.7) | 4.5 ± 1.7 (2.1~5.6) | 1.7 ± 0.2 (1.5~1.9) | |||
| IV | 4.1 ± 1.3 (2.0~5.5) | 4.3 ± 1.4 (2.1~5.6) | 1.1 ± 0.1 (1.0~1.2) |
| NMI | SEM Image | Concentration Mapping |
|---|---|---|
| Type I | 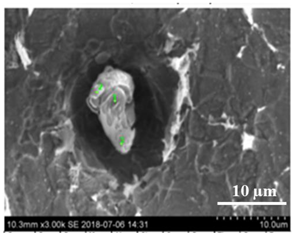 | 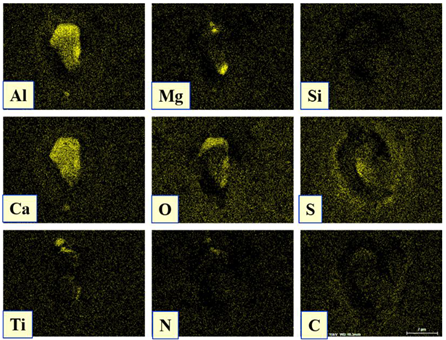 |
| Type II | 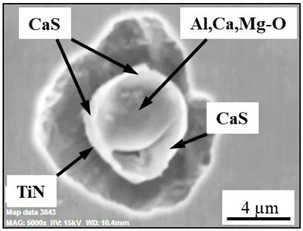 | 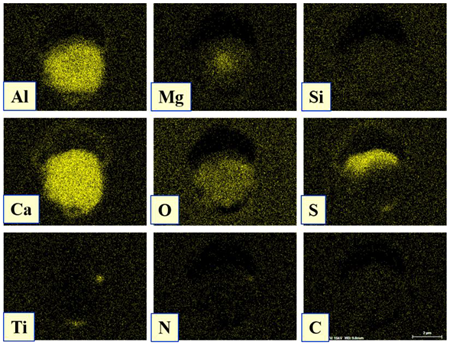 |
| Type III | 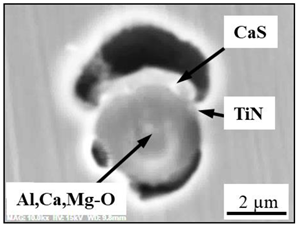 | 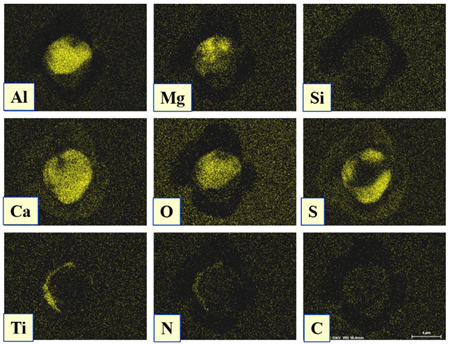 |
| Type IV | 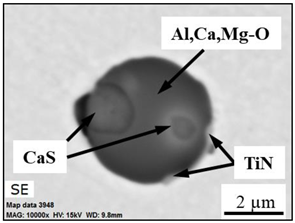 | 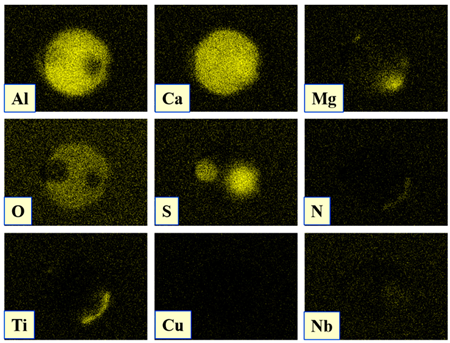 |
| Compounds | Poisson’s Ratio, vi | Young’s Modulus, Ei (GPa at 20 °C) | Thermal Expansion Coefficient, αi (×10−6/°C) | Residual Radial Stress, σr * (MPa) |
|---|---|---|---|---|
| CaS | 0.300 | 56.7 | 14.7 | −250.8 |
| 3CaO·Al2O3 | 0.234 | 115 | 10.0 | 285.0 |
| 12CaO·7Al2O3 | 0.234 | 115 | 7.8 | 684.0 |
| CaO·Al2O3 | 0.234 | 115 | 6.5 | 535.8 |
| CaO·2Al2O3 | 0.234 | 115 | 5.0 | 855.0 |
| Al2O3 | 0.250 | 402 | 8.0 | 513.0 |
| Al2O3·MgO | 0.260 | 271 | 8.4 | 467.4 |
| TiN | 0.192 | 323 | 9.4 | 353.4 |
| Steel matrix | 0.290 | 206 | 12.5 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidorova, E.; Karasev, A.; Kuznetsov, D.; Jönsson, P.G. Investigation of the Initial Corrosion Destruction of a Metal Matrix around Different Non-Metallic Inclusions on Surfaces of Pipeline Steels. Materials 2022, 15, 2530. https://doi.org/10.3390/ma15072530
Sidorova E, Karasev A, Kuznetsov D, Jönsson PG. Investigation of the Initial Corrosion Destruction of a Metal Matrix around Different Non-Metallic Inclusions on Surfaces of Pipeline Steels. Materials. 2022; 15(7):2530. https://doi.org/10.3390/ma15072530
Chicago/Turabian StyleSidorova, Elena, Andrey Karasev, Denis Kuznetsov, and Pär G. Jönsson. 2022. "Investigation of the Initial Corrosion Destruction of a Metal Matrix around Different Non-Metallic Inclusions on Surfaces of Pipeline Steels" Materials 15, no. 7: 2530. https://doi.org/10.3390/ma15072530
APA StyleSidorova, E., Karasev, A., Kuznetsov, D., & Jönsson, P. G. (2022). Investigation of the Initial Corrosion Destruction of a Metal Matrix around Different Non-Metallic Inclusions on Surfaces of Pipeline Steels. Materials, 15(7), 2530. https://doi.org/10.3390/ma15072530







