Preparation of High Thermo-Stability and Compactness Microencapsulated Phase Change Materials with Polyurea/Polyurethane/Polyamine Three-Composition Shells through Interfacial Polymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MicroPCMs
2.3. Characterization of MicroPCMs
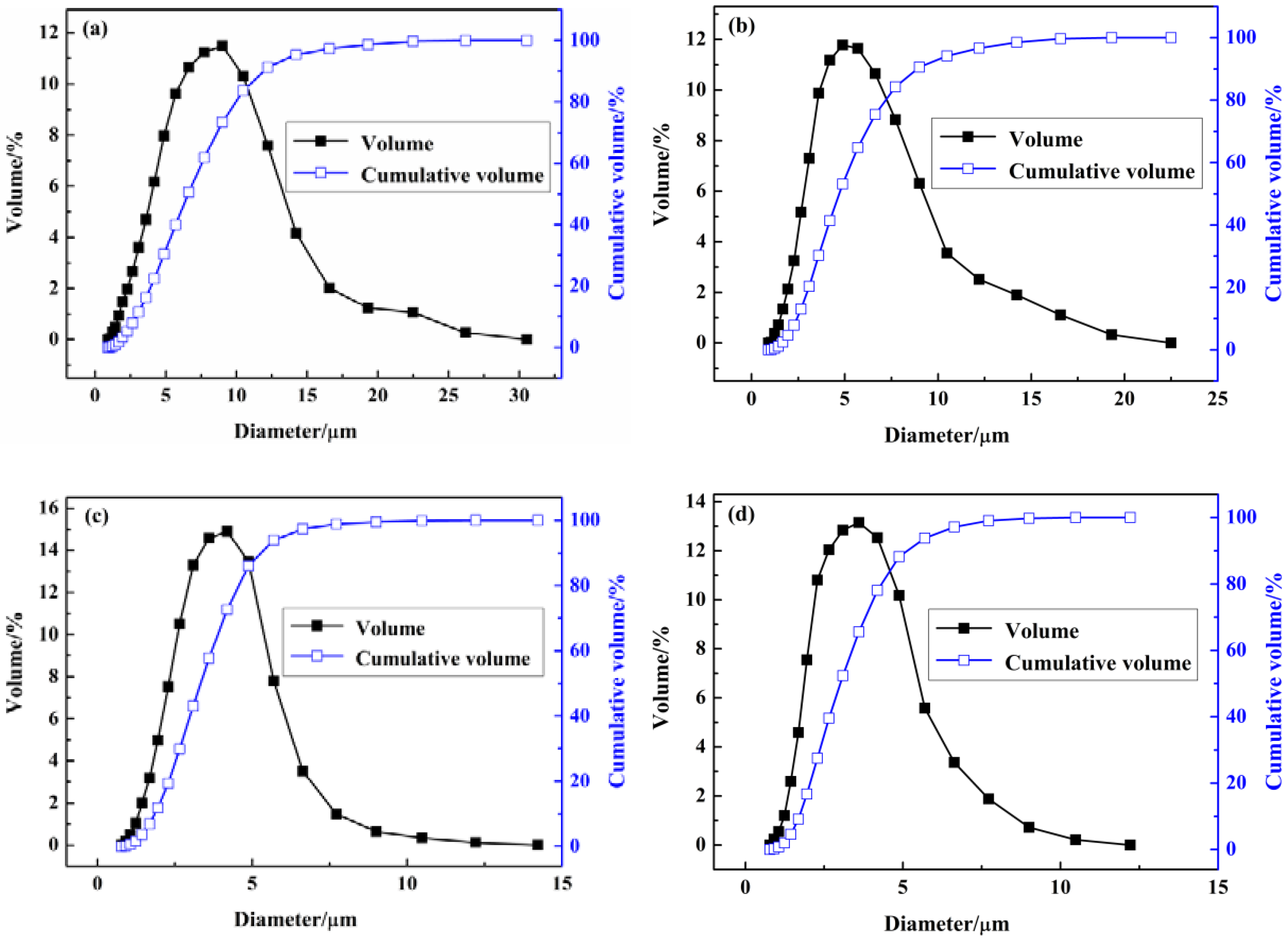
2.3.1. Particle Size Distribution
2.3.2. The Phase Change Properties
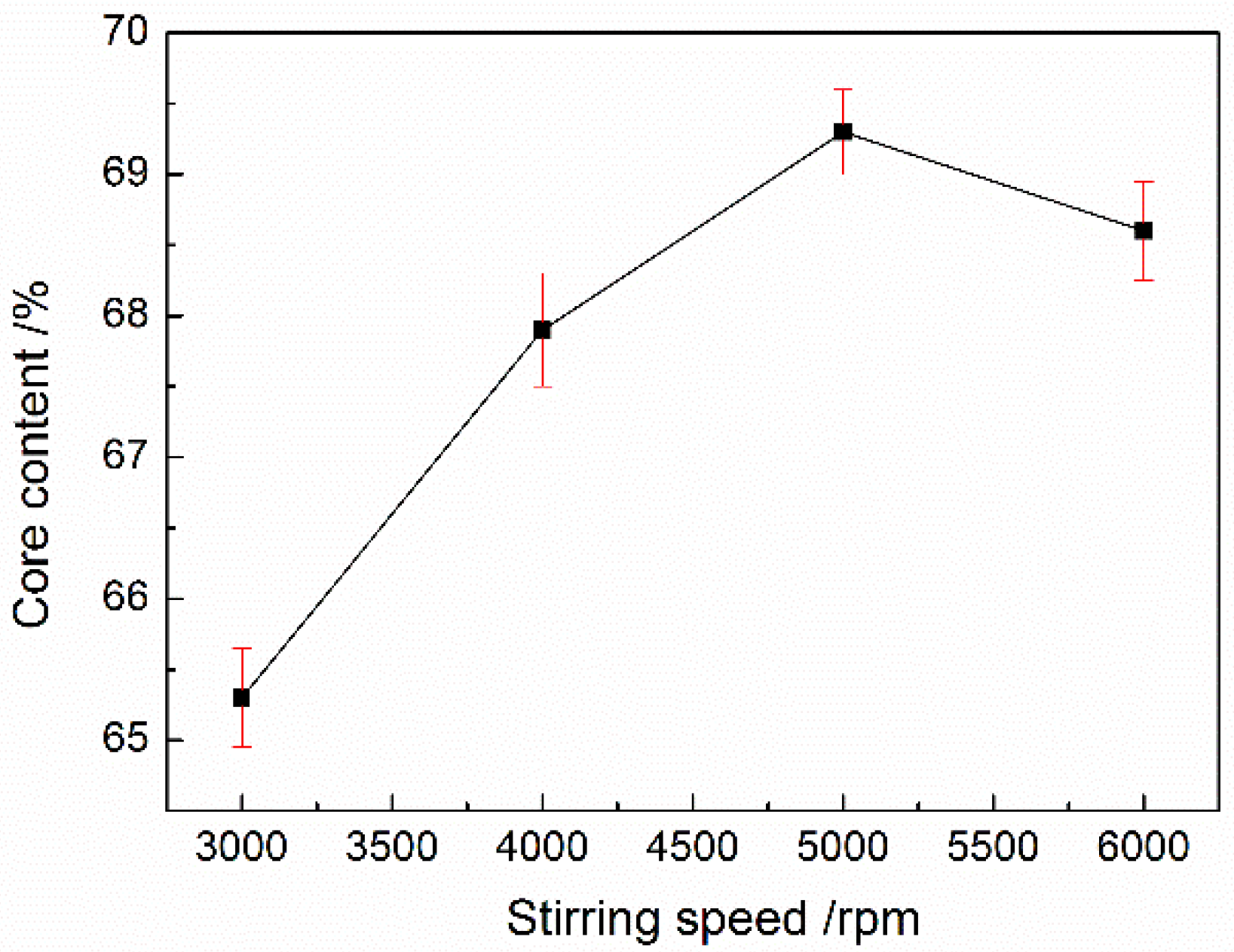
2.3.3. Core Content and Encapsulation Efficiency
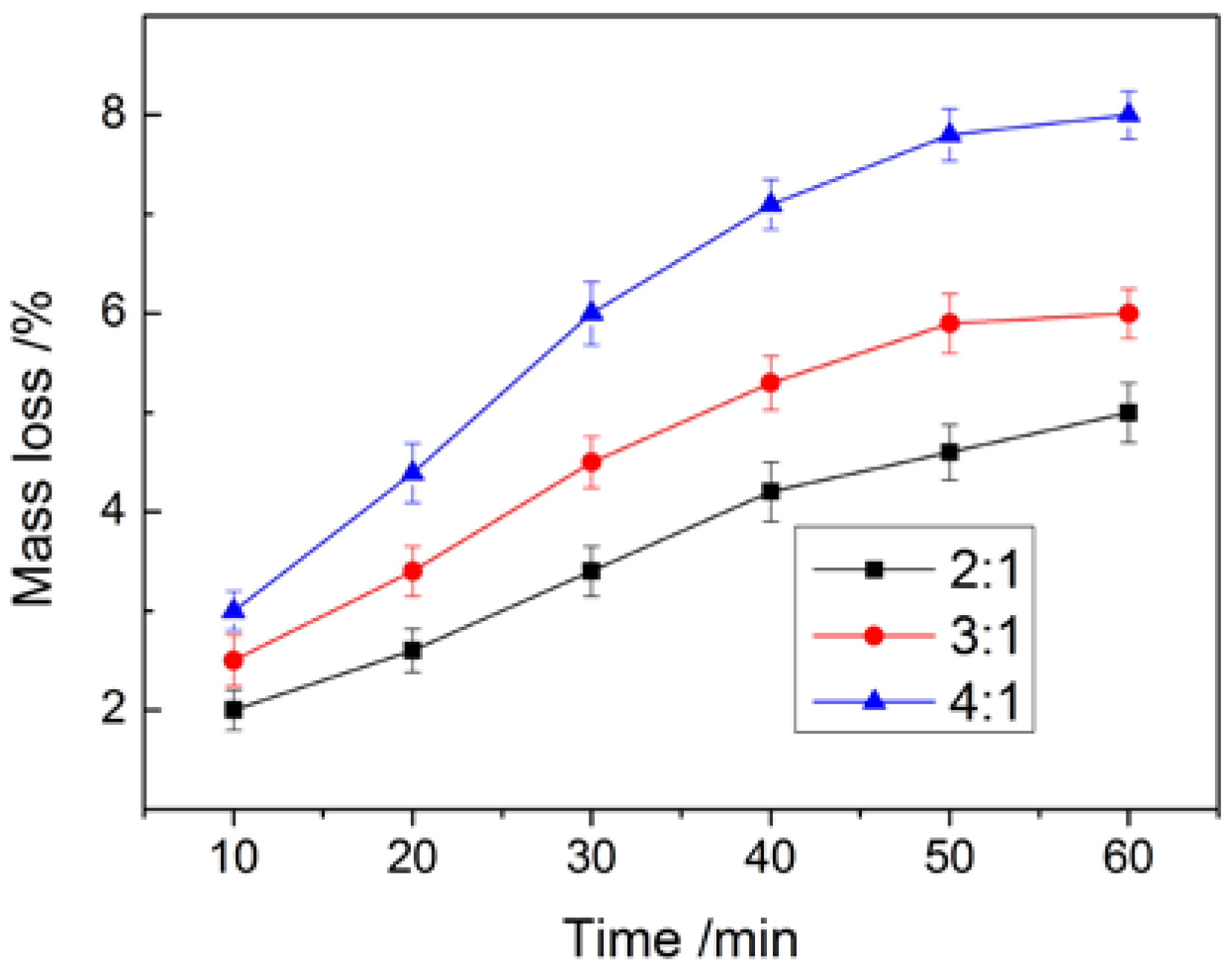
2.3.4. Compactness Analysis
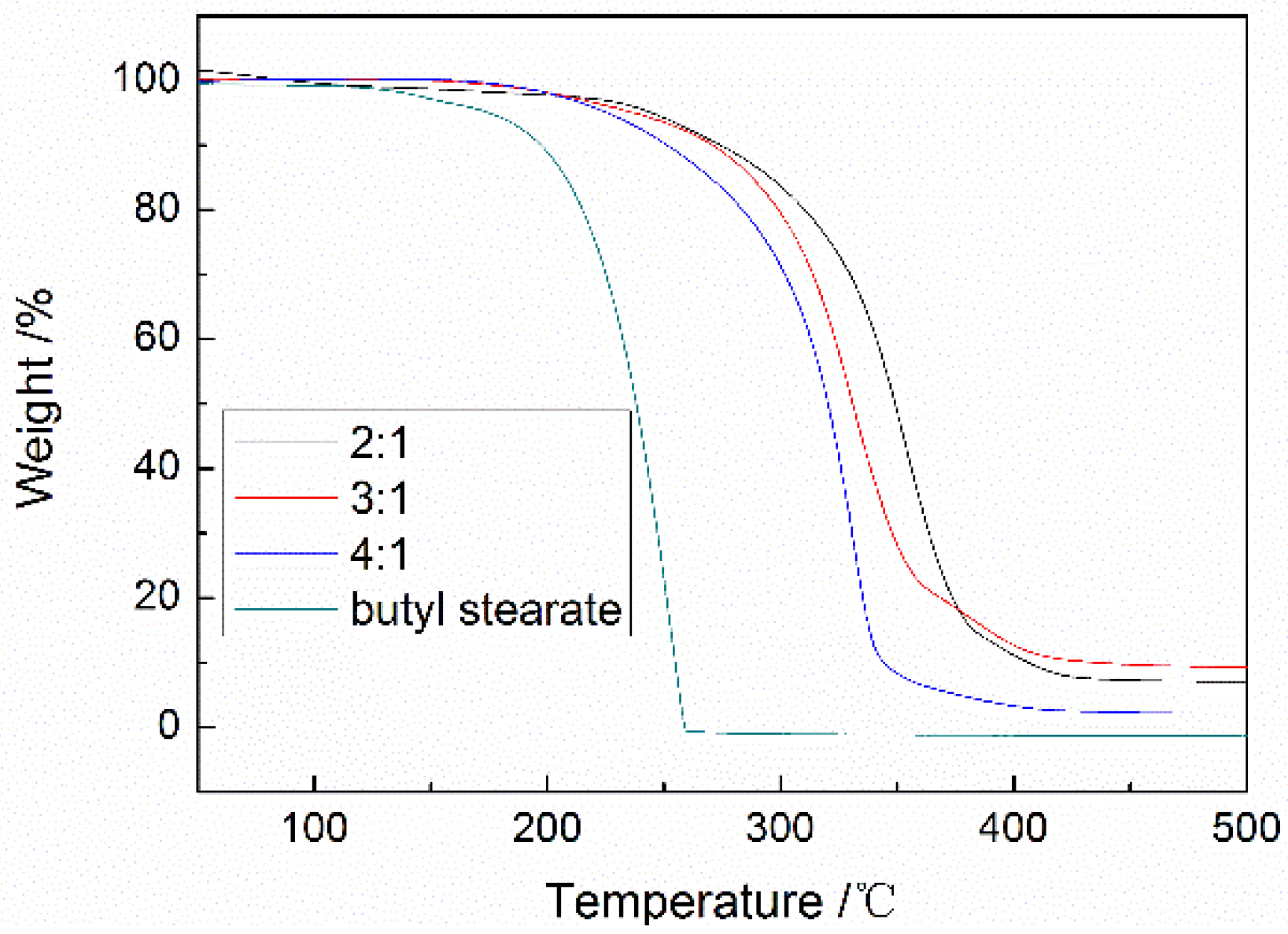
2.3.5. Thermal Gravimetric Analysis
2.3.6. Surface Characterization
2.3.7. Chemical Structure
3. Results and Discussion
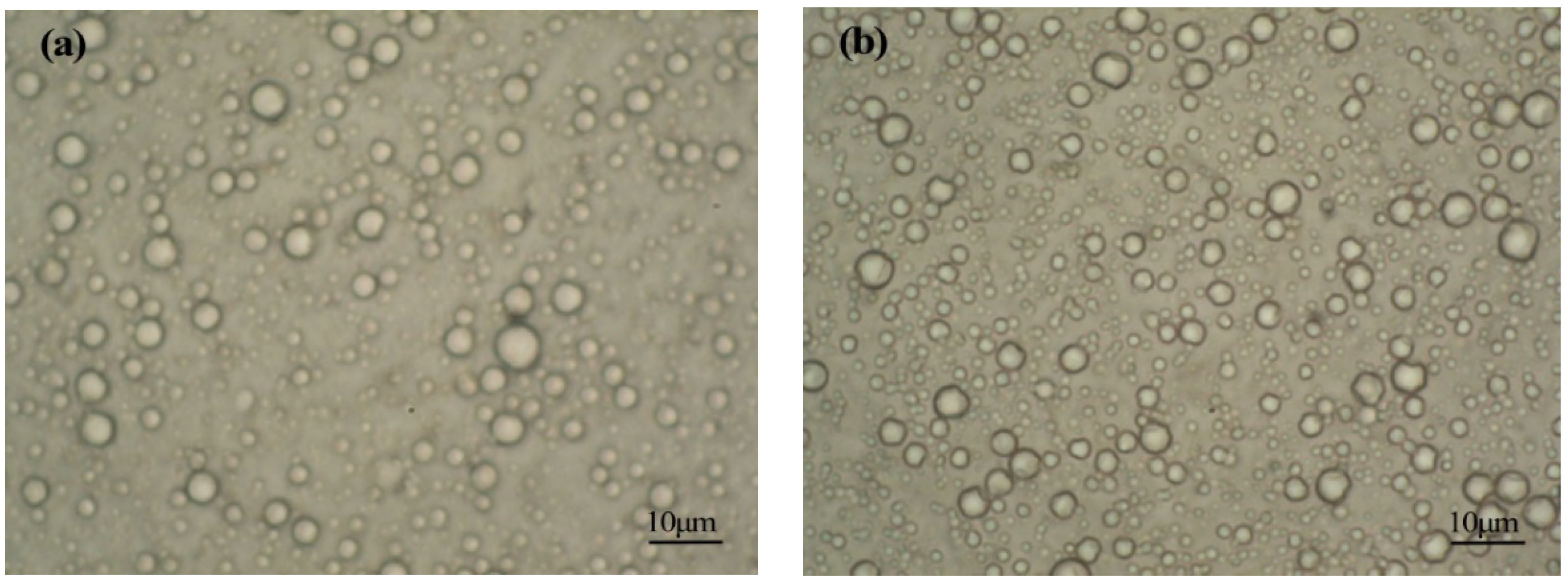
3.1. Analysis of Particle Size and the Distribution
3.2. Analysis of Core Content and Encapsulation Efficiency
3.3. Analysis of Thermal Stability and Compactness
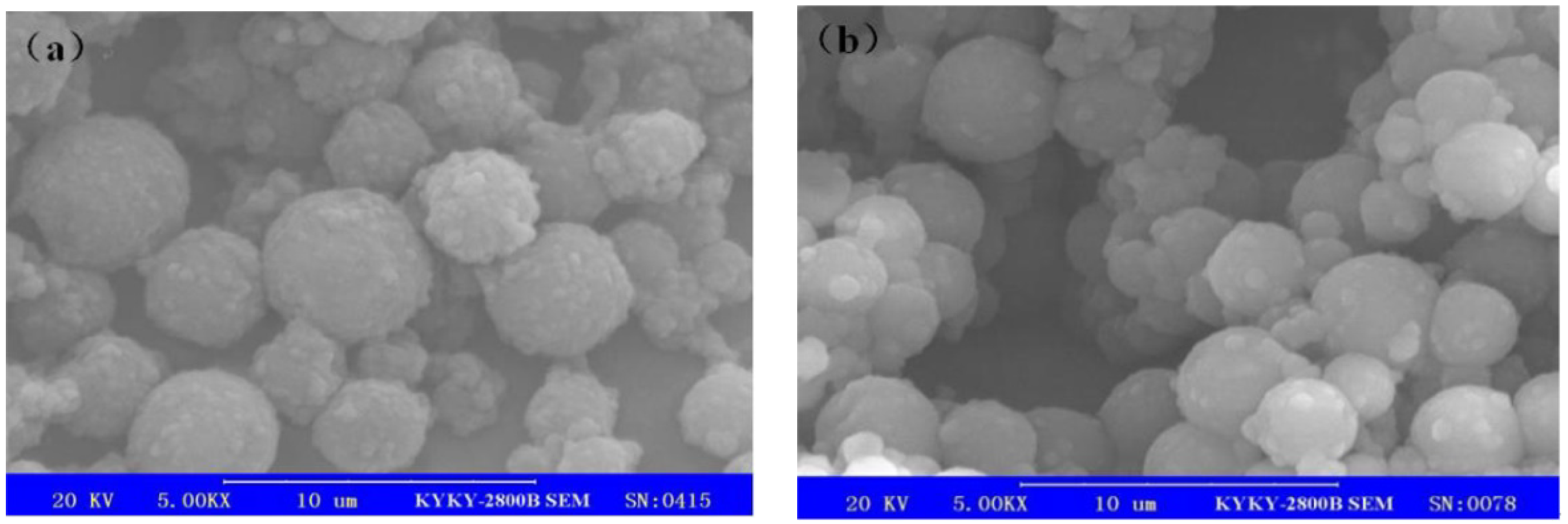
3.4. Analysis of Surface Morphology
3.5. Analysis of Thermal Storage Performance
3.6. Chemical Structure Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, P.M.; Qin, M.H.; Cui, S.Q.; Zu, K. Preparation and characterization of metal-organic framework/microencapsulated phase change material composites for indoor hygrothermal control. Build. Eng. 2020, 11, 101345. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Li, J.; Liu, H.L.; Chen, Z. Microencapsulated phase change materials composited Al2O3–SiO2 aerogel and the thermal regulation properties. Sol-Gel Sci. Technol. 2020, 96, 627–635. [Google Scholar] [CrossRef]
- Berk, K.; Kemal, C.; Halime, P. Preparation characterization and thermal properties of novel fire-resistant microencapsulated phase change materials based on paraffin and a polystyrene shell. RSC Adv. 2020, 10, 24134–24144. [Google Scholar]
- Esen, M. Thermal performance of a solar-aided latent heat store used for space heating by heat pump. Sol. Energy 2000, 69, 15–25. [Google Scholar] [CrossRef]
- Li, J.; Zhu, X.Y.; Wang, H.C.; Lin, P.C.; Jia, L.S.; Li, L.J. Synthesis and properties of multifunctional microencapsulated phase change material for intelligent textiles. Mater. Sci. 2020, 56, 2176–2191. [Google Scholar] [CrossRef]
- Ruiz, C.T.; Bonet, A.M.; Gisbert, P.J.; Bou, B.E. Analysis of the influence of graphene and phase change microcapsules on thermal behavior of cellulosic fabrics. Mater. Today Commun. 2020, 25, 101557. [Google Scholar] [CrossRef]
- Gu, M.Q.; Zhang, W.; Hao, S.; Liu, X.C.; Zhang, Z.C.; Shao, F.J. Ultraviolet light-initiated preparation of phase change material microcapsules and its infrared imaging effect on fabric. Pigment Resin Technol. 2020, 50, 129–135. [Google Scholar] [CrossRef]
- Su, W.G.; Jo, D.; Georgios, K. Numerical thermal evaluation of laminated binary microencapsulated phase change material drywall systems. Build. Simul. China 2020, 13, 89–98. [Google Scholar] [CrossRef]
- Gurbuz, E.; Erdem, S. Development and thermo-mechanical analysis of high-performance hybrid fibre engineered cementitious composites with microencapsulated phase change materials. Constr. Build. Mater. 2020, 263, 120139. [Google Scholar] [CrossRef]
- Wijesuriya, S.; Tabares, V.P.C. Experimental apparatus and methodology to test and quantify thermal performance of micro and macro-encapsulated phase change materials in building envelope applications. Energy Storage 2020, 32, 101770. [Google Scholar] [CrossRef]
- Dai, H.; Chen, W.; Cheng, Q.; Liu, Y.; Dong, X.B. Analysis of thermo-hydraulic characteristics in the porous-wall microchannel with microencapsulated phase change slurry. Int. J. Heat Mass Trans. 2021, 165, 120634. [Google Scholar] [CrossRef]
- Ran, F.G.; Chen, Y.K.; Cong, R.S.; Fang, G.Y. Flow and heat transfer characteristics of microencapsulated phase change slurry in thermal energy systems: A review. Renew. Sustain. Energy Rev. 2020, 134, 110101. [Google Scholar] [CrossRef]
- Gao, G.R.; Zhang, T.X.; Guo, C.L.; Jiao, S.K.; Rao, Z.H. Photo-thermal conversion and heat storage characteristics of multialled carbon nanotubes dispersed magnetic phase change microcapsules slurry. Int. J. Energy Res. 2020, 44, 6873–6884. [Google Scholar] [CrossRef]
- Li, W.Q.; Wan, H.; Jing, T.T.; Li, Y.B.; Liu, P.J.; He, G.Q. Microencapsulated phase change material (MEPCM) saturated in metal foam as an efficient hybrid PCM for passive thermal management: A numerical and experimental study. Appl. Therm. Eng. 2019, 146, 413–421. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Qin, Y.S.; Liang, S.E.; Chen, K.P.; Wei, C.S.; Tian, C.R. Nanoencapsulated phase change material with polydopamine-SiO2 hybrid shell for tough thermo-regulating rigid polyurethane foam. Thermochim. Acta 2019, 676, 104–114. [Google Scholar] [CrossRef]
- Liao, H.G.; Liu, Y.; Chen, R.; Wang, Q. Preparation and characterization of polyurethane foams containing microencapsulated phase change materials for thermal energy storage and thermal regulation. Polym. Int. 2020, 70, 619–627. [Google Scholar] [CrossRef]
- León, G.; Paret, N.; Fankhauser, P.; Grenno, D.; Erni, P.; Ouali, L. Formaldehyde-free melamine microcapsules as core/shell delivery systems for encapsulation of volatile active ingredients. RSC Adv. 2017, 7, 18962–18975. [Google Scholar] [CrossRef] [Green Version]
- Qiu, X.L.; Lu, L.X.; Tang, G.Y.; Song, G.L. Preparation and thermal properties of microencapsulated paraffin with polyurea/acrylic resin hybrid shells as phase change energy storage materials. Therm. Anal. Calorim. 2020, 143, 3023–3032. [Google Scholar] [CrossRef]
- Zhao, A.Q.; An, J.L.; Yang, J.L.; Yang, E.H. Microencapsulated phase change materials with composite titania-polyurea (TiO2-PUA) shell. Appl. Energy 2018, 215, 468–478. [Google Scholar] [CrossRef]
- Lu, S.F.; Shen, T.W.; Xing, J.W.; Song, Q.W.; Shao, J.F.; Zhang, J.; Xin, C. Preparation and Characterization of Cross-linked Polyurethane Shell Microencapsulated Phase Change Materials by Interfacial Polymerization. Mater. Lett. 2017, 211, 36–39. [Google Scholar] [CrossRef]
- Zhan, S.; Chen, S.; Chen, L.; Hou, W. Preparation and characterization of polyurea microencapsulated phase change material by interfacial polycondensation method. Powder Technol. 2016, 292, 217–222. [Google Scholar] [CrossRef]
- Bermudez, O.A.; Adam, C.I.; Aguado, H.A.; Landfester, K.; Muoz, E.R. Magnetic Polyurethane Microcarriers from Nanoparticle-Stabilized Emulsions for Thermal Energy Storage. ACS Sustain. Chem. Eng. 2020, 8, 17956–17966. [Google Scholar] [CrossRef]
- Shi, T.J.; Hu, P.; Wang, J.T. Preparation of Polyurea Microcapsules Containing Phase Change Materials Using Microfluidics. Chem. Select. 2020, 5, 2342–2347. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, W.; Wang, Y.N.; Shi, B. Preparation of polyurea microcapsules containing phase change materials in a rotating packed bed. RSC Adv. 2017, 7, 21196–21204. [Google Scholar] [CrossRef] [Green Version]
- Yin, Q.; Zhu, Z.; Li, W.; Guo, M.; Wang, Y.; Wang, J.; Zhang, X. Fabrication and performance of composite microencapsulated phase change materials with palmitic acid ethyl ester as core. Polymers 2018, 10, 726. [Google Scholar] [CrossRef] [Green Version]
- Hong, W.; Wang, R.; Li, N.; Gao, L.; Jiao, T. Facile Preparation and excellent ultraviolet shielding application of polyurethane-TiO2 composite microcapsules. Part. Part. Syst. Charact. 2020, 38, 2000265. [Google Scholar] [CrossRef]
- Lu, S.F.; Xing, J.W.; Zhang, Z.H.; Jia, G.P. Preparation and characterization of polyurea/polyurethane double-shell microcapsules containing butyl stearate through interfacial polymerization. Appl. Polym. Sci. 2011, 121, 3377–3383. [Google Scholar] [CrossRef]
- Lu, S.F.; Shen, T.W.; Xing, J.W.; Song, Q.W.; Xin, C. Preparation, characterization, and thermal stability of double-composition shell microencapsulated phase change material by interfacial polymerization. Colloid Polym. Sci. 2017, 295, 2061–2067. [Google Scholar] [CrossRef]
- Lu, S.F.; Xing, J.W.; Wu, Q. Preparation and characterization of high stability polyureaMicroPCMs using a two-step method of adding DETA by interfacial polymerization. Fiber Bioeng. Inform. 2013, 6, 185–194. [Google Scholar] [CrossRef]
- Zhang, X.X.; Fan, Y.F.; Wang, X.C.; Li, J.; Zhu, Q.B. Super-cooling prevention of microencapsulated phase change material. Thermochim. Acta 2004, 413, 1–6. [Google Scholar]
- Nan, G.H.; Wang, J.P.; Wang, Y.; Wang, H.; Wei, L.; Zhang, X.X. Preparation and properties of nanoencapsulated phase change materials containing polyaniline. Phys. Chem. 2014, 30, 338–344. [Google Scholar]





| Emu31lsification Speed/rpm | Tc | ΔHc/J·g−1 | |||
|---|---|---|---|---|---|
| Tcα/°C | Aα 1/% | Tcβ/°C | Aβ/% | ||
| 3000 | 21.3 | 71.2 | 17.5 | 28.7 | 80.2 |
| 4000 | 20.8 | 45.1 | 15.4 | 54.9 | 83.1 |
| 5000 | 20.7 | 44.7 | 14.1 | 55.3 | 83.8 |
| 6000 | 21.2 | 31.2 | 15.3 | 68.7 | 84.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, S.; Wang, Q.; Zhou, H.; Shi, W.; Zhang, Y.; Huang, Y. Preparation of High Thermo-Stability and Compactness Microencapsulated Phase Change Materials with Polyurea/Polyurethane/Polyamine Three-Composition Shells through Interfacial Polymerization. Materials 2022, 15, 2479. https://doi.org/10.3390/ma15072479
Lu S, Wang Q, Zhou H, Shi W, Zhang Y, Huang Y. Preparation of High Thermo-Stability and Compactness Microencapsulated Phase Change Materials with Polyurea/Polyurethane/Polyamine Three-Composition Shells through Interfacial Polymerization. Materials. 2022; 15(7):2479. https://doi.org/10.3390/ma15072479
Chicago/Turabian StyleLu, Shaofeng, Qiaoyi Wang, Hongjuan Zhou, Wenzhao Shi, Yongsheng Zhang, and Yayi Huang. 2022. "Preparation of High Thermo-Stability and Compactness Microencapsulated Phase Change Materials with Polyurea/Polyurethane/Polyamine Three-Composition Shells through Interfacial Polymerization" Materials 15, no. 7: 2479. https://doi.org/10.3390/ma15072479
APA StyleLu, S., Wang, Q., Zhou, H., Shi, W., Zhang, Y., & Huang, Y. (2022). Preparation of High Thermo-Stability and Compactness Microencapsulated Phase Change Materials with Polyurea/Polyurethane/Polyamine Three-Composition Shells through Interfacial Polymerization. Materials, 15(7), 2479. https://doi.org/10.3390/ma15072479





