A DFT-Based Descriptor to Predict the Water Vapor Corrosion Resistance of Rare-Earth Monosilicates
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
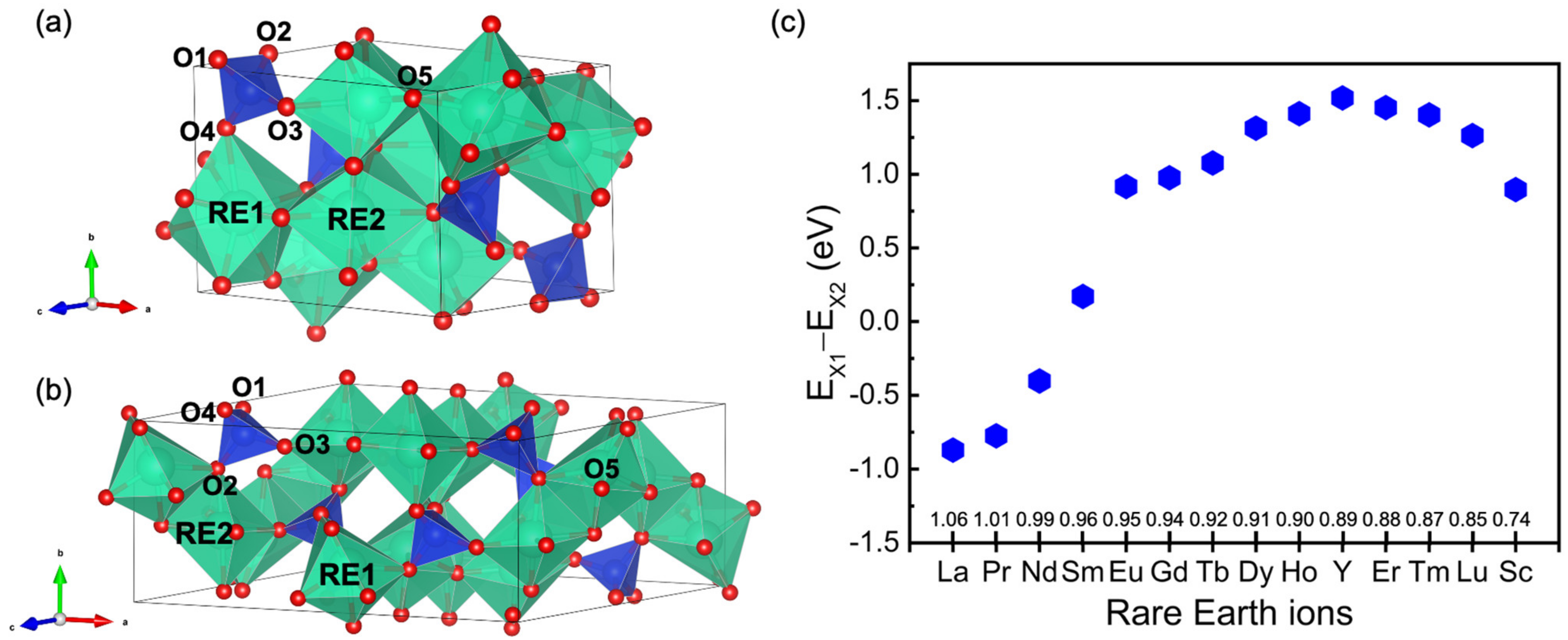
3.1. Crystal Structure of RE2SiO5
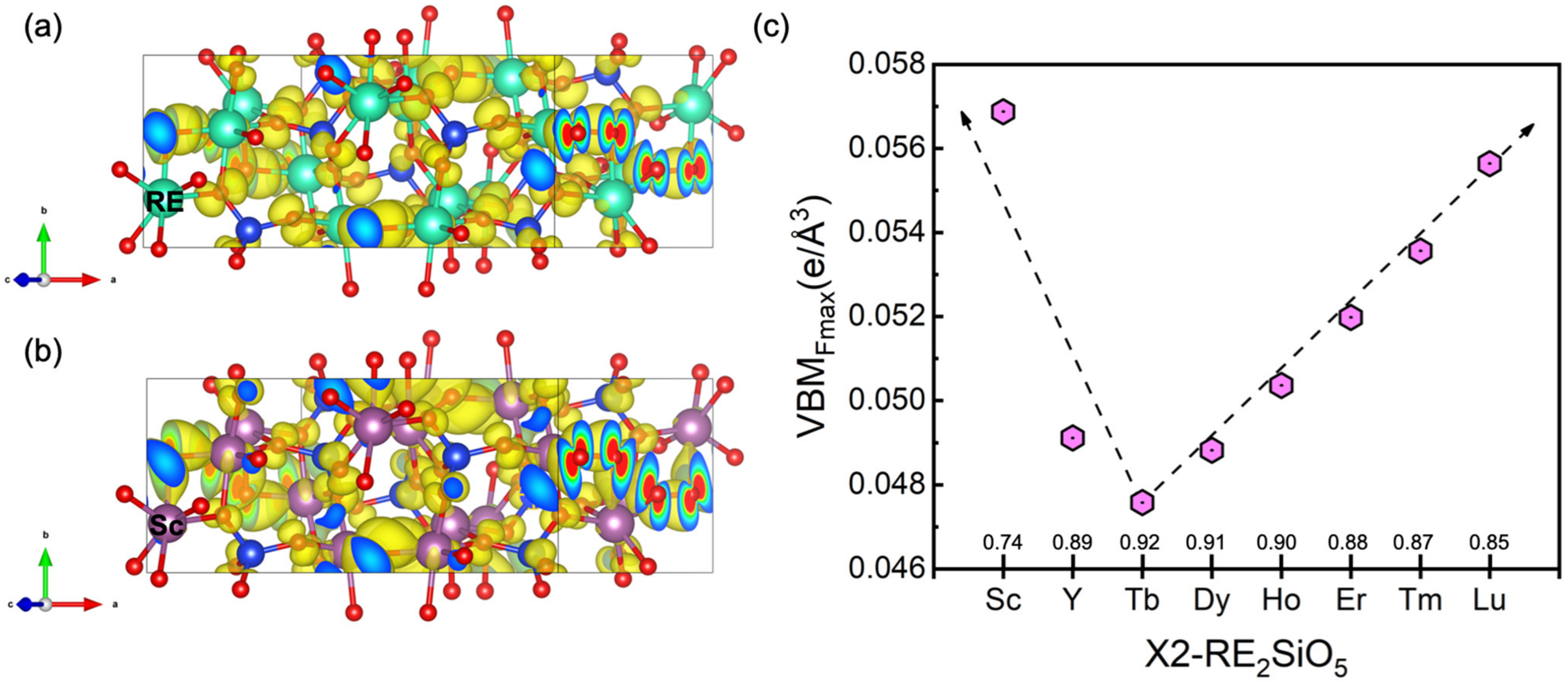
3.2. The Valence Band Maximum (VBM) of RE2SiO5
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, K.N.; Fox, D.S.; Bansal, N.P. Rare earth silicate environmental barrier coatings for SiC/SiC composites and Si3N4 ceramics. J. Eur. Ceram. Soc. 2005, 25, 1705–1715. [Google Scholar] [CrossRef]
- Tian, Z.L.; Wang, J.Y. Research Progress of Rare Earth Silicate Ceramics. J. Adv. Ceram. 2018, 39, 295–320. [Google Scholar]
- Wang, Y.; Niu, Y.; Zhong, X.; Shi, M.; Mao, F.; Zhang, L.; Li, Q.; Zheng, X. Water vapor corrosion behaviors of plasma sprayed RE2SiO5 (RE = Gd, Y, Er) coatings. Corros. Sci. 2020, 167, 108529. [Google Scholar] [CrossRef]
- Al Nasiri, N.; Patra, N.; Jayaseelan, D.D.; Lee, W.E. Water vapour corrosion of rare earth monosilicates for environmental barrier coating application. Ceram. Int. 2017, 43, 7393–7400. [Google Scholar] [CrossRef]
- Klemm, H. Silicon Nitride for High-Temperature Applications. J. Am. Ceram. Soc. 2010, 93, 1501–1522. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, J.; Sun, L.; Zheng, L.; Wang, J. Robust hydrophobicity and evaporation inertness of rare-earth monosilicates in hot steam at very high temperature. J. Am. Ceram. Soc. 2019, 102, 3076–3080. [Google Scholar] [CrossRef]
- Han, J.; Wang, Y.; Liu, R.; Jiang, D. Study on water vapor corrosion resistance of rare earth monosilicates RE2SiO5 (RE = Lu, Yb, Tm, Er, Ho, Dy, Y, and Sc) from first-principles calculations. Heliyon 2018, 4, e00857. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J. First-principles investigation on the corrosion resistance of rare earth disilicates in water vapor. J. Eur. Ceram. Soc. 2009, 29, 2163–2167. [Google Scholar] [CrossRef]
- Kresse, J.F.L.G. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Blochl, P.E.; Jepsen, O.; Andersen, O.K. Improved tetrahedron method for Brillouin-zone integrations. Phys. Rev. B 1994, 49, 16223–16233. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Wang, J.G.; Tian, S.J.; Li, G.B.; Liao, F.H.; Jing, X.P. Preparation and X-ray characterization of low-temperature phases of R2SiO5 (R = rare earth elements). Mater. Res. Bull. 2001, 36, 1855–1861. [Google Scholar] [CrossRef]
- Fukuda, K.; Iwata, T.; Champion, E. Crystal structure of lanthanum oxyorthosilicate, La2SiO5. Powder Diffr. 2006, 21, 300–303. [Google Scholar] [CrossRef][Green Version]
- Felsche, J. The crystal chemistry of the rare-earth silicates. Struct. Bond. 1973, 13, 99–197. [Google Scholar]
- León-Reina, L.; Porras-Vázquez, J.M.; Losilla, E.R.; Moreno-Real, L.; Aranda, M.A.G. Structure and oxide anion conductivity in Ln2(TO4)O (Ln=La, Nd; T=Ge, Si). J Solid State Chem. 2008, 181, 2501–2506. [Google Scholar] [CrossRef]
- Smolin, Y.I.; Tkachev, S.P. Determination of the structure of gadolinium oxyorthosilicate (Gd2O3)(SiO2). Kristallografiya 1969, 14, 22. [Google Scholar]
- Phanon, D.; Černý, R. Crystal Structure of the B-type Dierbium Oxideortho-Oxosilicate Er2O[SiO4]. Z. Anorg. Und Allg. Chem. 2008, 634, 1833–1835. [Google Scholar] [CrossRef]
- Gustafsson, T.; Kliintenberg, M.; Derenzo, S.E.; Weber, M.J.; Thomas, J.O. Structure of Lu2 Si O5 using single-crystal X-ray and neutron diffraction. Acta Crystallogr. C 2001, 57, 668–669. [Google Scholar] [CrossRef] [PubMed]
- DeKock, R.L.; Barbachyn, M.R. Proton Affinity, Ionization Energy, and the Nature of Frontier Orbital Electron Density. J. Am. Chem. Soc. 1979, 101, 6516–6519. [Google Scholar] [CrossRef]
- Huang, J.; Wang, B.; Yu, Y.; Valenzano, L.; Bauchy, M.; Sant, G. Electronic Origin of Doping-Induced Enhancements of Reactivity: Case Study of Tricalcium Silicate. J. Phys. Chem. C 2015, 119, 25991–25999. [Google Scholar] [CrossRef]


| Method | a (Å) | b (Å) | c (Å) | β (°) | Volume (Å3) | |
|---|---|---|---|---|---|---|
| La2SiO5 | Expt. [16] Calc. | 9.3320 9.3564 | 7.5088 7.7155 | 7.0332 7.0168 | 108.6790 109.0060 | 466.8700 478.9250 |
| Pr2SiO5 | Expt. [17] Calc. | 9.2530 9.3420 | 7.3010 7.5586 | 6.9340 6.9548 | 108.1500 108.7720 | 445.1000 464.9780 |
| Nd2SiO5 | Expt. [18] Calc. | 9.2295 9.3039 | 7.2848 7.4606 | 6.8744 6.9006 | 108.1990 108.5470 | 439.0800 454.1070 |
| Sm2SiO5 | Expt. [17] Calc. | 9.1610 9.2362 | 7.1120 7.2989 | 6.8210 6.8096 | 107.5100 108.1510 | 424.4000 436.2150 |
| Eu2SiO5 | Expt. [17] Calc. | 9.1420 9.1706 | 7.0540 7.2362 | 6.7900 6.7107 | 107.5300 107.7030 | 417.9000 424.2390 |
| Gd2SiO5 | Expt. [19] Calc. | 9.1200 9.1758 | 7.0600 7.0566 | 6.7300 6.7732 | 107.5800 107.2030 | 413.0900 419.0640 |
| Tb2SiO5 | Expt. Calc. | 14.3660 14.5834 | 6.6976 6.8319 | 10.3633 10.5585 | 122.2190 122.1380 | 843.5900 890.7910 |
| Dy2SiO5 | Expt. [17] Calc. | 14.3800 14.5296 | 6.7400 6.8106 | 10.4200 10.5085 | 122.0000 122.1140 | 856.5000 880.7640 |
| Ho2SiO5 | Expt. [17] Calc. | 14.3500 14.4802 | 6.7100 6.7785 | 10.3700 10.4563 | 122.2000 122.0950 | 843.0000 869.4740 |
| Er2SiO5 | Expt. [20] Calc. | 14.3660 14.4344 | 6.6976 6.7503 | 10.3633 10.4101 | 122.2190 122.1120 | 843.5900 859.1420 |
| Tm2SiO5 | Expt. [17] Calc. | 14.3020 14.3815 | 6.6620 6.7197 | 10.3130 10.3633 | 122.2100 122.0910 | 828.5000 848.4790 |
| Lu2SiO5 | Expt. [21] Calc. | 14.2774 14.2753 | 6.6398 6.6687 | 10.2465 10.2827 | 122.2240 121.9780 | 821.7400 830.3420 |
| Y2SiO5 | Expt. Calc. | 14.5643 14.5111 | 6.8354 6.8113 | 10.5570 10.5122 | 122.1320 122.0870 | 889.9930 880.3030 |
| Sc2SiO5 | Expt. Calc. | 13.8636 13.7566 | 6.4838 6.4896 | 9.9120 10.0833 | 121.5360 120.8350 | 759.3900 772.9450 |
| La2SiO5 | Pr2SiO5 | Nd2SiO5 | Sm2SiO5 | Eu2SiO5 | Gd2SiO5 | |
|---|---|---|---|---|---|---|
| VBMFmax(e/Å3) | 0.098 | 0.099 | 0.097 | 0.094 | 0.060 | 0.053 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Huang, J. A DFT-Based Descriptor to Predict the Water Vapor Corrosion Resistance of Rare-Earth Monosilicates. Materials 2022, 15, 2414. https://doi.org/10.3390/ma15072414
Wang Q, Huang J. A DFT-Based Descriptor to Predict the Water Vapor Corrosion Resistance of Rare-Earth Monosilicates. Materials. 2022; 15(7):2414. https://doi.org/10.3390/ma15072414
Chicago/Turabian StyleWang, Qianqian, and Jian Huang. 2022. "A DFT-Based Descriptor to Predict the Water Vapor Corrosion Resistance of Rare-Earth Monosilicates" Materials 15, no. 7: 2414. https://doi.org/10.3390/ma15072414
APA StyleWang, Q., & Huang, J. (2022). A DFT-Based Descriptor to Predict the Water Vapor Corrosion Resistance of Rare-Earth Monosilicates. Materials, 15(7), 2414. https://doi.org/10.3390/ma15072414





