Temperature Changes during Implant Osteotomy Preparations in Fresh Human Cadaver Tibiae, Comparing Straight with Tapered Drills †
Abstract
:1. Introduction
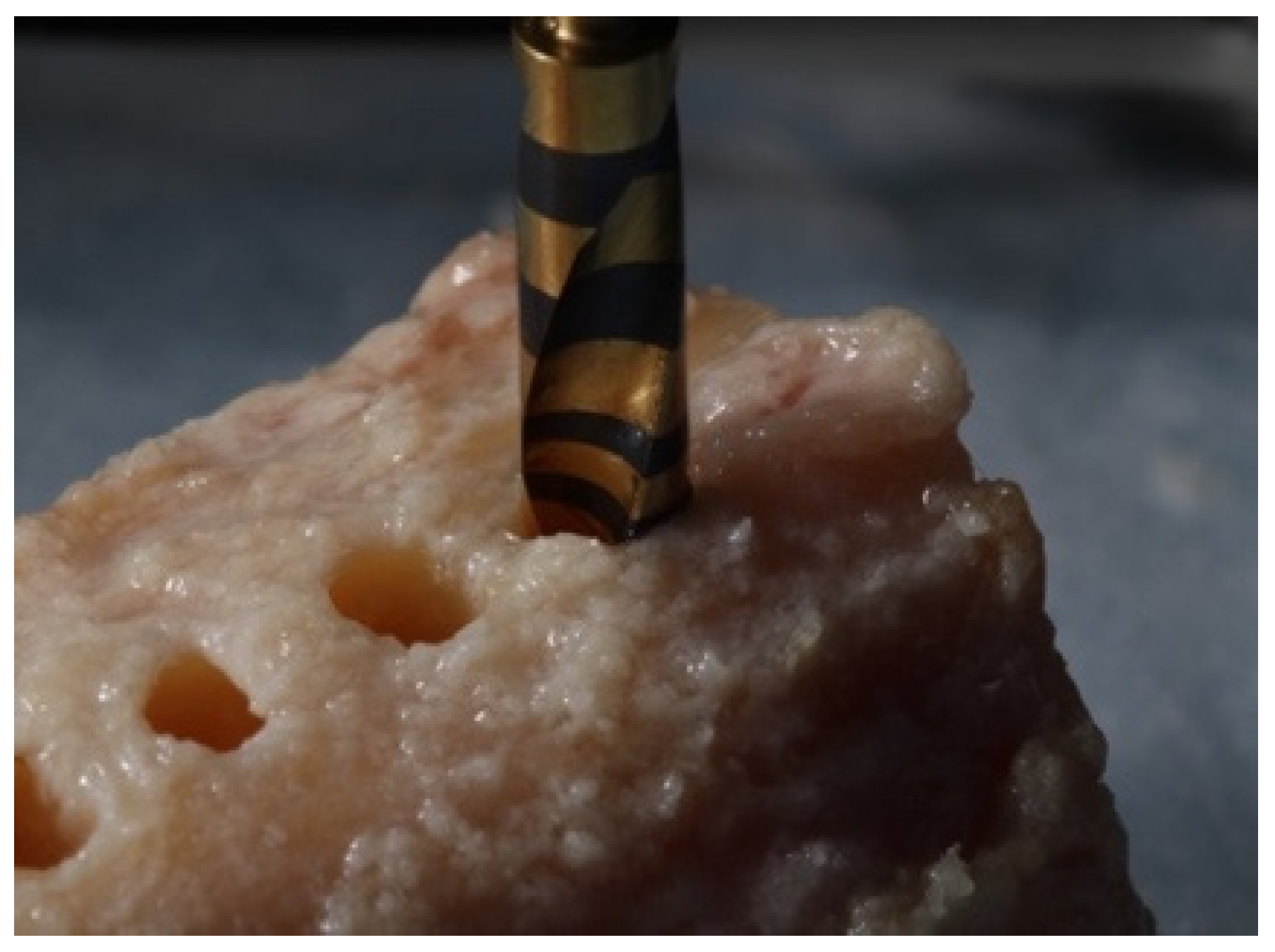
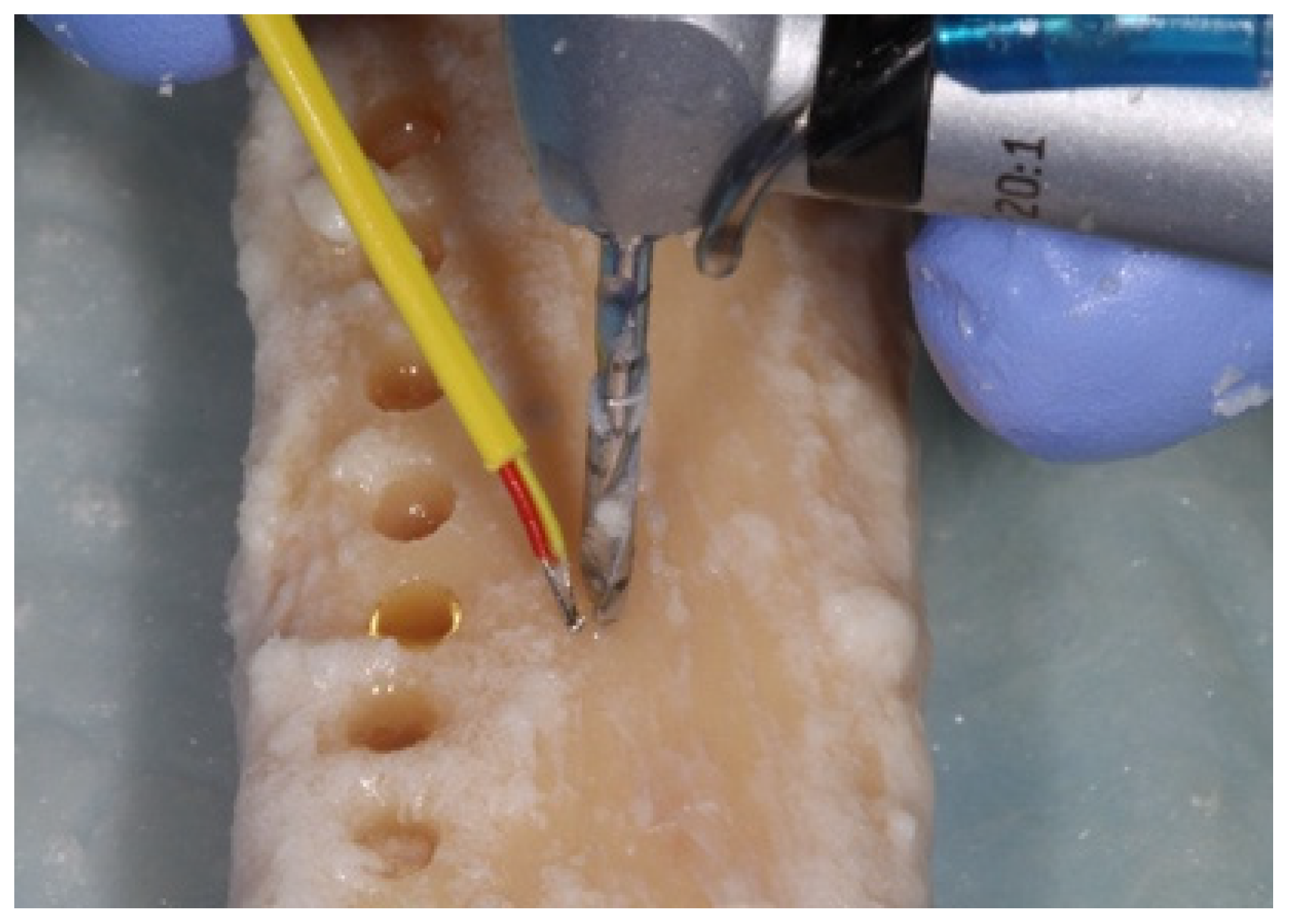
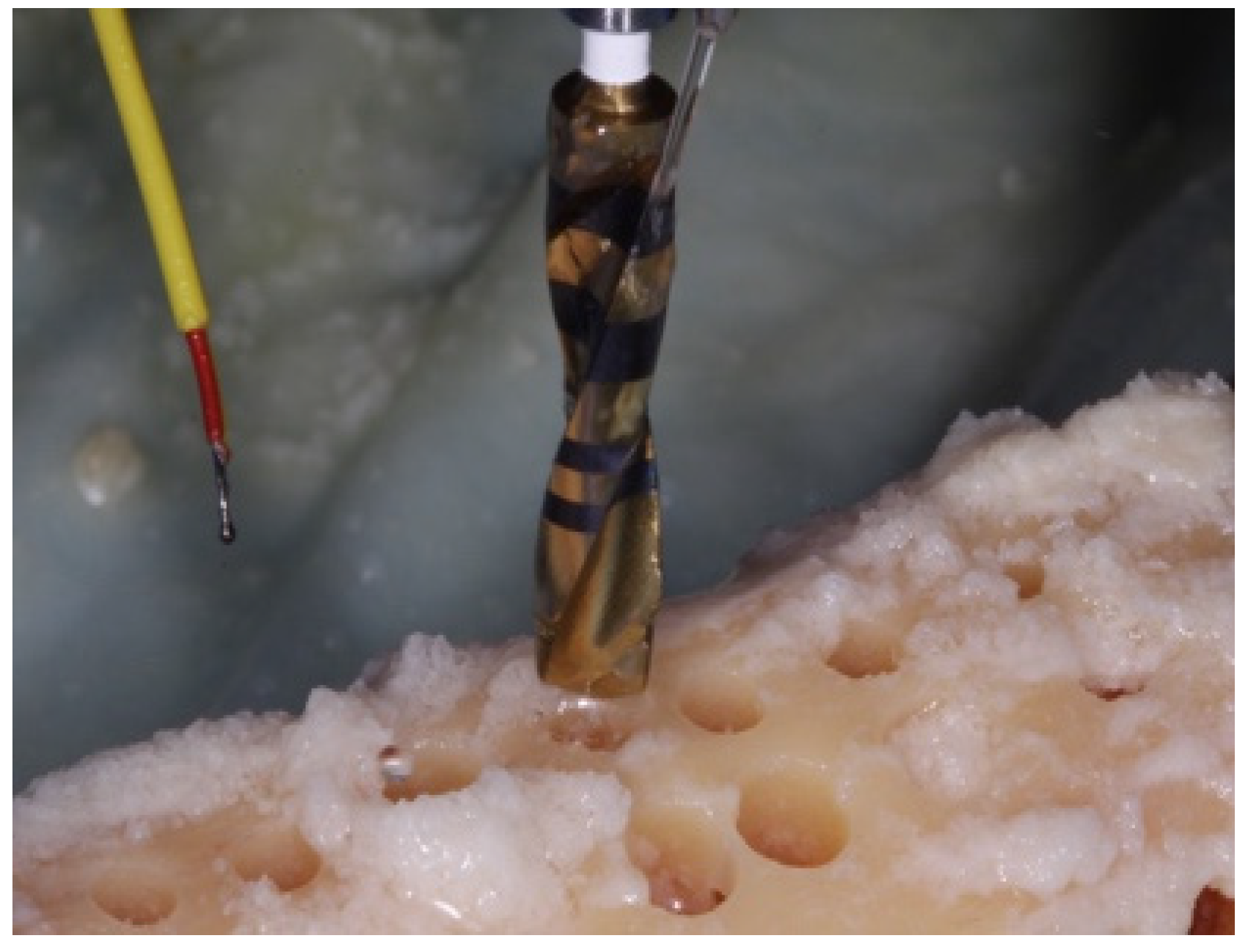
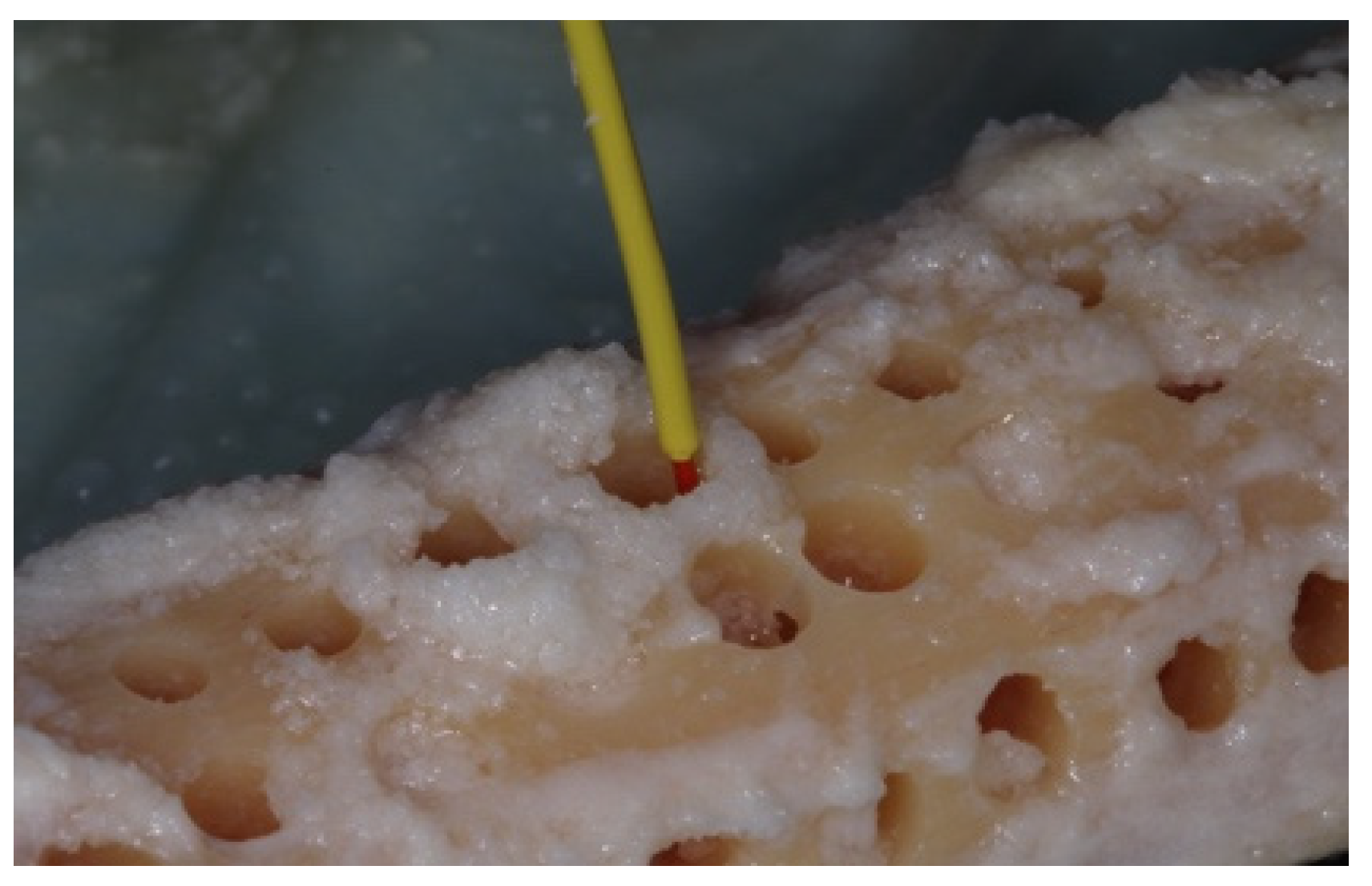
2. Materials and Methods
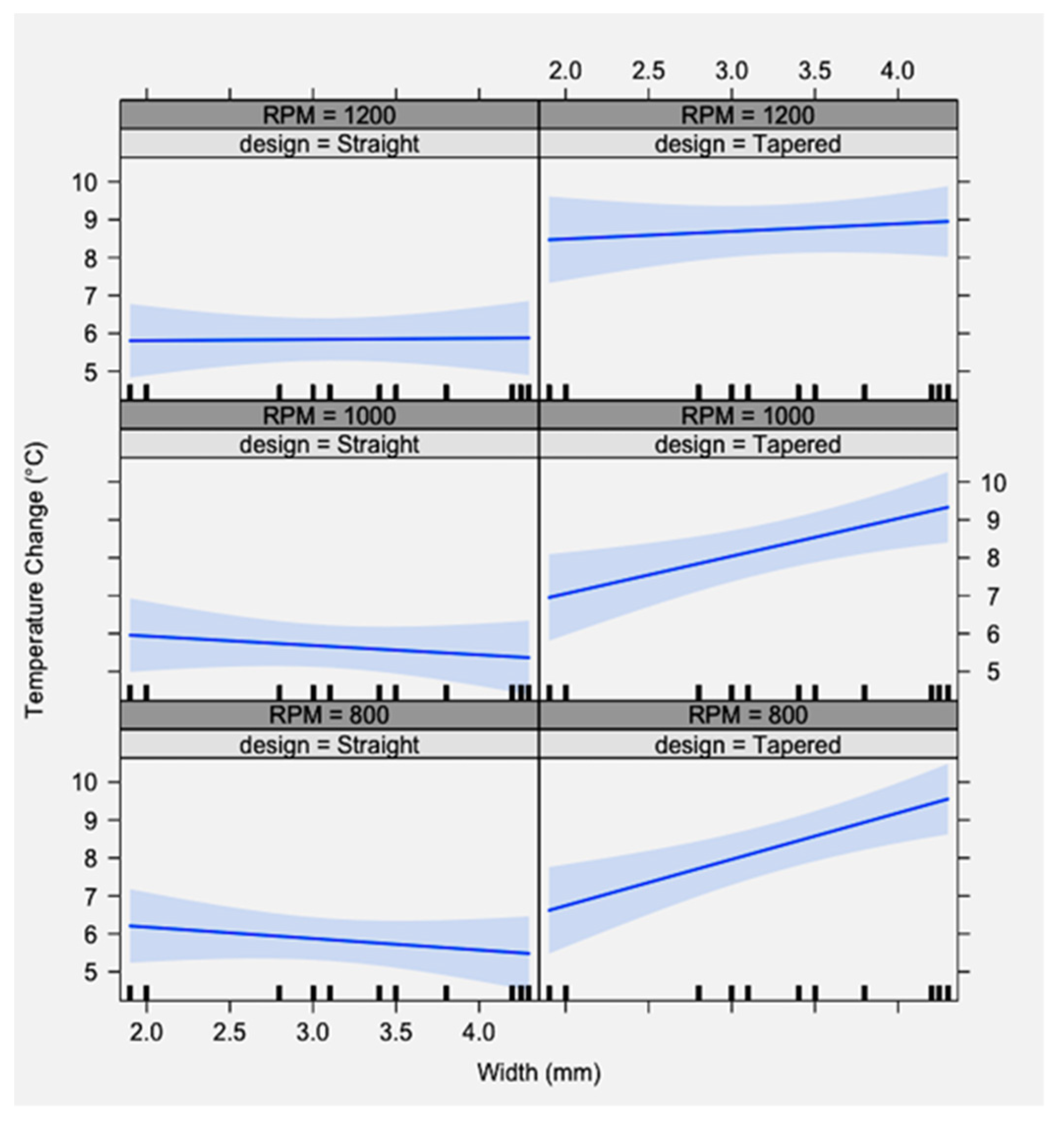
3. Results
4. Discussion
5. Conclusions
- The drill diameter and design had a significant effect on changes in bone temperature in a fresh human cadaver tibial model, which never exceeded the critical threshold of 47 °C.
- The drill speed did not play a significant role in altering temperature.
- The tapered drills caused significantly greater heat production compared to straight drills.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoshida, K.; Uoshima, K.; Oda, K.; Maeda, T. Influence of heat stress to matrix on bone formation. Clin. Oral Implant. Res. 2009, 20, 782–790. [Google Scholar] [CrossRef]
- Salonen, M.; Oikarinen, K.; Virtanen, K.; Pernu, H. Failures in the osseointegration of endosseous implants. Int. J. Oral Maxillofac. Implant. 1993, 8, 92–97. [Google Scholar] [CrossRef]
- Granström, G. Osseointegration in Irradiated Cancer Patients: An Analysis with Respect to Implant Failures. J. Oral Maxillofac. Surg. 2005, 63, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Cionca, N. Systemic diseases affecting osseointegration therapy. Clin. Oral Implant. Res. 2006, 17, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Isidor, F. Loss of osseointegration caused by occlusal load of Oral implants. Clin. Oral Implant. Res. 1996, 7, 143–152. [Google Scholar] [CrossRef]
- Esposito, M.; Hirsch, J.-M.; Lekholm, U. Thomsen P: Biological factors contributing to failures of osseointegrated Oral implants. (I) Success criteria and epidemiology. Eur. J. Oral Sci. 1998, 106, 527–551. [Google Scholar] [CrossRef]
- Jividen, G., Jr.; Misch, C. Reverse torque testing and early loading failures: Help or hindrance? J. Oral Implantol. 2000, 16, 82–90. [Google Scholar] [CrossRef]
- Eriksson, A.R.; Albrektsson, T.; Grane, B.; McQueen, D. Thermal injury to bone: A vital microscopic description of heat effects. Int. J. Oral Surg. 1982, 11, 115–121. [Google Scholar] [CrossRef]
- Oh, H.J.; Wikesjö, U.M.E.; Kang, H.-S.; Ku, Y.; Eom, T.-G.; Koo, K.-T. Effect of implant drill characteristics on heat generation in osteotomy sites: A pilot study. Clin. Oral Implant. Res. 2011, 22, 722–726. [Google Scholar] [CrossRef]
- Misir, A.F.; Sumer, M.; Yenisey, M.; Egioglu, E. Effect of Surgical Drill Guide on Heat Generated from Implant Drilling. J. Oral Maxillofac. Surg. 2009, 67, 2663–2668. [Google Scholar] [CrossRef]
- Tehemar, S.H. Factors affecting heat generation during implant site preparation: A review of biologic observations and future considerations. J. Oral Maxillofac. Implant. 1999, 14, 127–136. [Google Scholar]
- Akhabar, M.F.A.; Sulong, A.W. Surgical drill bit design and thermomechanical damage in bone drilling: A review. Ann. Biomed. Eng. 2021, 49, 29–56. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Eriksson, A. Thermally induced bone necrosis in rabbits: Relation to implant failure in humans. Clin. Orth. Rel. Res. 1985, 195, 311–312. [Google Scholar] [CrossRef]
- Wantable, F.; Tawada, Y.; Komatsu, S.; Hata, Y. Heat distribution in bone during preparation of implant sites: Heat analysis by real-time thermography. Int. J. Oral Maxillofac. Implant. 1992, 7, 212–219. [Google Scholar]
- Eriksson, A.R.; Albrektsson, T. Assessment of bone viability after heat trauma. Scand. J. Plast. Reconstr. Surg. 1984, 18, 261–268. [Google Scholar] [PubMed]
- Bashutski, J.D.; D’Silva, N.J.; Wang, H.-L. Implant Compression Necrosis: Current Understanding and Case Report. J. Periodontol. 2009, 80, 700–704. [Google Scholar] [CrossRef] [Green Version]
- Jochum, R.M.; Reichart, P.A. Influence of multiple use of Timedur-titanium cannon drills: Thermal Response and scanning electron microscopic findings. Clin. Oral Implant. Res. 2000, 11, 139–144. [Google Scholar] [CrossRef]
- Strbac, G.D.; Unger, E.; Donner, R.; Bijak, M.; Watzek, G.; Zechner, W. Thermal effects of a combined irrigation method during implant site drilling. A standardized in vitro study using a bovine rib model. Clin. Oral Implant. Res. 2014, 25, 665–674. [Google Scholar] [CrossRef]
- Calvo-Guirado, J.L.; Delgado-Peña, J.; Maté-Sánchez, J.E.; Mareque-Bueno, J.; Delgado-Ruiz, R.A.; Romanos, G.E. Novel hybrid drilling protocol. Evaluation for the implant healing. Thermal changes, crestal bone loss, and bone-to-implant contact. Clin. Oral Implant. Res. 2015, 26, 753–760. [Google Scholar] [CrossRef]
- Eriksson, A.R.; Albrektsson, T. The effect of heat on bone regeneration: An experimental study in the rabbit using the bone growth chamber. J. Oral Maxillofac. Surg. 1984, 42, 705–711. [Google Scholar] [CrossRef]
- Eriksson, A.R.; Adell, R. Temperatures during drilling for the placement of implants using the osseointegration technique. J. Oral Maxillofac. Surg. 1986, 44, 4–7. [Google Scholar] [CrossRef]
- Matthews, L.S.; Hirsch, C. Temperatures Measured in Human Cortical Bone when Drilling. J. Bone Jt. Surg. 1972, 54, 297–308. [Google Scholar] [CrossRef]
- Moss, R.W. Histopathologic reaction of bone to surgical cutting. J. Oral Surg. 1964, 17, 405–414. [Google Scholar] [CrossRef]
- Lavelle, C.; Wedgwood, D. Effect of Internal irrigation on frictional heat generated from bone drilling. J. Oral Surg. 1980, 38, 499–503. [Google Scholar] [PubMed]
- Krause, W.R.; Bradbury, D.W.; Kelly, J.E.; Lunceford, E.M. Temperature elevations in orthopedic cutting operations. J. Biomech. 1982, 15, 267–275. [Google Scholar] [CrossRef]
- Augustin, G.; Davila, S.; Mihoci, K.; Udiljak, T.; Vedrina, D.S.; Antabak, A. Thermal osteonecrosis and bone drilling parameters revisited. Arch. Orthop. Trauma Surg. 2007, 128, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, A.R.; Albrektsson, T.; Albrektsson, B. Heat caused by drilling cortical bone: Temperature measured in vivo in patients and animals. Acta Orthop. Scand. 1984, 55, 629.e6. [Google Scholar] [CrossRef]
- Strbac, G.D.; Giannis, K.; Unger, E.; Mittlböck, M.; Vasak, C.; Watzek, G.; Zechner, W. Drilling- and withdrawing-Related Thermal Changes during Implant Site Osteotomies. Clin. Implant Dent. Relat. Res. 2015, 17, 161–164. [Google Scholar] [CrossRef]
- Cordioli, G.; Majzoub, Z. Heat generation during implant site preparation: An in vitro study. Int. J. Oral Maxillofac. Implant. 1997, 12, 186–193. [Google Scholar]
- Natali, C.; Ingle, P.; Dowell, J. Orthopedic bone drills-can they be improved? Temperature changes near the drilling face. J. Bone Jt. Surg. 1996, 78, 357–362. [Google Scholar] [CrossRef]
- Stelzle, F.; Frenkel, C.; Riemann, M.; Knipfer, C.; Stockmann, P.; Nkenke, E. The effect of load on heat production, thermal effects and expenditure of time during implant site preparation-an experimental ex-vivo comparison between piezo surgery and conventional drilling. Clin. Oral Implant. Res. 2014, 25, e140–e148. [Google Scholar] [CrossRef] [PubMed]
- Soldatos, N.; Gozalo, D.; Font, K.; Moreno, D.; Powell, C. Temperature changes during implant osteotomies utilizing three different implant systems: A pilot study. J. Imp. Adv. Clin. Dent. 2016, 8, 34–43. [Google Scholar]
- Brisman, D.L. The effect of speed, pressure and time on bone temperature during the drilling of implant sites. Int. J. Oral Maxillofac. Implant. 1996, 11, 35–37. [Google Scholar]
- Sharawy, M.; Misch, C.E.; Weller, N.; Tehemar, S. Heat generation during implant drilling: The significance of motor speed. J. Oral Maxillofac. Surg. 2002, 60, 1160–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyer, S.; Weiss, C.; Mehta, A. Effects of drill speed on heat production and the rate and quality of bone formation in dental implant osteotomies. Part II: Relationship between drill speed and healing. Int. J. Prosthodont. 1997, 10, 536. [Google Scholar] [PubMed]
- Kim, S.-J.; Yoo, J.; Kim, Y.-S.; Shin, S.-W. Temperature change in pig rib bone during implant site preparation by low-speed drilling. J. Appl. Oral Sci. 2010, 18, 522–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romanos, G.E.; Bastardi, D.J.; Kakar, A.; Moore, R.; Delgado-Ruiz, R.A.; Javed, F. In vitro comparison of Resonance frequency analysis devices to evaluate implant stability of narrow diameter implants at varying drilling speeds in dense artificial bone blocks. Clin. Implant Dent. Relat. Res. 2019, 21, 1023–1027. [Google Scholar] [CrossRef]
- Abouzgia, M.B.; James, D.F. Temperature rise during drilling through bone. Int. J. Oral Maxillofac. Implant. 1997, 12, 342. [Google Scholar]
- Eriksson, A.R.; Albrektsson, T. Temperature threshold levels for heat-induced bone tissue injury: A vital-microscopic study in the rabbit. J. Prosthet. Dent. 1983, 50, 101–107. [Google Scholar] [CrossRef]
- Lundskog, J. Heat and bone tissue. An experimental investigation of the thermal properties of bone and threshold levels for thermal injury. Scand. J. Plast. Reconstr. Surg. 1972, 9, 72–74. [Google Scholar]
- Oliveira, N.; Alaejos-Algarra, F.; Mareque-Bueno, J.; Ferrés-Padró, E.; Hernández-Alfaro, F. Thermal changes and drill wear in bovine bone during implant site preparation. A comparative in vitro study: Twisted stainless steel and ceramic drills. Clin. Oral Implant. Res. 2013, 23, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.I.; Richards, R.G.; Milz, S.; Schneider, E.; Pearce, S.G. Animal models for implant biomaterial Research in bone: A review. Eur. Cell Mater. 2007, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Misch, C.; Qu, Z.; Bidez, W. Mechanical properties of trabecular bone in the human mandible: Implications for dental implant treatment planning and surgical placement. J. Oral Maxillofac. Surg. 1999, 57, 700–706. [Google Scholar] [CrossRef]
- Allsobrook, O.F.L.; Leichter, J.; Holborow, D.; Swain, M. Descriptive Study of the Longevity of Dental Implant Surgery Drills. Clin. Implant. Dent. Relat. Res. 2011, 13, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Chacon, G.E.; Bower, D.L.; Larsen, P.E.; McGlumphy, E.A.; Beck, F.M. Heat Production by 3 Implant Drill Systems after Repeated Drilling and Sterilization. J. Oral Maxillofac. Surg. 2006, 64, 265–269. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R. Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 5 January 2019).
- Scarano, A.; Piattelli, A.; Assenza, B.; Carinci, F.; Di Donato, L.; Romani, G.L.; Merla, A. Infrared Thermographic Evaluation of Temperature Modifications Induced during Implant Site Preparation with Cylindrical versus Conical Drills. Clin. Implant. Dent. Relat. Res. 2011, 13, 319–323. [Google Scholar] [CrossRef]
- Dekker, H.; Schulten, A.J.M.; Christiaan, M.; Bloemena, E.; van Ruijven, L.J.; Bravenboer, N. Regional differences in microarchitecture and mineralization of the atrophic edentulous mandible: A microcomputed tomography study. Arch. Oral Biol. 2022, 133, 105302. [Google Scholar] [CrossRef]
- Wang, S.-H.; Shen, Y.-W.; Fuh, L.-J.; Peng, S.-L.; Tsai, M.-T.; Huang, H.-L.; Hsu, J.-T. Relationship between Cortical Bone Thickness and Cancellous Bone Density at Dental Implant Sites in the Jawbone. Diagnostics 2020, 10, 710. [Google Scholar] [CrossRef]
- Mense, C.; Saliba-Serre, B.; Ferrandez, A.-M.; Hüe, O.; Ruquet, M.; Lalys, L. Cone beam computed tomography analysis of the edentulous mandibular symphysis. J. Dent. Sci. 2021, 16, 115–122. [Google Scholar] [CrossRef]
- Katranji, A.; Misch, K.; Wang, H.-L. Cortical Bone Thickness in Dentate and Edentulous Human Cadavers. J. Periodontol. 2007, 78, 874–878. [Google Scholar] [CrossRef]
- Gehrke, S.A.; Treichel, T.L.E.; Junior, J.A.; de Aza, P.N.; Prados-Frutos, J.C. Effects of the technique and drill design used durimg the osteotomy on the thermal and histological stimulation. Sci. Rep. 2020, 10, 20737. [Google Scholar] [CrossRef] [PubMed]
- Marzook, H.A.M.; Yousef, E.A.; Denewar, M.; Farahat, M.R.L. Response to the Letter to the Editor: Minimize damage of heat generated during drilling procedure. In-vitro Assessment of Bone viability with different Implant Drill Speeds. Br. J. Oral Maxillofac. Surg. 2020, 58, 301–306. [Google Scholar] [CrossRef] [PubMed]




| Implant Designs | Drilling Protocol | ||||
|---|---|---|---|---|---|
| ASTRA Tech® straight (York, PA, USA) | 1.9 mm | 2.5/3.1 mm | 3.7/4.3 mm | ||
| Nobel Biocare® tapered (Klote, Switzerland) | 2.0 mm | 3.5 mm | 4.3 mm | ||
| Sweden & Martina Implantology® straight (Premium) (Due Carrare, Italy) | 2.0 mm | 2.8 mm | 3.0 mm | 3.4 mm | 4.25 mm |
| Sweden & Martina Implantology® tapered (Shelta) (Due Carrare, Italy) | 2.0 mm | 3.8 mm | 4.2 mm | ||
| Source | SS | df | F | p Value |
|---|---|---|---|---|
| Design | 28.4 | 1 | 98.9 | <0.001 * |
| RPM | 0.2 | 2 | 0.4 | <0.68 |
| Width | 1.2 | 1 | 4.3 | <0.04 * |
| Design/RPM | 0.1 | 2 | 0.2 | <0.79 |
| Design/width | 2.2 | 1 | 7.5 | <0.01 * |
| RPM/width | 0.3 | 2 | 0.6 | <0.55 |
| Design/RPM/width | 0.9 | 2 | 1.7 | <0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soldatos, N.; Nelson-Rabe, L.; Palanker, N.; Angelov, N.; Romanos, G.; Weltman, R. Temperature Changes during Implant Osteotomy Preparations in Fresh Human Cadaver Tibiae, Comparing Straight with Tapered Drills. Materials 2022, 15, 2369. https://doi.org/10.3390/ma15072369
Soldatos N, Nelson-Rabe L, Palanker N, Angelov N, Romanos G, Weltman R. Temperature Changes during Implant Osteotomy Preparations in Fresh Human Cadaver Tibiae, Comparing Straight with Tapered Drills. Materials. 2022; 15(7):2369. https://doi.org/10.3390/ma15072369
Chicago/Turabian StyleSoldatos, Nikolaos, Laura Nelson-Rabe, Nathan Palanker, Nikola Angelov, Georgios Romanos, and Robin Weltman. 2022. "Temperature Changes during Implant Osteotomy Preparations in Fresh Human Cadaver Tibiae, Comparing Straight with Tapered Drills" Materials 15, no. 7: 2369. https://doi.org/10.3390/ma15072369
APA StyleSoldatos, N., Nelson-Rabe, L., Palanker, N., Angelov, N., Romanos, G., & Weltman, R. (2022). Temperature Changes during Implant Osteotomy Preparations in Fresh Human Cadaver Tibiae, Comparing Straight with Tapered Drills. Materials, 15(7), 2369. https://doi.org/10.3390/ma15072369








