Molecular Genetics and Cytotoxic Responses to Titanium Diboride and Zinc Borate Nanoparticles on Cultured Human Primary Alveolar Epithelial Cells
Abstract
:1. Introduction
2. Materials and Methods
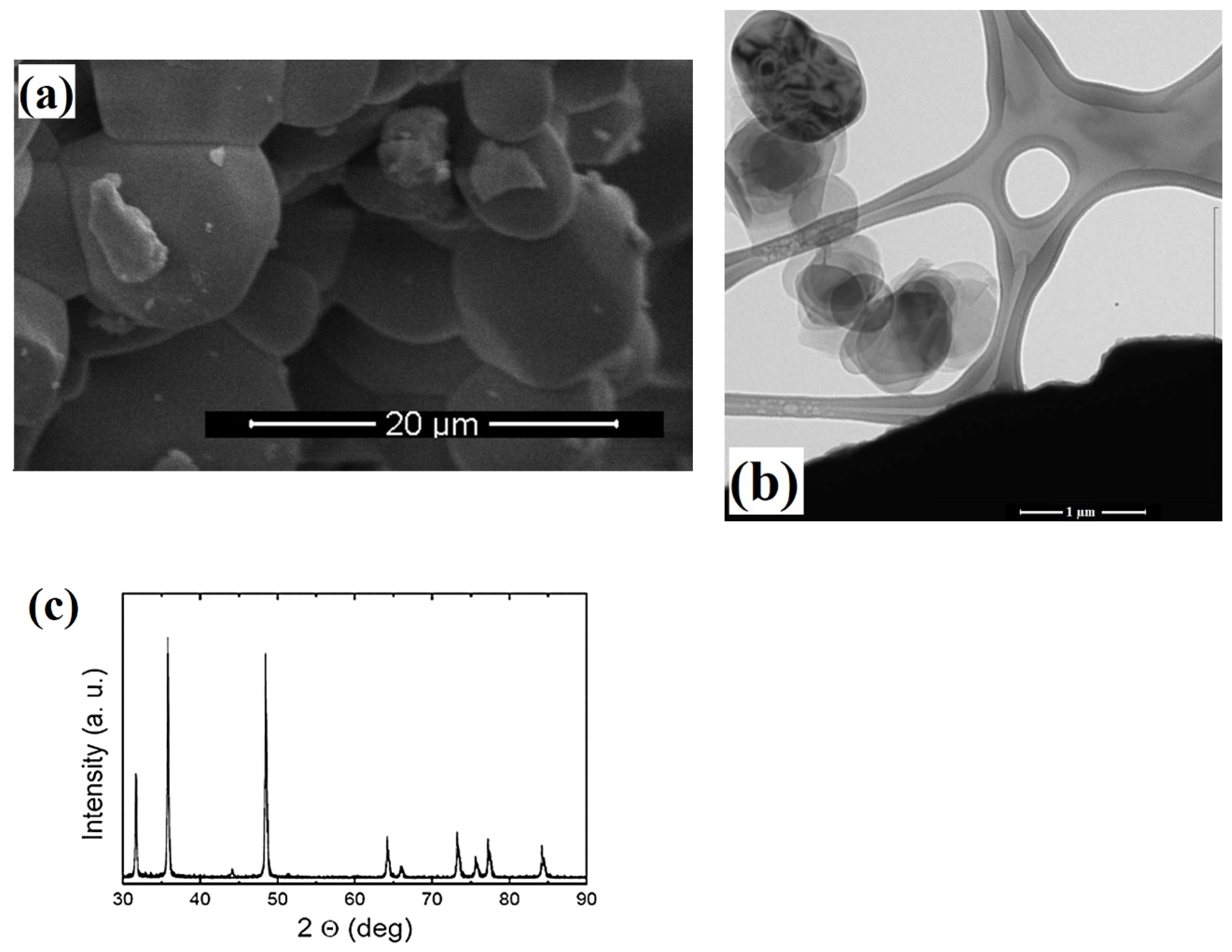
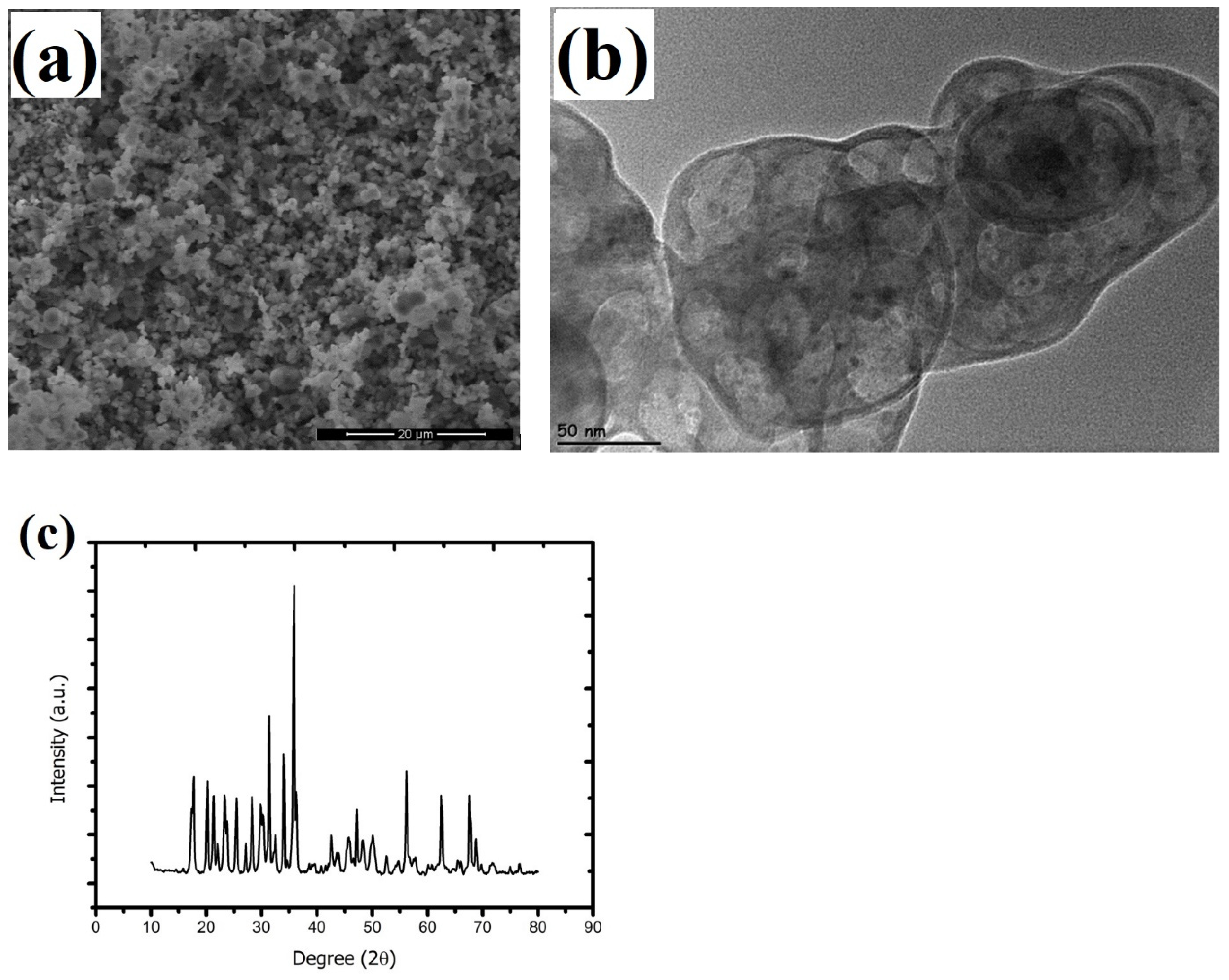
2.1. Characterizations of Nanoparticles
2.2. Cell Cultures and NPs Applications
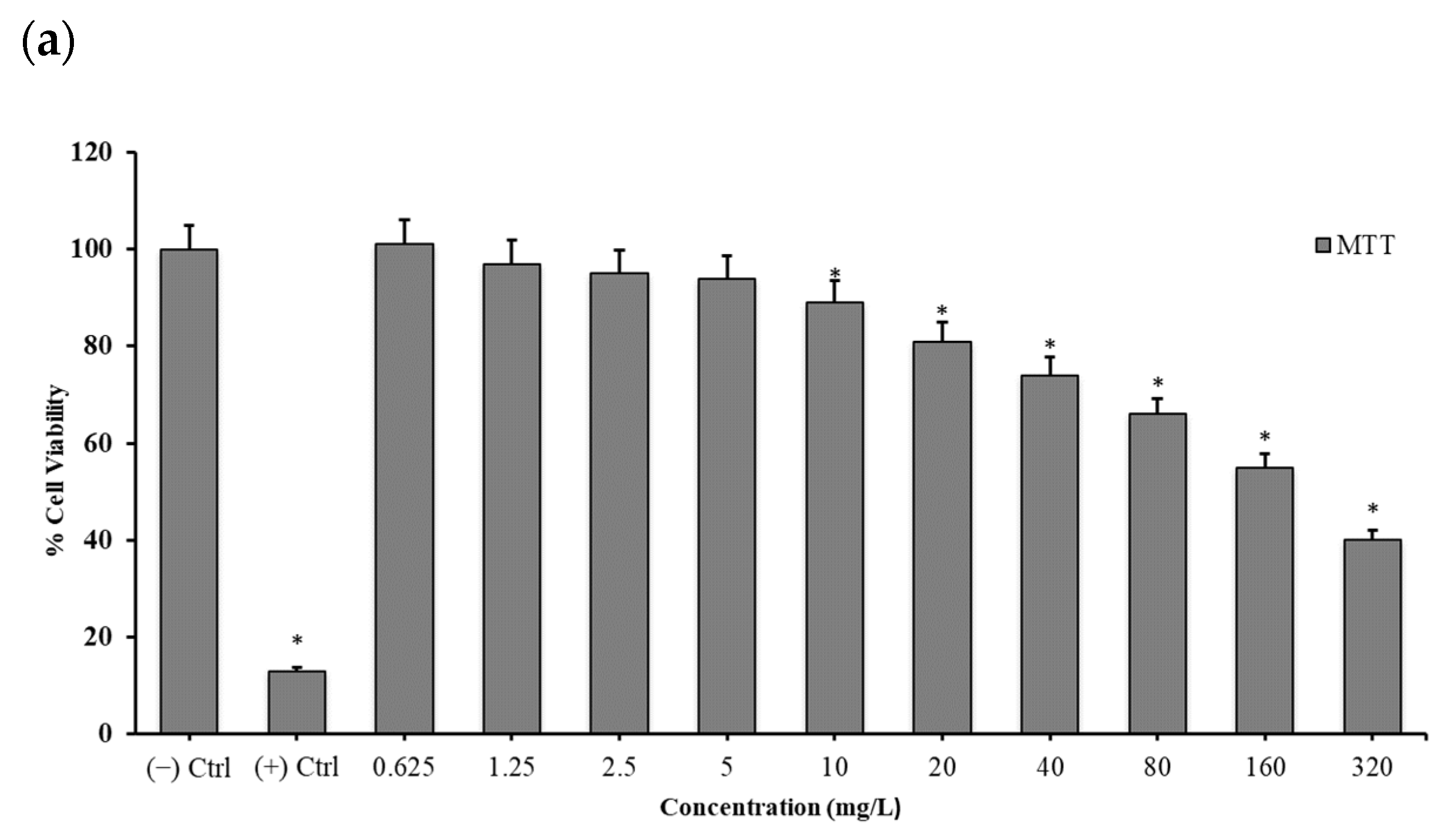
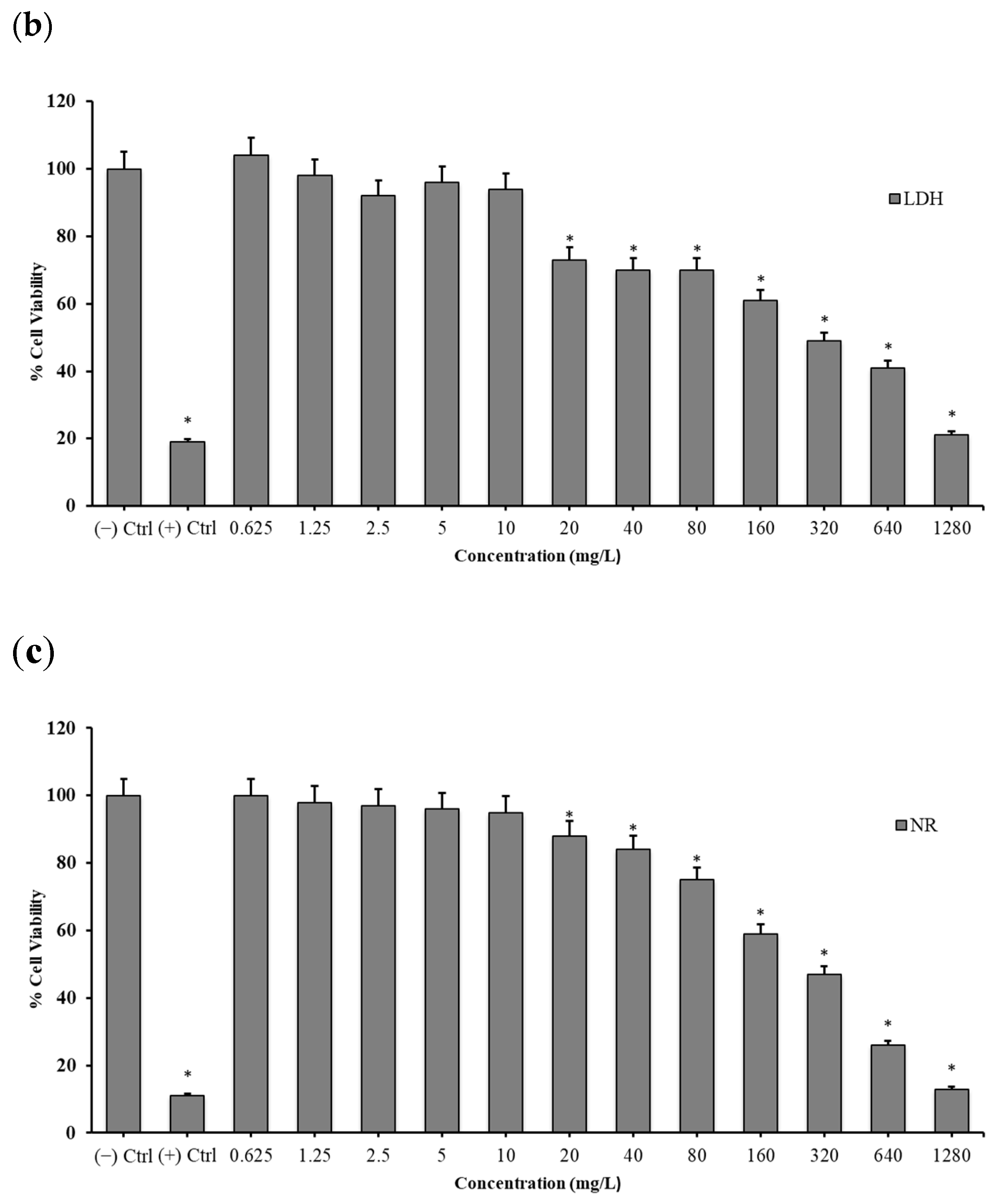
2.3. Cytotoxicity Testing
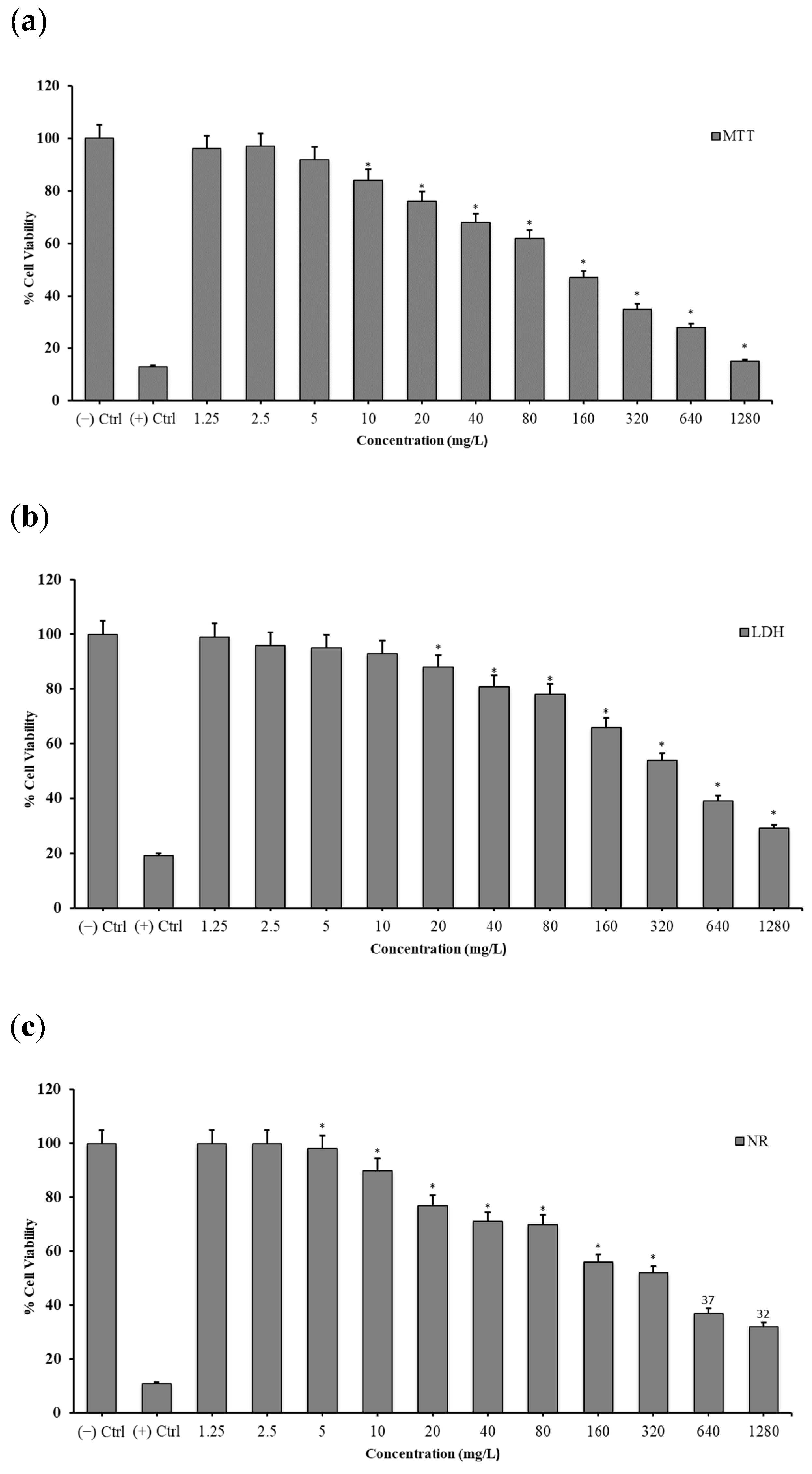
2.3.1. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) Assay
2.3.2. Lactate Dehydrogenase (LDH) Release Assay
2.3.3. Neutral Red (NR) Uptake Assay
2.4. Microarray Analysis
2.5. Data Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, M.A.M. DEAD-box RNA helicases: The driving forces behind RNA metabolism at the crossroad of viral replication and antiviral innate immunity. Virus Res. 2021, 296, 198352. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, G.; Sundar, I.K.; Hwang, J.-W.; Yao, H.; Rahman, I. Emphysema is associated with increased inflammation in lungs of atherosclerosis-prone mice by cigarette smoke: Implications in comorbidities of COPD. J. Inflamm. 2010, 7, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, B.; Raju, G.B.; Suri, A.K. Processing and properties of monolithic TiB2based materials. Int. Mater. Rev. 2006, 51, 352–374. [Google Scholar] [CrossRef]
- Benelli, R.; Venè, R.; Ferrari, N. Prostaglandin-endoperoxide synthase 2 (cyclooxygenase-2), a complex target for colorectal cancer prevention and therapy. Transl. Res. 2018, 196, 42–61. [Google Scholar] [CrossRef]
- Bilgi, E.; Camurlu, H.E.; Akgün, B.; Topkaya, Y.; Sevinç, N. Formation of TiB2 by volume combustion and mechanochemical process. Mater. Res. Bull. 2008, 43, 873–881. [Google Scholar] [CrossRef]
- Cacciatore, I.; Turkez, H.; Di Rienzo, A.; Ciulla, M.; Mardinoglu, A.; Di Stefano, A. Boron-based hybrids as novel scaffolds for the development of drugs with neuroprotective properties. RSC Med. Chem. 2021, 12, 1944–1949. [Google Scholar] [CrossRef] [PubMed]
- Chait, A.; Ginsberg, H.N.; Vaisar, T.; Heinecke, J.W.; Goldberg, I.J.; Bornfeldt, K.E. Remnants of the Triglyceride-Rich Lipoproteins, Diabetes, and Cardiovascular Disease. Diabetes 2020, 69, 508–516. [Google Scholar] [CrossRef]
- Chen, W.; Jiang, X.; Yang, Q. Glycoside hydrolase family 18 chitinases: The known and the unknown. Biotechnol. Adv. 2020, 43, 107553. [Google Scholar] [CrossRef]
- Chenel, V.; Boissy, P.; Cloarec, J.-P.; Patenaude, J. Effects of disciplinary cultures of researchers and research trainees on the acceptability of nanocarriers for drug delivery in different contexts of use: A mixed-methods study. J. Nanopart. Res. 2015, 17, 186. [Google Scholar] [CrossRef] [Green Version]
- Cohn, S.M.; Schloemann, S.; Tessner, T.; Seibert, K.; Stenson, W.F. Crypt stem cell survival in the mouse intestinal epithelium is regulated by prostaglandins synthesized through cyclooxygenase-1. J. Clin. Investig. 1997, 99, 1367–1379. [Google Scholar] [CrossRef]
- Cruz, K.J.C. Antioxidant role of zinc in diabetes mellitus. World J. Diabetes 2015, 6, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Currall, S.C.; King, E.B.; Lane, N.; Madera, J.; Turner, S. What drives public acceptance of nanotechnology? Nat. Nanotechnol. 2006, 1, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Mahaseni, Z.H.; Germi, M.D.; Ahmadi, Z.; Asl, M.S. Microstructural investigation of spark plasma sintered TiB2 ceramics with Si3N4 addition. Ceram. Int. 2018, 44, 13367–13372. [Google Scholar] [CrossRef]
- Han, J.; Hong, C.; Zhang, X.; Wang, B. Thermal shock resistance of TiB2–Cu interpenetrating phase composites. Compos. Sci. Technol. 2005, 65, 1711–1718. [Google Scholar] [CrossRef]
- Hegener, H.H.; Diehl, K.A.; Kurth, T.; Gaziano, J.M.; Ridker, P.M.; Zee, R.Y.L. Polymorphisms of prostaglandin-endoperoxide synthase 2 gene, and prostaglandin-E receptor 2 gene, C-reactive protein concentrations and risk of atherothrombosis: A nested case?control approach. J. Thromb. Haemost. 2006, 4, 1718–1722. [Google Scholar] [CrossRef] [PubMed]
- Ipek, Y. Effect of surfactant types on particle size and morphology of flame-retardant zinc borate powder. Turk. J. Chem. 2020, 44, 214–223. [Google Scholar] [CrossRef]
- Jensen, M.S.; Grande, T.; Einarsrud, M.-A. The Effect of Surface Oxides During Hot Pressing of TiB2. J. Am. Ceram. Soc. 2009, 92, 623–630. [Google Scholar] [CrossRef]
- Joseph, A.; Joshi, G.M. High performance of fluoro polymer modified by hexa-titanium boride nanocomposites. J. Mater. Sci. Mater. Electron. 2018, 29, 4749–4769. [Google Scholar] [CrossRef]
- Joshi, N.; Kumar, D.; Poluri, K.M. Elucidating the Molecular Interactions of Chemokine CCL2 Orthologs with Flavonoid Baicalin. ACS Omega 2020, 5, 22637–22651. [Google Scholar] [CrossRef]
- Kerkelä, E.; Saarialho-Kere, U. Matrix metalloproteinases in tumor progression: Focus on basal and squamous cell skin cancer. Exp. Dermatol. 2003, 12, 109–125. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Kumar, A.; Zhang, K.Y.J. Human Chitinases: Structure, Function, and Inhibitor Discovery. In Advances in Experimental Medicine and Biology; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2019; Volume 1142, pp. 221–251. [Google Scholar]
- Lamers, C.; Plüss, C.J.; Ricklin, D. The Promiscuous Profile of Complement Receptor 3 in Ligand Binding, Immune Modulation, and Pathophysiology. Front. Immunol. 2021, 12, 662164. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, Z.; Lu, W.; Chen, Z.; Chen, L.; Han, S.; Wu, X.; Cai, T.; Cai, Y. Chemokines and chemokine receptors: A new strategy for breast cancer therapy. Cancer Med. 2020, 9, 3786–3799. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef] [PubMed]
- Makau, F.M.; Morsi, K.; Gude, N.; Alvarez, R.; Sussman, M.; May-Newman, K. Viability of Titanium-Titanium Boride Composite as a Biomaterial. ISRN Biomater. 2013, 2013, 970535. [Google Scholar] [CrossRef] [Green Version]
- Mehta, A.; Shapiro, M.D. Apolipoproteins in vascular biology and atherosclerotic disease. Nat. Rev. Cardiol. 2022, 19, 168–179. [Google Scholar] [CrossRef]
- Mirzaei, R.; Mahdavi, F.; Badrzadeh, F.; Hosseini-Fard, S.R.; Heidary, M.; Jeda, A.S.; Mohammadi, T.; Roshani, M.; Yousefimashouf, R.; Keyvani, H.; et al. The emerging role of microRNAs in the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Int. Immunopharmacol. 2021, 90, 107204. [Google Scholar] [CrossRef]
- Mittal, R.; Patel, A.P.; Debs, L.H.; Nguyen, D.; Patel, K.; Grati, M.; Mittal, J.; Yan, D.; Chapagain, P.; Liu, X.Z. Intricate Functions of Matrix Metalloproteinases in Physiological and Pathological Conditions. J. Cell. Physiol. 2016, 231, 2599–2621. [Google Scholar] [CrossRef]
- Mlera, L.; Meade-White, K.; Dahlstrom, E.; Baur, R.; Kanakabandi, K.; Virtaneva, K.; Porcella, S.F.; Bloom, M.E. Peromyscus leucopus mouse brain transcriptome response to Powassan virus infection. J. Neuro Virol. 2018, 24, 75–87. [Google Scholar] [CrossRef] [Green Version]
- Mrugacz, M.; Bryl, A.; Falkowski, M.; Zorena, K. Integrins: An Important Link between Angiogenesis, Inflammation and Eye Diseases. Cells 2021, 10, 1703. [Google Scholar] [CrossRef]
- Murphy, G.; E Cawston, T.; A Galloway, W.; Barnes, M.J.; Bunning, R.A.D.; Mercer, E.; Reynolds, J.J.; E Burgeson, R. Metalloproteinases from rabbit bone culture medium degrade types IV and V collagens, laminin and fibronectin. Biochem. J. 1981, 199, 807–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nawaz, M.; Shah, N.; Zanetti, B.R.; Maugeri, M.; Silvestre, R.N.; Fatima, F.; Neder, L.; Valadi, H. Extracellular Vesicles and Matrix Remodeling Enzymes: The Emerging Roles in Extracellular Matrix Remodeling, Progression of Diseases and Tissue Repair. Cells 2018, 7, 167. [Google Scholar] [CrossRef] [Green Version]
- Nyska, A.; Lomnitski, L.; Maronpot, R.; Moomaw, C.; Brodsky, B.; Sintov, A.; Wormser, U. Effects of iodine on inducible nitric oxide synthase and cyclooxygenase-2 expression in sulfur mustard-induced skin injury in guinea pigs. Arch. Toxicol. 2001, 74, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Oancea, C.N.; Cîmpean, A. In Vitro Cytotoxicity of Zinc Fructoborate, a Novel Zinc-Boron Active Natural Complex. Curr. Health Sci. J. 2018, 44, 113–117. [Google Scholar] [CrossRef]
- Sharma, S.; Ebadi, M. Significance of metallothioneins in aging brain. Neurochem. Int. 2014, 65, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Pelletier, J. General and Target-Specific DExD/H RNA Helicases in Eukaryotic Translation Initiation. Int. J. Mol. Sci. 2020, 21, 4402. [Google Scholar] [CrossRef]
- Shen, W.; Li, Y.; Tang, Y.; Cummins, J.; Huard, J. NS-398, a Cyclooxygenase-2-Specific Inhibitor, Delays Skeletal Muscle Healing by Decreasing Regeneration and Promoting Fibrosis. Am. J. Pathol. 2005, 167, 1105–1117. [Google Scholar] [CrossRef] [Green Version]
- Stone, V.; Führ, M.; Feindt, P.H.; Bouwmeester, H.; Linkov, I.; Sabella, S.; Murphy, F.; Bizer, K.; Tran, L.; Ågerstrand, M.; et al. The Essential Elements of a Risk Governance Framework for Current and Future Nanotechnologies. Risk Anal. 2018, 38, 1321–1331. [Google Scholar] [CrossRef]
- Tang, B.; Li, X.; Liu, Y.; Chen, X.; Li, X.; Chu, Y.; Zhu, H.; Liu, W.; Xu, F.; Zhou, F.; et al. The Therapeutic Effect of ICAM-1-Overexpressing Mesenchymal Stem Cells on Acute Graft-Versus-Host Disease. Cell. Physiol. Biochem. 2018, 46, 2624–2635. [Google Scholar] [CrossRef] [Green Version]
- Titz, B.; Boue, S.; Phillips, B.; Talikka, M.; Vihervaara, T.; Schneider, T.; Nury, C.; Elamin, A.; Guedj, E.; Peck, M.J.; et al. Effects of Cigarette Smoke, Cessation, and Switching to Two Heat-Not-Burn Tobacco Products on Lung Lipid Metabolism in C57BL/6 and Apoe-/- Mice-An Integrative Systems Toxicology Analysis. Toxicol. Sci. 2016, 149, 441–457. [Google Scholar] [CrossRef] [Green Version]
- Türkez, H.; Arslan, M.E.; Sönmez, E.; Tatar, A.; Geyikoğlu, F.; Açikyildiz, M.; Mardinoğlu, A. Safety Assessments of Nickel Boride Nanoparticles on the Human Pulmonary Alveolar Cells by Using Cell Viability and Gene Expression Analyses. Biol. Trace Element Res. 2021, 199, 2602–2611. [Google Scholar] [CrossRef] [PubMed]
- Türkez, H.; Arslan, M.E.; Sönmez, E.; Geyikoğlu, F.; Açıkyıldız, M.; Tatar, A. Microarray assisted toxicological investigations of boron carbide nanoparticles on human primary alveolar epithelial cells. Chem. Interact. 2019, 300, 131–137. [Google Scholar] [CrossRef]
- Türkez, H.; Arslan, M.E.; Sönmez, E.; Tatar, A.; Açikyildiz, M.; Geyikoğlu, F. Toxicogenomic responses of human alveolar epithelial cells to tungsten boride nanoparticles. Chem. Interact. 2017, 273, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Ugur, A.; Ceylan, O.; Boran, R.; Ayrıkçil, S.; Sarac, N.; Yilmaz, D. A new approach for prevention the oxidations and mutations: Zinc borate. J. Boron 2019, 4, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Üreyen, M.E.; Kaynak, E. Effect of Zinc Borate on Flammability of PET Woven Fabrics. Adv. Polym. Technol. 2019, 2019, 7150736. [Google Scholar] [CrossRef] [Green Version]
- van Giesen, R.I.; Fischer, A.R.; van Trijp, H.C. Changes in the influence of affect and cognition over time on consumer attitude formation toward nanotechnology: A longitudinal survey study. Public Underst. Sci. 2018, 27, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Werynska, B.; Pula, B.; Kobierzycki, C.; Dziegiel, P.; Podhorska-Okolow, M. Metallothioneins in the lung cancer. Folia Histochem. Cytobiol. 2015, 53, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wlodarczyk, J.; Mukhina, I.; Kaczmarek, L.; Dityatev, A. Extracellular matrix molecules, their receptors, and secreted proteases in synaptic plasticity. Dev. Neurobiol. 2011, 71, 1040–1053. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-H.; Yeh, I.-J.; Phan, N.N.; Yen, M.-C.; Liu, H.-L.; Wang, C.-Y.; Hsu, H.-P. Severe acute respiratory syndrome coronavirus (SARS-CoV)-2 infection induces dysregulation of immunity: In silico gene expression analysis. Int. J. Med. Sci. 2021, 18, 1143–1152. [Google Scholar] [CrossRef]
- Yah, C.S.; Iyuke, S.E.; Simate, G.S. A review of nanoparticles toxicity and their routes of exposures. Iran. J. Pharm. Sci. 2012, 8, 299–314. [Google Scholar]
- Ziatabar, S.; Zepf, J.; Rich, S.; Danielson, B.T.; Bollyky, P.I.; Stern, R. Chitin, chitinases, and chitin lectins: Emerging roles in human pathophysiology. Pathophysiology 2018, 25, 253–262. [Google Scholar] [CrossRef] [PubMed]


| Titanium Diboride Fold Change (FC) | |||
|---|---|---|---|
| Upregulated Expressions | FC | Downregulated Expressions | FC |
| CXCL5 | 1.81 | FOS | −1.88 |
| GINS4 | 1.80 | MGC24103 | −1.69 |
| MMP3 | 1.67 | LOC100132564 | −1.67 |
| ANXA10 | 1.53 | MXRA5 | −1.58 |
| PTGS2 | 1.50 | RPS29 | −1.57 |
| PTGS2 | 1.47 | FOSB | −1.52 |
| BEX2 | 1.47 | INMT | −1.51 |
| CXCL5 | 1.45 | LOC100134259 | −1.50 |
| NEBL | 1.41 | MGC4677 | −1.50 |
| SERPINB2 | 1.41 | LOC728499 | −1.47 |
| IL13RA2 | 1.41 | FAM65C | −1.46 |
| DIRAS3 | 1.40 | C7orf54 | −1.45 |
| ASNS | 1.40 | BAALC | −1.41 |
| NGDN | 1.39 | STARD13 | −1.41 |
| CRLF1 | 1.38 | IFIT1 | −1.40 |
| PTPRH | 1.37 | SOCS3 | −1.39 |
| LOC143666 | 1.34 | PDGFRB | −1.38 |
| ICAM1 | 1.34 | TNFRSF11B | −1.37 |
| AGTR1 | 1.33 | HEYL | −1.37 |
| LOC100008588 | 1.32 | EGR3 | −1.37 |
| RPP25 | 1.32 | WDR33 | −1.36 |
| DDX52 | 1.32 | NEDD9 | −1.36 |
| LOC441253 | 1.32 | CRIP1 | −1.36 |
| RRAD | 1.32 | NDRG4 | −1.34 |
| SLC8A3 | 1.31 | HIST2H2BE | −1.34 |
| Zinc Borate Fold Change (FC) | |||
|---|---|---|---|
| Upregulated Expressions | FC | Downregulaed Expressions | FC |
| APOE | 22.44 | NPTX1 | −15.36 |
| ADH1A | 20.48 | SERPINB2 | −6.68 |
| IGFBP3 | 16.88 | ESM1 | −6.55 |
| IGFBP3 | 15.34 | SERPINB2 | −5.80 |
| CFD | 14.08 | LOC728285 | −5.54 |
| PTGDS | 13.67 | MMP1 | −4.63 |
| MFAP4 | 13.41 | CCL2 | −4.57 |
| RGS4 | 12.28 | NPTX1 | −4.57 |
| SERPINA3 | 11.94 | ESM1 | −4.48 |
| CXCL12 | 10.54 | INSIG1 | −4.45 |
| FOS | 10.25 | NPTX1 | −4.28 |
| PARM1 | 9.60 | KRT19 | −4.27 |
| AMPH | 9.38 | LOC100134073 | −4.16 |
| ANGPT1 | 8.62 | KRT34 | −4.12 |
| A2M | 8.47 | H2AFY2 | −4.09 |
| CXCL12 | 8.31 | LOC728255 | −4.08 |
| CHL1 | 7.68 | GPR68 | −4.04 |
| HSD17B2 | 7.58 | TNFSF4 | −3.94 |
| FMO2 | 7.50 | E2F7 | −3.92 |
| CFTR | 7.47 | HMGCS1 | −3.87 |
| H19 | 7.40 | RGMB | −3.77 |
| CHI3L1 | 7.39 | SC4MOL | −3.74 |
| FOSB | 7.37 | MMP3 | −3.73 |
| CORO6 | 7.36 | TMEM166 | −3.69 |
| FER1L4 | 7.32 | TNFSF4 | −3.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Türkez, H.; Arslan, M.E.; Tatar, A.; Özdemir, Ö.; Sönmez, E.; Çadirci, K.; Hacimüftüoğlu, A.; Ceylan, B.; Açikyildiz, M.; Kahraman, C.Y.; et al. Molecular Genetics and Cytotoxic Responses to Titanium Diboride and Zinc Borate Nanoparticles on Cultured Human Primary Alveolar Epithelial Cells. Materials 2022, 15, 2359. https://doi.org/10.3390/ma15072359
Türkez H, Arslan ME, Tatar A, Özdemir Ö, Sönmez E, Çadirci K, Hacimüftüoğlu A, Ceylan B, Açikyildiz M, Kahraman CY, et al. Molecular Genetics and Cytotoxic Responses to Titanium Diboride and Zinc Borate Nanoparticles on Cultured Human Primary Alveolar Epithelial Cells. Materials. 2022; 15(7):2359. https://doi.org/10.3390/ma15072359
Chicago/Turabian StyleTürkez, Hasan, Mehmet Enes Arslan, Arzu Tatar, Özlem Özdemir, Erdal Sönmez, Kenan Çadirci, Ahmet Hacimüftüoğlu, Bahattin Ceylan, Metin Açikyildiz, Cigdem Yuce Kahraman, and et al. 2022. "Molecular Genetics and Cytotoxic Responses to Titanium Diboride and Zinc Borate Nanoparticles on Cultured Human Primary Alveolar Epithelial Cells" Materials 15, no. 7: 2359. https://doi.org/10.3390/ma15072359
APA StyleTürkez, H., Arslan, M. E., Tatar, A., Özdemir, Ö., Sönmez, E., Çadirci, K., Hacimüftüoğlu, A., Ceylan, B., Açikyildiz, M., Kahraman, C. Y., Geyikoğlu, F., Tatar, A., & Mardinoglu, A. (2022). Molecular Genetics and Cytotoxic Responses to Titanium Diboride and Zinc Borate Nanoparticles on Cultured Human Primary Alveolar Epithelial Cells. Materials, 15(7), 2359. https://doi.org/10.3390/ma15072359







