Graphene-Oxide-Enriched Biomaterials: A Focus on Osteo and Chondroinductive Properties and Immunomodulation
Abstract
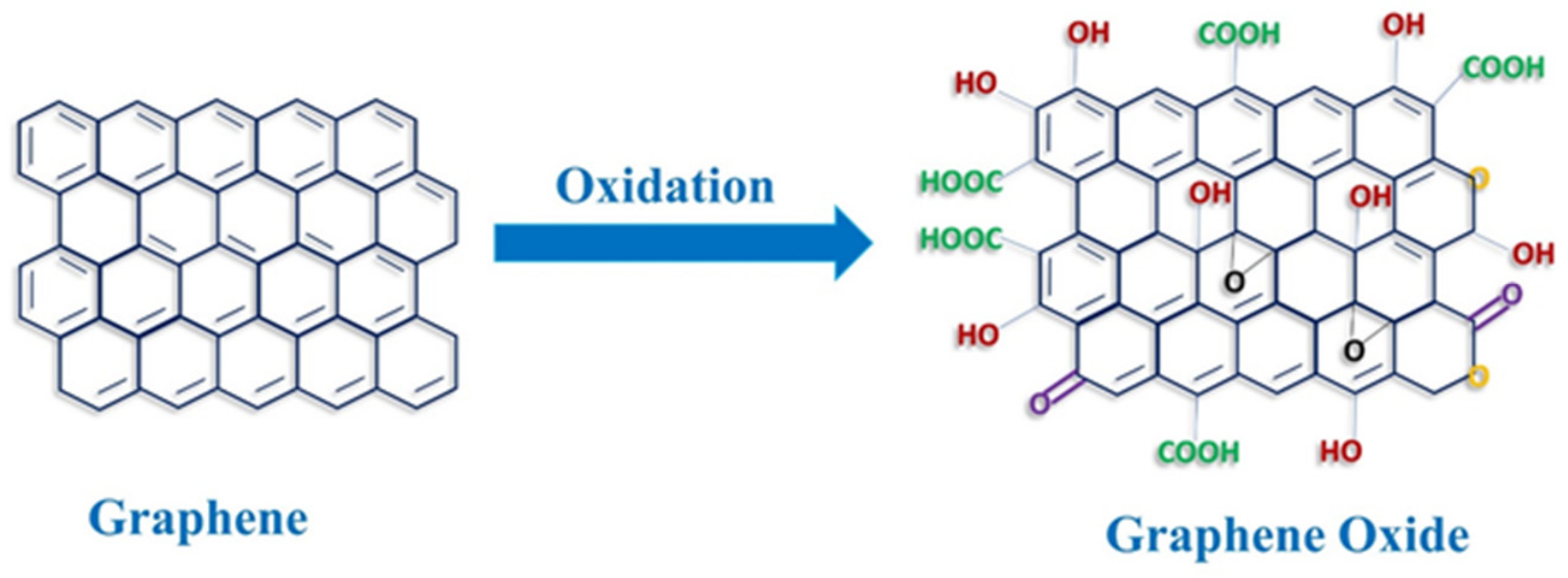
1. Introduction
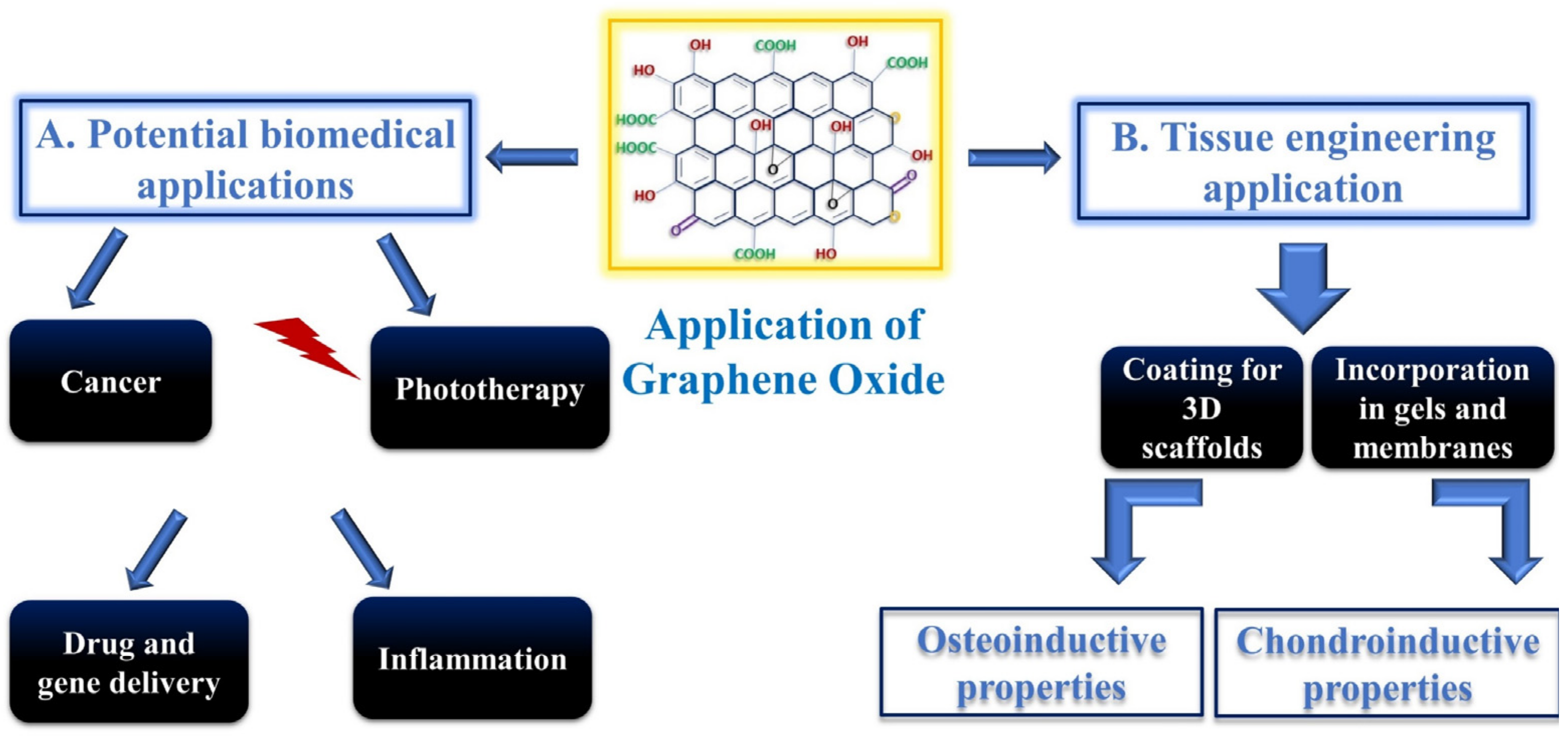
2. Biomedical Applications of GO
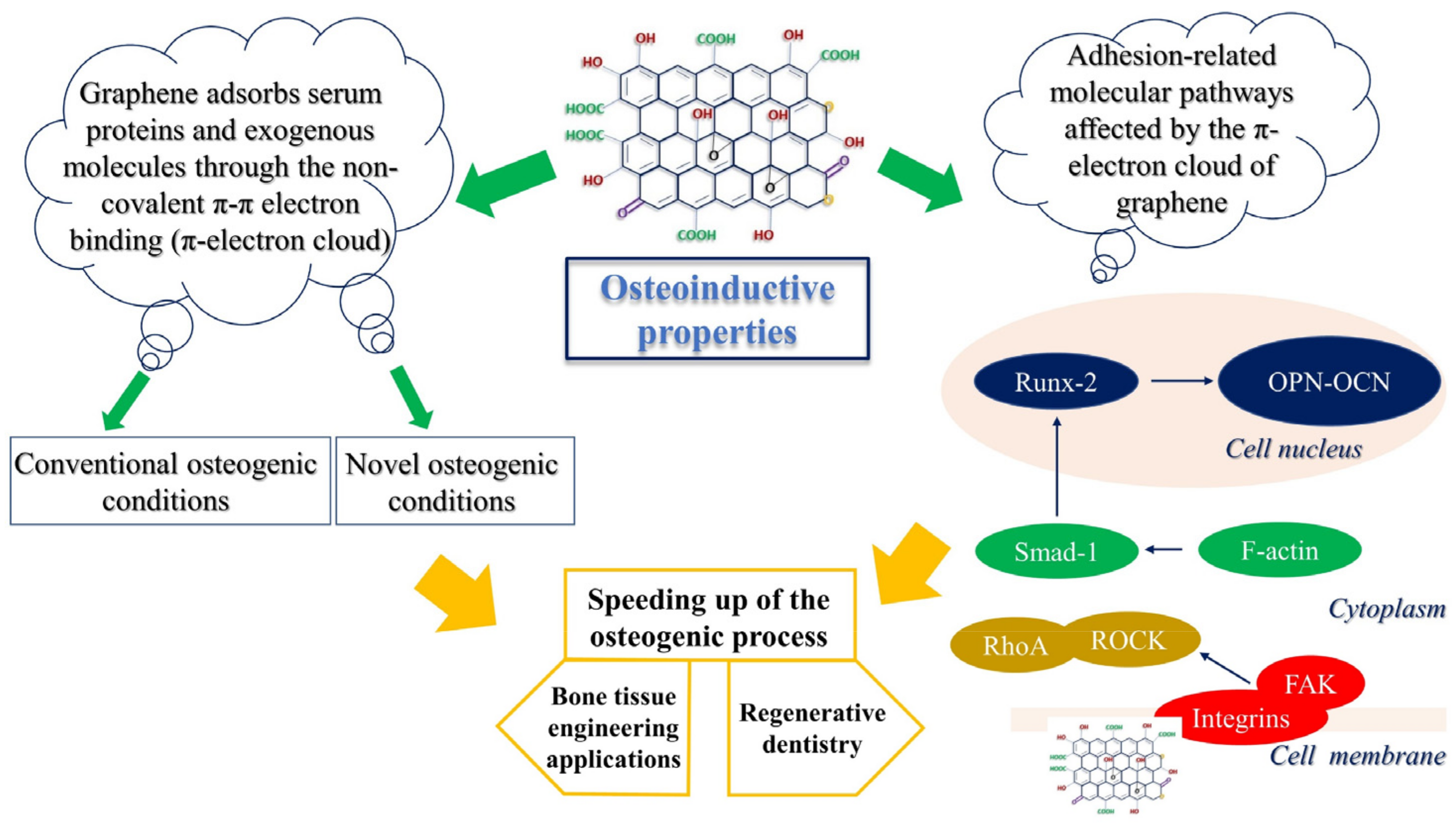
3. Osteoconductive Properties of Graphene
3.1. Enhanced Osteogenesis in the Presence of GO in Novel Differentiation Vehicles
3.2. Stimulation of FAK-Related Pathways by GO Induces MSC Adherence and Osteogenic Differentiation
3.3. GO in Dentistry
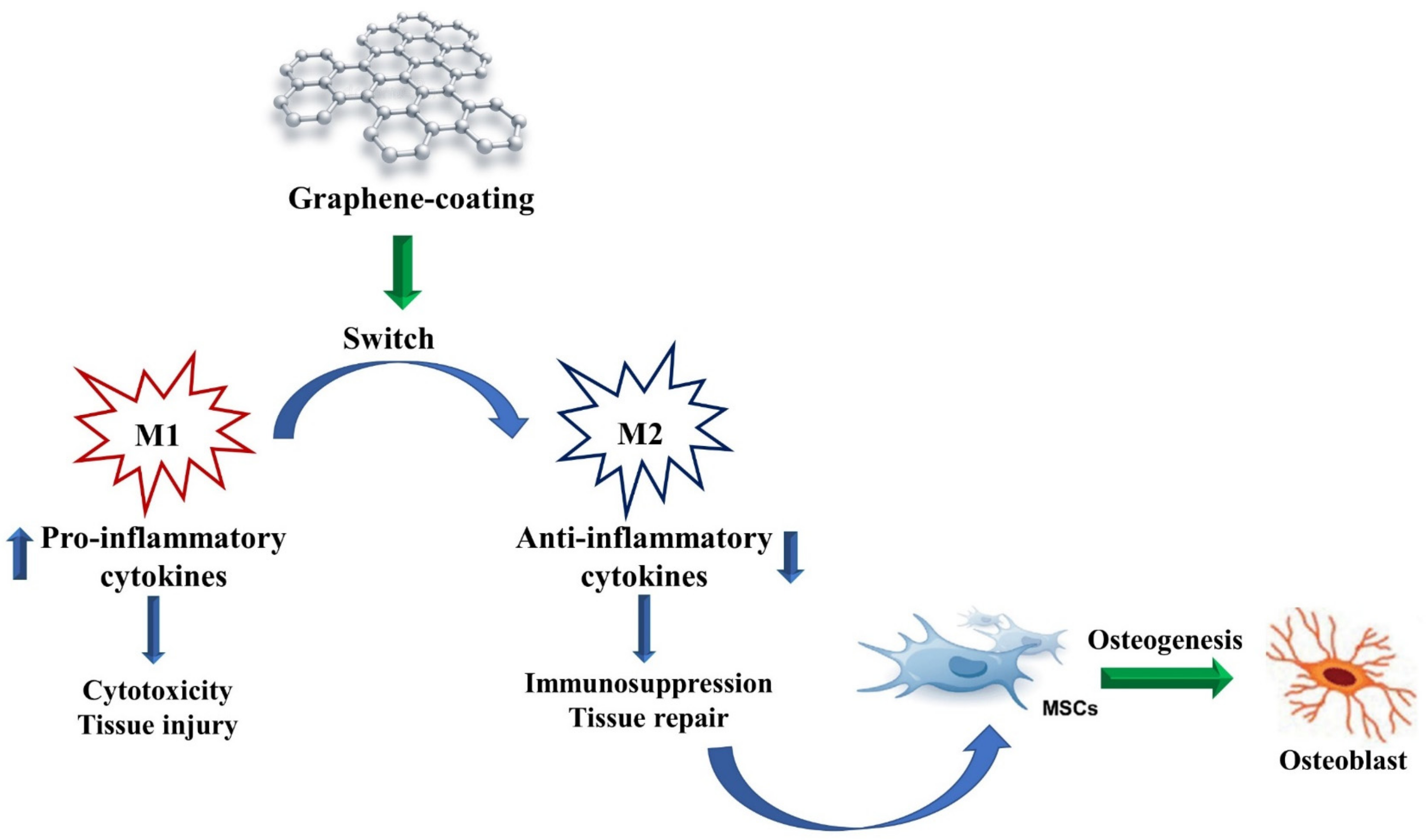
4. Immunomodulatory Properties of GO in Osteogenic Conditions
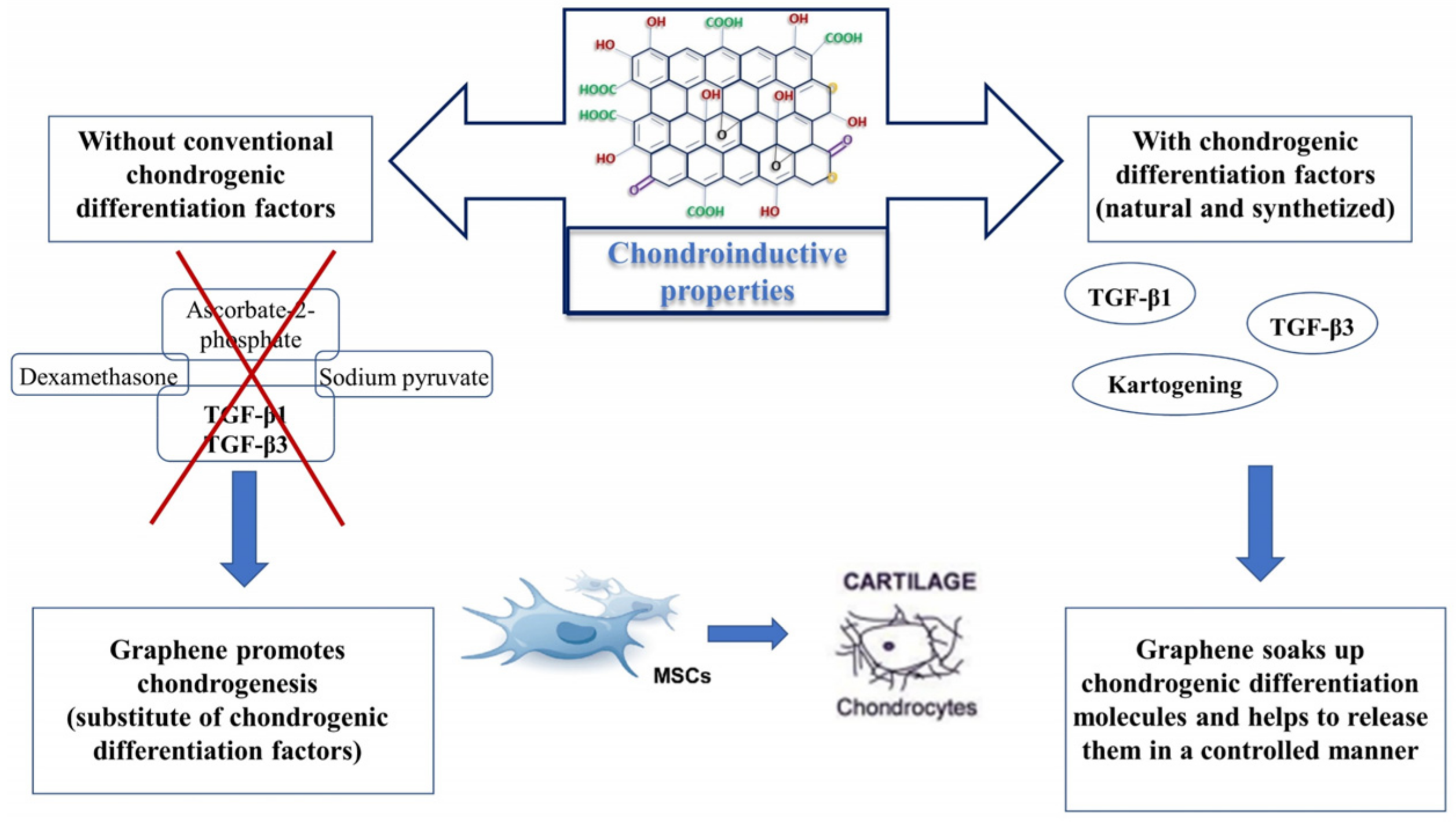
5. Chondroinductive Properties of Graphene
5.1. Graphene as a Substitute for Chondrogenic Differentiation Factors
5.2. Graphene as a Nanocarrier for Natural and Synthetized Chondrogenic Differentiation Factors
| Graphene Formulation | Biomedical Applications | ||
|---|---|---|---|
| De Marco, P. et al. [11] | Collagen membranes enriched with GO | Implementation of bone deposition | In vitro |
| Radunovic, M. et al. [12] | Collagen membranes enriched with GO | Implementation of bone formation and improvement of the clinical performance of collagen membranes | In vitro |
| Zarafu, I. et al. [16] | Amines-functionalized GO | Antimicrobial and antibiofilm activity | In vitro |
| Deng, X. et al. [17] | GO combined with polyethylene glycol (PEG) | Prevention of osteosarcoma invasion | In vitro and in vivo |
| Di Carlo, R. et al. [19] | GO-coated titanium surfaces | Improvement of properties related to dental implantation materials | In vitro |
| Jo, S.B. et al. [21] | Polyurethane–nanoGO fibers | Potential matrix for skeletal muscle engineering | In vitro |
| Bao, D. et al. [22] | Platelet-rich plasma gels with GO (PRP/GO) | Tendon–bone interface healing/supraspinatus tendon reconstruction | In vitro and in vivo |
| Sadeghianmaryan, A. et al. [23] | Electrospinning polyurethane–GO | Wound dressing | In vitro |
| Soliman, M. et al. [24] | GO–cellulose nanocomposite | Wound healing | In vitro and in vivo |
| Llewellyn, S.H. et al. [25] | GO substrates | Peripheral nerve regeneration | In vitro |
| Dinescu, S. et al. [29] | GO–Chitosan-based 3D scaffolds | Bone tissue engineering | In vitro and in vivo |
| Son, S.A. et al. [34] | Mesoporous bioactive glass combined with GO quantum dots | Dentin hypersensitivity | In vitro |
| Yilmaz, E. et al. [37] | HA/GO/COL bioactive composite coating on Ti16Nb | Antibacterial activity,improvement of cell adhesion and viability | In vitro |
| Kalbacova, M. et al. [38] | Single graphene layer | Improvement of osteoconductivity | In vitro |
| Nayak, T.R. et al. [39] | Graphene sheets | Acceleration of cell differentiation | In vitro |
| Arumugam, N. et al. [43] | GO quantum dots | Detection of ascorbic acid | In vitro |
| Krukiewicz, K. et al. [44] | GO–poly(methyl methacrylate) | Bone tissue engineering | In vitro |
| Kang, M.S. et al. [45] | rGO–titanium substrates | Dental and orthopaedic bone substitutes | In vitro |
| Li, Z. et al. [46] | Methacrylated gelatin–GO | Bone tissue engineering | In vitro and in vivo |
| Kang, E.S. et al. [47] | Gold nanostructure/peptide-nanopatterned GO | Treatment of disorders of bone tissue | In vitro |
| Zhou, C. et al. [49] | Collagen-functionalized GO | Enhancement of biomimetic mineralization | In vitro and in vivo |
| Bahrami, S.et al. [50] | rGO-coated collagen scaffolds | Bone tissue engineering | In vitro and in vivo |
| Fu, C. et al. [51] | L-lysine-functionalized GO nanoparticles on PLGA | Improvement of osseointegration of bone implants | In vitro and in vivo |
| Kim, J. et al. [52] | Glass slides coated with GO | Upregulation of osteogenic responses | In vitro |
| Arnold, A.M. et al. [54] | Phosphate–GO releasing inducerons (Ca2+ and PO43−) | Bone regeneration | In vitro and in vivo |
| Newby, S.D. et al. [56] | Functionalized graphene nanoparticles | Induction of specific ECM protein expression, bone repair, and regeneration | In vitro |
| Kim, H.D. et al. [61] | GO incorporated into cryogel-based scaffold | Improvement of osteogenic commitment | In vitro |
| Di Carlo, R. et al. [65] | GO-decorated cortical membrane | Bone regeneration | In vitro |
| Di Crescenzo, A. et al. [66] | GO foils | Bone regeneration | In vitro |
| Bordoni, V. et al. [70] | Monocytes activator GO complexed with calcium phosphate (maGO–CaP) | Immunomodulatory effects in osteogenesis | In vitro and in vivo |
| Su, J. et al. [71] | GO-coated titanium | Immunomodulatory effects in osteogenesis | In vitro |
| Chang, T.K. et al. [72] | Graphene and GO particles | Application in orthopaedic prostheses | In vitro and in vivo |
| Shen, H. et al. [76] | GO-incorporated hydrogel | Biologics-free approach for cartilage tissue engineering | In vitro |
| Deliormanlı, A.M. et al. [80] | Grid-like graphene/PCL composite scaffolds | Chondrogenic differentiation | In vitro |
| Olate-Moya, F. et al. [81] | Alginate-based hydrogel with GO | Chondroinductive capability | In vitro |
| Yoon H.H., et al. [84] | GO sheets | Chondroinductive capability | In vitro |
| Zhou, M. et al. [85] | Adsorbed TGF-β3 to GO flakes incorporated into collagen hydrogel | Delivering of growth factors and chondrogenic differentiation induction | In vitro |
| Jiao, D. et al. [83] | Biodegradable gelatin–rGO | Promoting chondrogenic differentiation through kartogenin delivery | In vitro |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-based materials: Synthesis, characterization, properties, and applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef] [PubMed]
- Castro-Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109. [Google Scholar] [CrossRef]
- Wallace, P.R. The Band Theory of Graphite. Phys. Rev. 1947, 71, 622–634. [Google Scholar] [CrossRef]
- Choi, W.; Lahiri, I.; Seelaboyina, R.; Kang, Y.S. Synthesis of Graphene and Its Applications: A Review. Crit. Rev. Solid State Mat. Sci. 2018, 35, 52–71. [Google Scholar] [CrossRef]
- Wang, X.Y.; Richter, M.; He, Y.; Björk, J.; Riss, A.; Rajesh, R.; Garnica, M.; Hennersdorf, F.; Weigand, J.J.; Narita, A.; et al. Exploration of pyrazine-embedded antiaromatic polycyclic hydrocarbons generated by solution and on-surface azomethine ylide homocoupling. Nat. Commun. 2017, 8, 1948. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Graphene Nanomaterials: Synthesis, Biocompatibility, and Cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564. [Google Scholar] [CrossRef]
- Sekiya, R.; Haino, T. Edge-Functionalized Nanographenes. Chemistry. 2021, 27, 187–199. [Google Scholar] [CrossRef]
- Dideikin, A.T.; Vul’, A.Y. Graphene Oxide and Derivatives: The Place in Graphene Family. Front. Phys. 2019, 6, 149. [Google Scholar] [CrossRef]
- Banerjee, A.N. Graphene and its derivatives as biomedical materials: Future prospects and challenges. Interface Focus. 2018, 8, 20170056. [Google Scholar] [CrossRef]
- Durán, N.; Martinez, D.S.; Silveira, C.P.; Durán, M.; de Moraes, A.C.; Simões, M.B.; Alves, O.L.; Fávaro, W.J. Graphene oxide: A carrier for pharmaceuticals and a scaffold for cell interactions. Curr. Top Med. Chem. 2015, 15, 309–327. [Google Scholar] [CrossRef]
- De Marco, P.; Zara, S.; De Colli, M.; Radunovic, M.; Lazović, V.; Ettorre, V.; Di Crescenzo, A.; Piattelli, A.; Cataldi, A.; Fontana, A. Graphene oxide improves the biocompatibility of collagen membranes in an in vitro model of human primary gingival fibroblasts. Biomed. Mater. 2017, 12, 055005. [Google Scholar] [CrossRef]
- Radunovic, M.; De Colli, M.; De Marco, P.; Di Nisio, C.; Fontana, A.; Piattelli, A.; Cataldi, A.; Zara, S. Graphene oxide enrichment of collagen membranes improves DPSCs differentiation and controls inflammation occurrence. J. Biomed. Mater. Res. A 2017, 105, 2312–2320. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Gaur, M.; Misra, C.; Yadav, A.B.; Swaroop, S.; Maolmhuaidh, F.Ó.; Bechelany, M.; Barhoum, A. Biomedical Applications of Carbon Nanomaterials: Fullerenes, Quantum Dots, Nanotubes, Nanofibers, and Graphene. Materials 2021, 14, 5978. [Google Scholar] [CrossRef]
- Yim, Y.; Shin, H.; Ahn, S.M.; Min, D.H. Graphene oxide-based fluorescent biosensors and their biomedical applications in diagnosis and drug discovery. Chem. Commun. 2021, 57, 9820–9833. [Google Scholar] [CrossRef] [PubMed]
- Zarafu, I.; Turcu, I.; Culiță, D.C.; Petrescu, S.; Popa, M.; Chifiriuc, M.C.; Limban, C.; Telehoiu, A.; Ioniță, P. Antimicrobial Features of Organic Functionalized Graphene-Oxide with Selected Amines. Materials 2018, 11, 1704. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Liang, H.; Yang, W.; Shao, Z. Polarization and function of tumor-associated macrophages mediate graphene oxide-induced photothermal cancer therapy. J. Photochem. Photobiol. B 2020, 208, 111913. [Google Scholar] [CrossRef]
- Daniyal, M.; Liu, B.; Wang, W. Comprehensive Review on Graphene Oxide for Use in Drug Delivery System. Curr. Med. Chem. 2020, 27, 3665–3685. [Google Scholar] [CrossRef]
- Di Carlo, R.; Di Crescenzo, A.; Pilato, S.; Ventrella, A.; Piattelli, A.; Recinella, L.; Chiavaroli, A.; Giordani, S.; Baldrighi, M.; Camisasca, A.; et al. Osteoblastic Differentiation on Graphene Oxide-Functionalized Titanium Surfaces: An In Vitro Study. Nanomaterials 2020, 10, 654. [Google Scholar] [CrossRef]
- Maleki, M.; Zarezadeh, R.; Nouri, M.; Sadigh, A.R.; Pouremamali, F.; Asemi, Z.; Kafil, H.S.; Alemi, F.; Yousefi, B. Graphene Oxide: A Promising Material for Regenerative Medicine and Tissue Engineering. Biomol. Concepts 2020, 11, 182–200. [Google Scholar] [CrossRef]
- Jo, S.B.; Erdenebileg, U.; Dashnyam, K.; Jin, G.Z.; Cha, J.R.; El-Fiqi, A.; Knowles, J.C.; Patel, K.D.; Lee, H.H.; Lee, J.H.; et al. Nano-graphene oxide/polyurethane nanofibers: Mechanically flexible and myogenic stimulating matrix for skeletal tissue engineering. J. Tissue Eng. 2020, 11, 2041731419900424. [Google Scholar] [CrossRef] [PubMed]
- Bao, D.; Sun, J.; Gong, M.; Shi, J.; Qin, B.; Deng, K.; Liu, G.; Zeng, S.; Xiang, Z.; Fu, S. Combination of graphene oxide and platelet-rich plasma improves tendon-bone healing in a rabbit model of supraspinatus tendon reconstruction. Regen. Biomater. 2021, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Sadeghianmaryan, A.; Sardroud, H.A.; Allafasghari, S.; Yazdanpanah, Z.; Naghieh, S.; Gorji, M.; Chen, X. Electrospinning of polyurethane/graphene oxide for skin wound dressing and its in vitro characterization. J. Biomater. Appl. 2020, 35, 135–145. [Google Scholar] [CrossRef]
- Soliman, M.; Sadek, A.A.; Abdelhamid, H.N.; Hussein, K. Graphene oxide-cellulose nanocomposite accelerates skin wound healing. Res. Vet. Sci. 2021, 137, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, S.H.; Faroni, A.; Iliut, M.; Bartlam, C.; Vijayaraghavan, A.; Reid, A.J. Graphene Oxide Substrate Promotes Neurotrophic Factor Secretion and Survival of Human Schwann-Like Adipose Mesenchymal Stromal Cells. Adv. Biol. 2021, 5, e2000271. [Google Scholar] [CrossRef] [PubMed]
- Soleymani Eil Bakhtiari, S.; Bakhsheshi-Rad, H.R.; Karbasi, S.; Tavakoli, M.; Razzaghi, M.; Ismail, A.F.; RamaKrishna, S.; Berto, F. Polymethyl Methacrylate-Based Bone Cements Containing Carbon Nanotubes and Graphene Oxide: An Overview of Physical, Mechanical, and Biological Properties. Polymers 2020, 12, 1469. [Google Scholar] [CrossRef]
- Cheng, A.; Schwartz, Z.; Kahn, A.; Li, X.; Shao, Z.; Sun, M.; Ao, Y.; Boyan, B.D.; Chen, H. Advances in Porous Scaffold Design for Bone and Cartilage Tissue Engineering and Regeneration. Tissue Eng. Part B Rev. 2019, 25, 14–29. [Google Scholar] [CrossRef]
- Blanco, F.J.; Ruiz-Romero, C. New targets for disease modifying osteoarthritis drugs: Chondrogenesis and Runx1. Ann. Rheum. Dis. 2013, 72, 631–634. [Google Scholar] [CrossRef]
- Dinescu, S.; Ionita, M.; Ignat, S.R.; Costache, M.; Hermenean, A. Graphene Oxide Enhances Chitosan-Based 3D Scaffold Properties for Bone Tissue Engineering. Int. J. Mol. Sci. 2019, 20, 5077. [Google Scholar] [CrossRef]
- Daneshmandi, L.; Barajaa, M.; Tahmasbi Rad, A.; Sydlik, S.A.; Laurencin, C.T. Graphene-Based Biomaterials for Bone Regenerative Engineering: A Comprehensive Review of the Field and Considerations Regarding Biocompatibility and Biodegradation. Adv. Healthc. Mater. 2021, 2001414. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Li, Z.; Liu, M.; Kinloch, I.A.; Young, R.J. Mechanisms of mechanical reinforcement by graphene and carbon nanotubes in polymer nanocomposites. Nanoscale 2020, 12, 2228–2267. [Google Scholar] [CrossRef] [PubMed]
- Mohammadrezaei, D.; Golzar, H.; Rezai Rad, M.; Omidi, M.; Rashedi, H.; Yazdian, F.; Khojasteh, A.; Tayebi, L. In vitro effect of graphene structures as an osteoinductive factor in bone tissue engineering: A systematic review. J. Biomed. Mater. Res. A 2018, 106, 2284–2343. [Google Scholar] [CrossRef] [PubMed]
- Aslam Khan, M.U.; Haider, A.; Abd Razak, S.I.; Abdul Kadir, M.R.; Haider, S.; Shah, S.A.; Hasan, A.; Khan, R.; Khan, S.D.; Shakir, I. Arabinoxylan/graphene-oxide/nHAp-NPs/PVA bionano composite scaffolds for fractured bone healing. J. Tissue Eng. Regen. Med. 2021, 15, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Son, S.A.; Kim, D.H.; Yoo, K.H.; Yoon, S.Y.; Kim, Y.I. Mesoporous Bioactive Glass Combined with Graphene Oxide Quantum Dot as a New Material for a New Treatment Option for Dentin Hypersensitivity. Nanomaterials 2020, 10, 621. [Google Scholar] [CrossRef]
- Oprea, M.; Voicu, S.I. Cellulose Composites with Graphene for Tissue Engineering Applications. Materials 2020, 13, 5347. [Google Scholar] [CrossRef]
- Zapata, M.E.V.; Tovar, C.D.G.; Hernandez, J.H.M. The Role of Chitosan and Graphene Oxide in Bioactive and Antibacterial Properties of Acrylic Bone Cements. Biomolecules 2020, 10, 1616. [Google Scholar] [CrossRef]
- Yılmaz, E.; Çakıroğlu, B.; Gökçe, A.; Findik, F.; Gulsoy, H.O.; Gulsoy, N.; Mutlu, Ö.; Özacar, M. Novel hydroxyapatite/graphene oxide/collagen bioactive composite coating on Ti16Nb alloys by electrodeposition. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 101, 292–305. [Google Scholar] [CrossRef]
- Kalbacova, M.; Broza, A.; Kong, J.; Kalbac, M. Graphene substrates promote adherence of human osteoblasts and mesenchymal stromal cells. Carbon 2010, 48, 4323–4329. [Google Scholar] [CrossRef]
- Nayak, T.R.; Andersen, H.; Makam, V.S.; Khaw, C.; Bae, S.; Xu, X.; Ee, P.L.; Ahn, J.H.; Hong, B.H.; Pastorin, G.; et al. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano 2011, 5, 4670–4678. [Google Scholar] [CrossRef]
- Gallorini, M.; Di Carlo, R.; Pilato, S.; Ricci, A.; Schweikl, H.; Cataldi, A.; Fontana, A.; Zara, S. Liposomes embedded with differentiating factors as a new strategy for enhancing DPSC osteogenic commitment. Eur. Cell Mater. 2021, 41, 108–120. [Google Scholar] [CrossRef]
- Paduano, F.; Aiello, E.; Cooper, P.R.; Marrelli, B.; Makeeva, I.; Islam, M.; Spagnuolo, G.; Maged, D.; De Vito, D.; Tatullo, M. A Dedifferentiation Strategy to Enhance the Osteogenic Potential of Dental Derived Stem Cells. Front. Cell Dev. Biol. 2021, 9, 668558. [Google Scholar] [CrossRef] [PubMed]
- Langenbach, F.; Handschel, J. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res. Ther. 2013, 4, 117. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, N.; Kim, J. Quantum dots attached to graphene oxide for sensitive detection of ascorbic acid in aqueous solutions. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 720–725. [Google Scholar] [CrossRef]
- Krukiewicz, K.; Putzer, D.; Stuendl, N.; Lohberger, B.; Awaja, F. Enhanced Osteogenic Differentiation of Human Primary Mesenchymal Stem and Progenitor Cultures on Graphene Oxide/Poly(methyl methacrylate) Composite Scaffolds. Materials 2020, 13, 2991. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Jeong, S.J.; Lee, S.H.; Kim, B.; Hong, S.W.; Lee, J.H.; Han, D.W. Reduced graphene oxide coating enhances osteogenic differentiation of human mesenchymal stem cells on Ti surfaces. Biomater. Res. 2021, 25, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xiang, S.; Lin, Z.; Li, E.N.; Yagi, H.; Cao, G.; Yocum, L.; Li, L.; Hao, T.; Bruce, K.K.; et al. Graphene oxide-functionalized nanocomposites promote osteogenesis of human mesenchymal stem cells via enhancement of BMP-SMAD1/5 signaling pathway. Biomaterials 2021, 277, 121082. [Google Scholar] [CrossRef]
- Kang, E.S.; Kim, H.; Han, Y.; Cho, Y.W.; Son, H.; Luo, Z.; Kim, T.H. Enhancing osteogenesis of adipose-derived mesenchymal stem cells using gold nanostructure/peptide-nanopatterned graphene oxide. Colloids Surf. B Biointerfaces 2021, 204, 111807. [Google Scholar] [CrossRef]
- Preethi Soundarya, S.; Haritha Menon, A.; Viji Chandran, S.; Selvamurugan, N. Bone tissue engineering: Scaffold preparation using chitosan and other biomaterials with different design and fabrication techniques. Int. J. Biol. Macromol. 2018, 119, 1228–1239. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, S.; Li, J.; Guo, K.; Yuan, Q.; Zhong, A.; Yang, J.; Wang, J.; Sun, J.; Wang, Z. Collagen Functionalized with Graphene Oxide Enhanced Biomimetic Mineralization and in Situ Bone Defect Repair. ACS Appl. Mater. Interfaces 2018, 10, 44080–44091. [Google Scholar] [CrossRef]
- Bahrami, S.; Baheiraei, N.; Shahrezaee, M. Biomimetic reduced graphene oxide coated collagen scaffold for in situ bone regeneration. Sci. Rep. 2021, 11, 16783. [Google Scholar] [CrossRef]
- Fu, C.; Jiang, Y.; Yang, X.; Wang, Y.; Ji, W.; Jia, G. Mussel-Inspired Gold Nanoparticle and PLGA/L-Lysine-g-Graphene Oxide Composite Scaffolds for Bone Defect Repair. Int. J. Nanomed. 2021, 16, 6693–6718. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.D.; Park, J.; Lee, E.S.; Kim, E.; Lee, S.S.; Yang, J.K.; Lee, Y.S.; Hwang, N.S. Enhanced osteogenic commitment of murine mesenchymal stem cells on graphene oxide substrate. Biomater. Res. 2018, 22, 1. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.; Awale, G.; Daneshmandi, L.; Umerah, O.; Lo, K.W. The roles of ions on bone regeneration. Drug Discov. Today 2018, 23, 879–890. [Google Scholar] [CrossRef]
- Arnold, A.M.; Holt, B.D.; Daneshmandi, L.; Laurencin, C.T.; Sydlik, S.A. Phosphate graphene as an intrinsically osteoinductive scaffold for stem cell-driven bone regeneration. Proc. Natl. Acad. Sci. USA 2019, 116, 4855–4860. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Jiang, D.; Wang, T.; Wang, Y.; Lou, Y.; Zhang, Y.; Ma, H.; Kang, Y. Mechanical Stress Regulates Osteogenesis and Adipogenesis of Rat Mesenchymal Stem Cells through PI3K/Akt/GSK-3β/β-Catenin Signaling Pathway. Biomed. Res. Int. 2017, 2017, 6027402. [Google Scholar] [CrossRef] [PubMed]
- Newby, S.D.; Masi, T.; Griffin, C.D.; King, W.J.; Chipman, A.; Stephenson, S.; Anderson, D.E.; Biris, A.S.; Bourdo, S.E.; Dhar, M. Functionalized Graphene Nanoparticles Induce Human Mesenchymal Stem Cells to Express Distinct Extracellular Matrix Proteins Mediating Osteogenesis. Int. J. Nanomed. 2020, 15, 2501–2513. [Google Scholar] [CrossRef]
- Felgueiras, H.P.; Evans, M.D.M.; Migonney, V. Contribution of fibronectin and vitronectin to the adhesion and morphology of MC3T3-E1 osteoblastic cells to poly(NaSS) grafted Ti6Al4V. Acta Biomater. 2015, 28, 225–233. [Google Scholar] [CrossRef]
- Steward, A.J.; Kelly, D.J. Mechanical regulation of mesenchymal stem cell differentiation. J. Anat. 2015, 227, 717–731. [Google Scholar] [CrossRef]
- Mathieu, P.S.; Loboa, E.G. Cytoskeletal and focal adhesion influences on mesenchymal stem cell shape, mechanical properties, and differentiation down osteogenic, adipogenic, and chondrogenic pathways. Tissue Eng. Part B Rev. 2012, 18, 436–444. [Google Scholar] [CrossRef]
- Gallorini, M.; Zara, S.; Ricci, A.; Mangano, F.G.; Cataldi, A.; Mangano, C. The Open Cell Form of 3D-Printed Titanium Improves Osteconductive Properties and Adhesion Behavior of Dental Pulp Stem Cells. Materials 2021, 14, 5308. [Google Scholar] [CrossRef]
- Kim, H.D.; Kim, J.; Koh, R.H.; Shim, J.; Lee, J.C.; Kim, T.I.; Hwang, N.S. Enhanced Osteogenic Commitment of Human Mesenchymal Stem Cells on Polyethylene Glycol-Based Cryogel with Graphene Oxide Substrate. ACS Biomater. Sci. Eng. 2017, 3, 2470–2479. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Cao, T.; Franco-Obregón, A.; Rosa, V. Graphene-Induced Osteogenic Differentiation Is Mediated by the Integrin/FAK Axis. Int. J. Mol. Sci. 2019, 20, 574. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Z. Involvement of FAK/P38 Signaling Pathways in Mediating the Enhanced Osteogenesis Induced by Nano-Graphene Oxide Modification on Titanium Implant Surface. Int. J. Nanomed. 2020, 15, 4659–4676. [Google Scholar] [CrossRef]
- Tahriri, M.; Del Monico, M.; Moghanian, A.; Tavakkoli Yaraki, M.; Torres, R.; Yadegari, A.; Tayebi, L. Graphene and its derivatives: Opportunities and challenges in dentistry. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, R.; Zara, S.; Ventrella, A.; Siani, G.; Da Ros, T.; Iezzi, G.; Cataldi, A.; Fontana, A. Covalent Decoration of Cortical Membranes with Graphene Oxide as a Substrate for Dental Pulp Stem Cells. Nanomaterials 2019, 9, 604. [Google Scholar] [CrossRef]
- Di Crescenzo, A.; Zara, S.; Di Nisio, C.; Ettorre, V.; Ventrella, A.; Zavan, B.; Di Profio, P.; Cataldi, A.; Fontana, A. Graphene Oxide Foils as an Osteoinductive Stem Cell Substrate. ACS Appl. Bio Mater. 2019, 2, 1643–1651. [Google Scholar] [CrossRef]
- Yang, N.; Liu, Y. The Role of the Immune Microenvironment in Bone Regeneration. Int. J. Med. Sci. 2021, 18, 3697–3707. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chen, G.; Liu, M.; Xu, Z.; Chen, H.; Yang, L.; Lv, Y. Scaffold strategies for modulating immune microenvironment during bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110411. [Google Scholar] [CrossRef]
- Omar, O.M.; Granéli, C.; Ekström, K.; Karlsson, C.; Johansson, A.; Lausmaa, J.; Wexell, C.L.; Thomsen, P. The stimulation of an osteogenic response by classical monocyte activation. Biomaterials 2011, 32, 8190–8204. [Google Scholar] [CrossRef][Green Version]
- Bordoni, V.; Reina, G.; Orecchioni, M.; Furesi, G.; Thiele, S.; Gardin, C.; Zavan, B.; Cuniberti, G.; Bianco, A.; Rauner, M.; et al. Stimulation of bone formation by monocyte-activator functionalized graphene oxide in vivo. Nanoscale 2019, 11, 19408–19421. [Google Scholar] [CrossRef]
- Su, J.; Du, Z.; Xiao, L.; Wei, F.; Yang, Y.; Li, M.; Qiu, Y.; Liu, J.; Chen, J.; Xiao, Y. Graphene oxide coated Titanium Surfaces with Osteoimmunomodulatory Role to Enhance Osteogenesis. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 113, 110983. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.K.; Lu, Y.C.; Yeh, S.T.; Lin, T.C.; Huang, C.H.; Huang, C.H. In vitro and in vivo Biological Responses to Graphene and Graphene Oxide: A Murine Calvarial Animal Study. Int. J. Nanomed. 2020, 15, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.M.; Jackson, D.W. Articular Cartilage: Injury Pathways and Treatment Options. Sports Med. Arthrosc. Rev. 2018, 26, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Armiento, A.R.; Stoddart, M.J.; Alini, M.; Eglin, D. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018, 65, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Solchaga, L.A.; Penick, K.J.; Welter, J.F. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells: Tips and tricks. Methods Mol. Biol. 2011, 698, 253–278. [Google Scholar]
- Shen, H.; Lin, H.; Sun, A.X.; Song, S.; Zhang, Z.; Dai, J.; Tuan, R.S. Chondroinductive factor-free chondrogenic differentiation of human mesenchymal stem cells in graphene oxide-incorporated hydrogels. J. Mater. Chem. B 2018, 6, 908–917. [Google Scholar] [CrossRef]
- Pogue, R.; Lyons, K. BMP signaling in the cartilage growth plate. Curr. Top. Dev. Biol. 2006, 76, 1–48. [Google Scholar]
- Song, B.; Estrada, K.D.; Lyons, K.M. Smad signaling in skeletal development and regeneration. Cytokine Growth Factor Rev. 2009, 20, 379–388. [Google Scholar] [CrossRef]
- Mueller, M.B.; Fischer, M.; Zellner, J.; Berner, A.; Dienstknecht, T.; Prantl, L.; Kujat, R.; Nerlich, M.; Tuan, R.S.; Angele, P. Hypertrophy in mesenchymal stem cell chondrogenesis: Effect of TGF-beta isoforms and chondrogenic conditioning. Cells Tissues Organs 2010, 192, 158–166. [Google Scholar] [CrossRef]
- Deliormanlı, A.M. Direct Write Assembly of Graphene/Poly(ε-Caprolactone) Composite Scaffolds and Evaluation of Their Biological Performance Using Mouse Bone Marrow Mesenchymal Stem Cells. Appl. Biochem. Biotechnol. 2019, 188, 1117–1133. [Google Scholar] [CrossRef]
- Olate-Moya, F.; Arens, L.; Wilhelm, M.; Mateos-Timoneda, M.A.; Engel, E.; Palza, H. Chondroinductive Alginate-Based Hydrogels Having Graphene Oxide for 3D Printed Scaffold Fabrication. ACS Appl. Mater. Interfaces 2020, 12, 4343–4357. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Silva, E.A.; Mooney, D.J. Growth factor delivery-based tissue engineering: General approaches and a review of recent developments. J. R. Soc. Interface 2011, 8, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.K.; Richard, C.; Bessodes, M.; Scherman, D.; Merten, O.W. Growth factor delivery approaches in hydrogels. Biomacromolecules 2009, 10, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.H.; Bhang, S.H.; Kim, T.; Yu, T.; Hyeon, T.; Kim, B.S. Dual roles of graphene oxide in chondrogenic differentiation of adult stem cells: Cell-adhesion substrate and growth factor-delivery carrier. Adv. Funct. Mater. 2014, 24, 6455–6464. [Google Scholar] [CrossRef]
- Zhou, M.; Lozano, N.; Wychowaniec, J.K.; Hodgkinson, T.; Richardson, S.M.; Kostarelos, K.; Hoyland, J.A. Graphene oxide: A growth factor delivery carrier to enhance chondrogenic differentiation of human mesenchymal stem cells in 3D hydrogels. Acta Biomater. 2019, 96, 271–280. [Google Scholar] [CrossRef]
- Jiao, D.; Wang, J.; Yu, W.; Zhang, N.; Zhang, K.; Bai, Y. Gelatin reduced Graphene Oxide Nanosheets as Kartogenin Nanocarrier Induces Rat ADSCs Chondrogenic Differentiation Combining with Autophagy Modification. Materials 2021, 14, 1053. [Google Scholar] [CrossRef]
- Cai, G.; Liu, W.; He, Y.; Huang, J.; Duan, L.; Xiong, J.; Liu, L.; Wang, D. Recent advances in kartogenin for cartilage regeneration. J. Drug Target. 2019, 27, 28–32. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricci, A.; Cataldi, A.; Zara, S.; Gallorini, M. Graphene-Oxide-Enriched Biomaterials: A Focus on Osteo and Chondroinductive Properties and Immunomodulation. Materials 2022, 15, 2229. https://doi.org/10.3390/ma15062229
Ricci A, Cataldi A, Zara S, Gallorini M. Graphene-Oxide-Enriched Biomaterials: A Focus on Osteo and Chondroinductive Properties and Immunomodulation. Materials. 2022; 15(6):2229. https://doi.org/10.3390/ma15062229
Chicago/Turabian StyleRicci, Alessia, Amelia Cataldi, Susi Zara, and Marialucia Gallorini. 2022. "Graphene-Oxide-Enriched Biomaterials: A Focus on Osteo and Chondroinductive Properties and Immunomodulation" Materials 15, no. 6: 2229. https://doi.org/10.3390/ma15062229
APA StyleRicci, A., Cataldi, A., Zara, S., & Gallorini, M. (2022). Graphene-Oxide-Enriched Biomaterials: A Focus on Osteo and Chondroinductive Properties and Immunomodulation. Materials, 15(6), 2229. https://doi.org/10.3390/ma15062229








