Structure and Photoluminescence Properties of Dy3+ Doped Phosphor with Whitlockite Structure
Abstract
:1. Introduction
2. Materials and Methods
3. Results
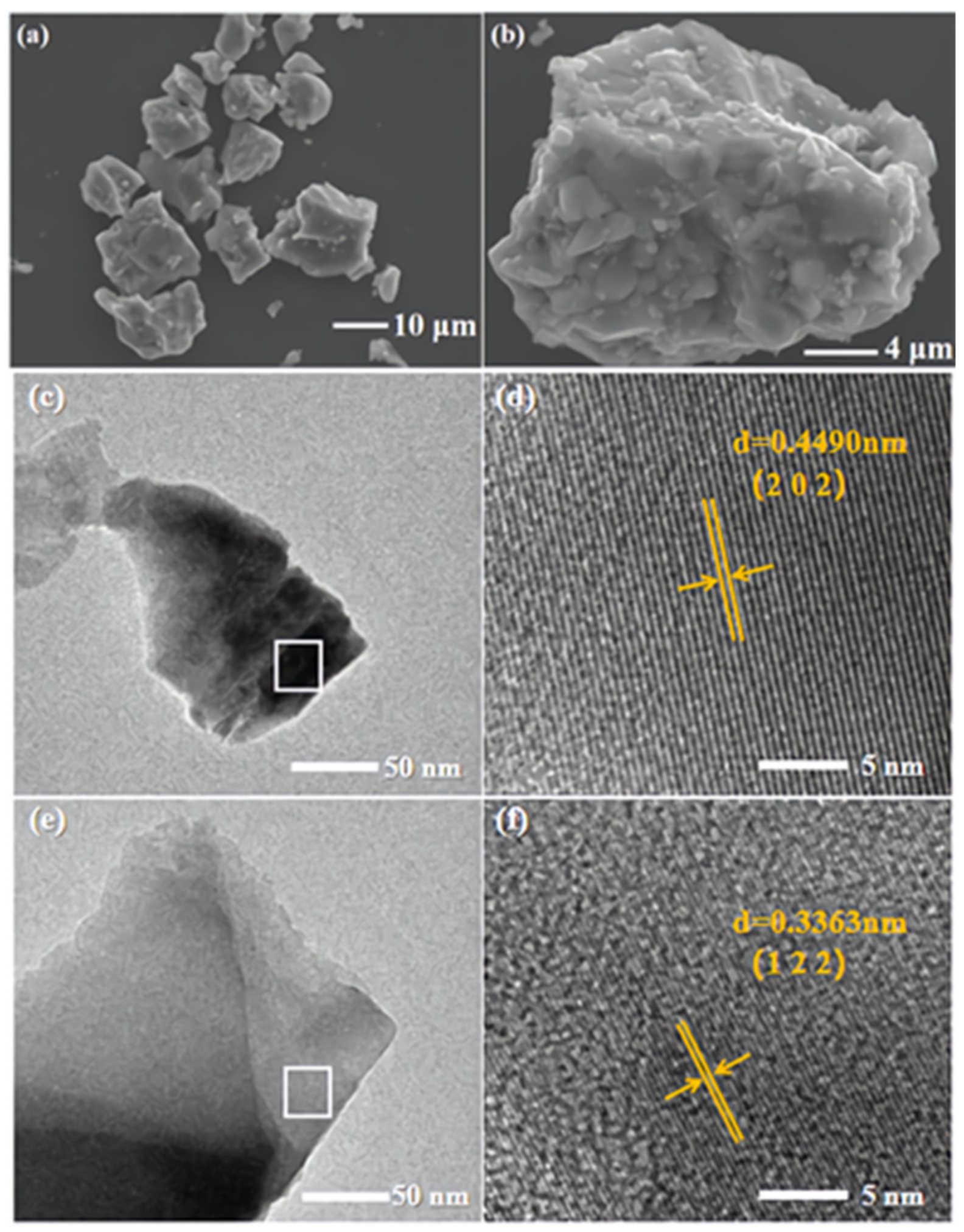
3.1. Crystal Structure
3.2. CIE
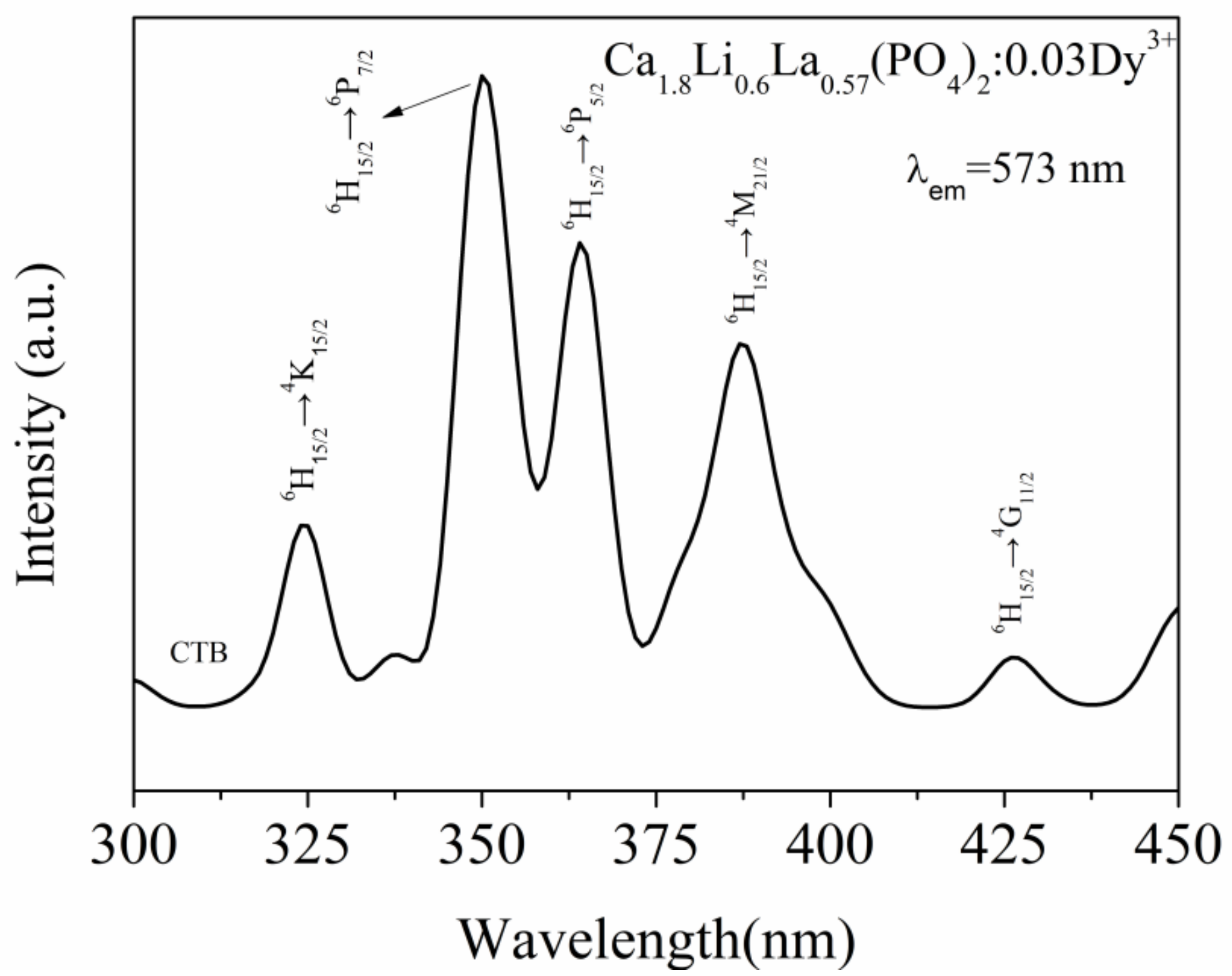
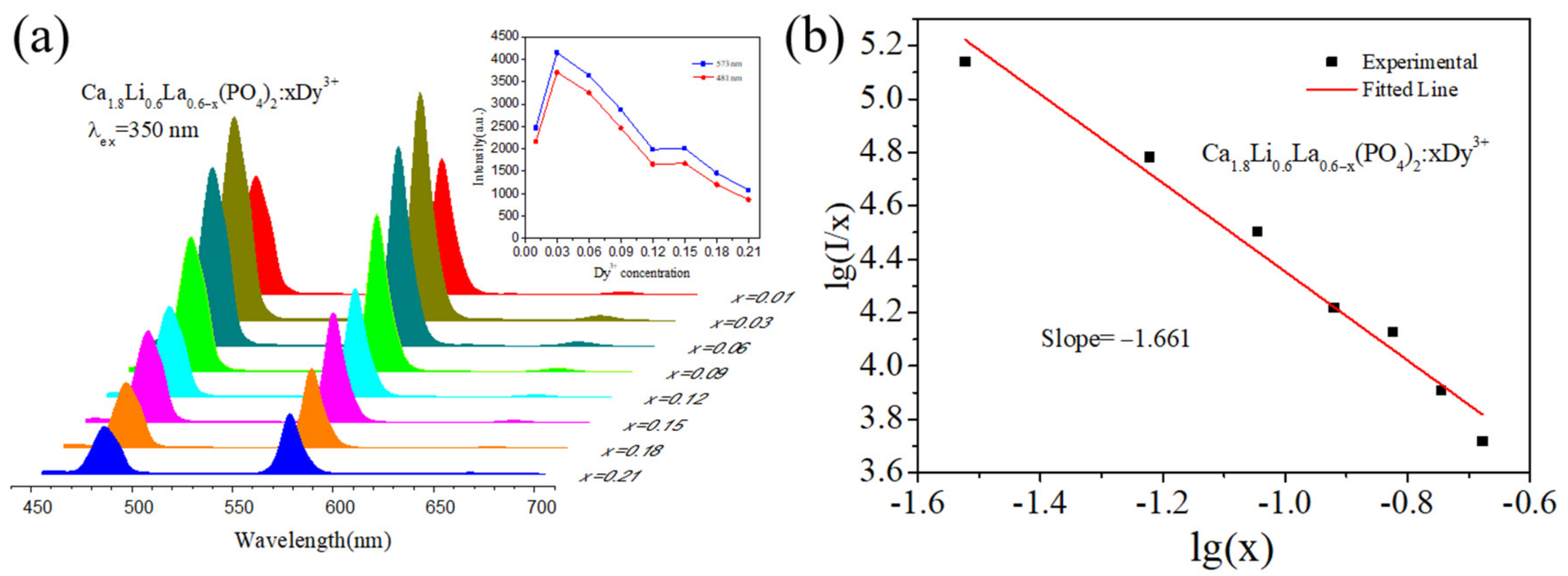
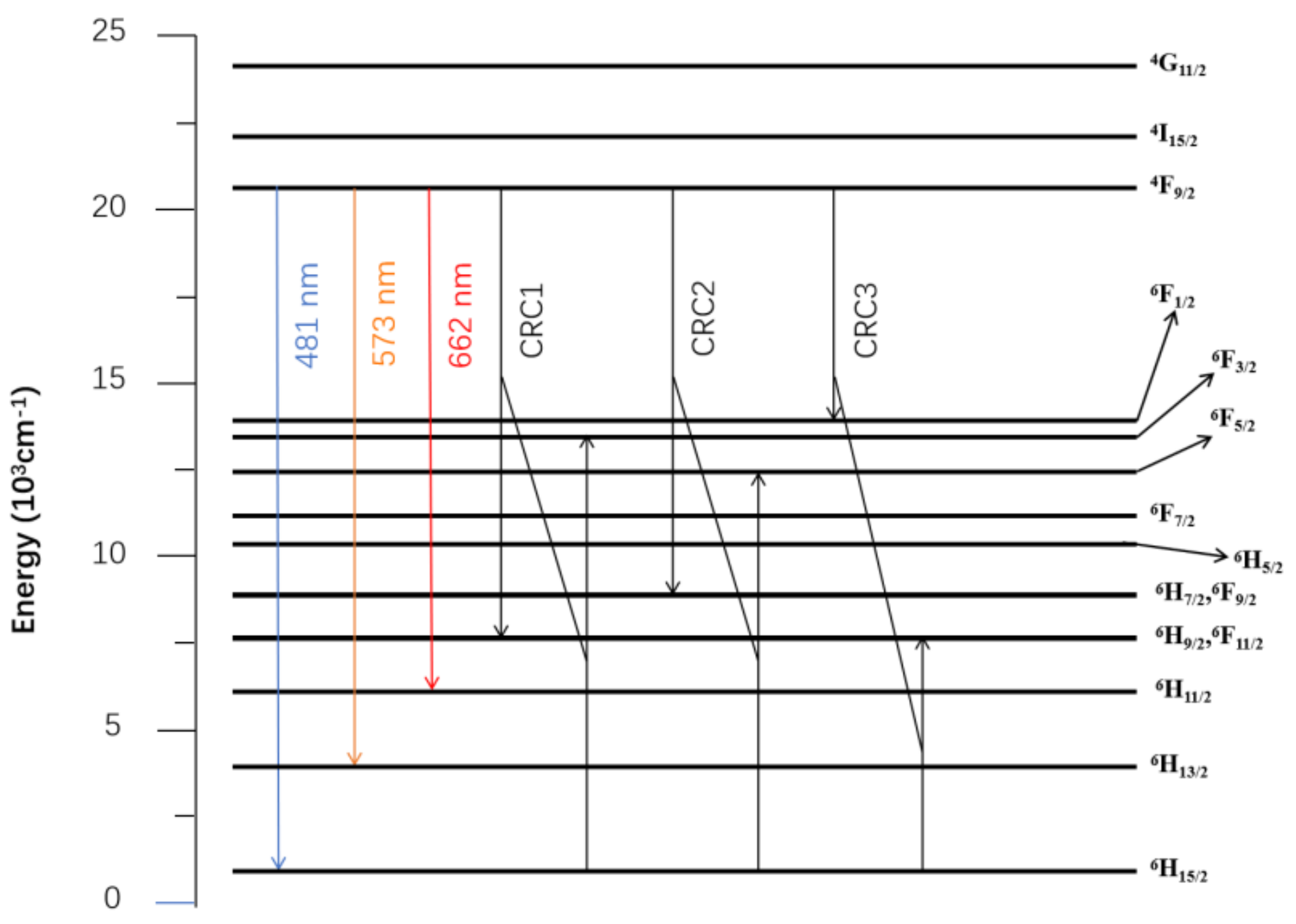
3.3. Photoluminescence Characteristics
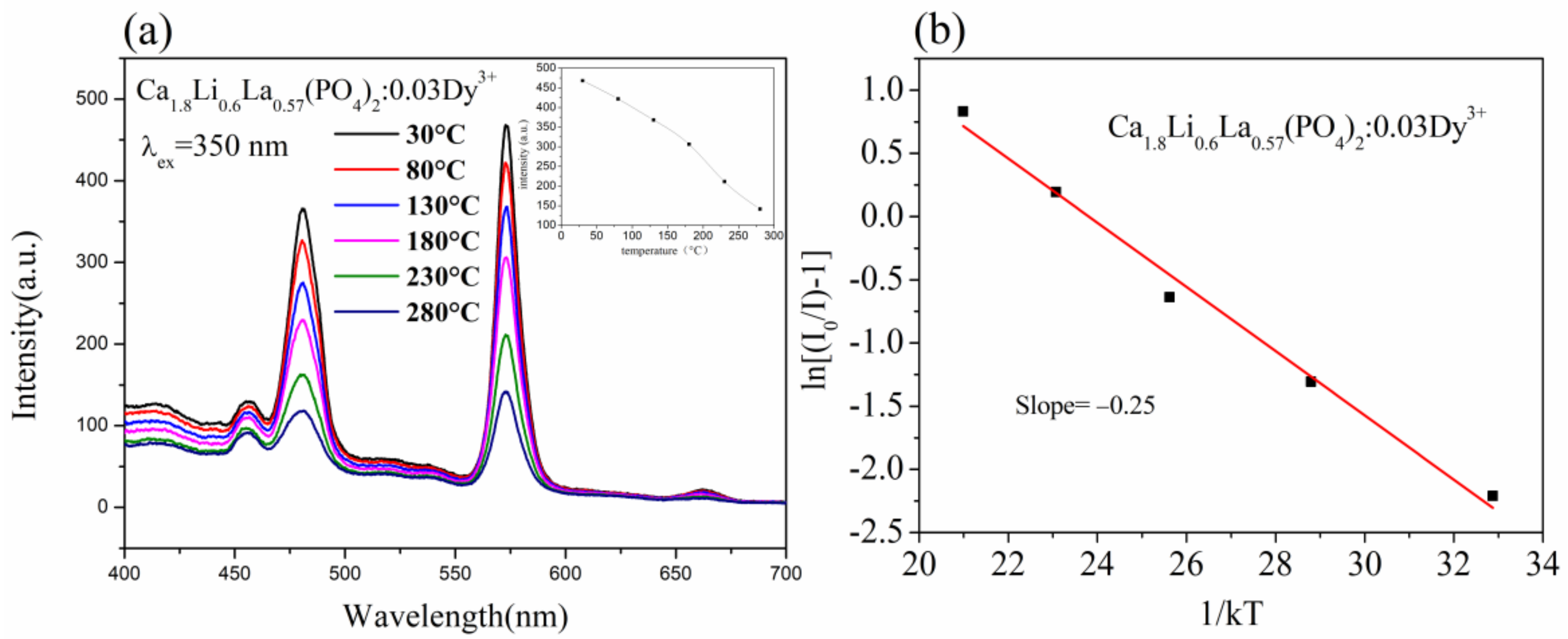
3.4. Thermal Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yi, J.H. Development of samarium–cobalt rare earth permanent magnetic materia. Rare Met. 2014, 33, 633–640. [Google Scholar] [CrossRef]
- Guo, Q.F.; Chen, Y.Y.; Hao, Q.; Stefan, L.; Liu, H.K.; Mei, L.F.; Liao, L.B. Crystal structure and luminescence properties of a thermally stable single-phase white emitting phosphor CaSr2(PO4)2:Dy3+,Li+. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Wang, T.; Hu, Y.; Chen, L.; Wang, X.; He, M. An intense single-component warm-white-light Sr3Lu(PO4)3:Dy3+ phosphor for white UV-LEDs. J. Mater. Sci. Mater. Electron. 2016, 27, 13235–13241. [Google Scholar] [CrossRef]
- Fan, G.; Ning, R.; Li, X.; Lin, X.; Du, B.; Luo, J.; Zhang, X. Mussel-Inspired Immobilization of Photocatalysts with Synergistic Photocatalytic–Photothermal Performance for Water Remediation. ACS Appl. Mater. Interfaces 2021, 13, 31066–31076. [Google Scholar] [CrossRef]
- Mahdavi, K.; Zinatloo-Ajabshir, S.; Yousif, Q.A.; Salavati-Niasari, M. Enhanced photocatalytic degradation of toxic contaminants using Dy2O3-SiO2 ceramic nanostructured materials fabricated by a new, simple and rapid sonochemical approach. Ultrason. Sonochem. 2021, 82, 105892. [Google Scholar] [CrossRef]
- Cani, D.; van der Waal, J.C.; Pescarmona, P.P. Highly-accessible, doped TiO2 nanoparticles embedded at the surface of SiO2 as photocatalysts for the degradation of pollutants under visible and UV radiation. Appl. Catal. A Gen. 2021, 621, 118179. [Google Scholar] [CrossRef]
- Janjua, M.R.S.A. Hydrogen as an energy currency: Encapsulation of inorganic Ga12N12 with alkali metals for efficient H2 adsorption as hydrogen storage materials. J. Phys. Chem. Solids 2021, 160, 110352. [Google Scholar] [CrossRef]
- Jung, G.W.; Park, K. Effect of monovalent charge compensators on the photoluminescence properties of Ca3(PO4)2:Tb3+, A+(A = Li, Na, K) phosphors. J. Mater. Sci. Technol. 2021, 82, 187–196. [Google Scholar] [CrossRef]
- Kim, D.; Bo, R.L.; Park, S.H. Study on Na3Lu1−xEux(PO4)2 phosphor: High efficient Na3Eu(PO4)2 red emitting phosphor with excellent thermal stability. J. Alloys Compd. 2019, 805, 346–354. [Google Scholar] [CrossRef]
- Park, K.; Kim, D.H.; Hakeem, D.A.; Jung, G.W.; Kim, S.W. Crystal structure and photoluminescence properties of garnet type as synthesized and aged Li7La3Zr2O12:xTb phosphors. Ceram. Int. 2020, 46, 10194–10202. [Google Scholar] [CrossRef]
- Park, K.; Hakeem, D.A.; Kim, D.H.; Jung, G.W.; Kim, S.W. Synthesis and Photoluminescence Properties of New Garnet-Type Red-Emitting Li7La3−XZr2O12:xEu3+ Phosphors. SSRN Electron. J. 2019, 179, 92–98. [Google Scholar]
- Han, Y.J.; Wang, S.; Liu, H.; Shi, L.; Ma, D.; Cui, X.Y.; Mao, Z.Y.; Wang, D.J.; Zhang, Z.W.; Lu, X.L. A novel Mn4+-activated garnet-type Li5La3Nb2O12 far red-emitting phosphor with high thermal stability for plant cultivation. J. Lumin. 2020, 219, 116888. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, W.; Li, X.; Pang, Y.; Wang, L.; Zhang, Q. Role of energy band structure on the luminescence performance of double perovskite La2LiMO6:Eu3+ (M = Sb, Ta, Nb) red emitting phosphors. Opt. Mater. 2020, 107, 110135. [Google Scholar] [CrossRef]
- Yu, B.; Li, Y.; Zhang, R.; Li, H.; Wang, Y. A novel thermally stable eulytite-type NaBaBi2(PO4)3:Eu3+ red-emitting phosphor for pc-WLEDs. J. Alloys Compd. 2021, 852, 157020. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, J.; Li, Z.; Yang, X.; Chen, J.; Chen, C. Luminescence properties of NaSrPO4:Tm3+ as novel blue emitting phosphors with high color purity. Optik 2018, 169, 257–263. [Google Scholar] [CrossRef]
- Nagaraj, R.; Rajagopal, V.; Raja, A.; Ranjith, S. Influence of Dy3+ ion concentration on photoluminescence and energy transfer mechanism of promising KBaScSi3O9 phosphors for warm white LEDs. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 264, 120212. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, C.; Liang, Z.; Wang, Q.; Feng, J.; Zhu, D.; Pan, S.; Lin, Z.; Tan, D.; Mu, Z. Photoluminescence properties of LaB3O6:Dy3+ phosphors for white light-emitting diodes. Optik 2020, 216, 164877. [Google Scholar] [CrossRef]
- Golim, O.P.; Huang, S.; Yang, T. Greenish-yellow emitting Ca9MgLi(PO4)7:Dy3+ phosphors-Photoluminescence and thermal stability. J. Lumin. 2021, 229, 117675. [Google Scholar] [CrossRef]
- Dai, T.; Ju, G.; Jin, Y.; Zhou, X.; Li, Y.; Wu, H.; Hu, Z.; Hu, Y. Novel yellow color-emitting BaY2O4:Dy3+ phosphors: Persistent luminescence from blue to red. Appl. Phys. A 2020, 126, 1–9. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Ping, D. Luminescent properties of Dy3+ doped Sr3Y(PO4)3 for white LEDs. Mater. Lett. 2013, 107, 96–98. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, C.L.; Shi, W.W. Enhanced novel white emission in Ca3(PO4)2:Dy3+ single-phase full-color phosphor by charge compensation. J. Mater. Sci. Mater. Electron. 2015, 26, 1923–1931. [Google Scholar] [CrossRef]
- Berends, A.C.; Haar, M.; Krames, M.R. YAG:Ce3+ Phosphor: From Micron-Sized Workhorse for General Lighting to a Bright Future on the Nanoscale. Chem. Rev. 2020, 120, 13461–13479. [Google Scholar] [CrossRef]
- Gao, S.; Xu, S.; Wang, Y. Spectral characteristics and White Emission of Dy3+/Tm3+-BaLaGa3O7 phosphors. J. Lumin. 2016, 178, 282–287. [Google Scholar] [CrossRef]
- Yu, X.; Yang, P.; Kang, W.; Song, R.; Zheng, Y.; Mi, X. Color-tunable luminescence and energy transfer properties of Ca1.5Sr1.5(PO4)2:Dy3+, Tb3+ phosphor via hydrothermal synthesis. J. Lumin. 2021, 241, 118478. [Google Scholar] [CrossRef]
- Blasse, G. Energy transfer in oxidic phosphors. Phys. Lett. A 1968, 28, 444–445. [Google Scholar] [CrossRef]
- Dexter, D.L.; Schulman, J.H. Theory of Concentration Quenching in Inorganic Phosphors. J. Chem. Phys. 1954, 22, 1063–1070. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Ding, X.; Wang, Y. A Potential Red-Emitting Phosphor BaZrGe3O9:Eu3+ for WLED and FED Applications: Synthesis, Structure, and Luminescence Properties. Inorg. Chem. 2017, 56, 6990–6998. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, C.; Lu, J.; Sun, R.; Wang, Z.; Yin, Y.; Liu, W.; Gu, C.; Li, Z.; Zhong, D.; et al. Single-phase white-emitting phosphors Ba3Bi(PO4)3:Dy3+, Eu3+ with tunable correlated color temperature and high thermal stability towards light emitting applications. J. Mater. Sci. Mater. Electron. 2021, 32, 28077–28087. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Q.; Fang, Y.; Zhang, N.; Yang, B.; Zhao, G. White light emission from NaLa(PO3)4:Dy3+ single-phase phosphors for light-emitting diodes. Ceram. Int. 2015, 41, 1917–1920. [Google Scholar] [CrossRef]
- Dillip, G.R.; Kumar, G.B.; Bandi, V.R.; Hareesh, M.; Raju, B.D.P.; Joo, S.W.; Bharat, L.K.; Yu, J.S. Versatile host-sensitized white light emission in a single-component K3ZnB5O10:Dy3+ phosphor for ultraviolet converted light-emitting diodes. J. Alloys Compd. 2017, 699, 1108–1117. [Google Scholar] [CrossRef]


| Sample | x = 0.00 | x = 0.01 | x = 0.03 | x = 0.06 | x = 0.09 | x = 0.12 | x = 0.15 | x = 0.18 | x = 0.21 |
|---|---|---|---|---|---|---|---|---|---|
| Li2CO3 (g) | 0.0443 | 0.0443 | 0.0443 | 0.0443 | 0.0443 | 0.0443 | 0.0443 | 0.0443 | 0.0443 |
| La2O3 (g) | 0.1955 | 0.1922 | 0.1857 | 0.1759 | 0.1662 | 0.1564 | 0.1466 | 0.1368 | 0.1271 |
| CaCO3 (g) | 0.3603 | 0.3603 | 0.3603 | 0.3603 | 0.3603 | 0.3603 | 0.3603 | 0.3603 | 0.3603 |
| Dy2O3 (g) | 0.0000 | 0.0037 | 0.0112 | 0.0224 | 0.0336 | 0.0448 | 0.0559 | 0.0671 | 0.0783 |
| NH4H2PO4 (g) | 0.4601 | 0.4601 | 0.4601 | 0.4601 | 0.4601 | 0.4601 | 0.4601 | 0.4601 | 0.4601 |
| Sample | Standard | x = 0 | x = 0.01 | x = 0.03 | x = 0.06 | x = 0.09 | x = 0.12 | x = 0.15 | x = 0.18 | x = 0.21 |
|---|---|---|---|---|---|---|---|---|---|---|
| a (Å) | 10.429 | 10.435 | 10.411 | 10.407 | 10.419 | 10.431 | 10.423 | 10.428 | 10.412 | 10.402 |
| c (Å) | 37.380 | 37.349 | 37.433 | 37.418 | 37.319 | 37.354 | 37.278 | 37.270 | 37.232 | 37.173 |
| Cell volume (Å) | 3520.90 | 3521.97 | 3513.70 | 3509.69 | 3508.22 | 3519.77 | 3507.12 | 3510.05 | 3495.80 | 3483.42 |
| Temperature (°C) | X | Y |
|---|---|---|
| 30 °C | 0.2750 | 0.3006 |
| 80 °C | 0.2753 | 0.3005 |
| 130 °C | 0.2756 | 0.3004 |
| 180 °C | 0.2738 | 0.2974 |
| 230 °C | 0.2668 | 0.2885 |
| 280 °C | 0.2568 | 0.2754 |
| Ca1.8Li0.6La0.6−x(PO4)2:xDy3+ | Ba3Bi(PO4)3:Dy3+, Eu3+ | SrLu(PO4)3:Dy3+ | K3ZnB5O10:Dy3+ | CaSr2(PO4)2:Dy3+, Li+ | NaLa(PO3)4:Dy3+ | |
|---|---|---|---|---|---|---|
| Concentration quenching | x = 0.03 | x = 0.08 | x = 0.08 | x = 0.05 | x = 0.06 | x = 0.06 |
| CIE Coordinate | (0.2750, 0.3006) | (0.3920, 0.3780) | (0.3740, 0.4070) | (0.2560, 0.2580) | (0.3450, 0.3787) | (0.2923, 0.3359) |
| Activation energy | 0.250 eV | 0.230 eV | 0.214 eV | 0.520 eV | Unspecified | Unspecified |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, W.; Guo, Q.; Su, K.; Liu, H.; Zhang, Y.; Mei, L.; Liao, L. Structure and Photoluminescence Properties of Dy3+ Doped Phosphor with Whitlockite Structure. Materials 2022, 15, 2177. https://doi.org/10.3390/ma15062177
Tang W, Guo Q, Su K, Liu H, Zhang Y, Mei L, Liao L. Structure and Photoluminescence Properties of Dy3+ Doped Phosphor with Whitlockite Structure. Materials. 2022; 15(6):2177. https://doi.org/10.3390/ma15062177
Chicago/Turabian StyleTang, Wanjuan, Qingfeng Guo, Ke Su, Haikun Liu, Yuanyuan Zhang, Lefu Mei, and Libing Liao. 2022. "Structure and Photoluminescence Properties of Dy3+ Doped Phosphor with Whitlockite Structure" Materials 15, no. 6: 2177. https://doi.org/10.3390/ma15062177
APA StyleTang, W., Guo, Q., Su, K., Liu, H., Zhang, Y., Mei, L., & Liao, L. (2022). Structure and Photoluminescence Properties of Dy3+ Doped Phosphor with Whitlockite Structure. Materials, 15(6), 2177. https://doi.org/10.3390/ma15062177







