Prediction of the Non-Isothermal Austenitization Kinetics of Fe-C-Cr Low Alloy Steels with Lamellar Pearlite Microstructure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental
2.2. Modeling
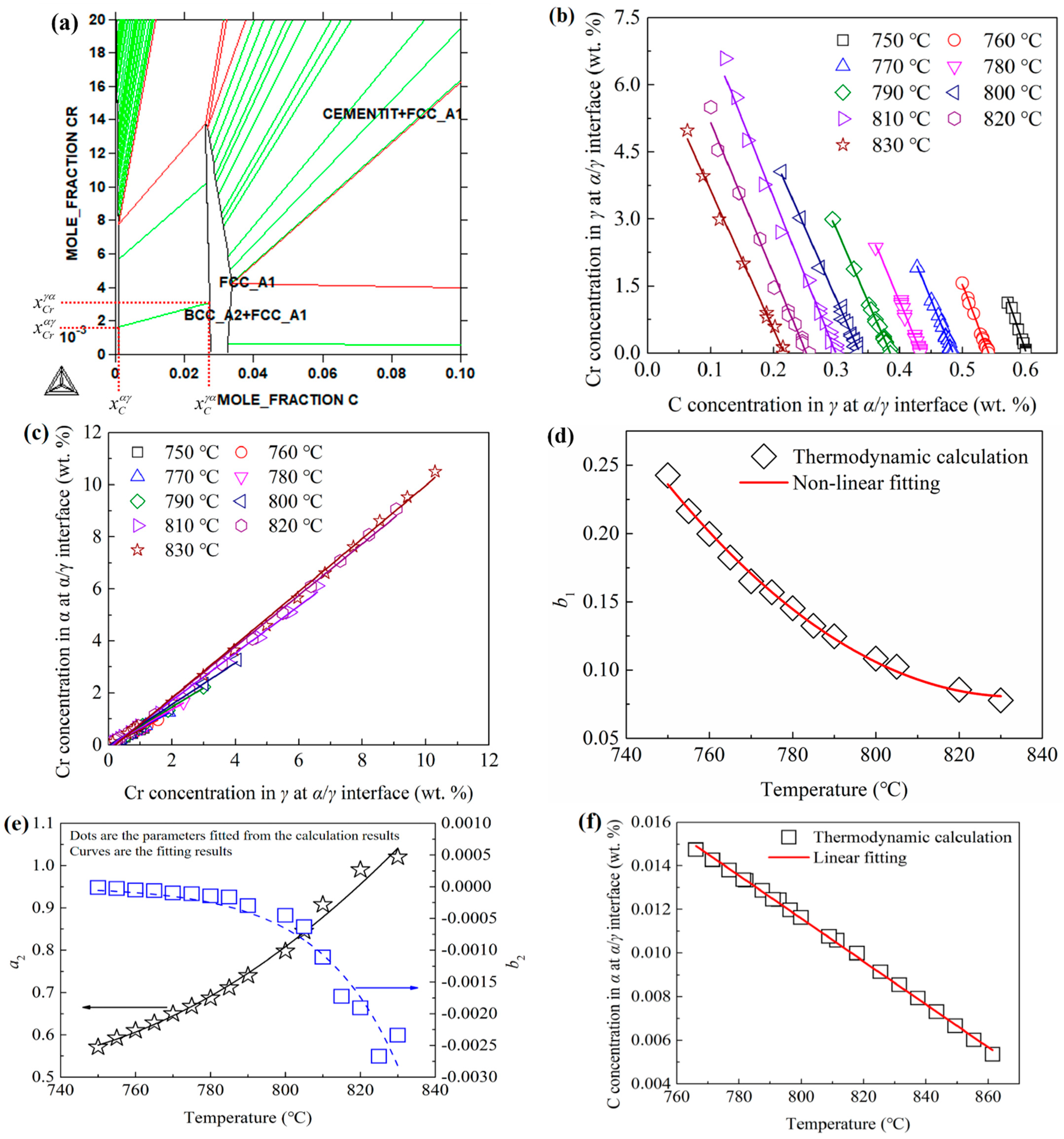
2.2.1. DICTRA Model
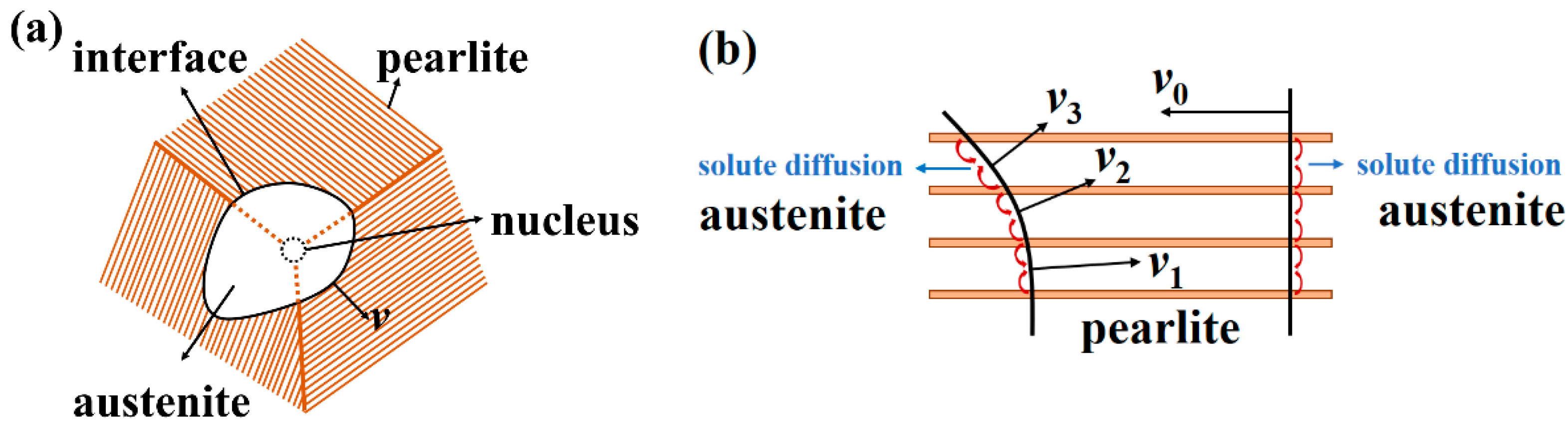
2.2.2. Cellular Automata Model
- Nucleation
- 2.
- Austenite/pearlite interface moving velocity
- 3.
- Cellular evolution rules
3. Results and Discussion
3.1. Experimental Results
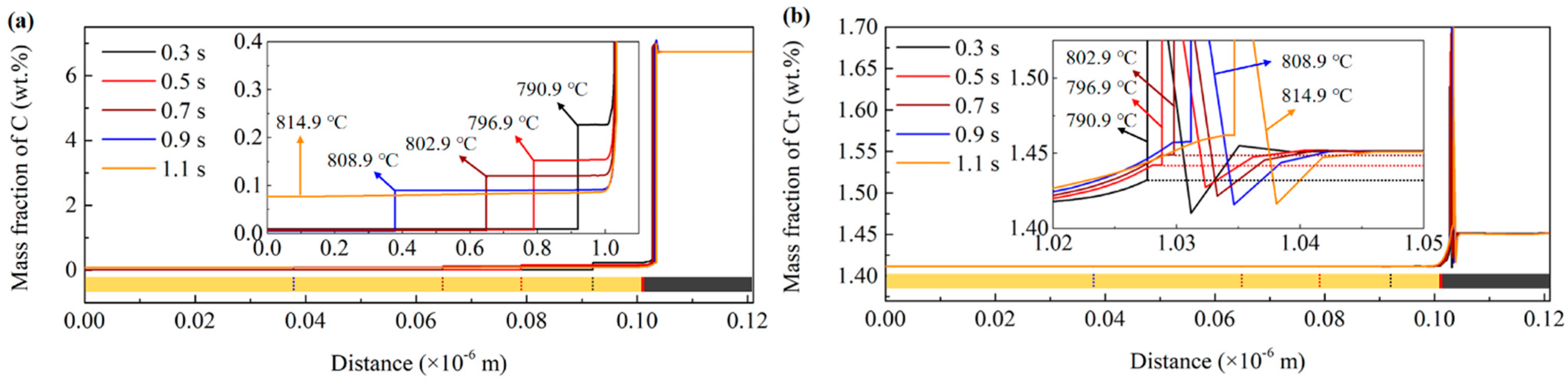
3.2. DICTRA Simulation Results
3.3. Cellular Automata Simulation Results
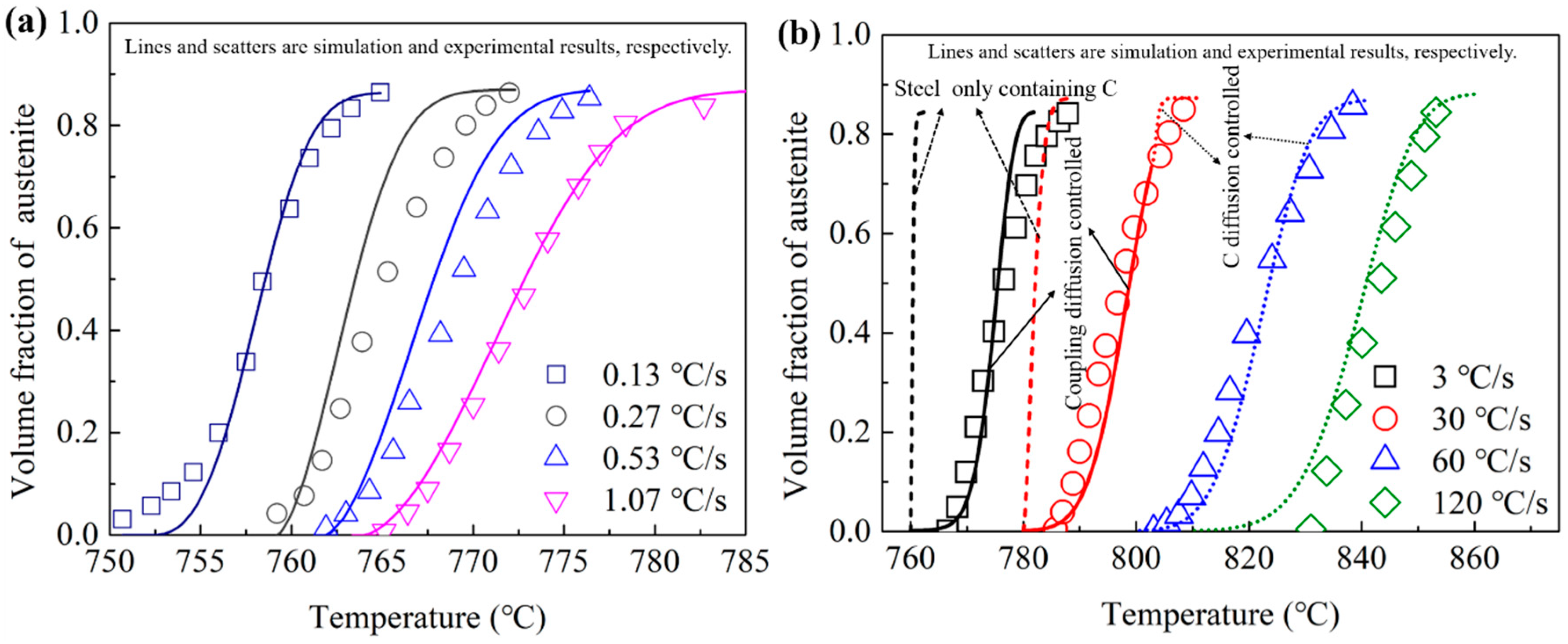
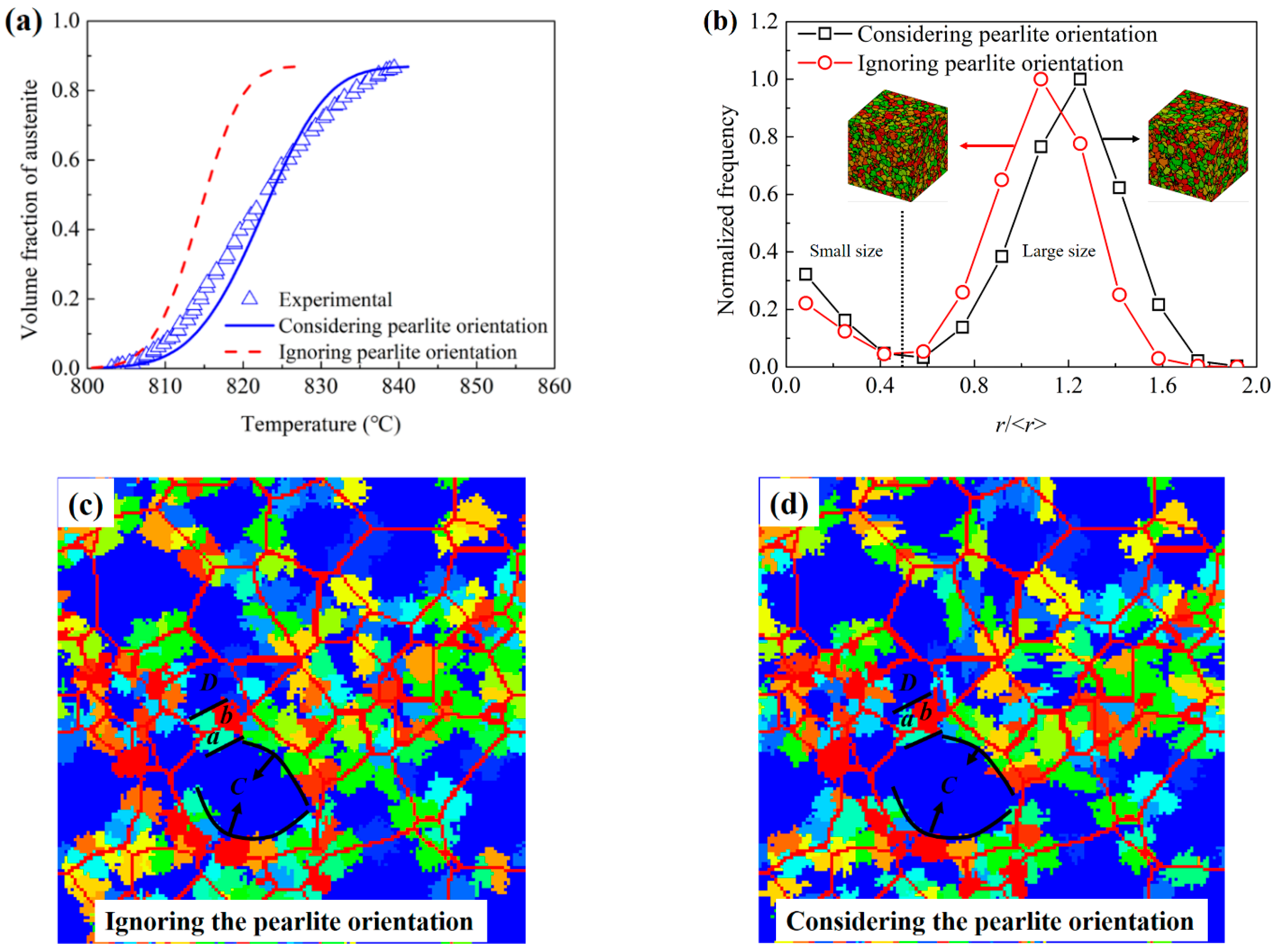
3.3.1. Comparisons between the Simulation Results and Experimental Results
3.3.2. Influences of Pearlite Lamellae Orientation
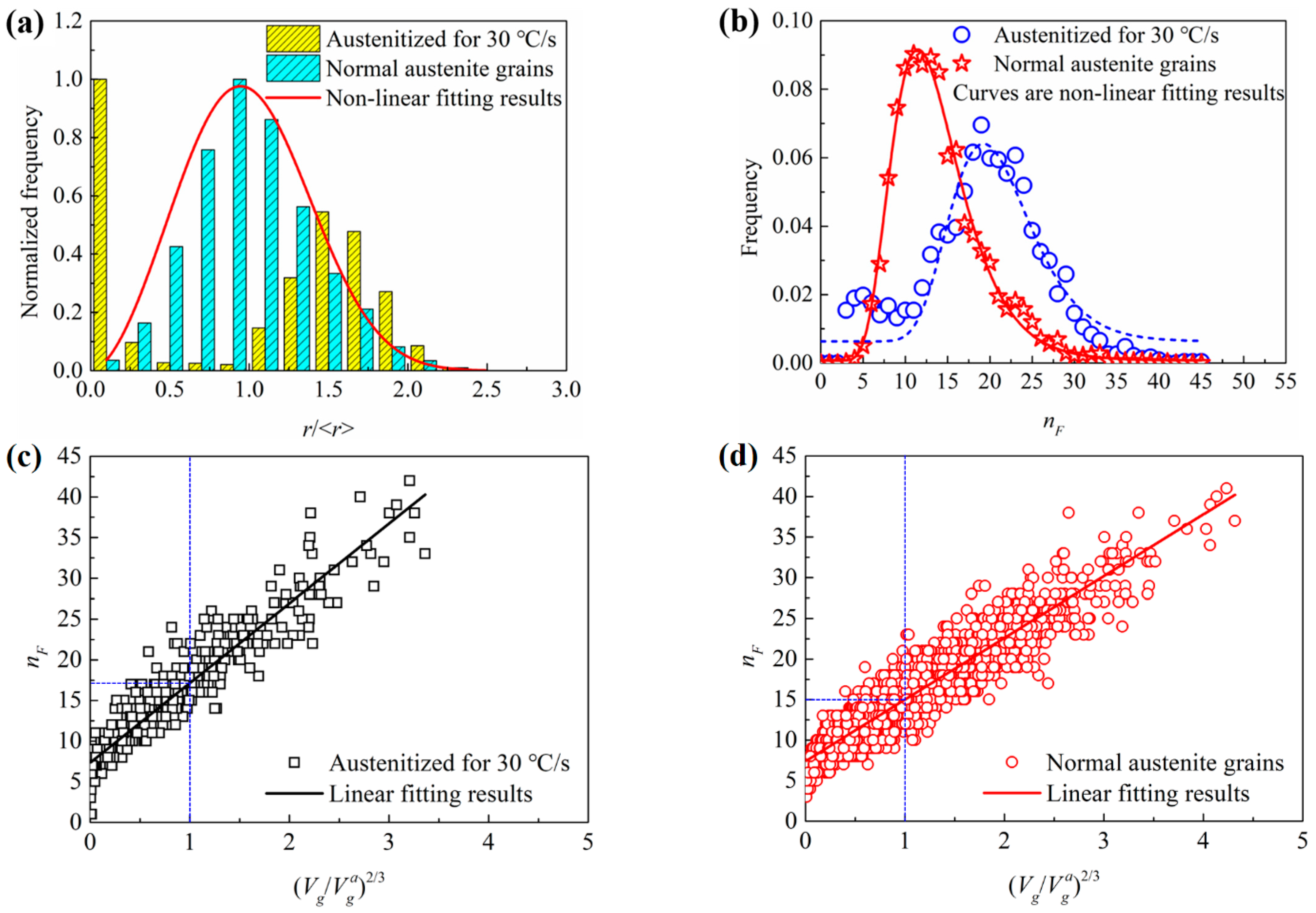
3.3.3. Comparison between the Rapidly Austenitized Grains and Normal Grains
4. Conclusions
- (a)
- The good agreements between the experimental results and the simulation results of the present cellular automata model indicate that the newly proposed model is reasonable.
- (b)
- The experimental results show an obvious change of the transition activity energy from low to high heating rates, which means a change of the transformation mechanisms. The activity energies of the austenitization processes were obtained to be 1.49 × 106 J·mol−1 and 2.98 × 105 J·mol−1 for the heating rate under and above 30 °C/s, respectively. The influences of the heating rate on the transformation mechanism could be clearly observed.
- (c)
- The DICTRA simulation results show that the austenitization transformation is controlled by the couple diffusion of C and Cr below the PNTT and by the diffusion of C above the PNTT. The PNTT of the present steel is calculated to be 803 °C. According to the experimental results, this temperature was located in the temperature range for the heating rate of 30 °C/s. The DICTRA simulation proved that the experimental observed mechanism transition was the partition to non-partition transition.
- (d)
- The lamellar orientation influences both the austenitization kinetics and the topological aspects of the austenite grains. Therefore, considering the influences of the lamellar orientation in an austenitization model is necessary.
- (e)
- The topological aspects of the austenite grains obtained via rapid heat treatment were very different from the normal grains. The rapidly austenitized grains have more rough surfaces, sharp crystal angles and irregular shapes than normal austenite grains.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Z.-X.; Tong, B.-Q.; Zhang, Q.-L.; Yao, J.-Q.; Kovalenko, V. Microstructure refinement and properties of 1.0C-1.5Cr steel in a duplex treatment combining double quenching and laser surface quenching. Mater. Sci. Eng. A 2020, 776, 138994. [Google Scholar] [CrossRef]
- Li, Z.; Wen, Z.; Su, F. Modeling research on laser quenching process of GCr15 bearing steel basing on material properties obtained with experimental methods. Mater. Res. Express. 2021, 8, 096516. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, M.; Chen, X.; Wei, L.; Wang, S.; Jiang, Y.; Jiang, N.; Wang, Z.; Lei, Z.; Chen, Y. Investigation of Weld Root Defects in High-Power Full-Penetration Laser Welding of High-Strength Steel. Materials 2022, 15, 1095. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-T.; Lei, W.-B.; Li, J.; Ma, Y.; Wang, W.-M.; Zhang, B.-H.; Liu, C.-S.; Cui, J.-Z. Laser cladding of high-entropy alloy on H13 steel. Rare Met. 2014, 33, 727–730. [Google Scholar] [CrossRef]
- Dai, J.; Meng, Q.; Zheng, H. An innovative pathway to produce high-performance quenching and partitioning steel through ultra-fast full austenitization annealing. Mater. Today Commun. 2020, 25, 101272. [Google Scholar] [CrossRef]
- Liu, G.; Dai, Z.; Yang, Z.; Zhang, C.; Li, J.; Chen, H. Kinetic transitions and Mn partitioning during austenite growth from a mixture of partitioned cementite and ferrite: Role of heating rate. J. Mater. Sci. Technol. 2020, 49, 70–80. [Google Scholar] [CrossRef]
- Knijf, D.D.; Puype, A.; Föjer, C.; Petrov, R. The influence of ultra-fast annealing prior to quenching and partitioning on the microstructure and mechanical properties. Mater. Sci. Eng. A 2015, 627, 182–190. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, S.; Li, J.; Wang, J.; Meng, Q. Fast-heating for intercritical annealing of cold-rolled quenching and partitioning steel. Mater. Sci. Eng. A 2016, 669, 387–395. [Google Scholar] [CrossRef]
- Miyamoto, G.; Usuki, H.; Li, Z.-D.; Furuhara, T. Effects of Mn, Si and Cr addition on reverse transformation at 1073 K from spheroidized cementite structure in Fe–0.6 mass% C alloy. Acta Mater. 2010, 58, 4492–4502. [Google Scholar] [CrossRef]
- Xia, Y.; Enomoto, M.; Yang, Z.G.; Li, Z.D.; Zhang, C. Effects of alloying elements on the kinetics of austenitization from pearlite in Fe–C–M alloys. Philos. Mag. 2013, 93, 1095–1109. [Google Scholar] [CrossRef]
- Yang, Z.N.; Xia, Y.; Enomoto, M.; Zhang, C.; Yang, Z.G. Effect of alloying element partition in pearlite on the growth of austenite in high-carbon low alloy steel. Metall. Mater. Trans. A 2016, 47, 1019–1027. [Google Scholar] [CrossRef]
- Emo, J.; Maugis, P.; Perlade, A. Austenite growth and stability in medium Mn, medium Al Fe-C-Mn-Al steels. Comput. Mater. Sci. 2016, 125, 206–217. [Google Scholar] [CrossRef]
- Lai, Q.; Gouné, M.; Perlade, A.; Pardoen, T.; Jacques, P.; Bouaziz, O.; Bréchet, Y. Mechanism of Austenite Formation from Spheroidized Microstructure in an Intermediate Fe-0.1C-3.5Mn Steel. Metall. Mater. Trans. A 2016, 47A, 3375–3386. [Google Scholar] [CrossRef]
- Enomoto, M.; Lib, S.; Yang, Z.N.; Zhang, C.; Yang, Z.G. Partition and non-partition transition of austenite growth from a ferrite and cementite mixture in hypo- and hypereutectoid Fe-C-Mn alloys. Calphad 2018, 61, 116–125. [Google Scholar] [CrossRef]
- Huyan, F.; Yan, J.-Y.; Höglund, L.; Ågren, J.; Borgenstam, A. Simulation of the growth of austenite from as-quenched martensite in medium Mn steels. Metall. Mater. Trans. A 2018, 49A, 1053–1060. [Google Scholar] [CrossRef] [Green Version]
- Rödel, J.; Spies, H.-J. Modelling of austenite formation during rapid heating. Surf. Eng. 1996, 12, 313–318. [Google Scholar] [CrossRef]
- Li, Z.; Wen, Z.; Su, F.; Zhang, R.; Zhou, Z. Dilatometric research on pearlite-to-austenite transformation of Fe–1C–1.44Cr low-alloy steel. J. Mater. Sci. 2018, 53, 1424–1436. [Google Scholar] [CrossRef]
- Yan, J.Y.; Ågren, J.; Jeppsson, J. Pearlite in multicomponent steels: Phenomenological steady-state modeling. Metall. Mater. Trans. A 2020, 51, 1978–2001. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Mayweg, D.; Tung, P.Y.; Pippan, R.; Herbig, M. Mechanism of cementite decomposition in 100Cr6 bearing steels during high pressure torsion. Acta Mater. 2020, 201, 79–93. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, Y.; Du, S. Preliminary Research on Response of GCr15 Bearing Steel under Cyclic Compression. Materials 2020, 13, 3443. [Google Scholar] [CrossRef]
- Su, F.; Liu, W.; Wen, Z. Three-Dimensional Cellular Automata Simulation of the Austenitizing Process in GCr15 Bearing Steel. Materials 2019, 12, 3022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Shi, X.; Guo, H.; Guo, J. Study on Precipitation and Growth of TiN in GCr15 Bearing Steel during Solidification. Materials 2019, 12, 1463. [Google Scholar] [CrossRef] [Green Version]
- Razik, N.A.; Lorimer, G.W.; Ridley, N. Chromium partitioning during the austenite-pearlite transformation. Metall. Trans. A 1976, 7A, 209–214. [Google Scholar] [CrossRef]
- Dirnfeld, S.F.; Korevaar, B.M.; Van’t Spijker, F. The transformation to austenite in fine grained tool steel. Metall. Trans. 1974, 5, 1437–1444. [Google Scholar] [CrossRef]
- Speiche, G.; Szirmae, A.; Richards, M.J. Formation of austenite from ferrite and ferrite-carbide aggregates. Trans. Met. Soc. AIME 1969, 245, 1063–1074. [Google Scholar]
- Gaude-Fugarolas, D.; Bhadeshia, H.K.D.H. A model for austenitisation of hypoeutectoid steels. J. Mater. Sci. 2003, 38, 1195–1201. [Google Scholar] [CrossRef]
- Hillert, M. The role of interfaces in phase transformations. In Mechanism of Phase Transformations in Crystalline Solids; Monograph and Report Series; Institute of Metals: London, UK, 1970; Volume 33, pp. 231–247. [Google Scholar]
- Lan, Y.J.; Li, D.Z.; Li, Y.Y. Modeling austenite decomposition into ferrite at different cooling rate in low-carbon steel with cellular automaton method. Acta Mater. 2004, 52, 1721–1729. [Google Scholar] [CrossRef]
- Pandit, A.S.; Bhadeshia, H.K.D.H. Mixed diffusion-controlled growth of pearlite in binary steel. Proc. R. Soc. A 2011, 467, 508–521. [Google Scholar] [CrossRef]
- Fridberg, J.; Törndahl, L.E.; Hillert, M. Diffusion in iron. Jernkonterets Ann. 1969, 153, 263–274. [Google Scholar]
- Chea, J.Y.; Jang, J.H.; Zhang, G.H.; Kim, K.H.; Bhadeshia, H.K.D.H.; Suh, D.W. Dilatometric analysis of cementite dissolu JS tion in hypereutectoid steels containing Cr. Scr. Mater. 2011, 65, 245–248. [Google Scholar] [CrossRef] [Green Version]
- Stuart, H.; Ridley, N. Thermal expansion of cementite and other phases. J. Iron Steel Inst. 1966, 204, 711–717. [Google Scholar]
- Ridley, N.; Stuart, H.; Zwell, L. Lattice parameters of Fe-C austenites at room temperature. Trans. AIME 1969, 245, 1834–1836. [Google Scholar]
- Dyson, D.J.; Holmes, B. Effect of alloying additions on the lattice parameter of austenite. J. Iron Steel Inst. 1970, 208, 469–474. [Google Scholar]
- Park, J.; Jung, M.; Lee, Y.K. Abnormal expansion during the ferro- to para-magnetic transition in pure iron. J. Magn. Magn. Mater. 2015, 377, 193–196. [Google Scholar] [CrossRef]
- Ding, H.L.; He, Y.Z.; Liu, L.F.; Ding, W.J. Cellular automata simulation of grain growth in three dimensions based on the lowest-energy principle. J. Cryst. Growth 2006, 293, 489–497. [Google Scholar] [CrossRef]
- Zhu, L.; Qu, S.; Guo, X.; Lu, J. Analysis of the twin spacing and grain size effects on mechanical properties in hierarchically nanotwinned face-centered cubic metals based on a mechanism-based plasticity model. J. Mech. Phys. Solids 2015, 76, 162–179. [Google Scholar] [CrossRef]
- Musinski, W.D.; McDowell, D.L. Simulating the effect of grain boundaries on microstructurally small fatigue crack growth from a focused ion beam notch through a three-dimensional array of grains. Acta Mater. 2016, 112, 20–39. [Google Scholar] [CrossRef]
- Wisniewski, A.; Beddoes, J. Influence of grain-boundary morphology on creep of a wrought Ni-base superalloy. Mater. Sci. Eng. A 2009, 510–511, 266–272. [Google Scholar] [CrossRef]





| α/γ Interface | θ/γ Interface |
|---|---|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhang, S.; He, Y.; Xiong, G.; Liu, Y.; Su, F. Prediction of the Non-Isothermal Austenitization Kinetics of Fe-C-Cr Low Alloy Steels with Lamellar Pearlite Microstructure. Materials 2022, 15, 2131. https://doi.org/10.3390/ma15062131
Li Z, Zhang S, He Y, Xiong G, Liu Y, Su F. Prediction of the Non-Isothermal Austenitization Kinetics of Fe-C-Cr Low Alloy Steels with Lamellar Pearlite Microstructure. Materials. 2022; 15(6):2131. https://doi.org/10.3390/ma15062131
Chicago/Turabian StyleLi, Zhiqiang, Shengyang Zhang, Yang He, Guangjie Xiong, Yude Liu, and Fuyong Su. 2022. "Prediction of the Non-Isothermal Austenitization Kinetics of Fe-C-Cr Low Alloy Steels with Lamellar Pearlite Microstructure" Materials 15, no. 6: 2131. https://doi.org/10.3390/ma15062131
APA StyleLi, Z., Zhang, S., He, Y., Xiong, G., Liu, Y., & Su, F. (2022). Prediction of the Non-Isothermal Austenitization Kinetics of Fe-C-Cr Low Alloy Steels with Lamellar Pearlite Microstructure. Materials, 15(6), 2131. https://doi.org/10.3390/ma15062131






