A Novel Process to Recover Gypsum from Phosphogypsum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
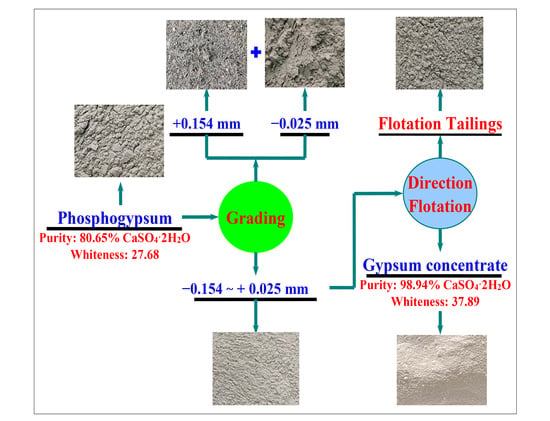
2.2. Experiment
2.3. Analyses
3. Results
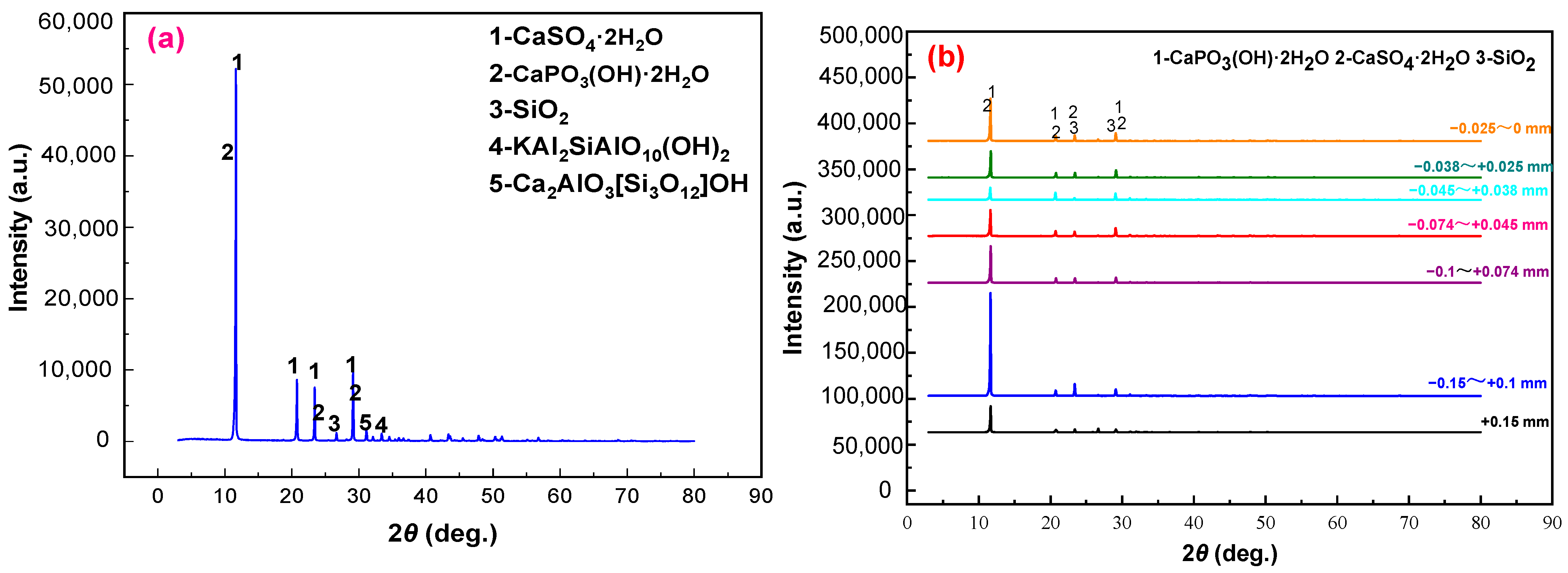
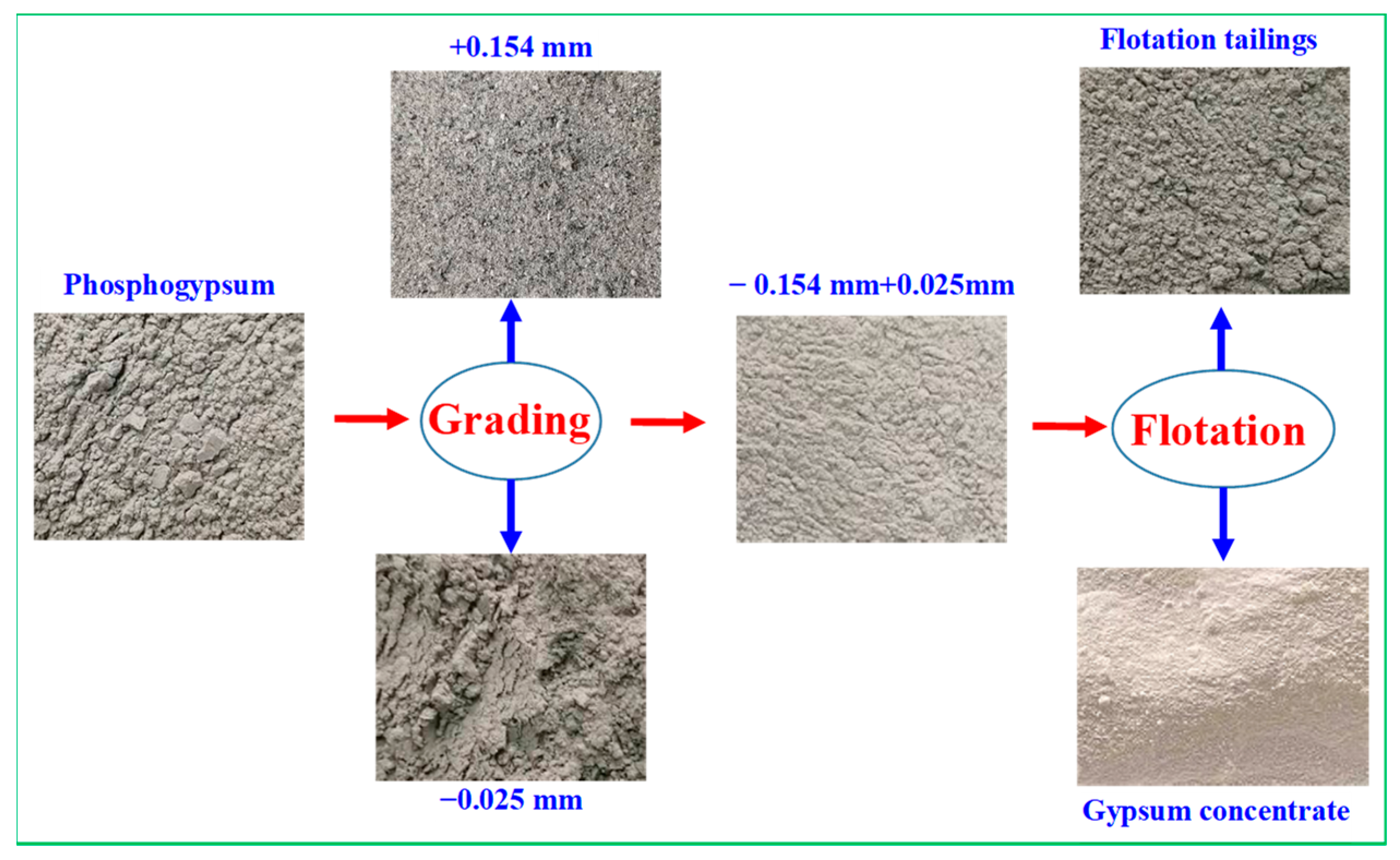
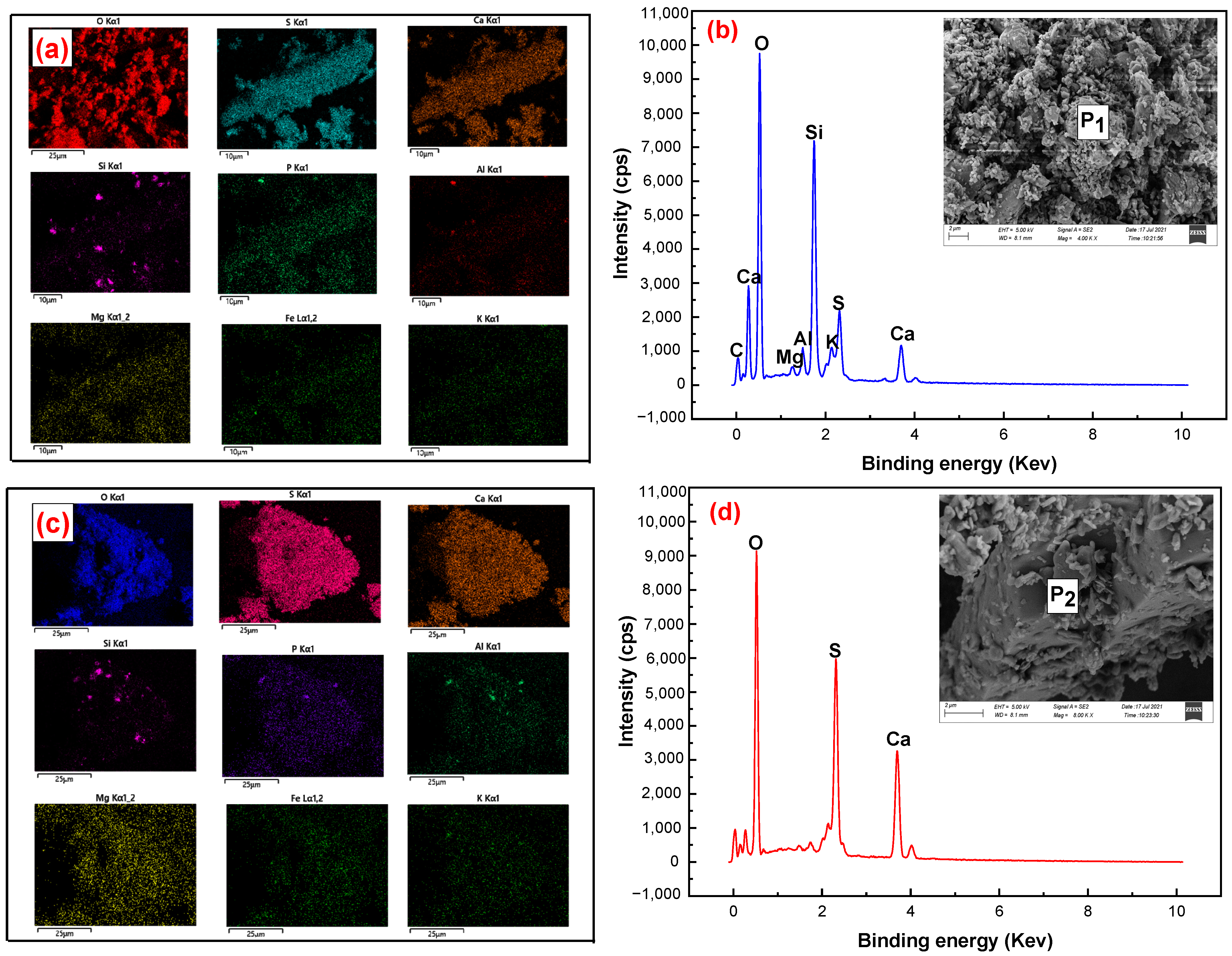
3.1. Process Mineralogical Analysis of Phosphogypsum
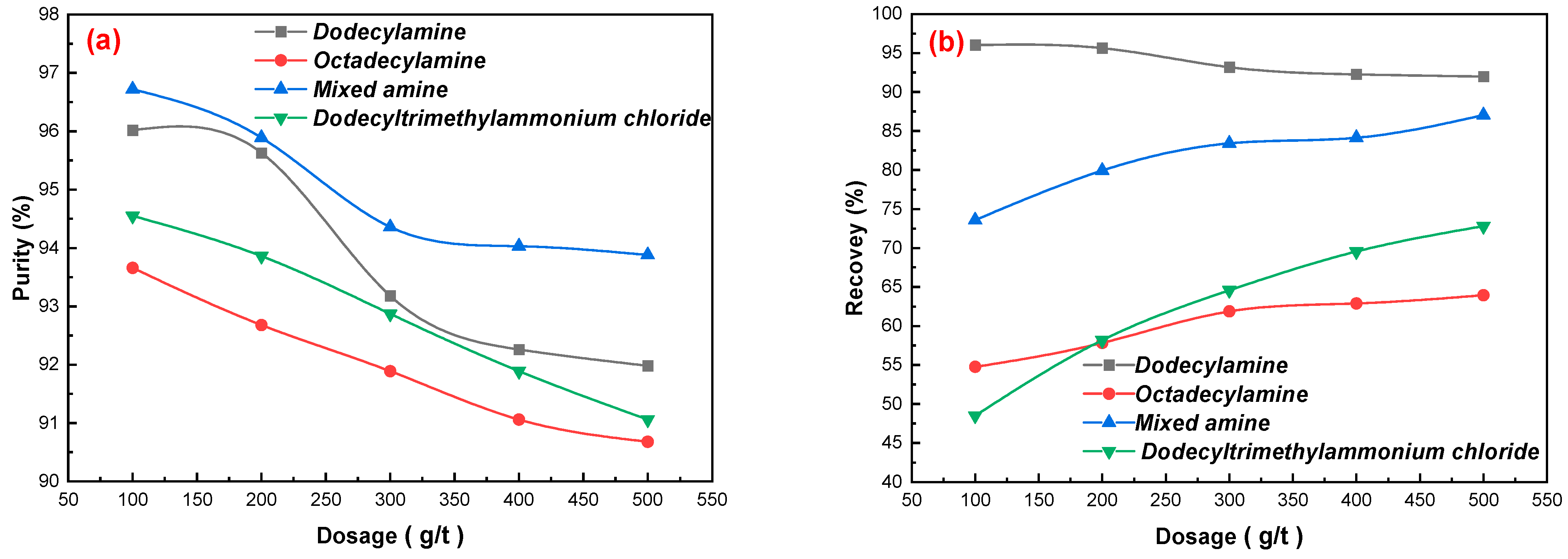
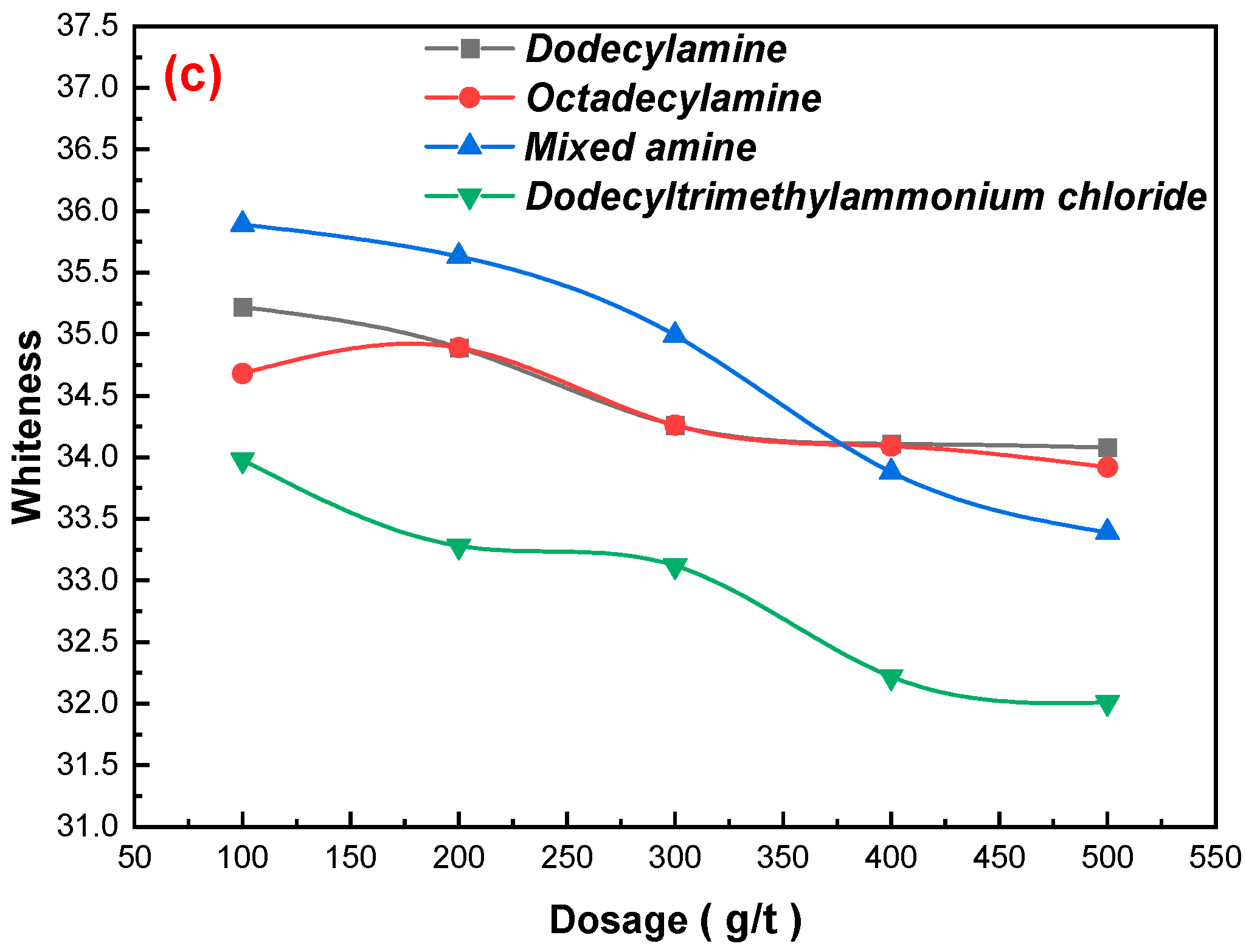
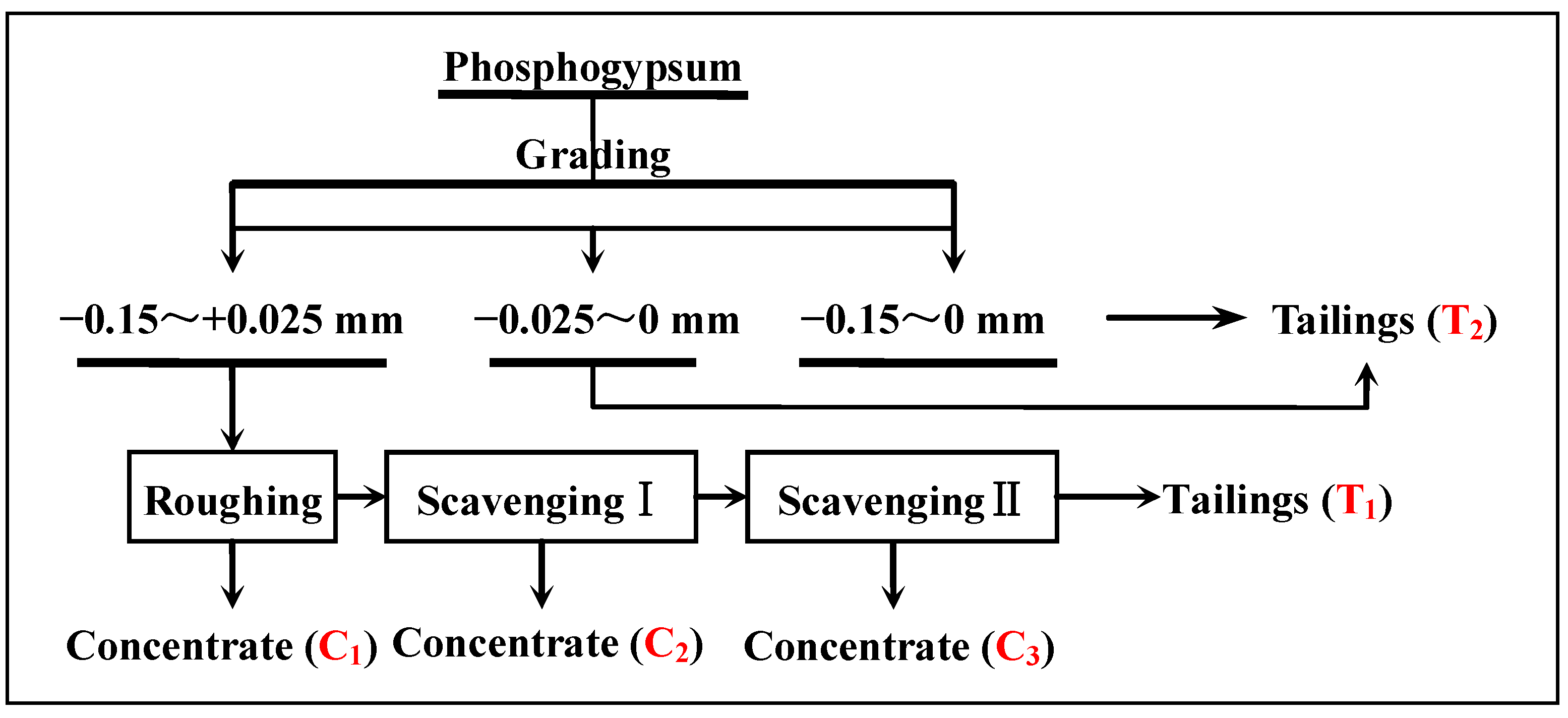
3.2. Effects of Flotation Flowsheet Parameters on Gypsum Purification
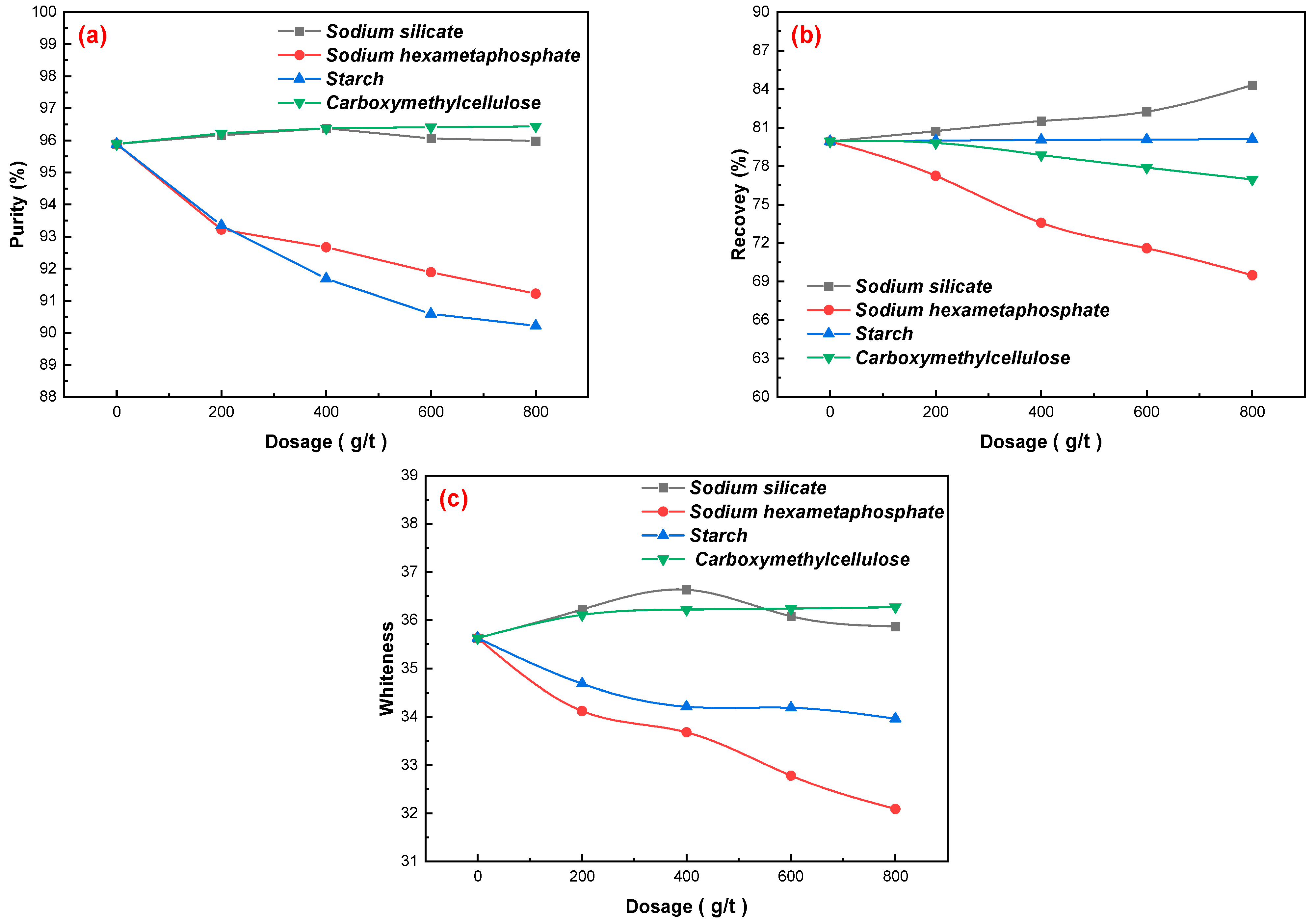
3.2.1. Collectors Dosages
3.2.2. Depressants Dosages
3.2.3. pH Value of Pulp
3.2.4. Flotation Concentration
3.2.5. Time of Flotation Scavenging
3.2.6. Time of Flotation Cleaning
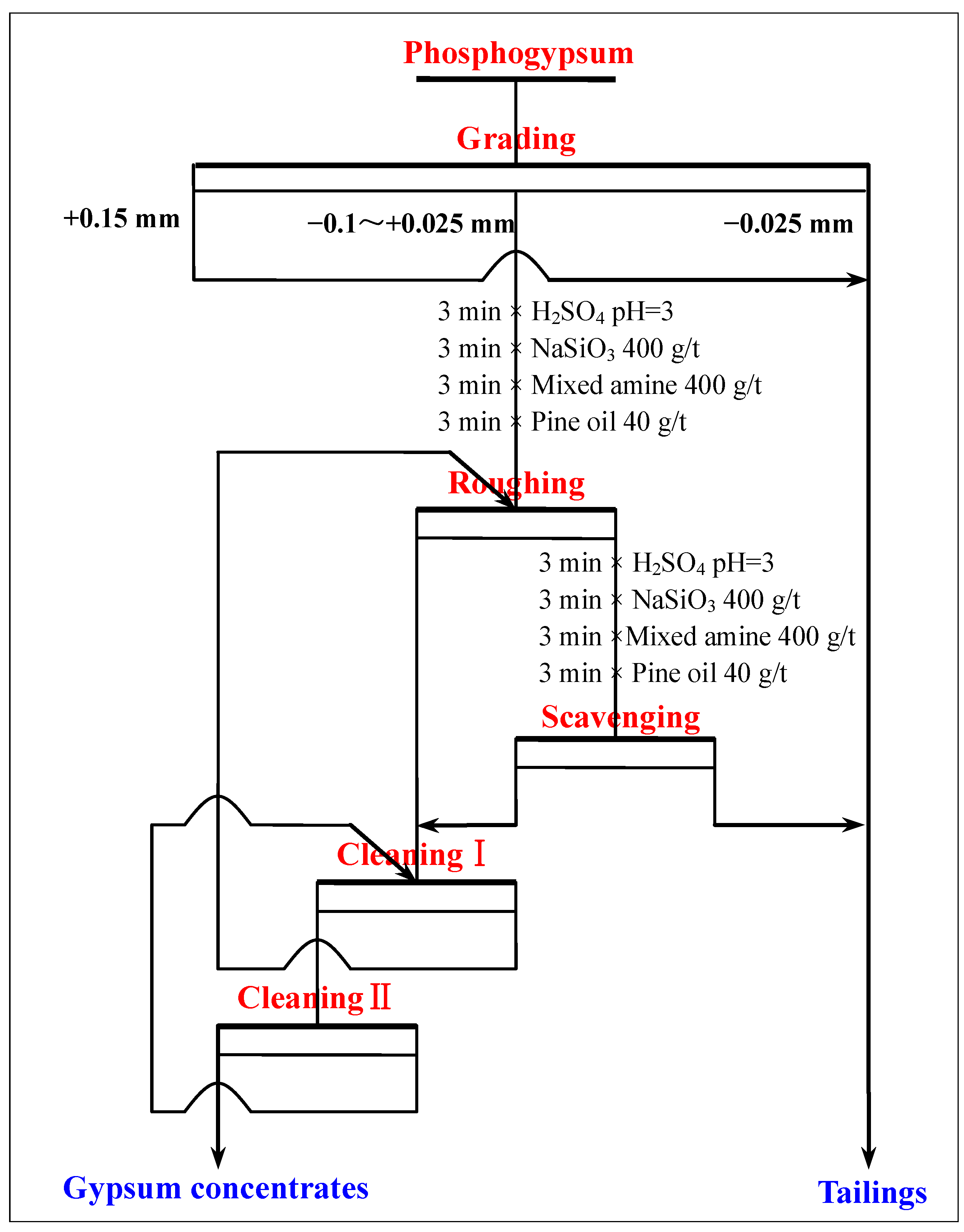
3.2.7. The Entire Flowsheet Test of Recovering Gypsum from Phosphogypsum
4. Discussion
5. Conclusions
- (1)
- Phosphogypsum contained 80.65% CaSO4·2H2O and whiteness of 27.68. The main mineral is CaSO4·2H2O with small amounts of brushite, quartz, muscovite, and zoisite in phosphogypsum. Harmful elements, such as silicon, phosphorus, and fluorine, are mainly concentrated in the +0.15 mm and −0.025 mm fraction; these can be pre-selected and removed by the grading method to increase the CaSO4·2H2O content and reduce the processing cost.
- (2)
- A novel direct flotation process, consisting of one roughing, one scavenging, and two cleaning operations, were employed to recover gypsum from the −0.15 to + 0.025 mm fraction materials. The addition of NaSiO3 enhanced the removal of gangue minerals. Furthermore, the addition of a mixed amine enhanced the capture of CaSO4·2H2O. The test results show that gypsum with a CaSO4·2H2O content of 98.94%, CaSO4·2H2O recovery of 80.02%, and whiteness of 37.05 was obtained under the following conditions: flotation roughing concentration of 30%, pH = 2.0 (H2SO4), mixed amine dosage of 400 g/t, pine dosage of 40 g/t, flotation scavenging of mixed amine dosage of 200 g/t, sodium silicate dosage of 400 g/t, and pine dosage of 20 g/t. Gypsum concentrate could be used as a high-quality raw material to prepare α-hemihydrate high-strength gypsum or β-hemihydrate-building gypsum.
- (3)
- The results of XRD and SEM-EDS demonstrated that the main mineral in the gypsum concentrate was gypsum; however, brushite mineral phase and quartz were not found. Meanwhile, a tiny amount of muscovite and zoisite entered the gypsum concentrate owing to the mechanical entrainment of the flotation process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chernysh, Y.; Yakhnenko, O.; Chubur, V.; Roubík, H. Phosphogypsum Recycling: A Review of Environmental Issues, Current Trends, and Prospects. Appl. Sci. 2021, 11, 1575. [Google Scholar] [CrossRef]
- Bakshi, P.; Pappu, A.; Gupta, M.K. A review on calcium-rich industrial wastes: A sustainable source of raw materials in India for civil infrastructure—opportunities and challenges to bond circular economy. J. Mater. Cycles Waste Manag. 2021, 24, 49–62. [Google Scholar] [CrossRef]
- Bouargane, B.; Marrouche, A.; El Issiouy, S.; Biyoune, M.G.; Mabrouk, A.; Atbir, A.; Bachar, A.; Bellajrou, R.; Boukbir, L.; Bakiz, B. Recovery of Ca(OH)2, CaCO3, and Na2SO4 from Moroccan phosphogypsum waste. J. Mater. Cycles Waste Manag. 2019, 21, 1563–1571. [Google Scholar] [CrossRef]
- Attallah, M.; Metwally, S.; Moussa, S.; Soliman, M. Environmental impact assessment of phosphate fertilizers and phosphogypsum waste: Elemental and radiological effects. Microchem. J. 2019, 146, 789–797. [Google Scholar] [CrossRef]
- Garbaya, H.; Jraba, A.; Khadimallah, M.A.; Elaloui, E. The Development of a New Phosphogypsum-Based Construction Material: A Study of the Physicochemical, Mechanical and Thermal Characteristics. Materials 2021, 14, 7369. [Google Scholar] [CrossRef]
- Rashad, A.M. Phosphogypsum as a construction material. J. Clean. Prod. 2017, 166, 732–743. [Google Scholar] [CrossRef]
- Patil, P.P.; Prabhu, M.; Mutnuri, S. A novel and sustainable approach for biotransformation of phosphogypsum to calcium carbonate using urease producing Lysinibacillus sphaericus strain GUMP2. Environ. Technol. 2021, 1–14. [Google Scholar] [CrossRef]
- Zhang, D.; Luo, H.; Zheng, L.; Wang, K.; Li, H.; Wang, Y.; Feng, H. Utilization of waste phosphogypsum to prepare hydroxyapatite nanoparticles and its application towards removal of fluoride from aqueous solution. J. Hazard. Mater. 2012, 241–242, 418–426. [Google Scholar] [CrossRef]
- Kuzmanović, P.; Todorović, N.; Forkapić, S.; Petrović, L.F.; Knežević, J.; Nikolov, J.; Miljević, B. Radiological characterization of phosphogypsum produced in Serbia. Radiat. Phys. Chem. 2020, 166, 108463. [Google Scholar] [CrossRef]
- Wei, J.; Gu, Y.; Lv, H.; Wu, X. A zero-emission method for recycling phosphogypsum using Na2SO4 electrolysis: Preliminary study. Sep. Purif. Technol. 2021, 259, 118168. [Google Scholar] [CrossRef]
- Kazanskaya, L.; Smirnova, O.; Palomo, Á.; Pidal, I.M.; Romana, M. Supersulfated Cement Applied to Produce Lightweight Concrete. Materials 2021, 14, 403. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, C.O.; Cheriaf, M.; Rocha, J.C. Production of Synthetic Phosphoanhydrite and Its Use as a Binder in Self-Leveling Underlayments (SLU). Materials 2017, 10, 958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hentati, O.; Abrantes, N.; Caetano, A.; Bouguerra, S.; Gonçalves, F.J.M.; Römbke, J.; Pereira, R. Phosphogypsum as a soil fertilizer: Ecotoxicity of amended soil and elutriates to bacteria, invertebrates, algae and plants. J. Hazard. Mater. 2015, 294, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Macías, F.; Cánovas, C.R.; Cruz-Hernández, P.; Carrero, S.; Asta, M.P.; Nieto, J.M.; Pérez-López, R. An anomalous metal-rich phosphogypsum: Characterization and classification according to international regulations. J. Hazard. Mater. 2017, 331, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Huang, Z.; Wang, H.; Liu, R.; Cheng, C.; Shuai, S.; Hu, Y.; Guo, Z.; Yu, X.; He, G.; et al. Flotation performance of a novel Gemini collector for kaolinite at low temperature. Int. J. Min. Sci. Technol. 2021, 31, 1145–1152. [Google Scholar] [CrossRef]
- El-Didamony, H.; Gado, H.; Awwad, N.; Fawzy, M.; Attallah, M. Treatment of phosphogypsum waste produced from phosphate ore processing. J. Hazard. Mater. 2013, 244–245, 596–602. [Google Scholar] [CrossRef]
- Yao, W.; Li, M.; Cui, R.; Jiang, X.; Jiang, H.; Deng, X.; Li, Y.; Zhou, S. Flotation Behavior and Mechanism of Anglesite with Salicyl Hydroxamic Acid as Collector. JOM 2018, 70, 2813–2818. [Google Scholar] [CrossRef]
- Marion, C.; Jordens, A.; Li, R.; Rudolph, M.; Waters, K. An evaluation of hydroxamate collectors for malachite flotation. Sep. Purif. Technol. 2017, 183, 258–269. [Google Scholar] [CrossRef]
- Ramirez, A.; Rojas, A.; Gutierrez, L.; Laskowski, J.S. Sodium hexametaphosphate and sodium silicate as dispersants to reduce the negative effect of kaolinite on the flotation of chalcopyrite in seawater. Miner. Eng. 2018, 125, 10–14. [Google Scholar] [CrossRef]
- Hussein, M.; Donaldson, A. Mixing strategy effect on dispersion of amine during reagent production for flotation applications. Can. J. Chem. Eng. 2018, 96, 360–366. [Google Scholar] [CrossRef]
- Özün, S.; Ulutaş, Ş. Interfacial Behavior of Anionic/Cationic Flotation Collectors in Mixed Aqueous Solutions and Their Effect on Flotation Recovery of Quartz. J. Surfactants Deterg. 2019, 22, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Kargupta, W.; Browne, C.; Verdugo, L.; Hunt, I.; Stack, K.; Batchelor, W.; Tanner, J. Flotation as a separation technology for recovering pulp fines and sustainable nanocellulose production. Sep. Purif. Technol. 2021, 270, 118810. [Google Scholar] [CrossRef]
- Bhondayi, C.; Moys, M.; Danha, G. Effect of pulp chemistry and solids on a froth bubble size measurement method. Powder Technol. 2016, 297, 202–210. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Forssberg, E. Physicochemical studies of smithsonite flotation using mixed anionic/cationic collector. Miner. Eng. 2007, 20, 621–624. [Google Scholar] [CrossRef]
- Marion, C.; Jordens, A.; McCarthy, S.; Grammatikopoulos, T.; Waters, K.E. An investigation into the flotation of muscovite with an amine collector and calcium lignin sulfonate depressant. Sep. Purif. Technol. 2015, 149, 216–227. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, S.; Li, J.; Weng, L.; Wu, C.; Liu, K. Improvement on combustible matter recovery in coal slime flotation with the addition of sodium silicate. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 603, 125220. [Google Scholar] [CrossRef]
- Feng, B.; Guo, W.; Xu, H.; Peng, J.; Luo, X.; Zhu, X. The combined effect of lead ion and sodium silicate in the flotation separation of scheelite from calcite. Sep. Sci. Technol. 2017, 52, 567–573. [Google Scholar] [CrossRef]
- Meng, Q.; Yuan, Z.; Xu, Y.; Du, Y. The effect of sodium silicate depressant on the flotation separation of fine wolframite from quartz. Sep. Sci. Technol. 2018, 54, 1400–1410. [Google Scholar] [CrossRef]
- Gao, Z.-Y.; Jiang, Z.-Y.; Sun, W.; Gao, Y.-S. Typical roles of metal ions in mineral flotation: A review. Trans. Nonferrous Met. Soc. China 2021, 31, 2081–2101. [Google Scholar] [CrossRef]
- Solongo, S.K.; Flores, A.G.; You, J.; Choi, S.; Heyes, G.W.; Ilyas, S.; Lee, J.; Kim, H. Cationic collector conformations on an oxide mineral interface: Roles of pH, ionic strength, and ion valence. Miner. Eng. 2020, 150, 106277. [Google Scholar] [CrossRef]
- Mechi, N.; Khiari, R.; Ammar, M.; Elaloui, E.; Belgacem, M.N. Preparation and application of Tunisian phosphogypsum as fillers in papermaking made from Prunus amygdalus and Tamarisk sp. Powder Technol. 2017, 312, 287–293. [Google Scholar] [CrossRef]
- Jara, A.D.; Woldetinsae, G.; Betemariam, A.; Kim, J.Y. Mineralogical and petrographic analysis on the flake graphite ore from Saba Boru area in Ethiopia. Int. J. Min. Sci. Technol. 2020, 30, 715–721. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, Y. Recovering Cobalt and Sulfur in Low Grade Cobalt-Bearing V–Ti Magnetite Tailings Using Flotation Process. Processes 2019, 7, 536. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Chen, C.; Ding, W.; Peng, Y.; Chen, T.; Zou, K. Extraction of Phosphorous from a Phosphorous-Containing Vanadium Titano-Magnetite Tailings by Direct Flotation. Processes 2020, 8, 874. [Google Scholar] [CrossRef]




| SO3 | CaO | SiO2 | P2O5 | Al2O3 | Fe2O3 | K2O | SrO | MgO | F | TiO2 | BaO | Na2O | Y2O3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 49.62 | 41.41 | 5.47 | 1.28 | 0.63 | 0.31 | 0.20 | 0.12 | 0.12 | 0.11 | 0.10 | 0.07 | 0.05 | 0.01 |
| Fraction (mm) | Yield | SO3 | CaO | SiO2 | P2O5 | Al2O3 | Fe2O3 | K2O | MgO | F |
|---|---|---|---|---|---|---|---|---|---|---|
| +0.15 | 7.60 | 41.17 | 38.35 | 12.89 | 5.08 | 1.00 | 0.57 | 0.34 | 0.10 | 0.41 |
| −0.15~+0.1 | 11.21 | 51.89 | 43.23 | 2.93 | 1.15 | 0.28 | 0.21 | 0.08 | 0.02 | 0.004 |
| −0.1~0.074 | 19.01 | 51.76 | 44.24 | 2.25 | 1.01 | 0.25 | 0.18 | 0.06 | 0.06 | 0.005 |
| −0.074~0.045 | 14.23 | 52.08 | 44.09 | 2.15 | 1.03 | 0.19 | 0.26 | 0.05 | 0.02 | 0.006 |
| −0.045~+0.038 | 17.35 | 51.86 | 43.93 | 2.34 | 1.03 | 0.26 | 0.28 | 0.05 | 0.02 | 0.005 |
| −0.038~+0.025 | 10.26 | 51.09 | 43.39 | 3.24 | 1.16 | 0.40 | 0.35 | 0.08 | 0.03 | 0.003 |
| −0.025~0 | 20.34 | 44.81 | 37.28 | 13.17 | 1.56 | 1.36 | 0.87 | 0.39 | 0.12 | 0.35 |
| Totals | 100.00 | 49.55 | 42.10 | 5.46 | 1.47 | 0.54 | 0.39 | 0.15 | 0.06 | 0.11 |
| Products | Mass Fraction (%) | Whiteness | ||||||
|---|---|---|---|---|---|---|---|---|
| Yield | Purity | Recovery | Individual | Cumulative | ||||
| Individual | Cumulative | Individual | Cumulative | Individual | Cumulative | |||
| C1 | 62.54 | 62.54 | 96.45 | 96.45 | 74.72 | 74.72 | 36.28 | 36.28 |
| C2 | 7.55 | 70.09 | 96.25 | 96.43 | 9.00 | 83.72 | 33.16 | 35.94 |
| C3 | 0.76 | 70.85 | 92.16 | 96.38 | 0.87 | 84.59 | 29.63 | 35.88 |
| T1 | 1.18 | 72.03 | 76.34 | 96.05 | 1.12 | 85.71 | 7.35 | 35.41 |
| T2 | 27.97 | 100 | 41.23 | 80.72 | 14.29 | 100.00 | 8.15 | 27.78 |
| Totals | 100.00 | 80.72 | 100.00 | 27.78 | ||||
| Products | Mass Fraction (%) | Whiteness | ||||||
|---|---|---|---|---|---|---|---|---|
| Yield | Purity | Recovery | Individual | Cumulative | ||||
| Individual | Cumulative | Individual | Cumulative | Individual | Cumulative | |||
| C | 64.56 | 64.56 | 98.98 | 98.98 | 79.11 | 79.11 | 37.07 | 37.07 |
| M3 | 0.19 | 64.75 | 95.88 | 98.97 | 0.22 | 79.33 | 35.75 | 37.07 |
| M2 | 0.46 | 65.21 | 94.65 | 98.94 | 0.54 | 79.87 | 35.26 | 37.05 |
| M1 | 0.96 | 66.17 | 93.12 | 98.86 | 1.11 | 80.98 | 32.37 | 36.98 |
| T1 | 5.87 | 72.04 | 65.25 | 91.31 | 4.74 | 85.72 | 6.66 | 34.52 |
| T2 | 27.96 | 100.00 | 41.24 | 80.77 | 14.28 | 100.00 | 8.67 | 27.72 |
| Totals | 100.00 | 80.77 | 100.00 | 27.72 | ||||
| Products | Mass Fraction (%) | Whiteness | ||
|---|---|---|---|---|
| Yield | Purity | Recovery | ||
| Gypsum concentrates | 65.27 | 98.92 | 80.02 | 37.12 |
| Tailings | 34.73 | 46.43 | 19.98 | 9.96 |
| Totals | 100.00 | 80.69 | 100.00 | 27.69 |
| SO3 | CaO | SiO2 | P2O5 | Al2O3 | Fe2O3 | K2O | SrO | MgO | F | TiO2 | BaO | Na2O | Y2O3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 52.52 | 44.88 | 0.32 | 0.05 | 0.05 | 0.02 | 0.05 | 0.01 | 0.06 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, J.; Lu, T.; Zhuang, Y.; Jin, H. A Novel Process to Recover Gypsum from Phosphogypsum. Materials 2022, 15, 1944. https://doi.org/10.3390/ma15051944
Xiao J, Lu T, Zhuang Y, Jin H. A Novel Process to Recover Gypsum from Phosphogypsum. Materials. 2022; 15(5):1944. https://doi.org/10.3390/ma15051944
Chicago/Turabian StyleXiao, Junhui, Tao Lu, Yuanfa Zhuang, and Huang Jin. 2022. "A Novel Process to Recover Gypsum from Phosphogypsum" Materials 15, no. 5: 1944. https://doi.org/10.3390/ma15051944
APA StyleXiao, J., Lu, T., Zhuang, Y., & Jin, H. (2022). A Novel Process to Recover Gypsum from Phosphogypsum. Materials, 15(5), 1944. https://doi.org/10.3390/ma15051944