Electrospun Nanofibers Revisited: An Update on the Emerging Applications in Nanomedicine
Abstract
1. Introduction
2. Electrospun NF Implants
2.1. Electrospun NF Implants for Cancer Therapy
2.2. Electrospun NF Implants as Antimicrobial Agents
2.3. Electrospun NF Implants Used in Regenerative Medicine
2.3.1. Wound Healing
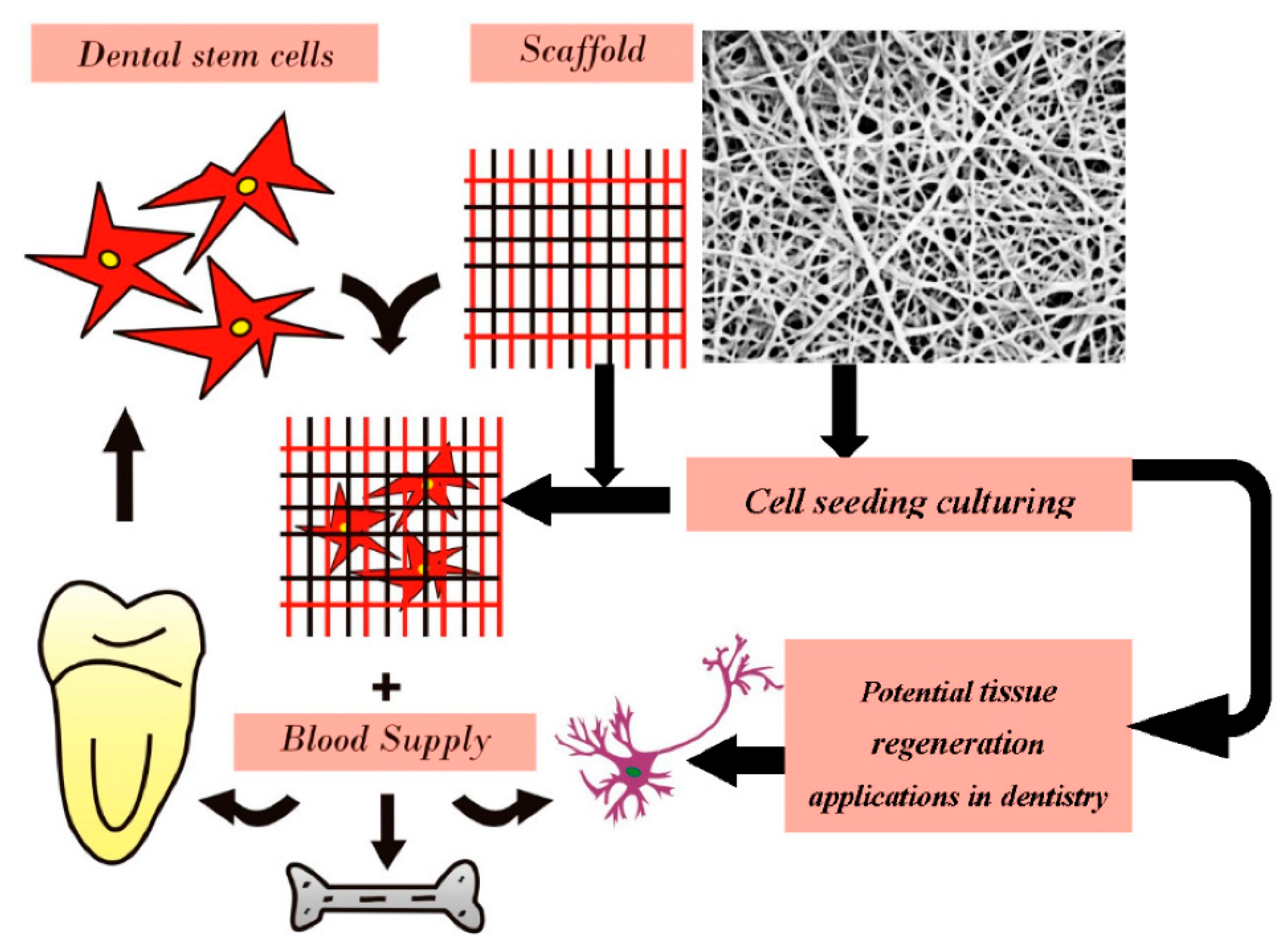
2.3.2. Dental Applications
2.3.3. THE Role of Electrospun NF Implants for the Treatment of Pelvic Organ Prolapse
2.3.4. Electrospun NF Implants for Cardiac Tissue Engineering
2.3.5. Orthopaedic Applications
2.3.6. Electrospun NFs as Antibacterial Coatings to Prevent Implant-Related Infections
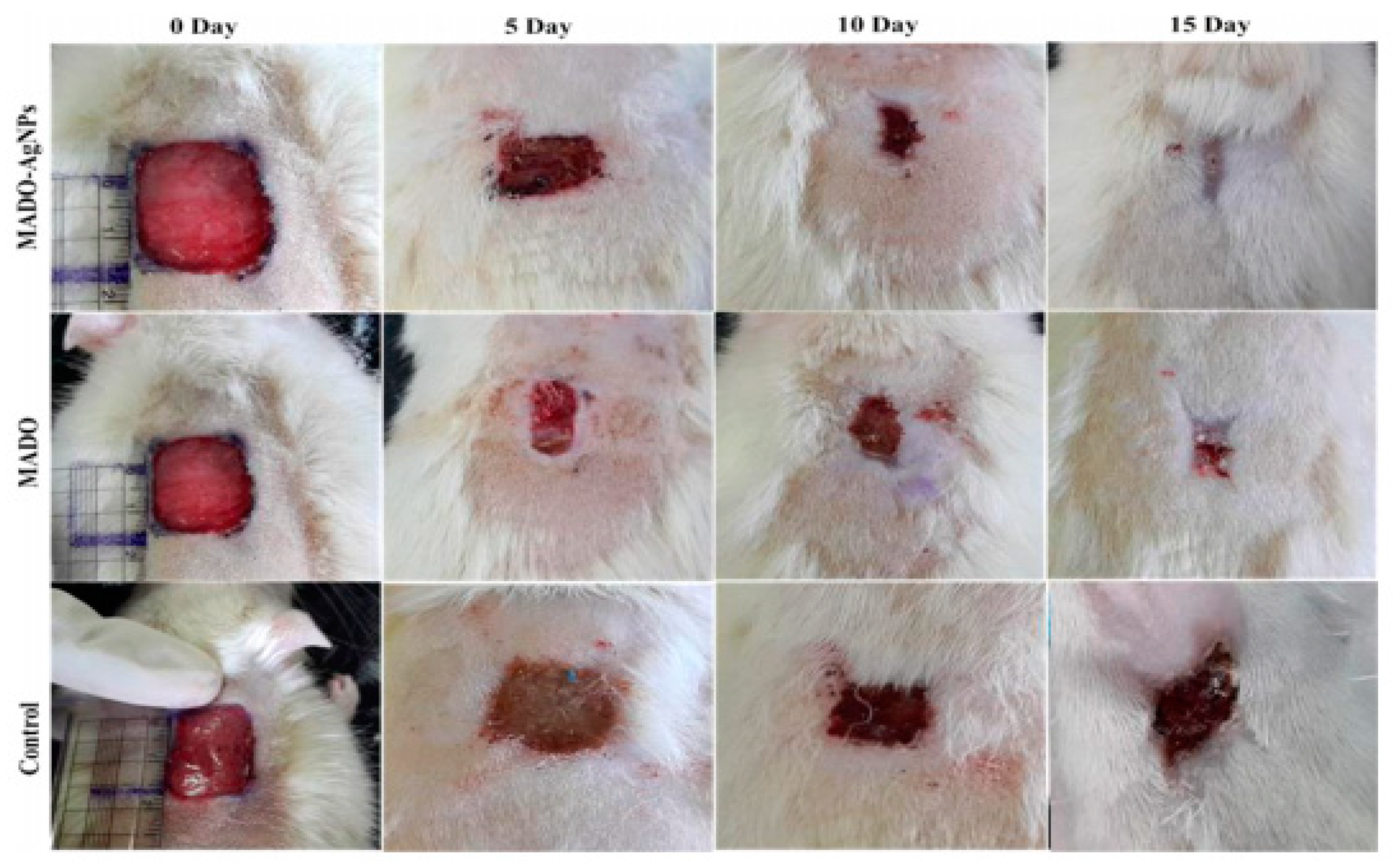
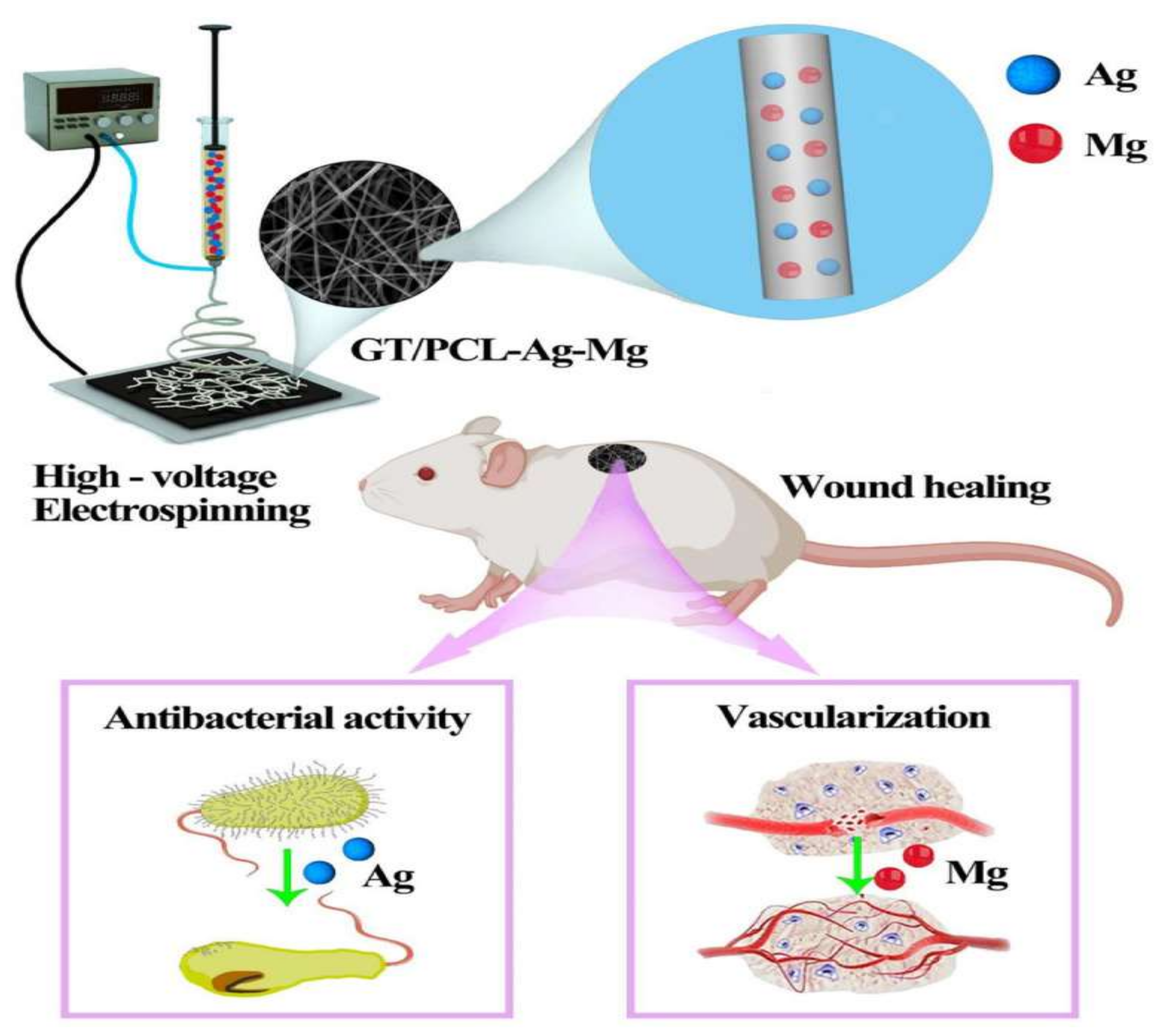
2.3.7. NF for Wound Healing Applications
Water Resistance and Breathable Wound Dressings
Electrospun NFs as a Drug Delivery Carrier for Wound Healing
3. Mechanical Properties of Electrospun NF Implants
4. Portable ES Device Applications
4.1. Wound Dressings with Skin Regenerating Properties
4.2. Rapid Hemostasis
Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.I. Disintegration of Water Drops in an Electric Field. Proc. R. Soc. Lond. Ser. A. Math. Phys. Sci. 1964, 280, 383–397. [Google Scholar] [CrossRef]
- Islam, M.S.; Ang, B.C.; Andriyana, A.; Afifi, A.M. A Review on Fabrication of Nanofibers via Electrospinning and Their Applications. SN Appl. Sci. 2019, 1, 1248. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for Drug Delivery Applications: A Review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Miletić, A.; Pavlić, B.; Ristić, I.; Zeković, Z.; Pilić, B. Encapsulation of Fatty Oils into Electrospun Nanofibers for Cosmetic Products with Antioxidant Activity. Appl. Sci. 2019, 9, 2955. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Wang, P.; Zhang, H. Electrospinning of Nanofibers: Potentials and Perspectives for Active Food Packaging. Compr. Rev. Food Sci. Food Saf. 2020, 19, 479–502. [Google Scholar] [CrossRef]
- Han, W.; Wang, Y.; Su, J.; Xin, X.; Guo, Y.; Long, Y.-Z.; Ramakrishna, S. Fabrication of Nanofibrous Sensors by Electrospinning. Sci. China Technol. Sci. 2019, 62, 886–894. [Google Scholar] [CrossRef]
- Mirjalili, M.; Zohoori, S. Review for Application of Electrospinning and Electrospun Nanofibers Technology in Textile Industry. J. Nanostruct. Chem. 2016, 6, 207–213. [Google Scholar] [CrossRef]
- Contreras-Cáceres, R.; Cabeza, L.; Perazzoli, G.; Díaz, A.; López-Romero, J.M.; Melguizo, C.; Prados, J. Electrospun Nanofibers: Recent Applications in Drug Delivery and Cancer Therapy. Nanomaterials 2019, 9, 656. [Google Scholar] [CrossRef]
- Calori, I.R.; Braga, G.; de Jesus, P.d.C.C.; Bi, H.; Tedesco, A.C. Polymer Scaffolds as Drug Delivery Systems. Eur. Polym. J. 2020, 129, 109621. [Google Scholar] [CrossRef]
- Bunggulawa, E.J.; Wang, W.; Yin, T.; Wang, N.; Durkan, C.; Wang, Y.; Wang, G. Recent Advancements in the Use of Exosomes as Drug Delivery Systems. J. Nanobiotechnol. 2018, 16, 81. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Rossi, S.; Bonferoni, M.C.; Caramella, C.; Ferrari, F. Electrospinning Technologies in Wound Dressing Applications. In Therapeutic Dressings and Wound Healing Applications; John Wieley & Sons Ltd.: Chichester, UK, 2020; pp. 315–336. ISBN 978-1-119-43331-6. [Google Scholar]
- Dong, Y.; Zheng, Y.; Zhang, K.; Yao, Y.; Wang, L.; Li, X.; Yu, J.; Ding, B. Electrospun Nanofibrous Materials for Wound Healing. Adv. Fiber Mater. 2020, 2, 212–227. [Google Scholar] [CrossRef]
- Chahal, S.; Kumar, A.; Hussian, F.S.J. Development of Biomimetic Electrospun Polymeric Biomaterials for Bone Tissue Engineering. A Review. J. Biomater. Sci. Polym. Ed. 2019, 30, 1308–1355. [Google Scholar] [CrossRef]
- Tan, G.Z.; Zhou, Y. Electrospinning of Biomimetic Fibrous Scaffolds for Tissue Engineering: A Review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 947–960. [Google Scholar] [CrossRef]
- Venugopal, J.; Prabhakaran, M.P.; Low, S.; Choon, A.T.; Zhang, Y.Z.; Deepika, G.; Ramakrishna, S. Nanotechnology for Nanomedicine and Delivery of Drugs. Curr. Pharm. Des. 2008, 14, 2184–2200. [Google Scholar] [CrossRef]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of Polymeric Nanofibers for Drug Delivery Applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef]
- Thakkar, S.; Misra, M. Electrospun Polymeric Nanofibers: New Horizons in Drug Delivery. Eur. J. Pharm. Sci. 2017, 107, 148–167. [Google Scholar] [CrossRef]
- Su, Y.; Su, Q.; Liu, W.; Jin, G.; Mo, X.; Ramakrishn, S. Dual-Drug Encapsulation and Release from Core–Shell Nanofibers. J. Biomater. Sci. Polym. Ed. 2012, 23, 861–871. [Google Scholar] [CrossRef]
- Rošic, R.; Kocbek, P.; Baumgartner, S.; Kristl, J. Electro-Spun Hydroxyethyl Cellulose Nanofibers: The Relationship between Structure and Process. J. Drug Deliv. Sci. Technol. 2011, 21, 229–236. [Google Scholar] [CrossRef]
- Pelipenko, J.; Kocbek, P.; Kristl, J. Critical Attributes of Nanofibers: Preparation, Drug Loading, and Tissue Regeneration. Int. J. Pharm. 2015, 484, 57–74. [Google Scholar] [CrossRef]
- Miguel, S.P.; Figueira, D.R.; Simões, D.; Ribeiro, M.P.; Coutinho, P.; Ferreira, P.; Correia, I.J. Electrospun Polymeric Nanofibres as Wound Dressings: A Review. Colloids Surf. B Biointerfaces 2018, 169, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yu, M.; Ramakrishna, S.; Russell, S.J.; Long, Y.-Z. Advances in Portable Electrospinning Devices for in Situ Delivery of Personalized Wound Care. Nanoscale 2019, 11, 19166–19178. [Google Scholar] [CrossRef] [PubMed]
- Keirouz, A.; Chung, M.; Kwon, J.; Fortunato, G.; Radacsi, N. 2D and 3D Electrospinning Technologies for the Fabrication of Nanofibrous Scaffolds for Skin Tissue Engineering: A Review. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1626. [Google Scholar] [CrossRef] [PubMed]
- Padmakumar, S.; Paul-Prasanth, B.; Pavithran, K.; Vijaykumar, D.K.; Rajanbabu, A.; Sivanarayanan, T.B.; Kadakia, E.; Amiji, M.M.; Nair, S.V.; Menon, D. Long-Term Drug Delivery Using Implantable Electrospun Woven Polymeric Nanotextiles. Nanomed. Nanotechnol. Biol. Med. 2019, 15, 274–284. [Google Scholar] [CrossRef]
- Ngweniform, P.; Abbineni, G.; Cao, B.; Mao, C. Self-Assembly of Drug-Loaded Liposomes on Genetically Engineered Target-Recognizing M13 Phage: A Novel Nanocarrier for Targeted Drug Delivery. Small 2009, 5, 1963–1969. [Google Scholar] [CrossRef]
- Li, W.; Peng, J.; Tan, L.; Wu, J.; Shi, K.; Qu, Y.; Wei, X.; Qian, Z. Mild Photothermal Therapy/Photodynamic Therapy/Chemotherapy of Breast Cancer by Lyp-1 Modified Docetaxel/IR820 Co-Loaded Micelles. Biomaterials 2016, 106, 119–133. [Google Scholar] [CrossRef]
- Jain, R.K.; Stylianopoulos, T. Delivering Nanomedicine to Solid Tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C. The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef]
- Chen, P.; Wu, Q.S.; Ding, Y.P.; Chu, M.; Huang, Z.M.; Hu, W. A Controlled Release System of Titanocene Dichloride by Electrospun Fiber and Its Antitumor Activity In Vitro. Eur. J. Pharm. Biopharm. 2010, 76, 413–420. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Z.; Zhang, A.; Hu, J.; Wang, X.; Yang, Z. Electrospun Nanofibers for Cancer Diagnosis and Therapy. Biomater. Sci. 2016, 4, 922–932. [Google Scholar] [CrossRef]
- Iannazzo, D.; Espro, C.; Celesti, C.; Ferlazzo, A.; Neri, G. Smart Biosensors for Cancer Diagnosis Based on Graphene Quantum Dots. Cancers 2021, 13, 3194. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Chen, Z.; Shin, D.M. Nanoparticle Therapeutics: An Emerging Treatment Modality for Cancer. Nat. Rev. Drug Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef]
- Wolinsky, J.B.; Colson, Y.L.; Grinstaff, M.W. Local Drug Delivery Strategies for Cancer Treatment: Gels, Nanoparticles, Polymeric Films, Rods, and Wafers. J. Control. Release 2012, 159, 14–26. [Google Scholar] [CrossRef]
- Torres-Martínez, E.J.; Bravo, J.M.C.; Medina, A.S.; González, G.L.P.; Gómez, L.J.V. A Summary of Electrospun Nanofibers as Drug Delivery System: Drugs Loaded and Biopolymers Used as Matrices. Curr. Drug Deliv. 2018, 15, 1360–1374. [Google Scholar] [CrossRef]
- Zong, S.; Wang, X.; Yang, Y.; Wu, W.; Li, H.; Ma, Y.; Lin, W.; Sun, T.; Huang, Y.; Xie, Z.; et al. The Use of Cisplatin-Loaded Mucoadhesive Nanofibers for Local Chemotherapy of Cervical Cancers in Mice. Eur. J. Pharm. Biopharm. 2015, 93, 127–135. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, G.; Liu, D.; Xie, Z.; Huang, Y.; Wang, X.; Wu, W.; Jing, X. Inhibition of Orthotopic Secondary Hepatic Carcinoma in Mice by Doxorubicin-Loaded Electrospun Polylactide Nanofibers. J. Mater. Chem. B 2013, 1, 101–109. [Google Scholar] [CrossRef]
- Steffens, L.; Morás, A.M.; Arantes, P.R.; Masterson, K.; Cao, Z.; Nugent, M.; Moura, D.J. Electrospun PVA-Dacarbazine Nanofibers as a Novel Nano Brain-Implant for Treatment of Glioblastoma: In Silico and In Vitro Characterization. Eur. J. Pharm. Sci. 2020, 143, 105183. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Zhou, Y.; Chang, J. Electrospun Nanofibrous Materials for Tissue Engineering and Drug Delivery. Sci. Technol. Adv. Mater. 2010, 11, 014108. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J.; Wang, Y.; Li, L.; Guo, X.; Zhou, S. An Implantable Active-Targeting Micelle-in-Nanofiber Device for Efficient and Safe Cancer Therapy. ACS Nano 2015, 9, 1161–1174. [Google Scholar] [CrossRef]
- Falde, E.J.; Freedman, J.D.; Herrera, V.L.M.; Yohe, S.T.; Colson, Y.L.; Grinstaff, M.W. Layered Superhydrophobic Meshes for Controlled Drug Release. J. Control. Release 2015, 214, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, M.; Alijani, S.; Montazeri, M.; Esmaeilizadeh, N.; Sadeghi-Soureh, S.; Pilehvar-Soltanahmadi, Y. Recent Advances in Electrospun Nanofiber-Mediated Drug Delivery Strategies for Localized Cancer Chemotherapy. J. Biomed. Mater. Res. Part A 2020, 108, 1444–1458. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.-Y.; Huang, Y.-C.; Yang, T.-C.; Yang, S.-T.; Liu, S.-C.; Chang, T.-M.; Kau, Y.-C.; Liu, S.-J. Concurrent Chemotherapy of Malignant Glioma in Rats by Using Multidrug-Loaded Biodegradable Nanofibrous Membranes. Sci. Rep. 2016, 6, 30630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Liu, T.; Liu, S.; Jing, X. Antitumor Activity of Electrospun Polylactide Nanofibers Loaded with 5-Fluorouracil and Oxaliplatin against Colorectal Cancer. Drug Deliv. 2016, 23, 794–800. [Google Scholar] [CrossRef]
- Sridhar, R.; Ravanan, S.; Venugopal, J.R.; Sundarrajan, S.; Pliszka, D.; Sivasubramanian, S.; Gunasekaran, P.; Prabhakaran, M.; Madhaiyan, K.; Sahayaraj, A.; et al. Curcumin- and Natural Extract-Loaded Nanofibres for Potential Treatment of Lung and Breast Cancer: In Vitro Efficacy Evaluation. J. Biomater. Sci. Polym. Ed. 2014, 25, 985–998. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, S.; Qi, Y.; Zhou, D.; Xie, Z.; Jing, X.; Chen, X.; Huang, Y. Time-Programmed DCA and Oxaliplatin Release by Multilayered Nanofiber Mats in Prevention of Local Cancer Recurrence Following Surgery. J. Control. Release 2016, 235, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhao, J.; Lin, Z.Y.; Pan, G.; Zhu, Y.; Cheng, Y.; Cui, W. Self-Coated Interfacial Layer at Organic/Inorganic Phase for Temporally Controlling Dual-Drug Delivery from Electrospun Fibers. Colloids Surf. B Biointerfaces 2015, 130, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, X.; Wang, Z.; Jing, X. Ultrafine PEG-PLA Fibers Loaded with Both Paclitaxel and Doxorubicin Hydrochloride and Their In Vitro Cytotoxicity. Eur. J. Pharm. Biopharm. 2009, 72, 18–25. [Google Scholar] [CrossRef]
- Yohe, S.T.; Herrera, V.L.M.; Colson, Y.L.; Grinstaff, M.W. 3D Superhydrophobic Electrospun Meshes as Reinforcement Materials for Sustained Local Drug Delivery against Colorectal Cancer Cells. J. Control. Release 2012, 162, 92–101. [Google Scholar] [CrossRef]
- Wei, J.; Hu, J.; Li, M.; Chen, Y.; Chen, Y. Multiple Drug-Loaded Electrospun PLGA/Gelatin Composite Nanofibers Encapsulated with Mesoporous ZnO Nanospheres for Potential Postsurgical Cancer Treatment. RSC Adv. 2014, 4, 28011–28019. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, H.; Chen, M.; Wei, J.; Zhang, Y.; Li, X. Antimetastasis and Antitumor Efficacy Promoted by Sequential Release of Vascular Disrupting and Chemotherapeutic Agents from Electrospun Fibers. Int. J. Pharm. 2014, 475, 438–449. [Google Scholar] [CrossRef]
- Li, J.; Xu, W.; Li, D.; Liu, T.; Zhang, Y.S.; Ding, J.; Chen, X. Locally Deployable Nanofiber Patch for Sequential Drug Delivery in Treatment of Primary and Advanced Orthotopic Hepatomas. ACS Nano 2018, 12, 6685–6699. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wei, J.; Wei, Y.; Chen, Y. Controlled Dual Drug Release and In Vitro Cytotoxicity of Electrospun Poly(Lactic-Co-Glycolic Acid) Nanofibers Encapsulated with Micelles. J. Biomed. Nanotechnol. 2015, 11, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wang, J.; Li, L.; Ding, S.; Zhou, S. Electrospun Micelles/Drug-Loaded Nanofibers for Time-Programmed Multi-Agent Release. Macromol. Biosci. 2014, 14, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Li, X.; Li, C.; Dai, Y.; Ma, P.; Zhang, X.; Kang, X.; Cheng, Z.; Lin, J. Electrospun Upconversion Composite Fibers as Dual Drugs Delivery System with Individual Release Properties. Langmuir 2013, 29, 9473–9482. [Google Scholar] [CrossRef]
- Tseng, Y.Y.; Yang, T.C.; Wang, Y.C.; Lee, W.H.; Chang, T.M.; Kau, Y.C.; Liu, S.J. Targeted Concurrent and Sequential Delivery of Chemotherapeutic and Antiangiogenic Agents to the Brain by Using Drug-Loaded Nanofibrous Membranes. Int. J. Nanomed. 2017, 12, 1265–1276. [Google Scholar] [CrossRef]
- Chen, M.; Feng, W.; Lin, S.; He, C.; Gao, Y.; Wang, H. Antitumor Efficacy of a PLGA Composite Nanofiber Embedded with Doxorubicin@ MSNs and Hydroxycamptothecin@ HANPs. RSC Adv. 2014, 4, 53344–53351. [Google Scholar] [CrossRef]
- Liu, D.; Wang, F.; Yue, J.; Jing, X.; Huang, Y. Metabolism Targeting Therapy of Dichloroacetate-Loaded Electrospun Mats on Colorectal Cancer. Drug Deliv. 2015, 22, 136–143. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Park, M.R.; Kim, M.S.; Kwon, O.H. Polyphenol-Loaded Polycaprolactone Nanofibers for Effective Growth Inhibition of Human Cancer Cells. Mater. Chem. Phys. 2012, 133, 674–680. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Zhang, Z.; Zhang, Y.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Use of Asymmetric Multilayer Polylactide Nanofiber Mats in Controlled Release of Drugs and Prevention of Liver Cancer Recurrence after Surgery in Mice. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1047–1056. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Zong, S.; Zhang, Z.; Xie, Z.; Huang, Y.; Yue, Y.; Liu, S.; Jing, X. Local, Combination Chemotherapy in Prevention of Cervical Cancer Recurrence after Surgery by Using Nanofibers Co-Loaded with Cisplatin and Curcumin. RSC Adv. 2015, 5, 106325–106332. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhao, X.; Zhao, J.; Pan, G.; Qiu, W.; Wang, X.; Zhu, Y.; Zheng, Q.; Cui, W. Synergistic Mediation of Tumor Signaling Pathways in Hepatocellular Carcinoma Therapy via Dual-Drug-Loaded PH-Responsive Electrospun Fibrous Scaffolds. J. Mater. Chem. B 2015, 3, 3436–3446. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Rashid, M.H.; Arbab, A.S.; Khan, M. Encapsulation of Anticancer Drugs (5-Fluorouracil and Paclitaxel) into Polycaprolactone (PCL) Nanofibers and In Vitro Testing for Sustained and Targeted Therapy. J. Biomed. Nanotechnol. 2017, 13, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, Y.; Hou, J.; Yang, G.; Zhou, S. A Time-Programmed Release of Dual Drugs from an Implantable Trilayer Structured Fiber Device for Synergistic Treatment of Breast Cancer. Small 2020, 16, 1902262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, Y.; Kuang, G.; Liu, S.; Zhou, D.; Chen, X.; Jing, X.; Huang, Y. Pt (Iv) Prodrug-Backboned Micelle and DCA Loaded Nanofibers for Enhanced Local Cancer Treatment. J. Mater. Chem. B 2017, 5, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhu, Y.; Ye, C.; Chen, Y.; Wang, S.; Zou, D.; Li, Z. Photothermal Transforming Agent and Chemotherapeutic Co-Loaded Electrospun Nanofibers for Tumor Treatment. Int. J. Nanomed. 2019, 14, 3893–3909. [Google Scholar] [CrossRef]
- Yao, Y.; Wei, W.; Sun, J.; Chen, L.; Deng, X.; Ma, L.; Hao, S. Proteomic Analysis of Exosomes Derived from Human Lymphoma Cells. Eur. J. Med. Res. 2015, 20, 8. [Google Scholar] [CrossRef]
- Giustini, A.J.; Petryk, A.A.; Cassim, S.M.; Tate, J.A.; Baker, I.; Hoopes, P.J. Magnetic nanoparticle hyperthermia in cancer treatment. Nano Life 2010, 1, 17–32. [Google Scholar] [CrossRef]
- Niiyama, E.; Uto, K.; Lee, C.M.; Sakura, K.; Ebara, M. Hyperthermia Nanofiber Platform Synergized by Sustained Release of Paclitaxel to Improve Antitumor Efficiency. Adv. Healthc. Mater. 2019, 8, 1900102. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Rivas-García, L.; Baptista, P.V.; Fernandes, A.R. Gene Therapy in Cancer Treatment: Why Go Nano? Pharmaceutics 2020, 12, 233. [Google Scholar] [CrossRef]
- Che, H.L.; Lee, H.J.; Uto, K.; Ebara, M.; Kim, W.J.; Aoyagi, T.; Park, I.K. Simultaneous Drug and Gene Delivery from the Biodegradable Poly(ε-Caprolactone) Nanofibers for the Treatment of Liver Cancer. J. Nanosci. Nanotechnol. 2015, 15, 7971–7975. [Google Scholar] [CrossRef]
- Cavalu, S.; Roiu, G.; Pop, O.; Heredea, D.A.P.; Costea, T.O.; Costea, C.F. Nano-Scale Modifications of Amniotic Membrane Induced by UV and Antibiotic Treatment: Histological, AFM and FTIR Spectroscopy Evidence. Materials 2021, 14, 863. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pornpattananangku, D.; Hu, C.M.; Huang, C.M. Development of Nanoparticles for Antimicrobial Drug Delivery. Curr. Med. Chem. 2010, 17, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.A.A.; Saleh, A.M. Applications of Nanoparticle Systems in Drug Delivery Technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Cavalu, S.; Banica, F.; Gruian, C.; Vanea, E.; Goller, G.; Simon, V. Microscopic and Spectroscopic Investigation of Bioactive Glasses for Antibiotic Controlled Release. J. Mol. Struct. 2013, 1040, 47–52. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Bowlin, G.L.; Mansfield, K.; Layman, J.; Simpson, D.G.; Sanders, E.H.; Wnek, G.E. Release of Tetracycline Hydrochloride from Electrospun Poly(Ethylene-Co-Vinylacetate), Poly(Lactic Acid), and a Blend. J. Control. Release 2002, 81, 57–64. [Google Scholar] [CrossRef]
- Han, J.; Chen, T.-X.; Branford-White, C.J.; Zhu, L.-M. Electrospun Shikonin-Loaded PCL/PTMC Composite Fiber Mats with Potential Biomedical Applications. Int. J. Pharm. 2009, 382, 215–221. [Google Scholar] [CrossRef]
- Gilchrist, S.E.; Lange, D.; Letchford, K.; Bach, H.; Fazli, L.; Burt, H.M. Fusidic Acid and Rifampicin Co-Loaded PLGA Nanofibers for the Prevention of Orthopedic Implant Associated Infections. J. Control. Release 2013, 170, 64–73. [Google Scholar] [CrossRef]
- Ratiu, C.; Brocks, M.; Costea, T.; Moldovan, L.; Cavalu, S. PRGF-Modified Collagen Membranes for Guided Bone Regeneration: Spectroscopic, Microscopic and Nano-Mechanical Investigations. Appl. Sci. 2019, 9, 1035. [Google Scholar] [CrossRef]
- Cavalu, S.; Ratiu, C.; Ponta, O.; Simon, V.; Rugina, D.; Miclaus, V.; Akin, I.; Goller, G. Improving osseointegration of alumina/zirconia ceramic implants by fluoride surface treatment. Dig. J. Nanomater. Biostructures 2014, 9, 797–808. [Google Scholar]
- Xue, J.; He, M.; Niu, Y.; Liu, H.; Crawford, A.; Coates, P.; Chen, D.; Shi, R.; Zhang, L. Preparation and in Vivo Efficient Anti-Infection Property of GTR/GBR Implant Made by Metronidazole Loaded Electrospun Polycaprolactone Nanofiber Membrane. Int. J. Pharm. 2014, 475, 566–577. [Google Scholar] [CrossRef]
- Kataria, K.; Gupta, A.; Rath, G.; Mathur, R.B.; Dhakate, S. In Vivo Wound Healing Performance of Drug Loaded Electrospun Composite Nanofibers Transdermal Patch. Int. J. Pharm. 2014, 469, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, M.X.; Shao, X.; Wang, X.; Zhang, L.; Xu, P.; Zhong, W.; Zhang, L.; Xing, M.M.Q.; Zhang, L. A Laminin Mimetic Peptide SIKVAV-Conjugated Chitosan Hydrogel Promoting Wound Healing by Enhancing Angiogenesis, Re-Epithelialization and Collagen Deposition. J. Mater. Chem. B 2015, 3, 6798–6804. [Google Scholar] [CrossRef]
- Madison, K.C. Barrier Function of the Skin: “La Raison d’être” of the Epidermis. J. Investig. Dermatol. 2003, 121, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The Skin: An Indispensable Barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Broughton, G., 2nd; Janis, J.E.; Attinger, C.E. The Basic Science of Wound Healing. Plast. Reconstr. Surg. 2006, 117, 12s–34s. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human Skin Wounds: A Major and Snowballing Threat to Public Health and the Economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef]
- Dagogo-Jack, S. Primary Prevention of Type-2 Diabetes in Developing Countries. J. Natl. Med. Assoc. 2006, 98, 415–419. [Google Scholar]
- O’Reilly, G.; Cameron, P.; Joshipura, M. Global Trauma Registry Mapping: A Scoping Review. Injury 2012, 43, 1148–1153. [Google Scholar] [CrossRef]
- Kim, T.G.; Park, T.G. Biomimicking Extracellular Matrix: Cell Adhesive RGD Peptide Modified Electrospun Poly(D,L-Lactic-Co-Glycolic Acid) Nanofiber Mesh. Tissue Eng. 2006, 12, 221–233. [Google Scholar] [CrossRef]
- Keshel, S.H.; Biazar, E.; Rezaei Tavirani, M.; Rahmati Roodsari, M.; Ronaghi, A.; Ebrahimi, M.; Rad, H.; Sahebalzamani, A.; Rakhshan, A.; Afsordeh, K. The Healing Effect of Unrestricted Somatic Stem Cells Loaded in Collagen-Modified Nanofibrous PHBV Scaffold on Full-Thickness Skin Defects. Artif. Cells Nanomed. Biotechnol. 2014, 42, 210–216. [Google Scholar] [CrossRef]
- Singer, A.J.; Clark, R.A.F. Cutaneous Wound Healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U.; Meyer, T.; Handschel, J.; Wiesmann, H.-P. Fundamentals of Tissue Engineering and Regenerative Medicine; Springer: Berlin/Heidelberg, Germany, 2009; p. 1076. ISBN 978-3-540-77754-0. [Google Scholar]
- Greiner, A.; Wendorff, J.H. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.A.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of Collagen Nanofibers. Biomacromolecules 2002, 3, 232–238. [Google Scholar] [CrossRef]
- Chakraborty, S.; Liao, I.C.; Adler, A.; Leong, K.W. Electrohydrodynamics: A Facile Technique to Fabricate Drug Delivery Systems. Adv. Drug Deliv Rev. 2009, 61, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H.; Gharehaghaji, A.A.; Toliyat, T.; Dijkstra, P.J. Drug Release Behavior of Electrospun Twisted Yarns as Implantable Medical Devices. Biofabrication 2016, 8, 035019. [Google Scholar] [CrossRef] [PubMed]
- Katti, D.S.; Robinson, K.W.; Ko, F.K.; Laurencin, C.T. Bioresorbable Nanofiber-Based Systems for Wound Healing and Drug Delivery: Optimization of Fabrication Parameters. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 70, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Charernsriwilaiwat, N.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T. Lysozyme-Loaded, Electrospun Chitosan-Based Nanofiber Mats for Wound Healing. Int. J. Pharm. 2012, 427, 379–384. [Google Scholar] [CrossRef]
- Lanno, G.-M.; Ramos, C.; Preem, L.; Putrinš, M.; Laidmäe, I.; Tenson, T.; Kogermann, K. Antibacterial Porous Electrospun Fibers as Skin Scaffolds for Wound Healing Applications. ACS Omega 2020, 5, 30011–30022. [Google Scholar] [CrossRef]
- Xing, Z.-C.; Meng, W.; Yuan, J.; Moon, S.; Jeong, Y.; Kang, I.-K. In Vitro Assessment of Antibacterial Activity and Cytocompatibility of Quercetin-Containing PLGA Nanofibrous Scaffolds for Tissue Engineering. J. Nanomater. 2012, 2012, 202608. [Google Scholar] [CrossRef]
- Ruckh, T.T.; Oldinski, R.A.; Carroll, D.A.; Mikhova, K.; Bryers, J.D.; Popat, K.C. Antimicrobial Effects of Nanofiber Poly(Caprolactone) Tissue Scaffolds Releasing Rifampicin. J. Mater. Sci Mater. Med. 2012, 23, 1411–1420. [Google Scholar] [CrossRef]
- Bhattarai, D.P.; Kim, M.H.; Park, H.; Park, W.H.; Kim, B.S.; Kim, C.S. Coaxially Fabricated Polylactic Acid Electrospun Nanofibrous Scaffold for Sequential Release of Tauroursodeoxycholic Acid and Bone Morphogenic Protein2 to Stimulate Angiogenesis and Bone Regeneration. Chem. Eng. J. 2020, 389, 123470. [Google Scholar] [CrossRef]
- Chen, S.; Liu, B.; Carlson, M.A.; Gombart, A.F.; Reilly, D.A.; Xie, J. Recent Advances in Electrospun Nanofibers for Wound Healing. Nanomed. 2017, 12, 1335–1352. [Google Scholar] [CrossRef] [PubMed]
- Rho, K.S.; Jeong, L.; Lee, G.; Seo, B.-M.; Park, Y.J.; Hong, S.-D.; Roh, S.; Cho, J.J.; Park, W.H.; Min, B.-M. Electrospinning of Collagen Nanofibers: Effects on the Behavior of Normal Human Keratinocytes and Early-Stage Wound Healing. Biomaterials 2006, 27, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, J.R.; Zhang, Y.; Ramakrishna, S. In Vitro Culture of Human Dermal Fibroblasts on Electrospun Polycaprolactone Collagen Nanofibrous Membrane. Artif. Organs 2006, 30, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Simman, R.; Phavixay, L. Split-Thickness Skin Grafts Remain the Gold Standard for the Closure of Large Acute and Chronic Wounds. J. Am. Coll. Certif. Wound Spec. 2011, 3, 55–59. [Google Scholar] [CrossRef] [PubMed][Green Version]
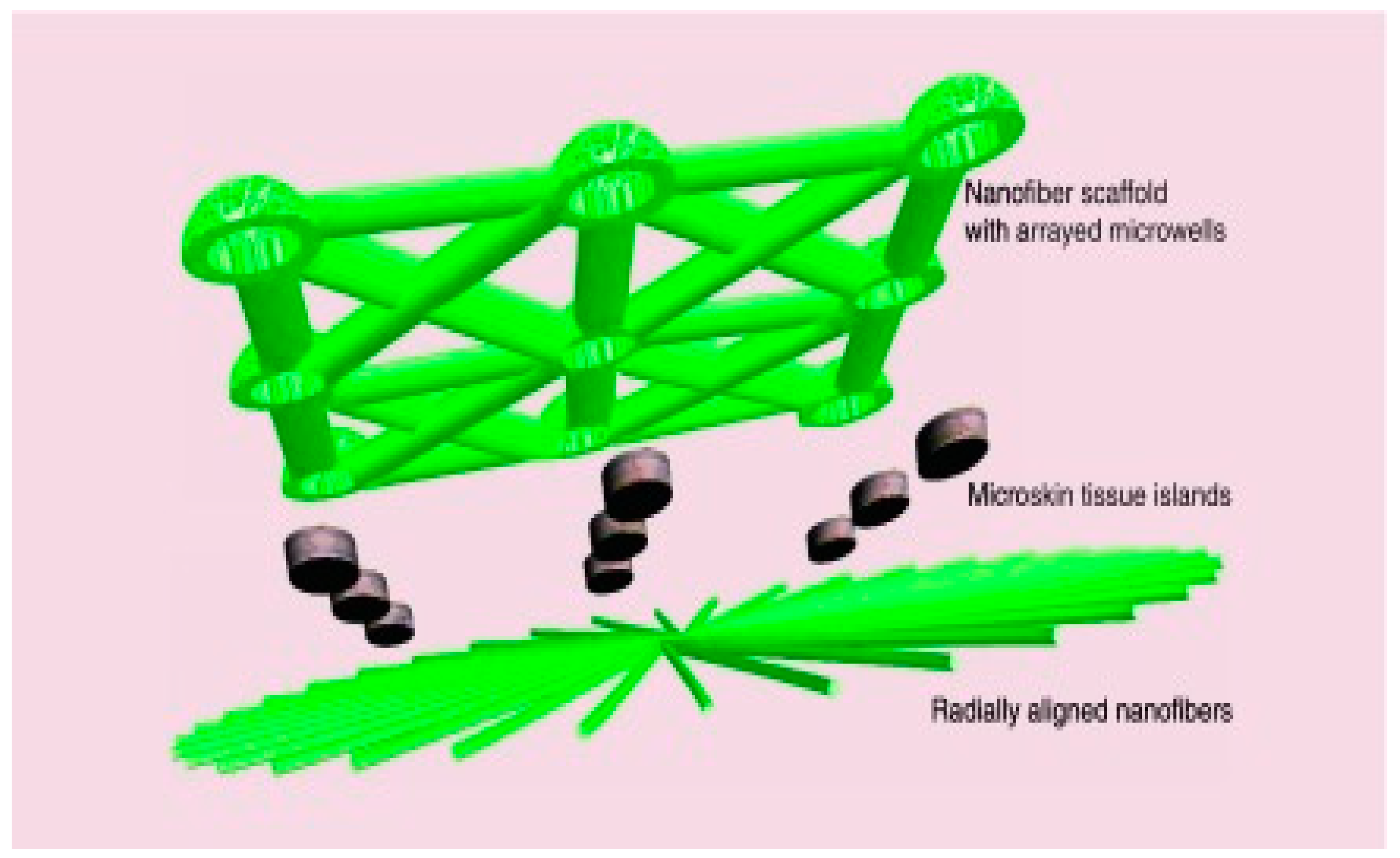
- Ma, B.; Xie, J.; Jiang, J.; Wu, J. Sandwich-Type Fiber Scaffolds with Square Arrayed Microwells and Nanostructured Cues as Microskin Grafts for Skin Regeneration. Biomaterials 2014, 35, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.; Najeeb, S.; Khurshid, Z.; Vazirzadeh, M.; Zohaib, S.; Najeeb, B.; Sefat, F. Potential of Electrospun Nanofibers for Biomedical and Dental Applications. Materials 2016, 9, 73. [Google Scholar] [CrossRef]
- Damle, S.G.; Bhattal, H.; Loomba, A. Apexification of Anterior Teeth: A Comparative Evaluation of Mineral Trioxide Aggregate and Calcium Hydroxide Paste. J. Clin. Pediatric Dent. 2012, 36, 263–268. [Google Scholar] [CrossRef]
- Odabaş, M.E.; Alaçam, A.; Sillelioğlu, H.; Deveci, C. Clinical and Radiographic Success Rates of Mineral Trioxide Aggregate and Ferric Sulphate Pulpotomies Performed by Dental Students. Eur. J. Paediatr. Dent. 2012, 13, 118–122. [Google Scholar]
- Kim, G.M.; Asran, A.S.; Michler, G.H.; Simon, P.; Kim, J.S. Electrospun PVA/HAp Nanocomposite Nanofibers: Biomimetics of Mineralized Hard Tissues at a Lower Level of Complexity. Bioinspiration Biomim. 2008, 3, 046003. [Google Scholar] [CrossRef]
- Kim, J.J.; Bae, W.J.; Kim, J.M.; Kim, J.J.; Lee, E.J.; Kim, H.W.; Kim, E.C. Mineralized Polycaprolactone Nanofibrous Matrix for Odontogenesis of Human Dental Pulp Cells. J. Biomater. Appl. 2014, 28, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, I.; Barber, M.D.; Burgio, K.L.; Kenton, K.; Meikle, S.; Schaffer, J.; Spino, C.; Whitehead, W.E.; Wu, J.; Brody, D.J. Prevalence of Symptomatic Pelvic Floor Disorders in US Women. JAMA 2008, 300, 1311–1316. [Google Scholar] [CrossRef]
- Mukherjee, S.; Darzi, S.; Paul, K.; Cousins, F.L.; Werkmeister, J.A.; Gargett, C.E. Electrospun Nanofiber Meshes with Endometrial MSCs Modulate Foreign Body Response by Increased Angiogenesis, Matrix Synthesis, and Anti-Inflammatory Gene Expression in Mice: Implication in Pelvic Floor. Front. Pharmacol. 2020, 11, 353. [Google Scholar] [CrossRef] [PubMed]
- Albouaini, K.; Egred, M.; Rao, A.; Alahmar, A.; Wright, D.J. Cardiac Resynchronisation Therapy: Evidence Based Benefits and Patient Selection. Eur. J. Intern. Med. 2008, 19, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, P.; Wang, H.; Wang, Y.; Du, Y.; Cheng, W.; Xu, Z.; Zhou, N.; Wang, L.; Yang, Z. Necroptosis Induced by Ad-HGF Activates Endogenous C-Kit+ Cardiac Stem Cells and Promotes Cardiomyocyte Proliferation and Angiogenesis in the Infarcted Aged Heart. Cell. Physiol. Biochem. 2016, 40, 847–860. [Google Scholar] [CrossRef]
- Ho, C.M.B.; Mishra, A.; Lin, P.T.P.; Ng, S.H.; Yeong, W.Y.; Kim, Y.; Yoon, Y. 3D Printed Polycaprolactone Carbon Nanotube Composite Scaffolds for Cardiac Tissue Engineering. Macromol. Biosci. 2017, 17, 1600250. [Google Scholar] [CrossRef] [PubMed]
- Kitsara, M.; Agbulut, O.; Kontziampasis, D.; Chen, Y.; Menasché, P. Fibers for Hearts: A Critical Review on Electrospinning for Cardiac Tissue Engineering. Acta Biomater. 2017, 48, 20–40. [Google Scholar] [CrossRef]
- Hanas, T.; Sampath Kumar, T.S.; Perumal, G.; Doble, M. Tailoring Degradation of AZ31 Alloy by Surface Pre-Treatment and Electrospun PCL Fibrous Coating. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 65, 43–50. [Google Scholar] [CrossRef]
- Yasin, A.S.; Mohamed, I.M.A.; Mousa, H.M.; Park, C.H.; Kim, C.S. Facile Synthesis of TiO2/ZrO2 Nanofibers/Nitrogen Co-Doped Activated Carbon to Enhance the Desalination and Bacterial Inactivation via Capacitive Deionization. Sci. Rep. 2018, 8, 541. [Google Scholar] [CrossRef]
- Kim, H.-W.; Lee, H.-H.; Chun, G.-S. Bioactivity and Osteoblast Responses of Novel Biomedical Nanocomposites of Bioactive Glass Nanofiber Filled Poly(Lactic Acid). J. Biomed. Mater. Res. Part A 2008, 85A, 651–663. [Google Scholar] [CrossRef]
- Bianco, A.; di Federico, E.; Moscatelli, I.; Camaioni, A.; Armentano, I.; Campagnolo, L.; Dottori, M.; Kenny, J.M.; Siracusa, G.; Gusmano, G. Electrospun Poly(ε-Caprolactone)/Ca-Deficient Hydroxyapatite Nanohybrids: Microstructure, Mechanical Properties and Cell Response by Murine Embryonic Stem Cells. Mater. Sci. Eng. C Biomim. Mater. Sens. Syst. 2009, 29, 2063–2071. [Google Scholar] [CrossRef]
- Fabbri, P.; Bondioli, F.; Messori, M.; Bartoli, C.; Dinucci, D.; Chiellini, F. Porous Scaffolds of Polycaprolactone Reinforced with in Situ Generated Hydroxyapatite for Bone Tissue Engineering. J. Mater. Sci. Mater. Med. 2010, 21, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Mousa, H.M.; Abdal-Hay, A.; Bartnikowski, M.; Mohamed, I.M.A.; Yasin, A.S.; Ivanovski, S.; Park, C.H.; Kim, C.S. A Multifunctional Zinc Oxide/Poly(Lactic Acid) Nanocomposite Layer Coated on Magnesium Alloys for Controlled Degradation and Antibacterial Function. ACS Biomater. Sci. Eng. 2018, 4, 2169–2180. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Xu, D.; Liu, X. Mussel-Inspired Functionalization of PEO/PCL Composite Coating on a Biodegradable AZ31 Magnesium Alloy. Colloids Surf. B Biointerfaces 2016, 141, 327–337. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Kasiri-Asgarani, M.; Jabbarzare, S.; Iqbal, N.; Abdul Kadir, M.R. Deposition of Nanostructured Fluorine-Doped Hydroxyapatite-Polycaprolactone Duplex Coating to Enhance the Mechanical Properties and Corrosion Resistance of Mg Alloy for Biomedical Applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 526–537. [Google Scholar] [CrossRef]
- Cavalu, S.; Simon, V. Microstructure and Bioactivity of Acrylic Bone Cements for Prosthetic Surgery. J. Optoelectron. Adv. Mater. 2006, 8, 1520. [Google Scholar]
- Mordike, B.L.; Ebert, T. Magnesium: Properties—Applications—Potential. Mater. Sci. Eng. A 2001, 302, 37–45. [Google Scholar] [CrossRef]
- Rezk, A.I.; Mousa, H.M.; Lee, J.; Park, C.H.; Kim, C.S. Composite PCL/HA/Simvastatin Electrospun Nanofiber Coating on Biodegradable Mg Alloy for Orthopedic Implant Application. J. Coat. Technol. Res. 2019, 16, 477–489. [Google Scholar] [CrossRef]
- Romanò, C.L.; Tsuchiya, H.; Morelli, I.; Battaglia, A.G.; Drago, L. Antibacterial Coating of Implants: Are We Missing Something? Bone Jt. Res. 2019, 8, 199–206. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, J.; Yin, Z.; Tang, C.; Guo, Y.; Li, D.; Wei, B.; Xu, Y.; Gu, Q.; Wang, L. Electrospun Vancomycin-Loaded Coating on Titanium Implants for the Prevention of Implant-Associated Infections. Int. J. Nanomed. 2014, 9, 3027–3036. [Google Scholar] [CrossRef]
- Kranthi Kiran, A.S.; Kizhakeyil, A.; Ramalingam, R.; Verma, N.K.; Lakshminarayanan, R.; Kumar, T.S.S.; Doble, M.; Ramakrishna, S. Drug Loaded Electrospun Polymer/Ceramic Composite Nanofibrous Coatings on Titanium for Implant Related Infections. Ceram. Int. 2019, 45, 18710–18720. [Google Scholar] [CrossRef]
- Wu, S.; Liu, X.; Yeung, K.W.K.; Liu, C.; Yang, X. Biomimetic Porous Scaffolds for Bone Tissue Engineering. Mater. Sci. Eng. R Rep. 2014, 80, 1–36. [Google Scholar] [CrossRef]
- Diefenbeck, M.; Mückley, T.; Hofmann, G.O. Prophylaxis and Treatment of Implant-Related Infections by Local Application of Antibiotics. Injury 2006, 37, S95–S104. [Google Scholar] [CrossRef]
- Hake, M.E.; Young, H.; Hak, D.J.; Stahel, P.F.; Hammerberg, E.M.; Mauffrey, C. Local Antibiotic Therapy Strategies in Orthopaedic Trauma: Practical Tips and Tricks and Review of the Literature. Injury 2015, 46, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Jahanmard, F.; Croes, M.; Castilho, M.; Majed, A.; Steenbergen, M.J.; Lietaert, K.; Vogely, H.C.; van der Wal, B.C.H.; Stapels, D.A.C.; Malda, J.; et al. Bactericidal Coating to Prevent Early and Delayed Implant-Related Infections. J. Control. Release 2020, 326, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Ashbaugh, A.G.; Jiang, X.; Zheng, J.; Tsai, A.S.; Kim, W.-S.; Thompson, J.M.; Miller, R.J.; Shahbazian, J.H.; Wang, Y.; Dillen, C.A.; et al. Polymeric Nanofiber Coating with Tunable Combinatorial Antibiotic Delivery Prevents Biofilm-Associated Infection in Vivo. Proc. Natl. Acad. Sci. USA 2016, 113, E6919. [Google Scholar] [CrossRef]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R. Executive Summary: Diagnosis and Management of Prosthetic Joint Infection: Clinical Practice Guidelines by the Infectious Diseases Society of Americaa. Clin. Infect. Dis. 2013, 56, 1–10. [Google Scholar] [CrossRef]
- Sandoe, J.A.T.; Barlow, G.; Chambers, J.B.; Gammage, M.; Guleri, A.; Howard, P.; Olson, E.; Perry, J.D.; Prendergast, B.D.; Spry, M.J.; et al. Guidelines for the Diagnosis, Prevention and Management of Implantable Cardiac Electronic Device Infection. Report of a Joint Working Party Project on Behalf of the British Society for Antimicrobial Chemotherapy (BSAC, Host Organization), British Heart Rhythm Society (BHRS), British Cardiovascular Society (BCS), British Heart Valve Society (BHVS) and British Society for Echocardiography (BSE). J. Antimicrob. Chemother. 2015, 70, 325–359. [Google Scholar] [CrossRef]
- Shahi, R.G.; Albuquerque, M.T.P.; Münchow, E.A.; Blanchard, S.B.; Gregory, R.L.; Bottino, M.C. Novel Bioactive Tetracycline-Containing Electrospun Polymer Fibers as a Potential Antibacterial Dental Implant Coating. Odontology 2017, 105, 354–363. [Google Scholar] [CrossRef]
- Sadri, M.; Pashmfroosh, N.; Samadieh, S. Implants Modified with Polymeric Nanofibers Coating Containing the Antibiotic Vancomycin. Nanomed. Res. J. 2017, 2, 208–215. [Google Scholar] [CrossRef]
- Cochis, A.; Ferraris, S.; Sorrentino, R.; Azzimonti, B.; Novara, C.; Geobaldo, F.; Truffa Giachet, F.; Varesano, A.; Vineis, C.; Mahmoud, A.; et al. Silver Doped Keratin Nanofibres Preserve Titanium Surface from Biofilm Contamination and Favour Soft Tissue Healing. J. Mater. Chem. B 2017, 5, 8366–8377. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Seta, J.; Chen, L.; Bergum, C.; Zhou, Z.; Kanneganti, P.; Kast, R.E.; Auner, G.W.; Shen, M.; Markel, D.C.; et al. Doxycycline-Loaded Coaxial Nanofiber Coating of Titanium Implants Enhances Osseointegration and Inhibits Staphylococcus Aureus Infection. Biomed. Mater. 2017, 12, 045008. [Google Scholar] [CrossRef] [PubMed]
- Maver, T.; Mastnak, T.; Mihelič, M.; Maver, U.; Finšgar, M. Clindamycin-Based 3D-Printed and Electrospun Coatings for Treatment of Implant-Related Infections. Materials 2021, 14, 1464. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L.; Wang, L.M.; Xu, Y.; Lv, L.X. Preparation of Gentamicin-Loaded Electrospun Coating on Titanium Implants and a Study of Their Properties In Vitro. Arch. Orthop. Trauma Surg. 2012, 132, 897–903. [Google Scholar] [CrossRef]
- Chopra, H.; Kumar, S.; Singh, I. Strategies and Therapies for Wound Healing: A Review. Curr. Drug Targets 2022, 23, 87–98. [Google Scholar] [CrossRef]
- Chopra, H.; Gandhi, S.; Gautam, R.K.; Kamal, M.A. Bacterial Nanocellulose Based Wound Dressings: Current and Future Prospects. Curr. Pharm. Des. 2022, 28, 570–580. [Google Scholar] [CrossRef]
- Wang, X.; Ding, B.; Yu, J.; Wang, M. Engineering Biomimetic Superhydrophobic Surfaces of Electrospun Nanomaterials. Nano Today 2011, 6, 510–530. [Google Scholar] [CrossRef]
- Yu, X.; Li, Y.; Yin, X.; Wang, X.; Han, Y.; Si, Y.; Yu, J.; Ding, B. Corncoblike, Superhydrophobic, and Phase-Changeable Nanofibers for Intelligent Thermoregulating and Water-Repellent Fabrics. ACS Appl. Mater. Interfaces 2019, 11, 39324–39333. [Google Scholar] [CrossRef]
- Sheng, J.; Li, Y.; Wang, X.; Si, Y.; Yu, J.; Ding, B. Thermal Inter-Fiber Adhesion of the Polyacrylonitrile/Fluorinated Polyurethane Nanofibrous Membranes with Enhanced Waterproof-Breathable Performance. Sep. Purif. Technol. 2016, 158, 53–61. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, W.; Wang, X.; Liu, L.; Yu, J.; Ding, B. Environmentally Benign Modification of Breathable Nanofibrous Membranes Exhibiting Superior Waterproof and Photocatalytic Self-Cleaning Properties. Nanoscale Horiz. 2019, 4, 867–873. [Google Scholar] [CrossRef]
- Li, Y.; Yang, F.; Yu, J.; Ding, B. Hydrophobic Fibrous Membranes with Tunable Porous Structure for Equilibrium of Breathable and Waterproof Performance. Adv. Mater. Interfaces 2016, 3, 1600516. [Google Scholar] [CrossRef]
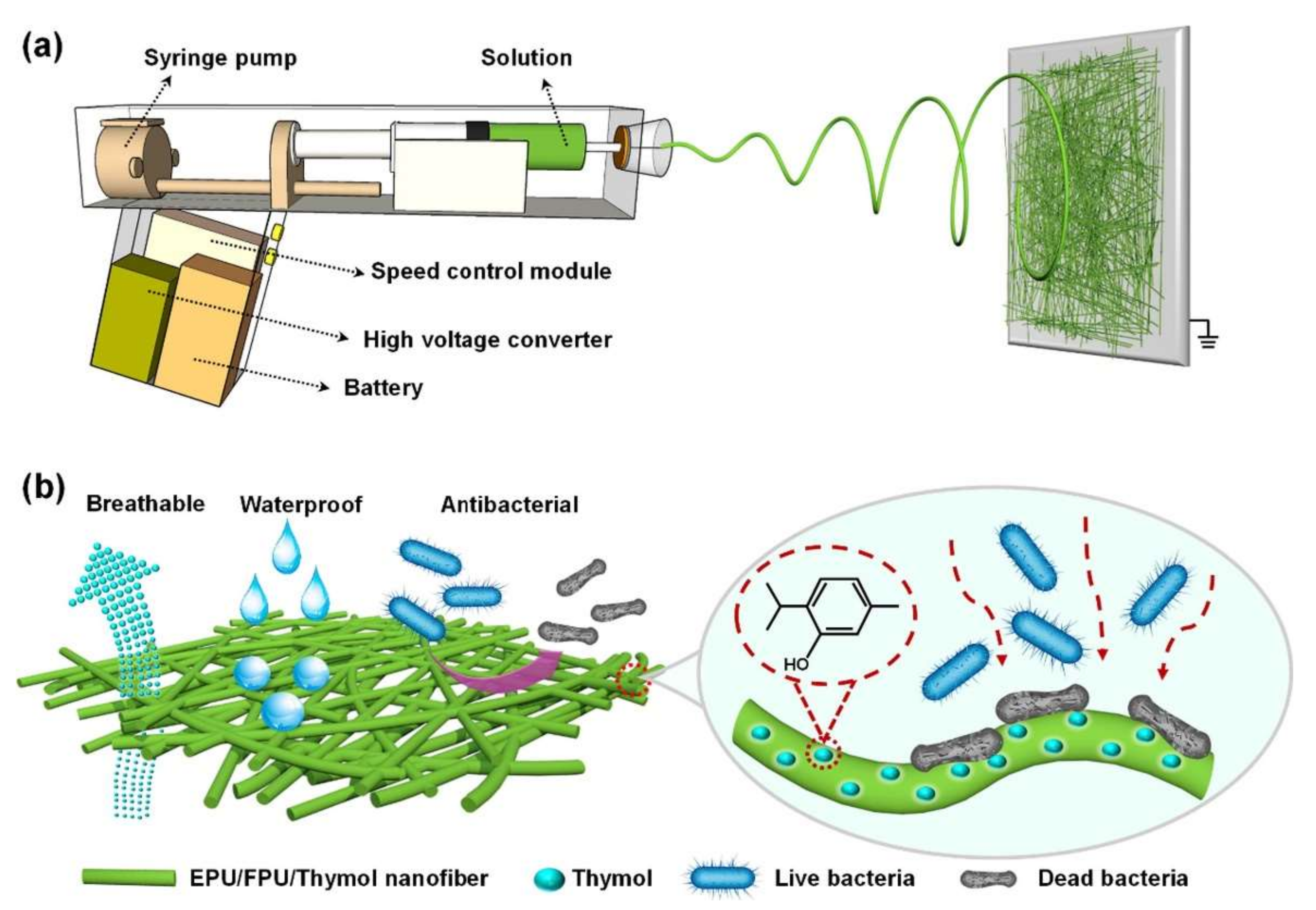
- Yue, Y.; Gong, X.; Jiao, W.; Li, Y.; Yin, X.; Si, Y.; Yu, J.; Ding, B. In-Situ Electrospinning of Thymol-Loaded Polyurethane Fibrous Membranes for Waterproof, Breathable, and Antibacterial Wound Dressing Application. J. Colloid Interface Sci. 2021, 592, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Guo, Y.; Li, X.; Yu, J.; Ding, B. Asymmetric Wettable, Waterproof, and Breathable Nanofibrous Membranes for Wound Dressings. ACS Appl. Bio Mater. 2021, 4, 3287–3293. [Google Scholar] [CrossRef]
- Guo, Y.; Zhou, W.; Wang, L.; Dong, Y.; Yu, J.; Li, X.; Ding, B. Stretchable PDMS Embedded Fibrous Membranes Based on an Ethanol Solvent System for Waterproof and Breathable Applications. ACS Appl. Bio Mater. 2019, 2, 5949–5956. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, F.; Liu, Y.; Zheng, Y.; Xin, B.; Jiang, Z.; Peng, X.; Jin, S. Waterproof and Breathable Polyacrylonitrile/(Polyurethane/Fluorinated-Silica) Composite Nanofiber Membrane via Side-by-Side Electrospinning. J. Mater. Res. 2020, 35, 1173–1181. [Google Scholar] [CrossRef]
- Xu, Y.; Sheng, J.; Yin, X.; Yu, J.; Ding, B. Functional Modification of Breathable Polyacrylonitrile/Polyurethane/TiO2 Nanofibrous Membranes with Robust Ultraviolet Resistant and Waterproof Performance. J. Colloid Interface Sci. 2017, 508, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.R.; Rabbani, S.; Bahrami, S.H.; Joghataei, M.T.; Moayer, F. Antibacterial Performance and in Vivo Diabetic Wound Healing of Curcumin Loaded Gum Tragacanth/Poly(ε-Caprolactone) Electrospun Nanofibers. Mater. Sci. Eng. C 2016, 69, 1183–1191. [Google Scholar] [CrossRef]
- Merrell, J.G.; McLaughlin, S.W.; Tie, L.; Laurencin, C.T.; Chen, A.F.; Nair, L.S. Curcumin-Loaded Poly(ε-Caprolactone) Nanofibres: Diabetic Wound Dressing with Anti-Oxidant and Anti-Inflammatory Properties. Clin. Exp. Pharmacol. Physiol. 2009, 36, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar-Mohammadi, M.; Zamani, M.; Prabhakaran, M.P.; Bahrami, S.H.; Ramakrishna, S. Electrospinning of PLGA/Gum Tragacanth Nanofibers Containing Tetracycline Hydrochloride for Periodontal Regeneration. Mater. Sci. Eng. C 2016, 58, 521–531. [Google Scholar] [CrossRef]
- Golchin, A.; Hosseinzadeh, S.; Jouybar, A.; Staji, M.; Soleimani, M.; Ardeshirylajimi, A.; Khojasteh, A. Wound Healing Improvement by Curcumin-Loaded Electrospun Nanofibers and BFP-MSCs as a Bioactive Dressing. Polym. Adv. Technol. 2020, 31, 1519–1531. [Google Scholar] [CrossRef]
- GhavamiNejad, A.; Rajan Unnithan, A.; Ramachandra Kurup Sasikala, A.; Samarikhalaj, M.; Thomas, R.G.; Jeong, Y.Y.; Nasseri, S.; Murugesan, P.; Wu, D.; Hee Park, C.; et al. Mussel-Inspired Electrospun Nanofibers Functionalized with Size-Controlled Silver Nanoparticles for Wound Dressing Application. ACS Appl. Mater. Interfaces 2015, 7, 12176–12183. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Cao, R.; Zhang, Y.; Chen, R. Metallic Ions Encapsulated in Electrospun Nanofiber for Antibacterial and Angiogenesis Function to Promote Wound Repair. Front. Cell Dev. Biol. 2021, 9, 707. [Google Scholar] [CrossRef] [PubMed]
- Vargas, E.A.T.; do Vale Baracho, N.C.; de Brito, J.; de Queiroz, A.A.A. Hyperbranched Polyglycerol Electrospun Nanofibers for Wound Dressing Applications. Acta Biomater. 2010, 6, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, P.; Karami, Z.; Rezaeian, I.; Jafari, S.H.; Mahdaviani, P.; Abdolghaffari, A.H.; Abdollahi, M. Preparation and Performance Evaluation of Tetracycline Hydrochloride Loaded Wound Dressing Mats Based on Electrospun Nanofibrous Poly(Lactic Acid)/Poly(Ïμ-Caprolactone) Blends. J. Appl. Polym. Sci. 2012, 124, 4174–4183. [Google Scholar] [CrossRef]
- Kim, K.; Luu, Y.K.; Chang, C.; Fang, D.; Hsiao, B.S.; Chu, B.; Hadjiargyrou, M. Incorporation and Controlled Release of a Hydrophilic Antibiotic Using Poly(Lactide-Co-Glycolide)-Based Electrospun Nanofibrous Scaffolds. J. Control. Release 2004, 98, 47–56. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, F.; Huang, Y.; Fang, Y.; Shen, M.; Zhu, M.; Shi, X. Encapsulation of Amoxicillin within Laponite-Doped Poly(Lactic-Co-Glycolic Acid) Nanofibers: Preparation, Characterization, and Antibacterial Activity. ACS Appl. Mater. Interfaces 2012, 4, 6393–6401. [Google Scholar] [CrossRef]
- Thakur, R.A.; Florek, C.A.; Kohn, J.; Michniak, B.B. Electrospun Nanofibrous Polymeric Scaffold with Targeted Drug Release Profiles for Potential Application as Wound Dressing. Int. J. Pharm. 2008, 364, 87–93. [Google Scholar] [CrossRef]
- Toncheva, A.; Paneva, D.; Maximova, V.; Manolova, N.; Rashkov, I. Antibacterial Fluoroquinolone Antibiotic-Containing Fibrous Materials from Poly(l-Lactide-Co-d,l-Lactide) Prepared by Electrospinning. Eur. J. Pharm. Sci. 2012, 47, 642–651. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Martinez-Abad, A.; Gimeno-Alcañiz, J.V.; Ocio, M.J.; Lagaron, J.M. Controlled Delivery of Gentamicin Antibiotic from Bioactive Electrospun Polylactide-Based Ultrathin Fibers. Adv. Eng. Mater. 2012, 14, B112–B122. [Google Scholar] [CrossRef]
- Su, Y.; Xiaoqiang, A.E.; Ae, L.; Wang, H.; Chuanglong, A.E.; Ae, H.; Mo, X. Fabrication and Characterization of Biodegradable Nanofibrous Mats by Mix and Coaxial Electrospinning. J. Mater. Sci. Mater. Med. 2009, 20, 2285. [Google Scholar] [CrossRef]
- Sohrabi, A.; Shaibani, P.M.; Etayash, H.; Kaur, K.; Thundat, T. Sustained Drug Release and Antibacterial Activity of Ampicillin Incorporated Poly(Methyl Methacrylate)-Nylon6 Core/Shell Nanofibers. Polymer 2013, 54, 2699–2705. [Google Scholar] [CrossRef]
- Khampieng, T.; Wnek, G.E.; Supaphol, P. Electrospun DOXY-h Loaded-Poly(Acrylic Acid) Nanofiber Mats: In Vitro Drug Release and Antibacterial Properties Investigation. J. Biomater. Sci. Polym. Ed. 2014, 25, 1292–1305. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, A.; Umu, O.C.O.; Tekinay, T.; Uyar, T. Antibacterial Electrospun Nanofibers from Triclosan/Cyclodextrin Inclusion Complexes. Colloids Surf. B Biointerfaces 2014, 116, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, L.J.; Díaz, A.; Royo, M.; Rodríguez-Galán, A.; Puiggalí, J. Biodegradable Polyesters Reinforced with Triclosan Loaded Polylactide Micro/Nanofibers: Properties, Release and Biocompatibility. Express Polym. Lett. 2012, 6, 266–282. [Google Scholar] [CrossRef]
- Kayaci, F.; Umu, O.C.O.; Tekinay, T.; Uyar, T. Antibacterial Electrospun Poly(Lactic Acid) (PLA) Nanofibrous Webs Incorporating Triclosan/Cyclodextrin Inclusion Complexes. J. Agric. Food Chem. 2013, 61, 3901–3908. [Google Scholar] [CrossRef]
- Ren, X.; Kocer, H.B.; Worley, S.D.; Broughton, R.M.; Huang, T.S. Biocidal Nanofibers via Electrospinning. J. Appl. Polym. Sci. 2013, 127, 3192–3197. [Google Scholar] [CrossRef]
- Gliścińska, E.; Gutarowska, B.; Brycki, B.; Krucińska, I. Electrospun Polyacrylonitrile Nanofibers Modified by Quaternary Ammonium Salts. J. Appl. Polym. Sci. 2013, 128, 767–775. [Google Scholar] [CrossRef]
- Chen, L.; Bromberg, L.; Hatton, T.A.; Rutledge, G.C. Electrospun Cellulose Acetate Fibers Containing Chlorhexidine as a Bactericide. Polymer 2008, 49, 1266–1275. [Google Scholar] [CrossRef]
- Liu, X.; Lin, T.; Gao, Y.; Xu, Z.; Huang, C.; Yao, G.; Jiang, L.; Tang, Y.; Wang, X. Antimicrobial Electrospun Nanofibers of Cellulose Acetate and Polyester Urethane Composite for Wound Dressing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100 B, 1556–1565. [Google Scholar] [CrossRef]
- Spasova, M.; Manolova, N.; Paneva, D.; Rashkov, I. Preparation of Chitosan-Containing Nanofibres by Electrospinning of Chitosan/Poly(Ethylene Oxide) Blend Solutions. E-Polymers 2004, 4, 56. [Google Scholar] [CrossRef]
- Mahapatra, A.; Garg, N.; Nayak, B.P.; Mishra, B.G.; Hota, G. Studies on the Synthesis of Electrospun PAN-Ag Composite Nanofibers for Antibacterial Application. J. Appl. Polym. Sci. 2012, 124, 1178–1185. [Google Scholar] [CrossRef]
- Au, H.T.; Pham, L.N.; Vu, T.H.T.; Park, J.S. Fabrication of an Antibacterial Non-Woven Mat of a Poly(Lactic Acid)/Chitosan Blend by Electrospinning. Macromol. Res. 2012, 20, 51–58. [Google Scholar] [CrossRef]
- An, J.; Zhang, H.; Zhang, J.; Zhao, Y.; Yuan, X. Preparation and Antibacterial Activity of Electrospun Chitosan/ Poly(Ethylene Oxide) Membranes Containing Silver Nanoparticles. Colloid Polym. Sci. 2009, 287, 1425–1434. [Google Scholar] [CrossRef]
- Abdelgawad, A.M.; Hudson, S.M.; Rojas, O.J. Antimicrobial Wound Dressing Nanofiber Mats from Multicomponent (Chitosan/Silver-NPs/Polyvinyl Alcohol) Systems. Carbohydr. Polym. 2014, 100, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Heunis, T.; Bshena, O.; Klumperman, B.; Dicks, L. Release of Bacteriocins from Nanofibers Prepared with Combinations of Poly(D,L-Lactide) (PDLLA) and Poly(Ethylene Oxide) (PEO). Int. J. Mol. Sci. 2011, 12, 2158–2173. [Google Scholar] [CrossRef] [PubMed]
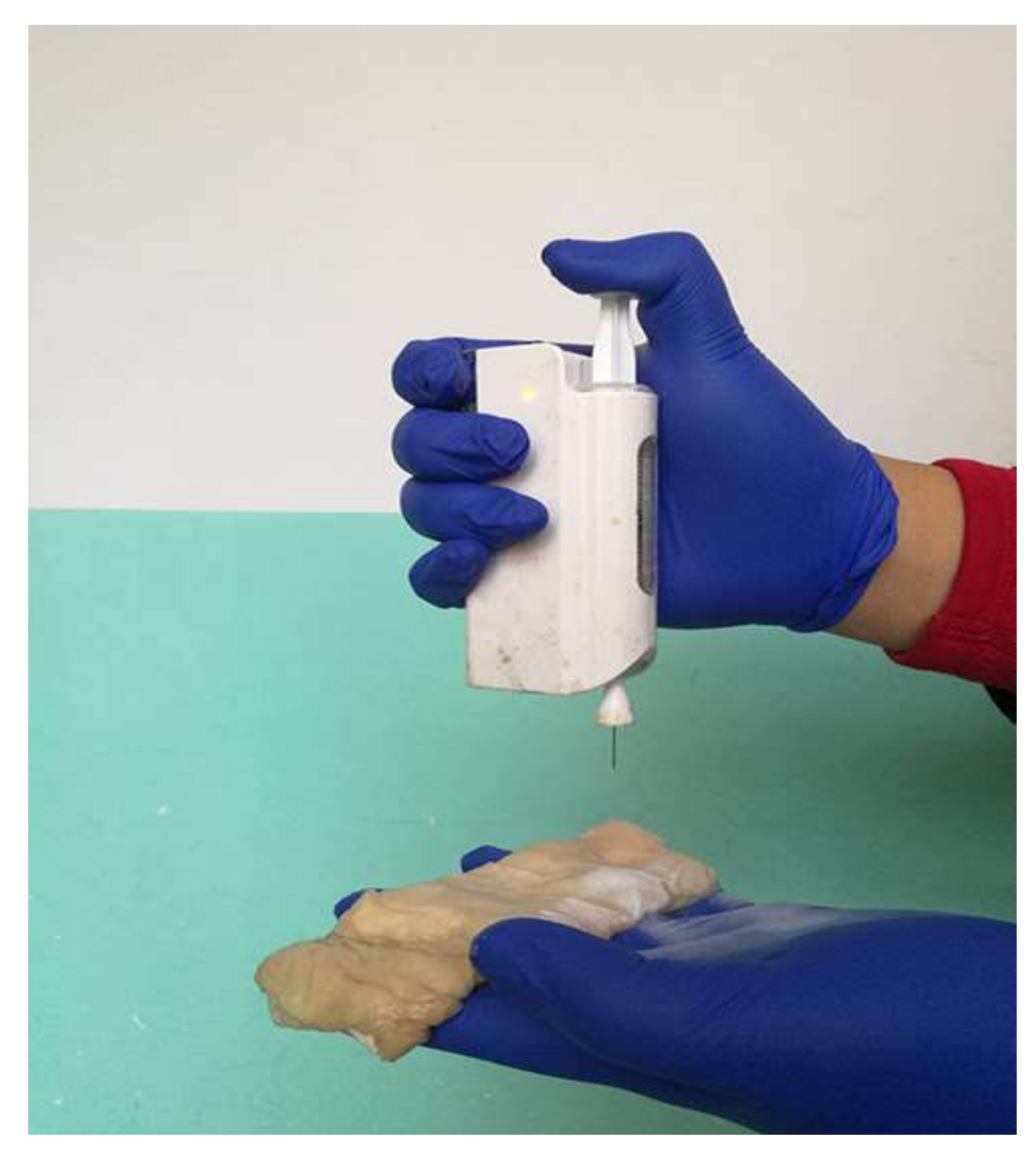
- Brako, F.; Luo, C.; Craig, D.Q.M.; Edirisinghe, M. An Inexpensive, Portable Device for Point-of-Need Generation of Silver-Nanoparticle Doped Cellulose Acetate Nanofibers for Advanced Wound Dressing. Macromol. Mater. Eng. 2018, 303, 1700586. [Google Scholar] [CrossRef]
- Liu, G.-S.; Yan, X.; Yan, F.-F.; Chen, F.-X.; Hao, L.-Y.; Chen, S.-J.; Lou, T.; Ning, X.; Long, Y.-Z. In Situ Electrospinning Iodine-Based Fibrous Meshes for Antibacterial Wound Dressing. Nanoscale Res. Lett. 2018, 13, 309. [Google Scholar] [CrossRef]
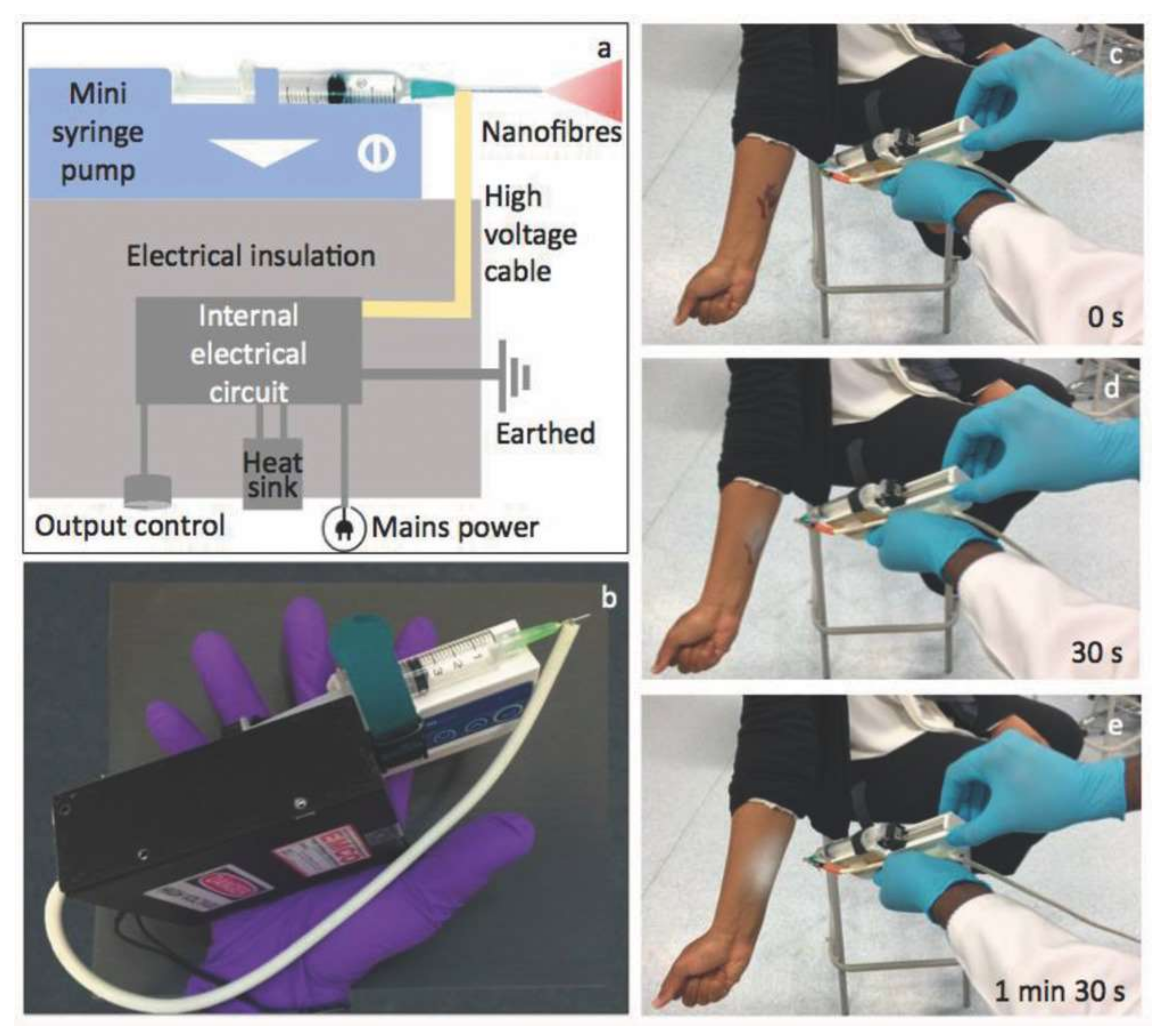
- Haik, J.; Kornhaber, R.; Blal, B.; Harats, M. The Feasibility of a Handheld Electrospinning Device for the Application of Nanofibrous Wound Dressings. Adv. Wound Care 2017, 6, 166–174. [Google Scholar] [CrossRef]
- Dong, W.-H.; Liu, J.-X.; Mou, X.-J.; Liu, G.-S.; Huang, X.-W.; Yan, X.; Ning, X.; Russell, S.J.; Long, Y.-Z. Performance of Polyvinyl Pyrrolidone-Isatis Root Antibacterial Wound Dressings Produced in Situ by Handheld Electrospinner. Colloids Surf. B Biointerfaces 2020, 188, 110766. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, Y.; Lei, F.; Yu, J. In-Situ Electrospinning for Intestinal Hemostasis. Int. J. Nanomed. 2020, 15, 3869. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.-T.; Hu, P.-Y.; Liu, J.-J.; Liu, X.-F.; Hu, M.; Cui, Z.; Wang, N.; Niu, Z.; Xiang, H.-F.; et al. Laparoscopic Electrospinning for in Situ Hemostasis in Minimally Invasive Operation. Chem. Eng. J. 2020, 395, 125089. [Google Scholar] [CrossRef]
- Liu, X.-F.; Zhang, J.; Liu, J.-J.; Zhou, Q.-H.; Liu, Z.; Hu, P.-Y.; Yuan, Z.; Ramakrishna, S.; Yang, D.-P.; Long, Y.-Z. Bifunctional CuS Composite Nanofibers via in Situ Electrospinning for Outdoor Rapid Hemostasis and Simultaneous Ablating Superbug. Chem. Eng. J. 2020, 401, 126096. [Google Scholar] [CrossRef]
- Xu, S.-C.; Qin, C.-C.; Yu, M.; Dong, R.-H.; Yan, X.; Zhao, H.; Han, W.-P.; Zhang, H.-D.; Long, Y.-Z. A Battery-Operated Portable Handheld Electrospinning Apparatus. Nanoscale 2015, 7, 12351–12355. [Google Scholar] [CrossRef] [PubMed]
- Sofokleous, P.; Stride, E.; Bonfield, W.; Edirisinghe, M. Design, Construction and Performance of a Portable Handheld Electrohydrodynamic Multi-Needle Spray Gun for Biomedical Applications. Mater. Sci. Eng. C 2013, 33, 213–223. [Google Scholar] [CrossRef]
- Lau, W.K.; Sofokleous, P.; Day, R.; Stride, E.; Edirisinghe, M. A Portable Device for in Situ Deposition of Bioproducts. Bioinspired Biomim. Nanobiomater. 2014, 3, 94–105. [Google Scholar] [CrossRef]
- Perng, J.-W.; Kuo, Y.-C.; Lu, S.-P. Grounding System Cost Analysis Using Optimization Algorithms. Energies 2018, 11, 3484. [Google Scholar] [CrossRef]
- Liu, J.-X.; Dong, W.-H.; Mou, X.-J.; Liu, G.-S.; Huang, X.-W.; Yan, X.; Zhou, C.-F.; Jiang, S.; Long, Y.-Z. In Situ Electrospun Zein/Thyme Essential Oil-Based Membranes as an Effective Antibacterial Wound Dressing. ACS Appl. Bio Mater. 2019, 3, 302–307. [Google Scholar] [CrossRef]
- Qin, M.; Mou, X.; Dong, W.; Liu, J.; Liu, H.; Dai, Z.; Huang, X.; Wang, N.; Yan, X. In Situ Electrospinning Wound Healing Films Composed of Zein and Clove Essential Oil. Macromol. Mater. Eng. 2020, 305, 1900790. [Google Scholar] [CrossRef]
- Qin, M.; Liu, D.; Dai, Z.; Meng, X.; Liu, G.; Liu, H.; Huang, X.; Yan, X.; Chen, S. One Step Fabrication and Application of Antibacterial Electrospun Zein/Cinnamon Oil Membrane Wound Dressing via In Situ Electrospinning Process. Chem. Res. Chin. Univ. 2021, 37, 464–469. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, H.; Shen, Y.; Dai, X.; Wang, X.; Deng, K.; Long, X.; Liu, L.; Zhang, X.; Li, Y. Instant In-Situ Tissue Repair by Biodegradable PLA/Gelatin Nanofibrous Membrane Using a 3D Printed Handheld Electrospinning Device. Front. Bioeng. Biotechnol. 2021, 9, 684105. [Google Scholar] [CrossRef]
- Han, W.-P.; Huang, Y.-Y.; Yu, M.; Zhang, J.-C.; Yan, X.; Yu, G.-F.; Zhang, H.-D.; Yan, S.-Y.; Long, Y.-Z. Self-Powered Electrospinning Apparatus Based on a Hand-Operated Wimshurst Generator. Nanoscale 2015, 7, 5603–5606. [Google Scholar] [CrossRef] [PubMed]
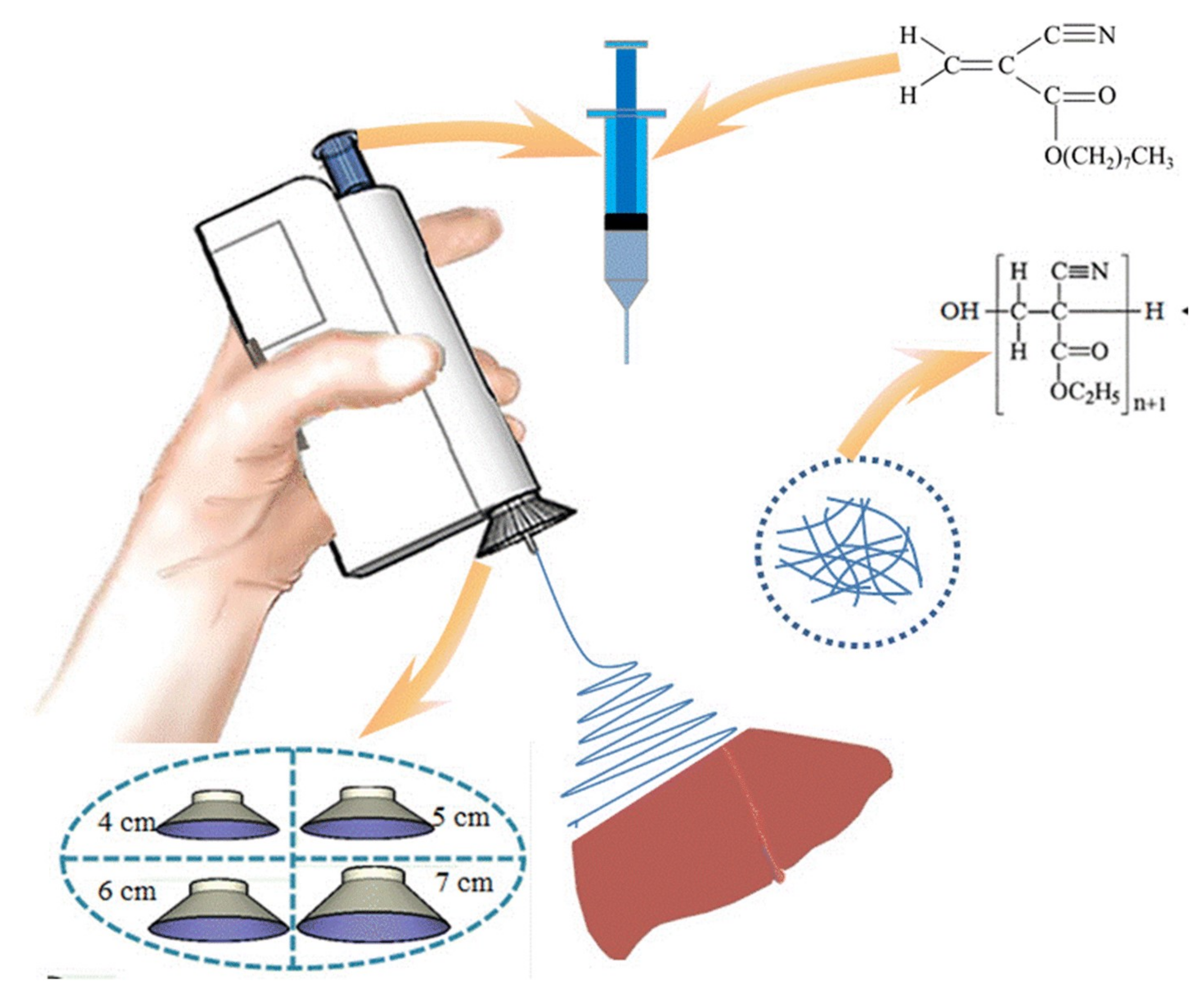
- Luo, W.-L.; Zhang, J.; Qiu, X.; Chen, L.-J.; Fu, J.; Hu, P.-Y.; Li, X.; Hu, R.-J.; Long, Y.-Z. Electric-Field-Modified in Situ Precise Deposition of Electrospun Medical Glue Fibers on the Liver for Rapid Hemostasis. Nanoscale Res. Lett. 2018, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
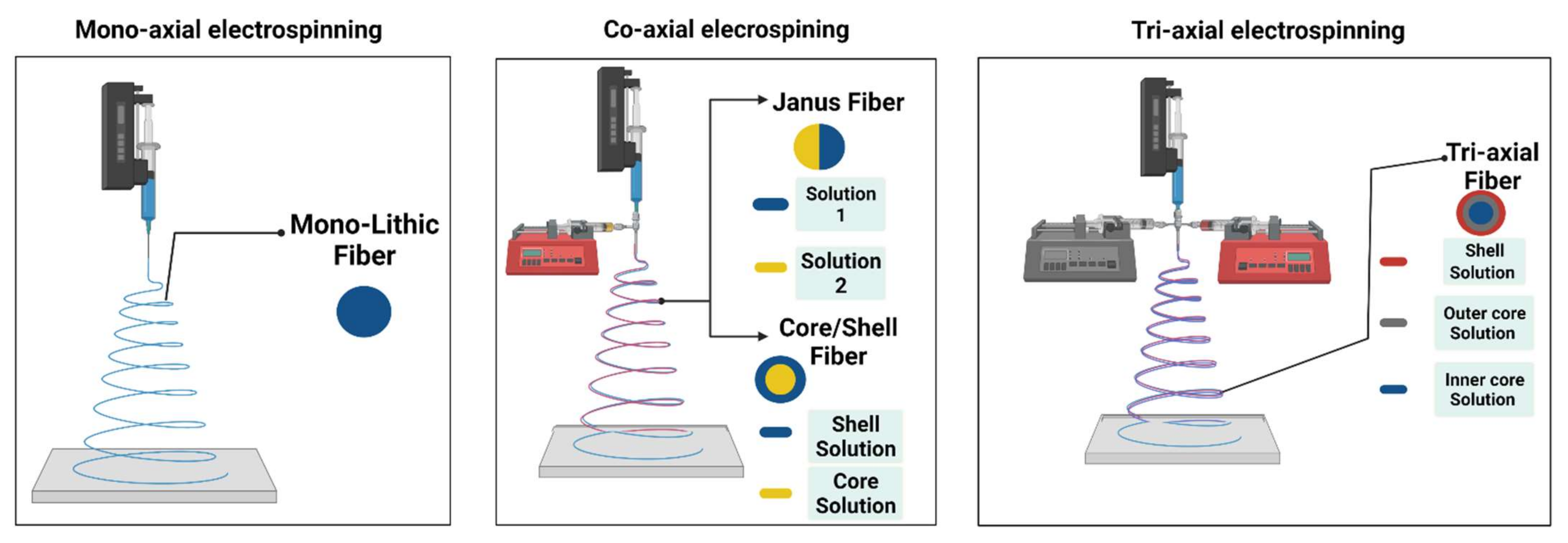
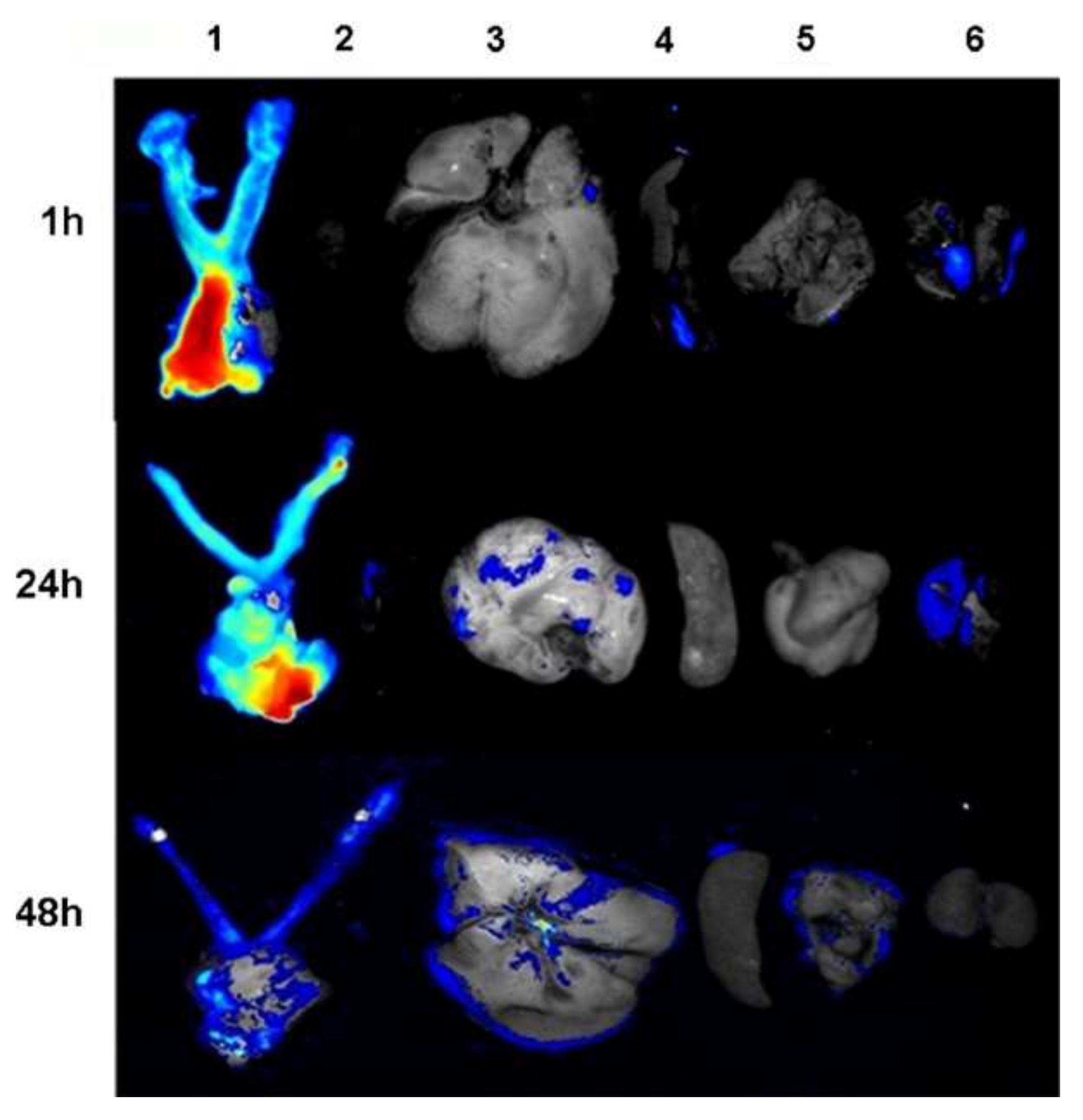
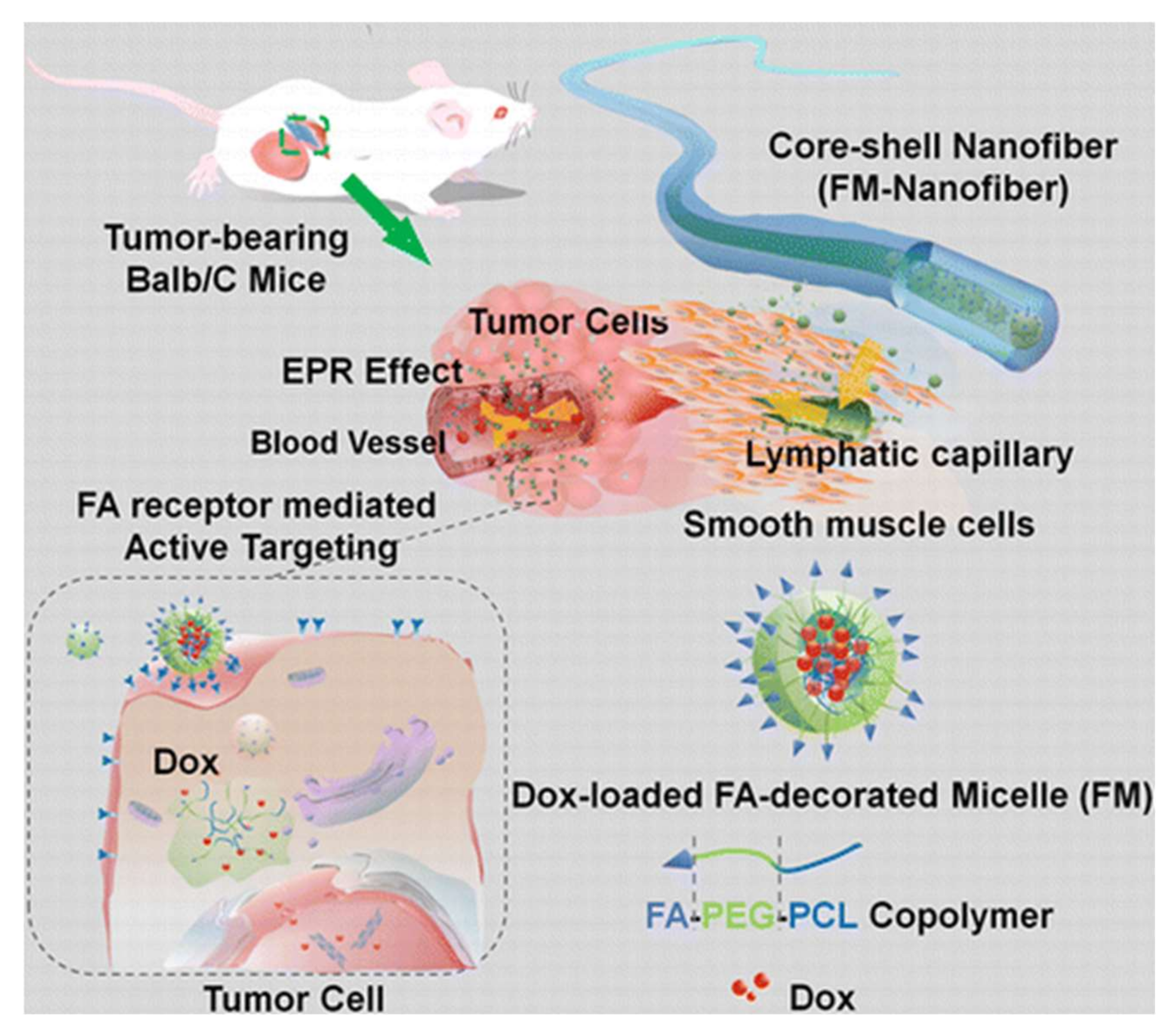
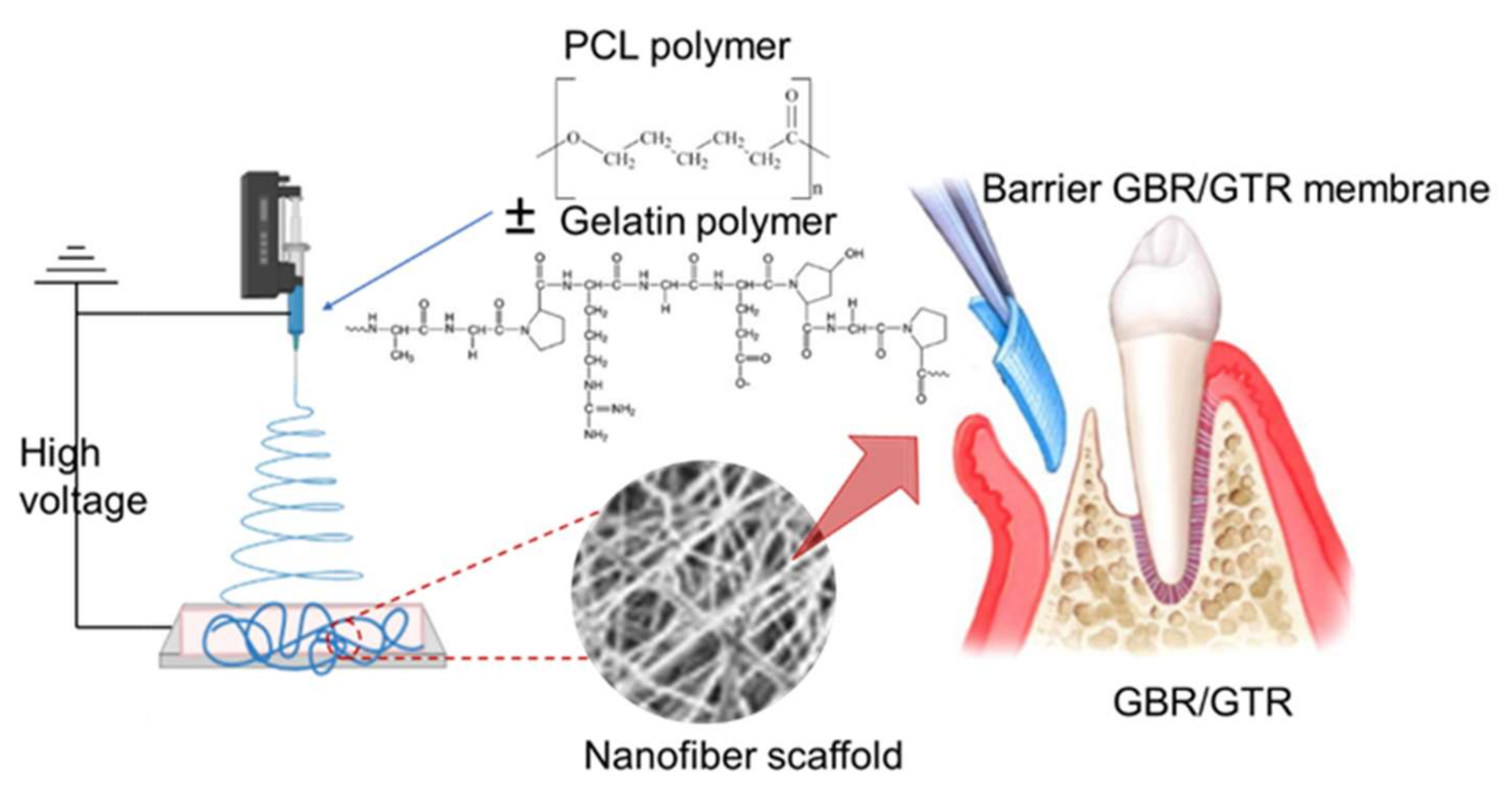
| Polymeric Fiber | ES Technique | Drugs | Type of Cancer | In Vivo | In Vitro | Ref. |
|---|---|---|---|---|---|---|
| BIC/(PLGA) | Blend ES | CAR, CIS and CPT-11 | Brain cancer (C6 glioma) | + | [43] | |
| Blend ES | 5-FU and oxaliplatin | Colorectal cancer (HCT8 and CT26 cell lines) | + | + | [44] | |
| PGC-C18/PCL | Blend ES | CPT-11 and SN-38 | Human colorectal (HT-29) cell line | + | [45] | |
| PLGA/gelatin | Blend ES | DOX-encapsulated mesoporous zinc oxide microspheres /camptothecin | Liver cancer (HepG2) cell line | + | [46] | |
| PEG/PLA | Blend ES | CBT A-4 and HCPT | Breast (4T1) tumor model | + | + | [47] |
| Emulsion ES | Paclitaxel and DOX | Brain cancer (C6 glioma) | + | [48] | ||
| Dextran/PLGA | Emulsion ES | HCPT and tea polyphenol | Orthotopic liver (H22) carcinoma cell line | [49] | ||
| PCL/gelatin | Second carrier ES (core/shell silica nanoparticles) | DOX and Indomethacin | L929 fibroblast cells | [50] | ||
| PLGA | Sequential ES | CAR, CPT-11, CIS and CBT | Brain cancer (C6 glioma) | + | [51] | |
| Emulsion ES | Paclitaxel and Brefeldin A | Human liver (HepG2) cancer cell line | + | [52] | ||
| PLLA | Sequential ES | DCA and oxaliplatin | Cervical cancer (Hela cancer and U14 cancer cell lines) | + | + | [53] |
| Second carrier ES (mesoporous silica nanoparticles) | DOX/Ibuprofen | Cervical cancer (Hela cell line) | + | [54] | ||
| Blend ES | DOX and HCPT | Human cervical cancer (HeLa cells) | + | [55] | ||
| DCA and diisopropylamine dichloroacetate | Colorectal cancer (C26 cells) | + | + | [56] | ||
| Oxaliplatin and cyclophosphamide | Human hepatocellular cancer (HCC cells) | + | + | [57] | ||
| PCL | Blend ES | (−)-epigallocatechin-3-O-gallate and caffeic acid | Human gastric cancer MKN28 cells | + | [58] | |
| Cisplatin and CUR | Human cervical cancer (HeLa cells) | + | + | [59] | ||
| CUR and aloe-vera or neem-extract | Lung carcinoma (A549) and breast cancer (MCF-7) | + | [60] | |||
| Core-sheath ES | Ibuprofen and DOX | Human hepatocellular carcinoma cell line (HuH-7) | + | [61] | ||
| 5-FU and paclitaxel | TNBC cells human triple-negative breast cancer | + | + | [62] | ||
| PLLA/PCL | Microfluidic ES | DOX and angiogenesis inhibitor apatinib | Breast cancer (4T1 cells) | + | + | [63] |
| PVA | Second carrier ES (mPEG-PCL micelles) | DOX and CUR | Cervical cancer (Hela) cell line | + | [64] | |
| Blend ES | Dichloroacetate and Pt(IV) prodrug-backboned micelle | HeLa human cervical cancer cells | + | + | [65] | |
| 5-FU: 5-fluorouracil; CAR: Carmustine; CBT: combretastatin; CIS: Cisplatin; CPT-11: irinotecan; CUR: Curcumin; DCA: Sodium dichloroacetate; DOX: Doxorubicin; HCPT: Hydroxycamptothecin; PGC-C18: Poly(glycerol monostearate-co-ε-caprolactone); PLLA: Poly (l-lactic acid); PLGA: (D, L-lactic acid-co-glycolic acid); and SN-38: irinotecan metabolite. | ||||||
| Polymeric Fiber | Therapeutic Agent | Purpose | Ref. |
|---|---|---|---|
| PLLA/PVA | Cefazoline | Antibacterial | [97] |
| PLGA | [98] | ||
| Chitosan/PVA | Lysozyme | Antimicrobial | [99] |
| PCL | Chloramphenicol | Antibacterial | [100] |
| PLGA | Quercetin | Cell proliferation and adhesion/antibacterial | [101] |
| PCL | Rifampicin | Antimicrobial | [102] |
| PCL/Gelatin | Metronidazole | Antibacterial | [81] |
| PCL | Tauroursodeoxycholic acid | Angiogenesis | [103] |
| Polymeric Fiber | Antibacterial Agent | Implant | Antibacterial Action against | Ref. |
|---|---|---|---|---|
| PCL/HA (Polymer/Ceramic) | Rifampicin | Titanium (orthopedic) | Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa and MRSA | [133] |
| PLGA/PCL | Vancomycin and Rifampicin | Titanium (orthopedic) | Staphylococcus aureus | [137] |
| PLGA/PCL | Vancomycin/Rifampicin Linezolid/rifampicin Daptomycin/rifampicin | Titanium (orthopedic) | MRSA | [138] |
| PLGA | Vancomycin | Titanium (orthopedic) | Staphylococcus aureus | [132] |
| PLA/PCL/gelatin | Tetracycline | Titanium (dental) | Prevotella intermedia, Porphyromonas gingivalis, Fusobacterium nucleatum and Porphyromonas gingivalis | [141] |
| Chitosan/polyethylene oxide (PEO) | Vancomycin | Titanium (orthopedic) | Staphylococcus aureus | [142] |
| Keratin | Silver | Titanium (dental) | Staphylococcus aureus | [143] |
| PCL/PVA | Doxycycline | Titanium (orthopedic) | Staphylococcus aureus | [144] |
| Carboxymethylcellulose (CMC)/ PEO | Clindamycin | AISI 316LVM (stainless steel) and Ti90Al6V4 (alloy) (orgopedic) | Staphylococci, streptococci, pneumococci, and bacteroides species | [145] |
| PLGA/PEO | Gentamycin | Titanium (orthopedic) | Staphylococcus aureus | [146] |
| Polymeric Chain | Active Agents | Antibacterial Action against | Ref. |
|---|---|---|---|
| PLA/PCL | Tetracycline hydrochloride | Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa | [166] |
| PLGA | Cefoxitin sodium | Staphylococcus aureus | [167] |
| Amoxicillin | Staphylococcus aureus | [168] | |
| PLA | Mupirocin | Staphylococcus aureus | [169] |
| coPLA/PEG | Ciprofloxacin hydrochloride, levofloxacin hemihydrate or moxifloxacin hydrochloride | Staphylococcus aureus | [170] |
| PLA, PLA/Collagen | Gentamicin | Escherichia coli, Staphylococcus epidermidis, Pseudomonas aeruginosa | [171] |
| PLLACL | Tetracycline hydrochloride | Escherichia coli | [172] |
| PMMA/Nylon 6 | Ampicillin | Listeria innocua | [173] |
| PAA | Doxycycline hyclate | Staphylococcus aureus, Streptococcus agalactiae, Pseudomonas aeruginosa | [174] |
| Cyclodextrin complex | Triclosan | Staphylococcus aureus, Escherichia coli | [175] |
| PCL/PLA | N-halamine | Escherichia coli, Staphylococcus epidermidis | [176] |
| PLA | Staphylococcus aureus, Escherichia coli | [177] | |
| PAN | Staphylococcus aureus, Escherichia coli | [178] | |
| CAc | Quaternary ammonium salts | Staphylococcus aureus, Escherichia coli | [179] |
| Chlorhexidine | Escherichia coli, Staphylococcus epidermidis | [180] | |
| CAc/PEU | Polyhexamethylene biguanide | Escherichia coli | [181] |
| PEO/Chitosan | Potassium 5-nitro-8-quinolinolate | Staphylococcus aureus, Escherichia coli, Candida albicans | [182] |
| PAN | Silver NPs | Staphylococcus aureus, Escherichia coli, Bacillus subtilis | [183] |
| PLA/Chitosan | Staphylococcus aureus, Escherichia coli | [184] | |
| PEO/Chitosan | Antimicrobial peptides (Plantaricin 423 and bacteriocin ST4SA) | Escherichia coli | [185] |
| PVA/Chitosan | Escherichia coli | [186] | |
| PDLLA/PEO | Enterococcus faecium | [187] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsadek, N.E.; Nagah, A.; Ibrahim, T.M.; Chopra, H.; Ghonaim, G.A.; Emam, S.E.; Cavalu, S.; Attia, M.S. Electrospun Nanofibers Revisited: An Update on the Emerging Applications in Nanomedicine. Materials 2022, 15, 1934. https://doi.org/10.3390/ma15051934
Elsadek NE, Nagah A, Ibrahim TM, Chopra H, Ghonaim GA, Emam SE, Cavalu S, Attia MS. Electrospun Nanofibers Revisited: An Update on the Emerging Applications in Nanomedicine. Materials. 2022; 15(5):1934. https://doi.org/10.3390/ma15051934
Chicago/Turabian StyleElsadek, Nehal E., Abdalrazeq Nagah, Tarek M. Ibrahim, Hitesh Chopra, Ghada A. Ghonaim, Sherif E. Emam, Simona Cavalu, and Mohamed S. Attia. 2022. "Electrospun Nanofibers Revisited: An Update on the Emerging Applications in Nanomedicine" Materials 15, no. 5: 1934. https://doi.org/10.3390/ma15051934
APA StyleElsadek, N. E., Nagah, A., Ibrahim, T. M., Chopra, H., Ghonaim, G. A., Emam, S. E., Cavalu, S., & Attia, M. S. (2022). Electrospun Nanofibers Revisited: An Update on the Emerging Applications in Nanomedicine. Materials, 15(5), 1934. https://doi.org/10.3390/ma15051934









