Structure of Sewage Sludge-Clay Multiscale Composite Particles to Control the Mechanism of SO2 and H2S Gas Release
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. TG
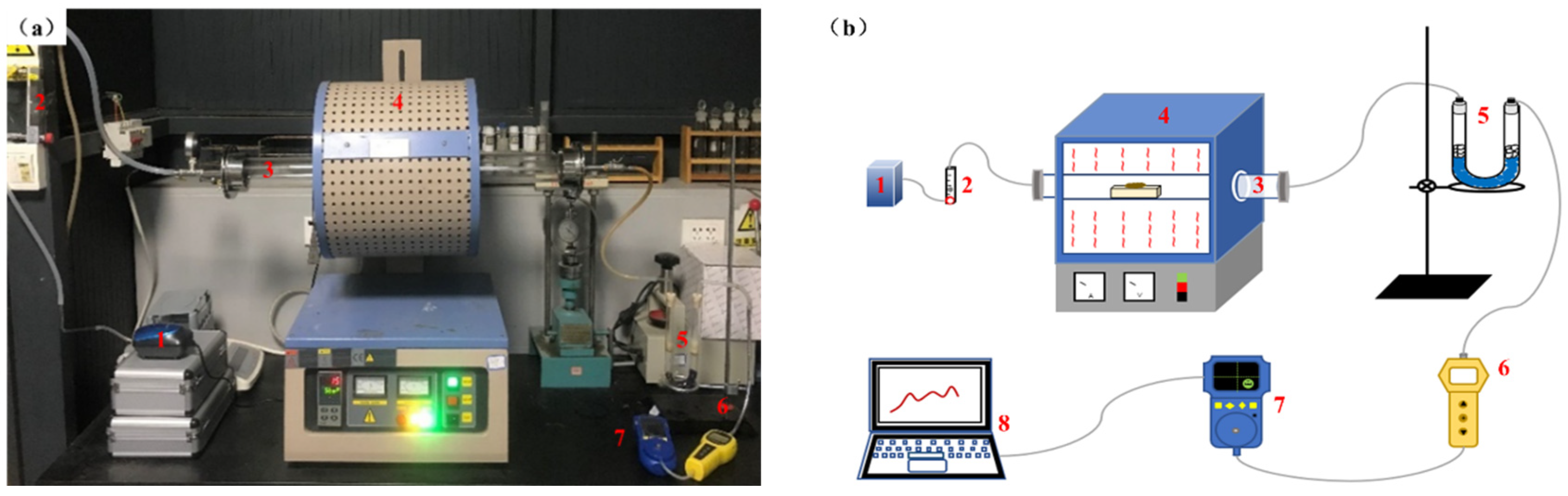
2.2.2. Sewage Sludge-Clay Multiscale Composite Particles with Sulfur Gas Online Monitoring Test
3. Results and Discussion
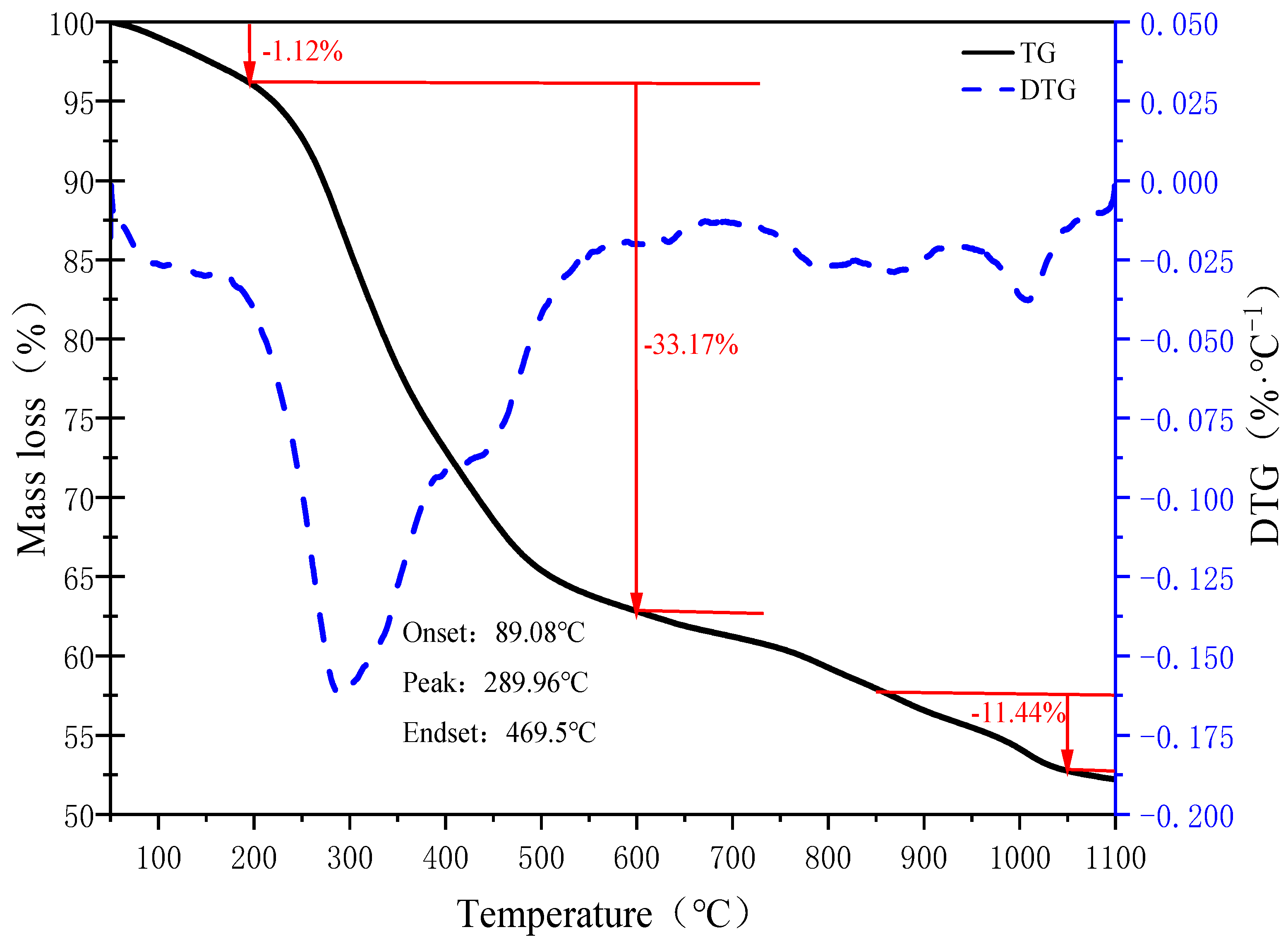
3.1. TG-DTG Analysis of Sludge
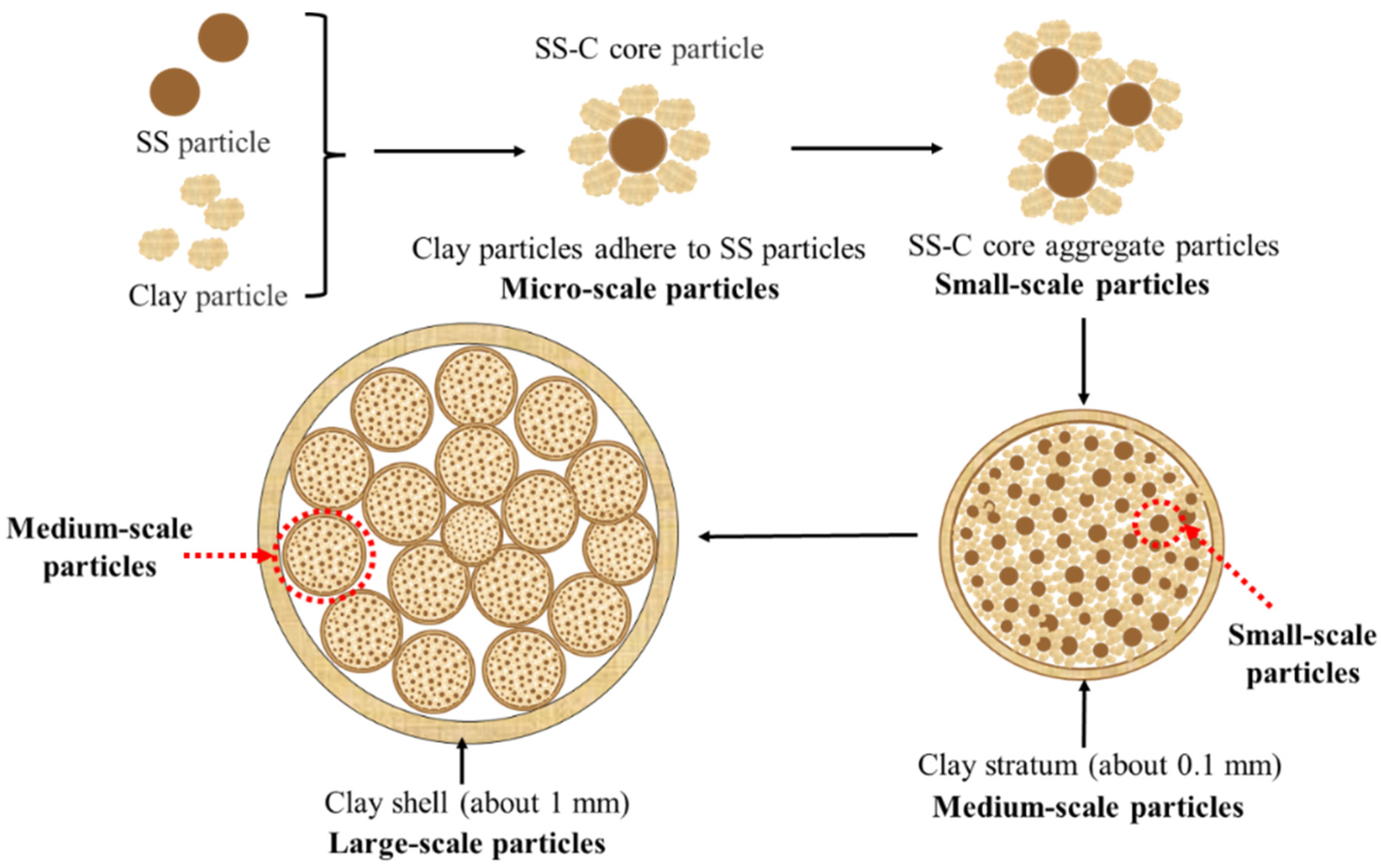
3.2. Preparation of Sludge-Clay Multiscale Composite Particles
3.3. Changes in the Internal Structure of Sewage Sludge-Clay Multiscale Composite Particles during Heating
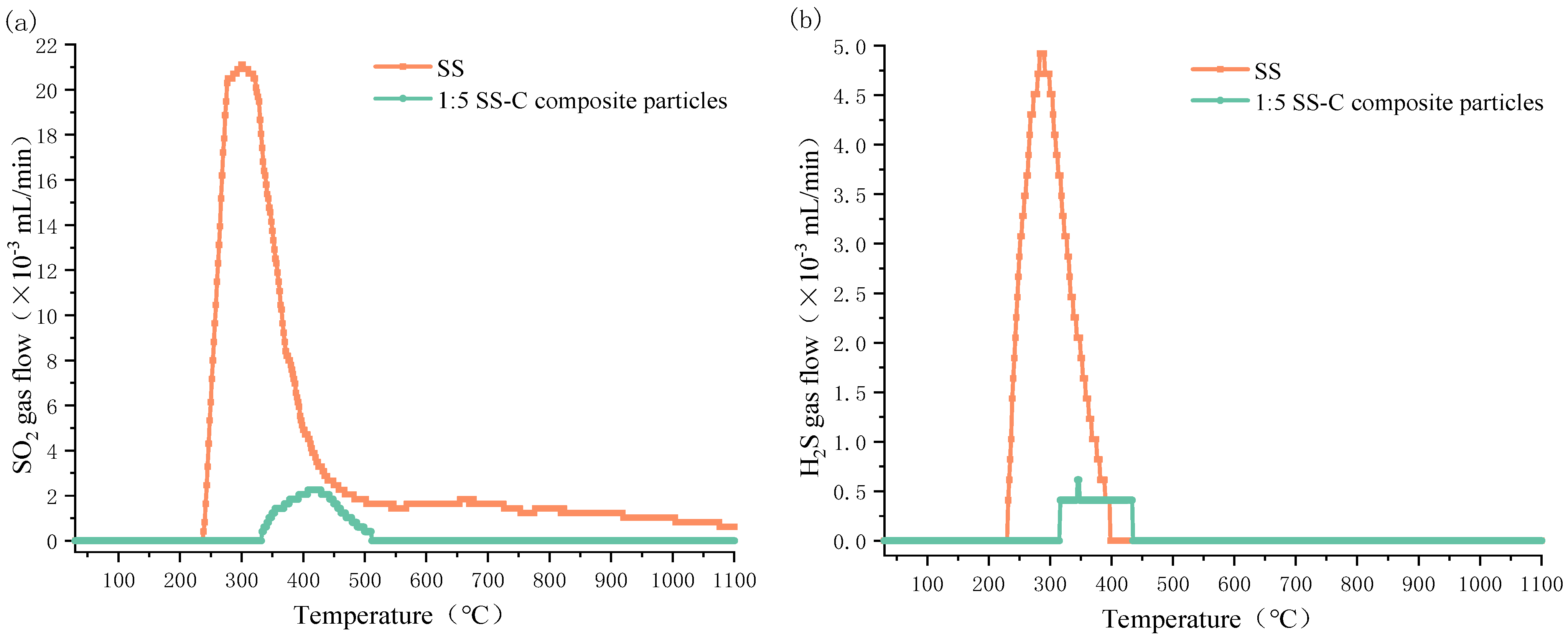
3.4. Characteristics of SO2 and H2S Release from Sewage Sludge-Clay Multiscale Composite Particles
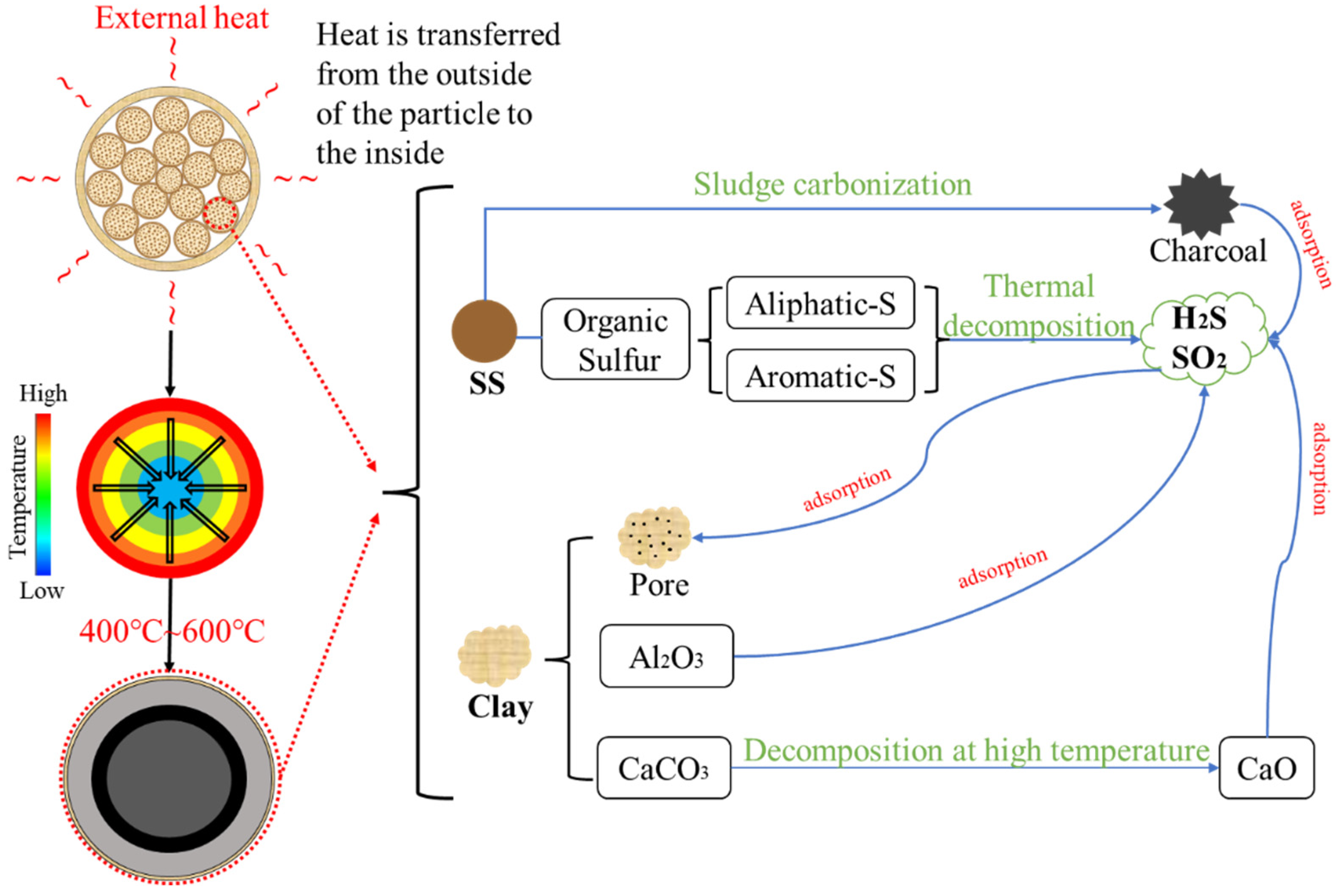
3.5. Mechanism of Sulfur-Containing-Gas Release from Sewage Sludge-Clay Multiscale Composite Particles
4. Conclusions
- (1)
- The mass loss in the temperature range of 200 °C to 600 °C is the largest in the sludge-drying process, and the mass loss amounts to 33.17%.
- (2)
- The special structure of sewage sludge-clay multiscale composite particles and the composition of clay can prevent the diffusion of sludge pyrolysis gas. Furthermore, it can form a double-layer structure of “gray surface layer-dark gray mixed layer”, “gray surface layer-black mixed layer-dark gray spherical core”, and “light yellow surface layer-yellow spherical core” within the composite particles at different temperature intervals.
- (3)
- The “large scale-medium scale-small scale-micro scale” structure of the sewage sludge-clay multiscale composite particles can inhibit the sulfur gas release of SO2 and H2S.
- (4)
- Sewage sludge-clay multiscale composite particles are also 80 °C and 70 °C higher than the minimum temperature at which SO2 and H2S gas are, respectively, released from pure sludge. Moreover, the peak temperature point is also 120 °C and 80 °C higher than that of pure sludge, and the maximum release is 90% and 91% lower than that of pure sludge.
- (5)
- The double-layer structure of “gray surface layer-dark gray mixed layer” and the three-layer structure of “gray surface layer-black mixed layer-dark gray spherical core” formed by sewage sludge-clay multiscale composite particles are the best internal structures to control the release of sulfur-containing gases.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kosov, V.F.; Umnova, O.M.; Zaichenko, V.M. The pyrolysis process of sewage sludge. J. Phys. Conf. Ser. 2015, 653, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Bibby, K.; Peccia, J. Identification of viral pathogen diversity in sewage sludge by metagenome analysis. Environ. Sci. Technol. 2013, 47, 1945–1951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronda, A.; Gómez-Barea, A.; Haro, P.; de Almeida, V.F.; Salinero, J. Elements partitioning during thermal conversion of sewage sludge. Fuel Process. Technol. 2019, 186, 156–166. [Google Scholar] [CrossRef]
- Xin, L.; Xiujuan, H.; Fengde, T. Analysis of the current situation of sludge generation, treatment and disposal in typical cities in China and trends in economics. Environ. Prot. Circ. Econ. 2021, 41, 88–93. (In Chinese) [Google Scholar] [CrossRef]
- Xiaohu, D. Status and development trend of sludge treatment and disposal in China. Science 2020, 72, 30–34. (In Chinese) [Google Scholar] [CrossRef]
- Świerczek, L.; Cieślik, B.M.; Konieczka, P. Challenges and opportunities related to the use of sewage sludge ash in cement-based building materials–A review. J. Clean. Prod. 2021, 287. [Google Scholar] [CrossRef]
- Taki, K.; Gahlot, R.; Kumar, M. Utilization of fly ash amended sewage sludge as brick for sustainable building material with special emphasis on dimensional effect. J. Clean. Prod. 2020, 275. [Google Scholar] [CrossRef]
- Amin, S.K.; Abdel Hamid, E.M.; El-Sherbiny, S.A.; Sibak, H.A.; Abadir, M.F. The use of sewage sludge in the production of ceramic floor tiles. HBRC J. 2019, 14, 309–315. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.; Fan, X.; Jiang, Z.; Wang, X.; Chen, L.; Li, J.; Zhu, L.; Wan, C.; Chen, Z. Differential impact of clay minerals and organic matter on pore structure and its fractal characteristics of marine and continental shales in China. Appl. Clay Sci. 2021. [Google Scholar] [CrossRef]
- Cai, J.; Du, J.; Song, M.; Lei, T.; Wang, X.; Li, Y. Control of clay mineral properties on hydrocarbon generation of organo-clay complexes: Evidence from high-temperature pyrolysis experiments. Appl. Clay Sci. 2022, 216. [Google Scholar] [CrossRef]
- Zheng, A.; Li, L.; Tippayawong, N.; Huang, Z.; Zhao, K.; Wei, G.; Zhao, Z.; Li, H. Reducing emission of NOx and SOx precursors while enhancing char production from pyrolysis of sewage sludge by torrefaction pretreatment. Energy 2020, 192. [Google Scholar] [CrossRef]
- Wang, M.; Pan, X.; Xia, Y.; Zhu, A.; Wu, Y.; Fu, C.; Zhang, P.; Zhao, J.; Li, J.; Fu, J. Effect of dewatering conditioners on pollutants with nitrogen, sulfur, and chlorine releasing characteristics during sewage sludge pyrolysis. Fuel 2022, 307. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Q.; Hu, H.; Xiao, R.; Li, A.; Qiao, Y.; Yao, H.; Naruse, I. Dual role of conditioner CaO in product distributions and sulfur transformation during sewage sludge pyrolysis. Fuel 2014, 134, 514–520. [Google Scholar] [CrossRef]
- Cheng, S.; Qiao, Y.; Huang, J.; Cao, L.; Yang, H.; Liu, H.; Yu, Y.; Xu, M. Effect of alkali addition on sulfur transformation during low temperature pyrolysis of sewage sludge. Proc. Combust. Inst. 2017, 36, 2253–2261. [Google Scholar] [CrossRef]
- Arsenović, M.; Radojević, Z.; Jakšić, Ž.; Pezo, L. Mathematical approach to application of industrial wastes in clay brick production—Part II: Optimization. Ceram. Int. 2015, 41, 4899–4905. [Google Scholar] [CrossRef]
- Otsuki, A.; Hayagan, N.L. Zeta potential of inorganic fine particle-Na-bentonite binder mixture systems. Electrophoresis 2020, 41, 1405–1412. [Google Scholar] [CrossRef]
- Sivrikaya, O.; Arol, A.İ. The bonding/strengthening mechanism of colemanite added organic binders in iron ore pelletization. Int. J. Miner. Process. 2012, 110–111, 90–100. [Google Scholar] [CrossRef]
- Magdziarz, A.; Werle, S. Analysis of the combustion and pyrolysis of dried sewage sludge by TGA and MS. Waste Manag. 2014, 34, 174–179. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, J.; Zuo, W.; Chen, L.; Cui, Y.; Tan, T. Nitrogen conversion in relation to NH3 and HCN during microwave pyrolysis of sewage sludge. Environ. Sci. Technol. 2013, 47, 3498–3505. [Google Scholar] [CrossRef]
- Zhuang, X.; Huang, Y.; Song, Y.; Zhan, H.; Yin, X.; Wu, C. The transformation pathways of nitrogen in sewage sludge during hydrothermal treatment. Bioresour. Technol. 2017, 245, 463–470. [Google Scholar] [CrossRef]
- Wu, J.; Liu, J.; Yuan, S.; Zhang, X.; Liu, Y.; Wang, Z.; Zhou, J. Sulfur Transformation during Hydrothermal Dewatering of Low Rank Coal. Energy Fuels 2015, 29, 6586–6592. [Google Scholar] [CrossRef]
- Wang, Z.; Zhai, Y.; Wang, T.; Peng, C.; Li, S.; Wang, B.; Liu, X.; Li, C. Effect of temperature on the sulfur fate during hydrothermal carbonization of sewage sludge. Environ. Pollut. 2020, 260, 114067. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, L.; Ledakowicz, S. Comprehensive characterization of thermal decomposition of sewage sludge by TG–MS. J. Anal. Appl. Pyrolysis 2014, 110, 220–228. [Google Scholar] [CrossRef]
- Ischia, M.; Perazzolli, C.; Maschio, R.D.; Campostrini, R. Pyrolysis study of sewage sludge by TG-MS and TG-GC-MS coupled analyses. J. Therm. Anal. Calorim. 2007, 87, 567–574. [Google Scholar] [CrossRef]
- Ischia, M.; Dal Maschio, R.; Grigiante, M.; Baratieri, M. Clay-sewage sludge co-pyrolysis. A TG-MS and Py-GC study on potential advantages afforded by the presence of clay in the pyrolysis of wastewater sewage sludge. Waste Manag. 2011, 31, 71–77. [Google Scholar] [CrossRef]
- Wedd, M.; Ward-Smith, S.; Rawle, A. Particle Size Analysis. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Academic Press: Oxford, UK, 2019; pp. 144–157. [Google Scholar]
- Gong, H.; Shao, W.; Ma, X.; Cui, Z. Absorption properties of a multilayer composite nanoparticle for solar thermal utilization. Opt. Laser Technol. 2022, 150. [Google Scholar] [CrossRef]
- Xiao, L.P.; Shi, Z.J.; Xu, F.; Sun, R.C. Hydrothermal carbonization of lignocellulosic biomass. Bioresour. Technol. 2012, 118, 619–623. [Google Scholar] [CrossRef]
- Qie, Z.; Zhang, Z.; Sun, F.; Wang, L.; Pi, X.; Gao, J.; Zhao, G. Effect of pore hierarchy and pore size on the combined adsorption of SO2 and toluene in activated coke. Fuel 2019, 257. [Google Scholar] [CrossRef]
- Gasquet, V.; Kim, B.; Bonhomme, A.; Benbelkacem, H. Sewage sludge ash-derived materials for H2S removal from a landfill biogas. Waste Manag. 2021, 136, 230–237. [Google Scholar] [CrossRef]
- Yang, K.; Su, B.; Shi, L.; Wang, H.; Cui, Q. Adsorption mechanism and regeneration performance of 13X for H2S. Energy Fuels 2018, 32, 12742–12749. [Google Scholar] [CrossRef]
- Yi, K.; Liu, H.; Wang, J.; Lu, G.; Jin, M.; Hu, H.; Yao, H. The adsorption and transformation of SO2, H2S and NH3 by using sludge gasification ash: Effects of Fenton oxidation and CaO pre-conditioning. Chem. Eng. J. 2019, 360, 1498–1508. [Google Scholar] [CrossRef]


| Ma | Vd | Ad | FC | Qb,ad (MJ/kg) |
|---|---|---|---|---|
| 78.1 | 41.64 | 52.46 | 5.91 | 15.09 |
| SiO2 | Al2O3 | Fe2O3 | P2O5 | CaO | K2O | MgO | SO3 | Na2O | TiO2 |
|---|---|---|---|---|---|---|---|---|---|
| 37.076 | 20 | 14.99 | 12.076 | 5.792 | 2.543 | 2.336 | 2.215 | 1.038 | 0.843 |
| SiO2 | CaO | Al2O3 | Fe2O3 | MgO | K2O | Na2O | TiO2 | P2O5 | MnO |
|---|---|---|---|---|---|---|---|---|---|
| 37.66 | 19.87 | 10.2 | 4.733 | 2.746 | 2.02 | 0.693 | 0.595 | 0.206 | 0.0824 |
| Number | 1# | 2# | 3# | 4# | 5# | 6# |
|---|---|---|---|---|---|---|
| SS (g):Clay (g) | 1:5 | 1:10 | 1:15 | 1:20 | 1:25 | 1:30 |
| NO. | 1# | 2# | 3# | 4# | 5# | 6# | |
|---|---|---|---|---|---|---|---|
| T | |||||||
| 105 °C |  |  |  |  |  |  | |
| 200 °C |  |  |  |  |  |  | |
| 300 °C |  |  |  |  |  |  | |
| 360 °C |  |  |  |  |  |  | |
| 400 °C |  |  |  |  |  |  | |
| 460 °C |  |  |  |  |  |  | |
| 500 °C |  |  |  |  |  |  | |
| 560 °C |  |  |  |  |  |  | |
| 600 °C |  |  |  |  |  |  | |
| 660 °C |  |  |  |  |  |  | |
| 700 °C |  |  |  |  |  |  | |
| 800 °C |  |  |  |  |  |  | |
| 900 °C |  |  |  |  |  |  | |
| 1000 °C |  |  |  |  |  |  | |
| 1100 °C |  |  |  |  |  |  | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, H.; Li, L.; Li, Z.; Shang, S. Structure of Sewage Sludge-Clay Multiscale Composite Particles to Control the Mechanism of SO2 and H2S Gas Release. Materials 2022, 15, 1855. https://doi.org/10.3390/ma15051855
Fan H, Li L, Li Z, Shang S. Structure of Sewage Sludge-Clay Multiscale Composite Particles to Control the Mechanism of SO2 and H2S Gas Release. Materials. 2022; 15(5):1855. https://doi.org/10.3390/ma15051855
Chicago/Turabian StyleFan, Haihong, Lin Li, Zhou Li, and Shuo Shang. 2022. "Structure of Sewage Sludge-Clay Multiscale Composite Particles to Control the Mechanism of SO2 and H2S Gas Release" Materials 15, no. 5: 1855. https://doi.org/10.3390/ma15051855
APA StyleFan, H., Li, L., Li, Z., & Shang, S. (2022). Structure of Sewage Sludge-Clay Multiscale Composite Particles to Control the Mechanism of SO2 and H2S Gas Release. Materials, 15(5), 1855. https://doi.org/10.3390/ma15051855






