Ultrafine Grinded and Silane Grafted Iron Ore Tailings as Reinforcing Filler of Styrene Butadiene Rubber
Abstract
:1. Introduction
2. Experiments
2.1. Chemicals and Materials
2.2. Characterizations
2.3. Preparation of G-IOT/Si69-G-IOT Powders
2.3.1. Wet Ultrafine Grinding of IOTs
2.3.2. Surface Modification of G-IOTs
2.4. Preparation of SBR Composites
2.5. Model of Grinding Simulation
3. Results and Discussion
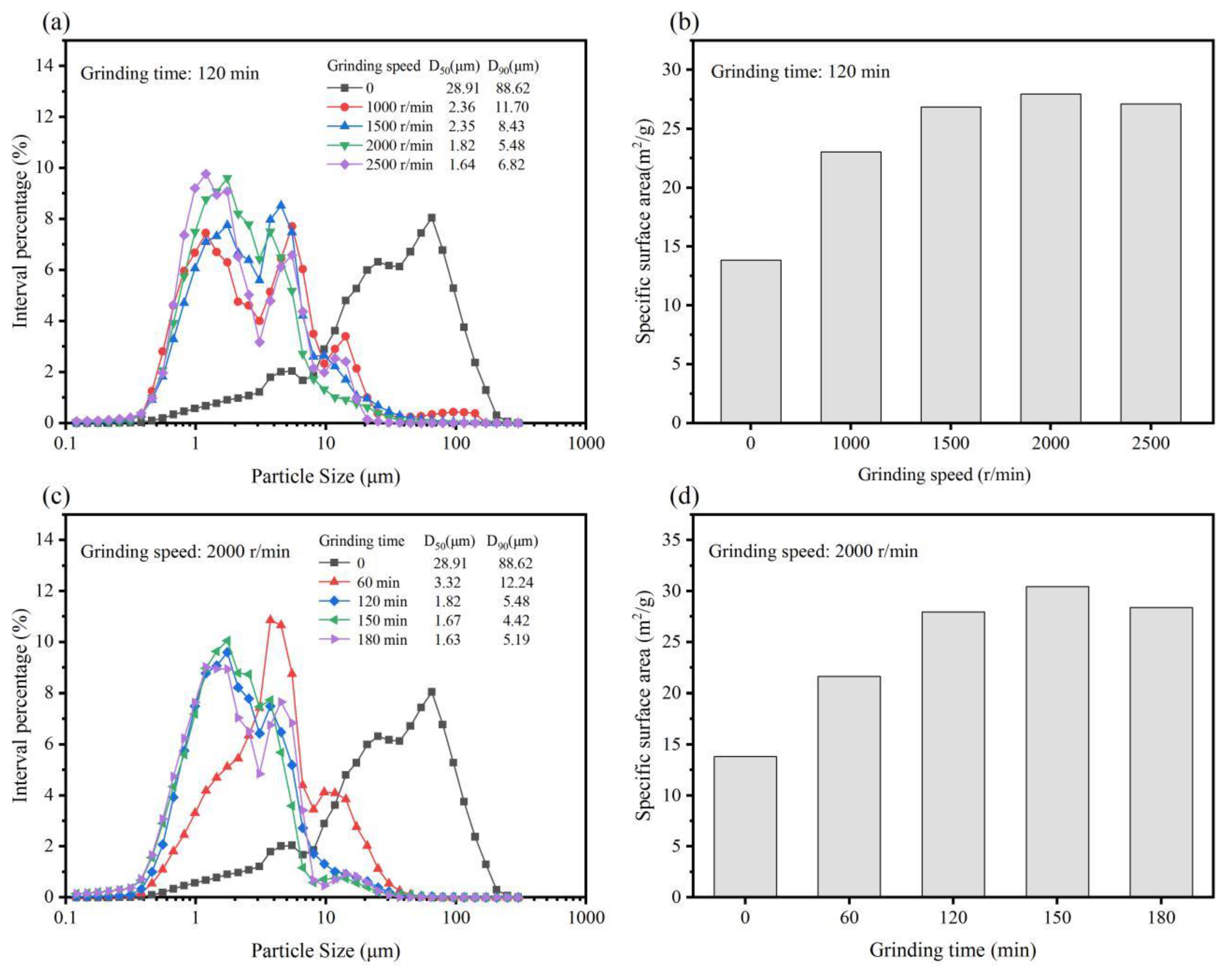
3.1. Particle Size Distribution and Specific Surface Area of G-IOTs
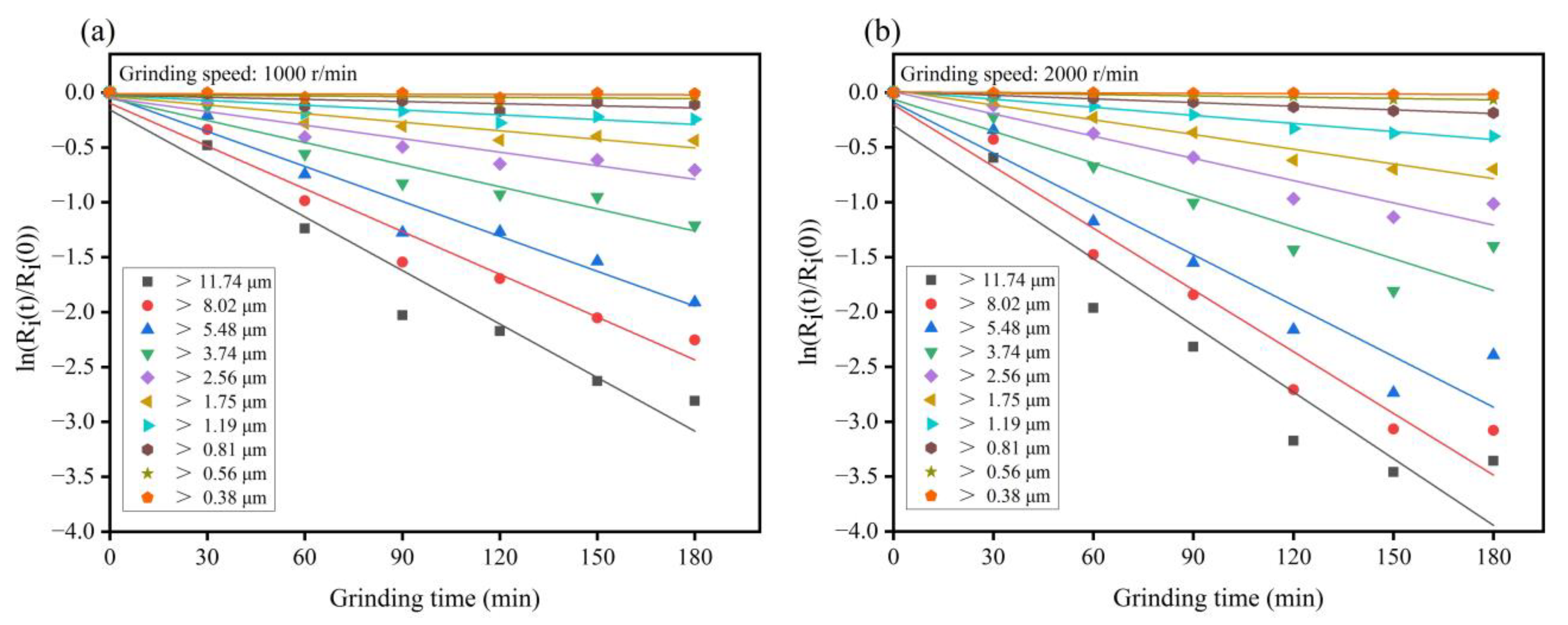
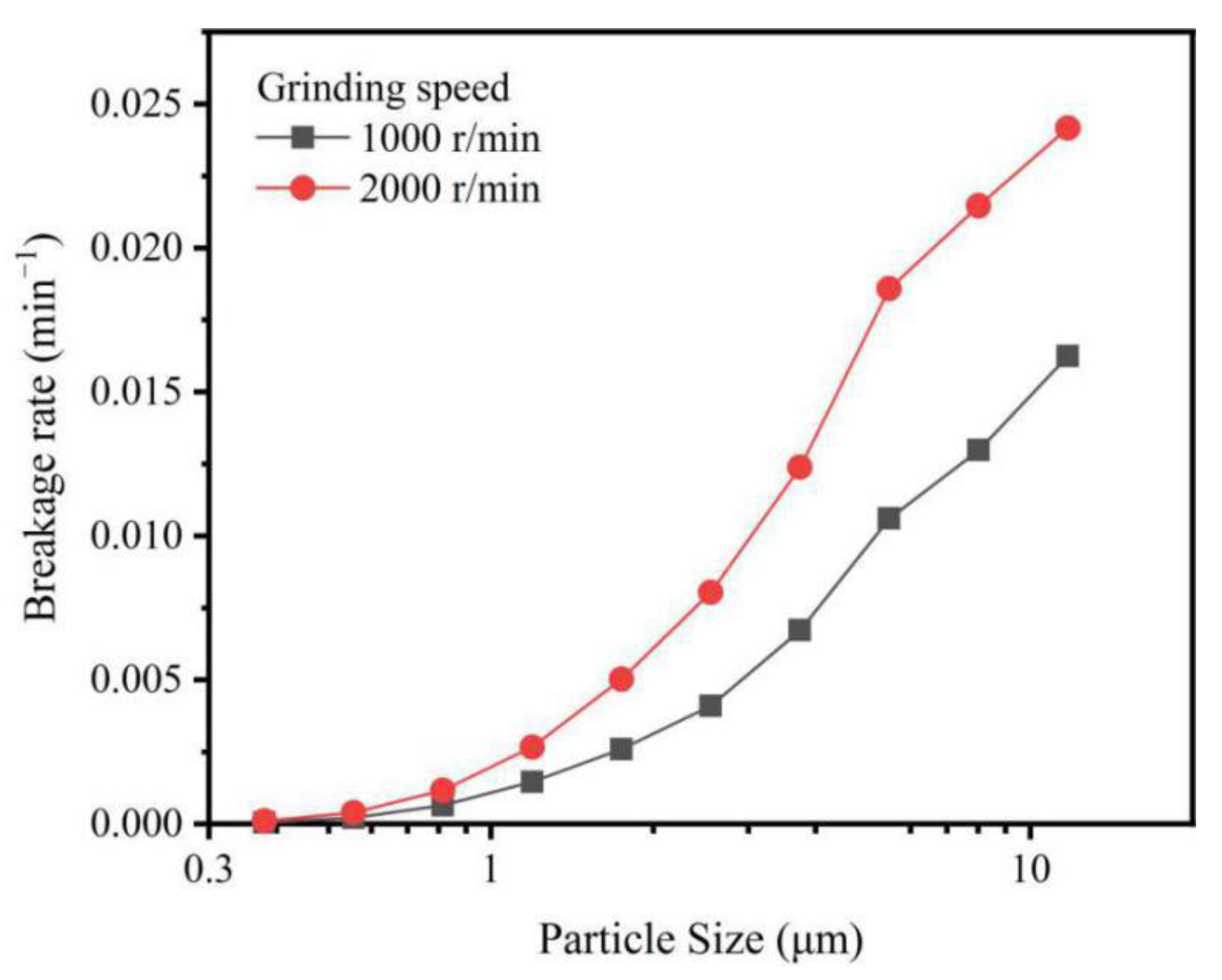
3.2. Simulation and Grinding Mechanism of IOTs
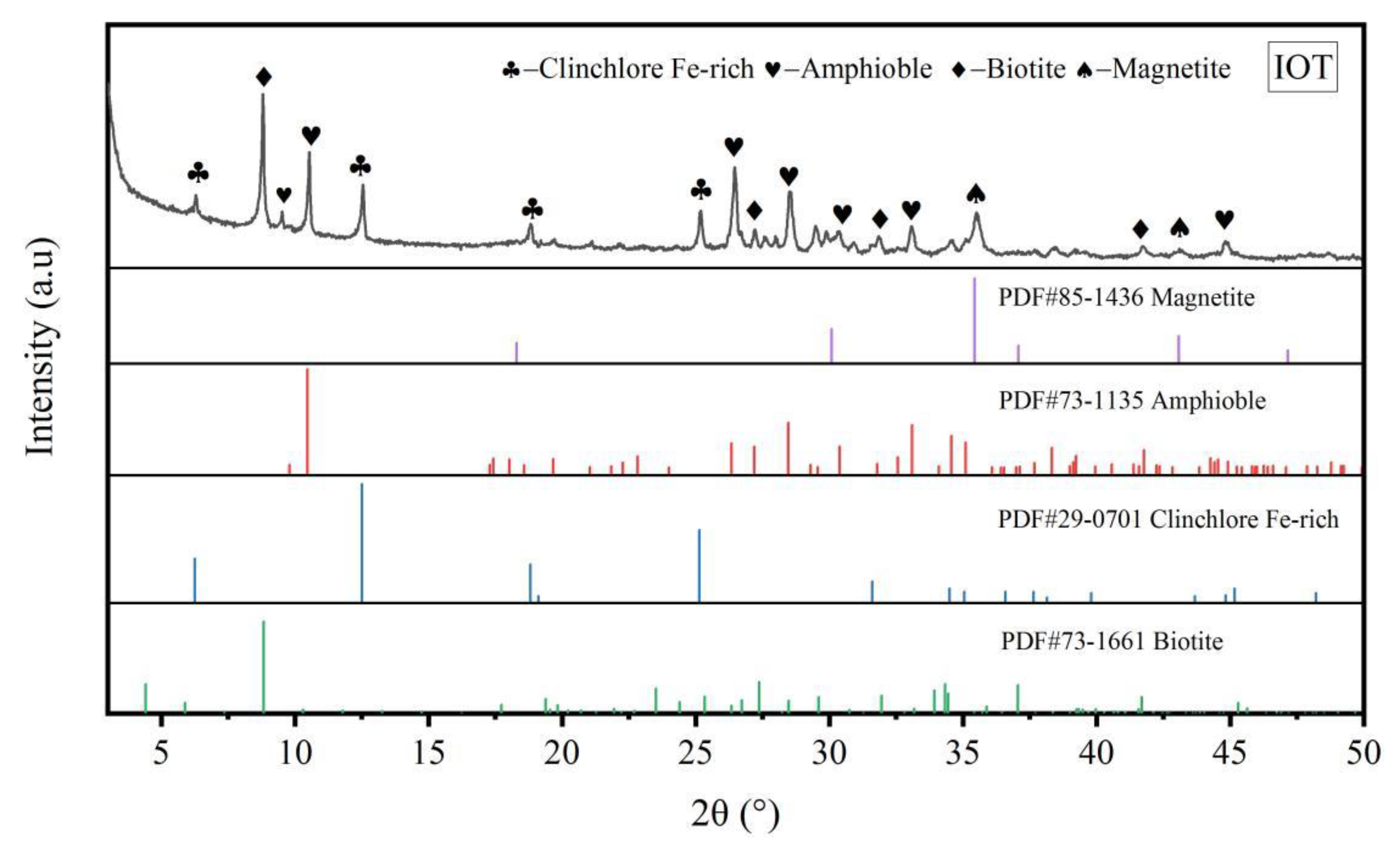
3.3. X-ray Diffraction Analysis of G-IOTs
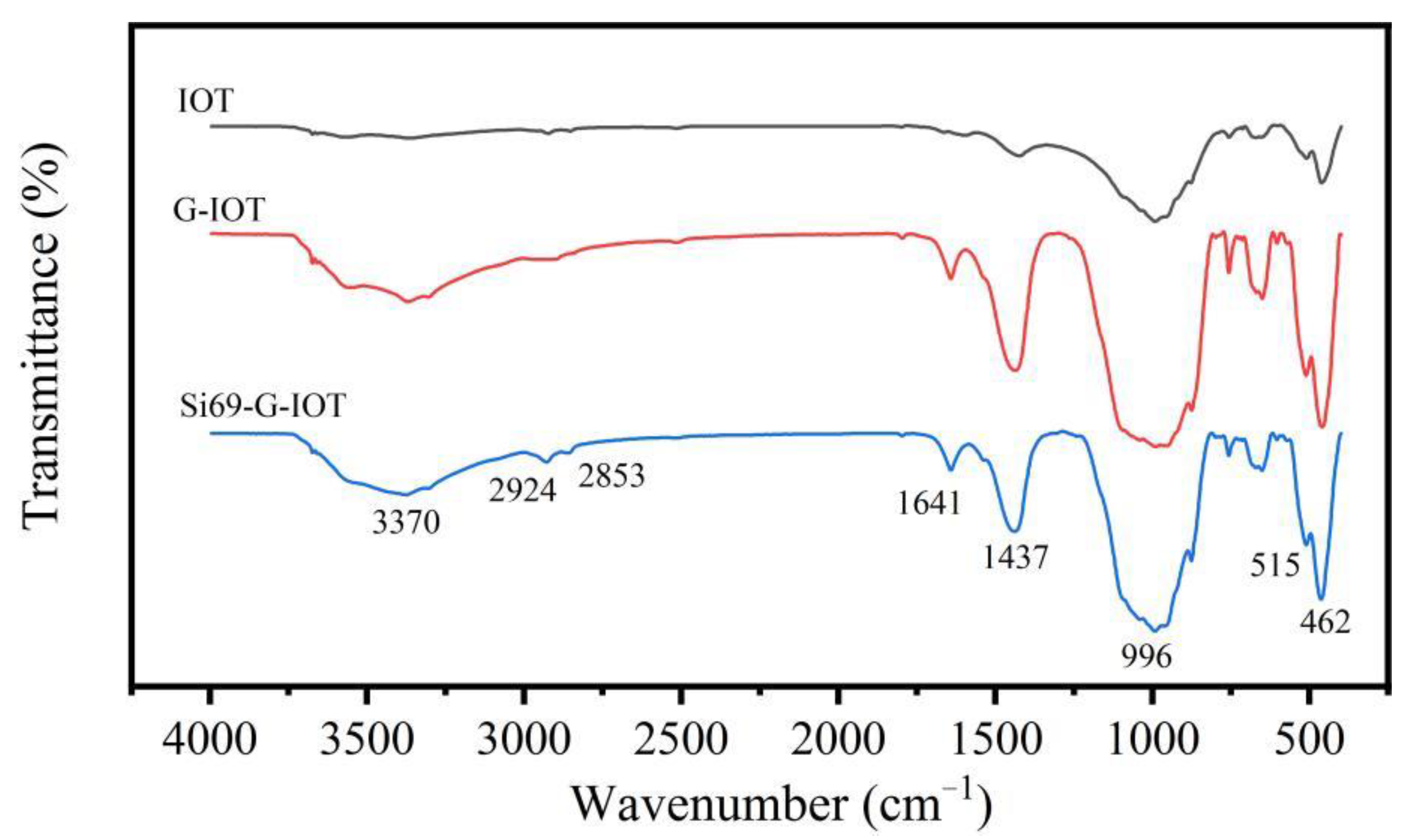
3.4. Fourier Transform Infrared Spectroscopy of Si69-G-IOTs
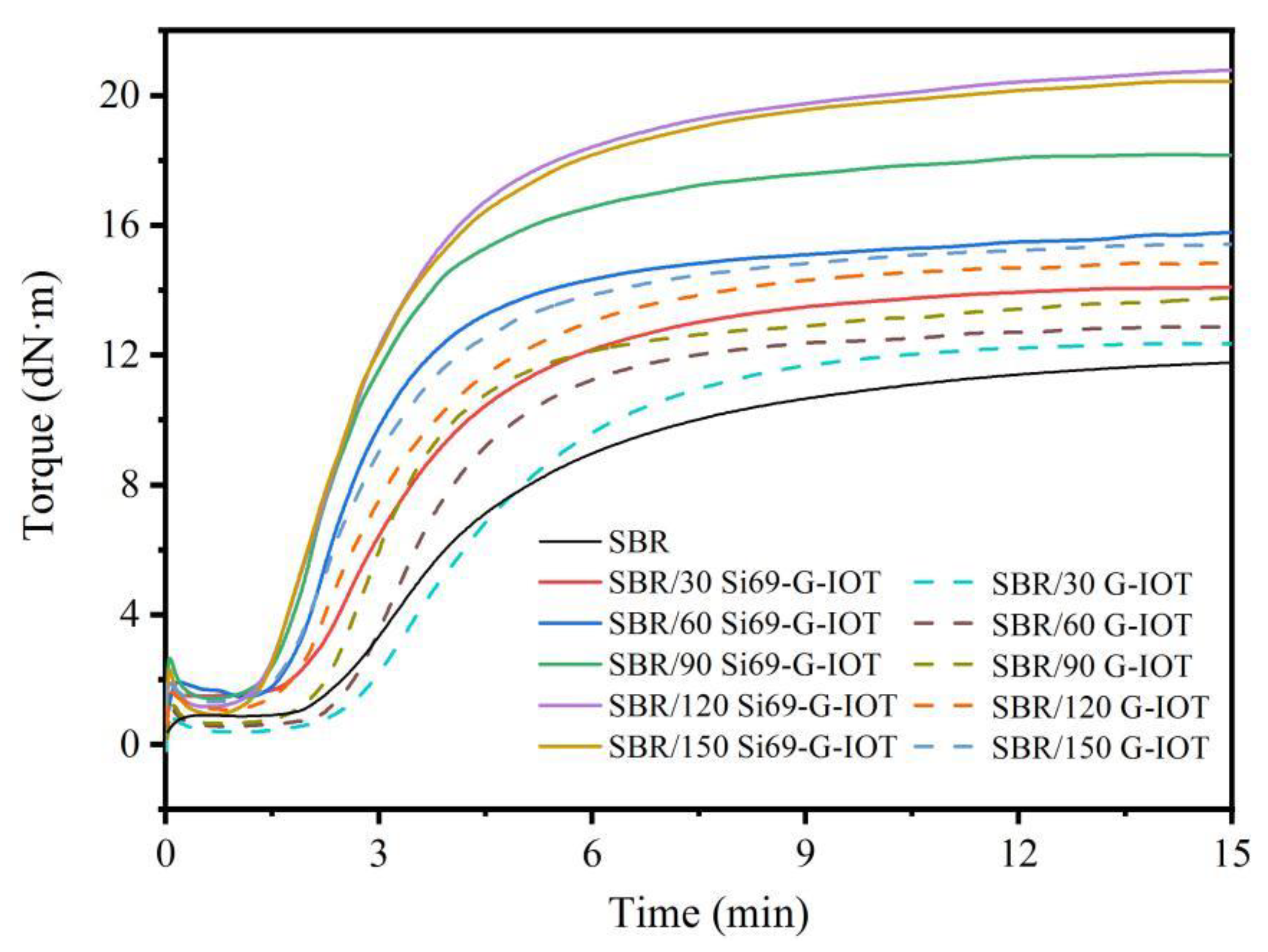
3.5. Cure Characteristics of SBR Composites
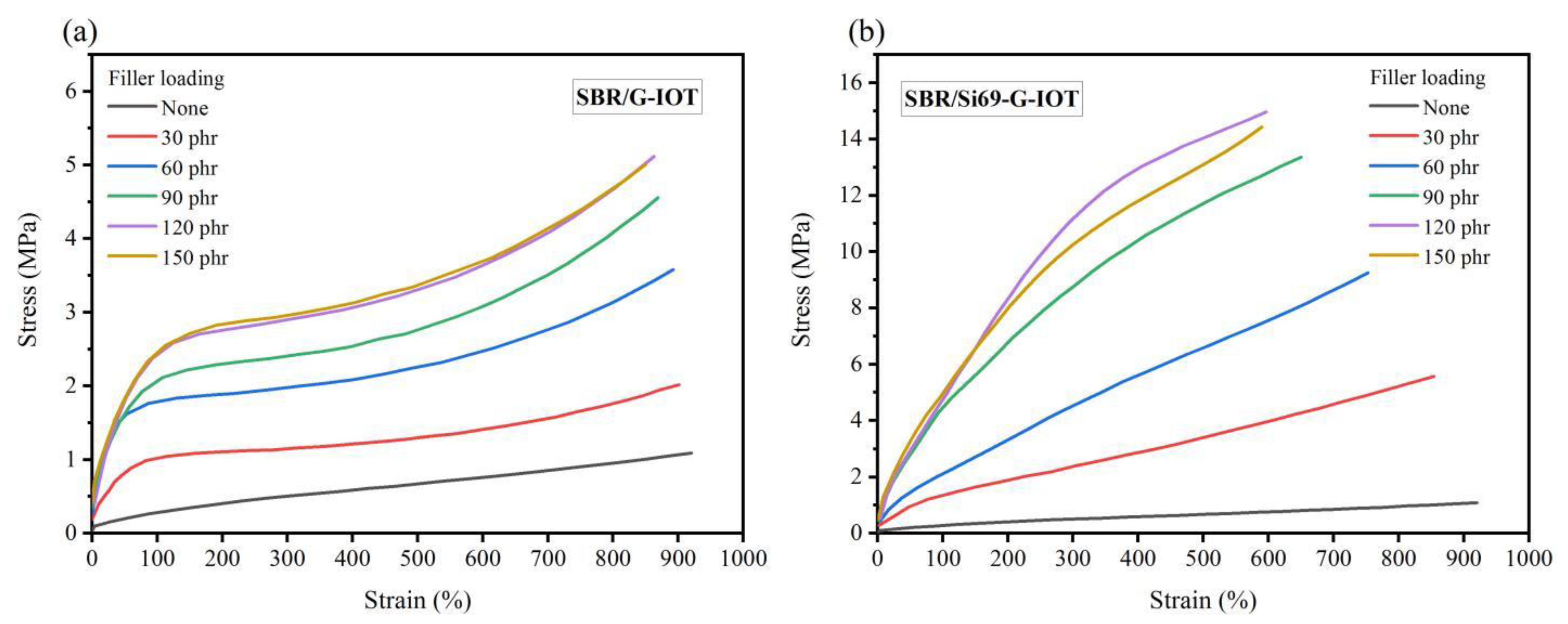
3.6. Static Mechanical Properties of SBR Composites
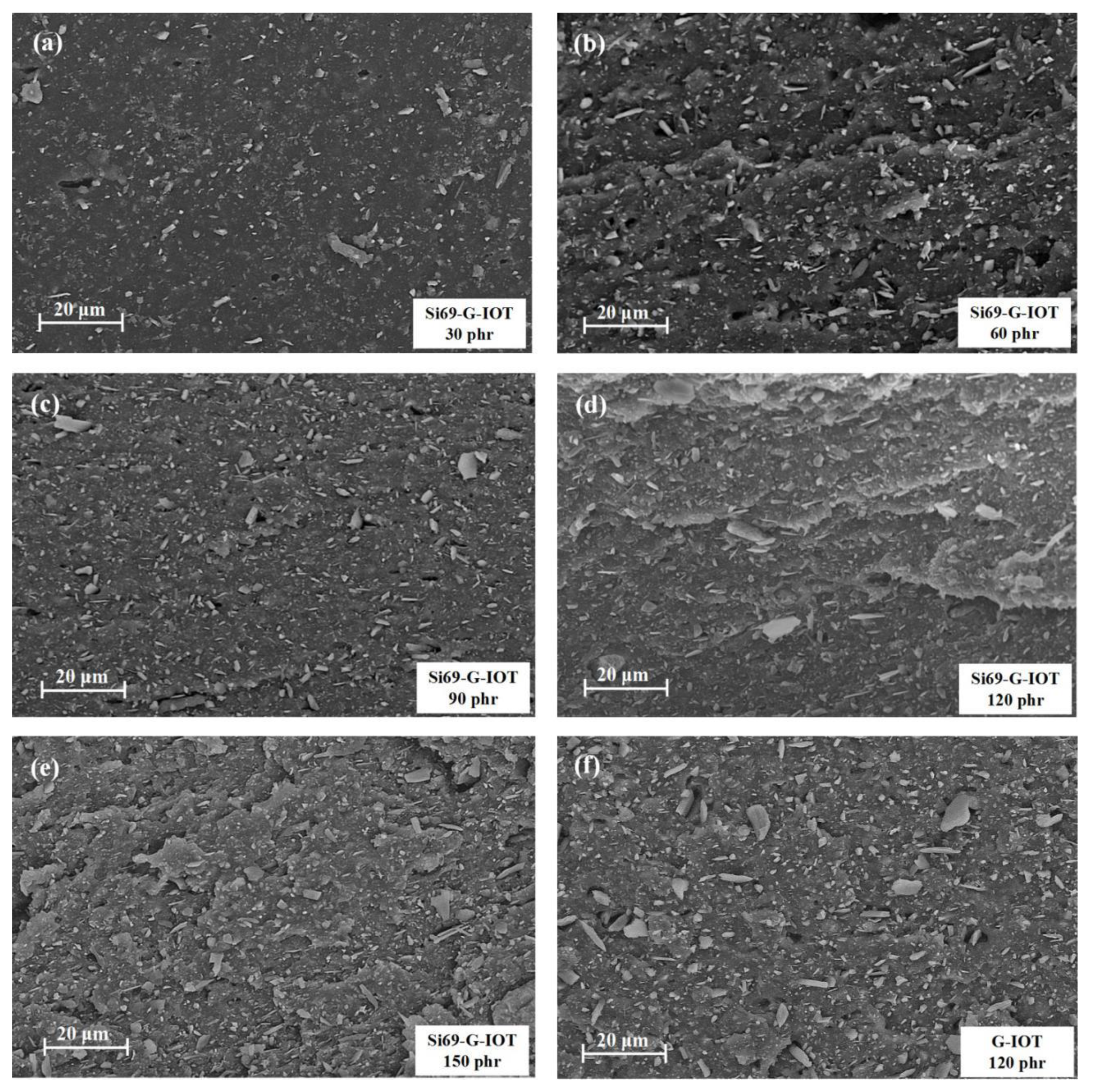
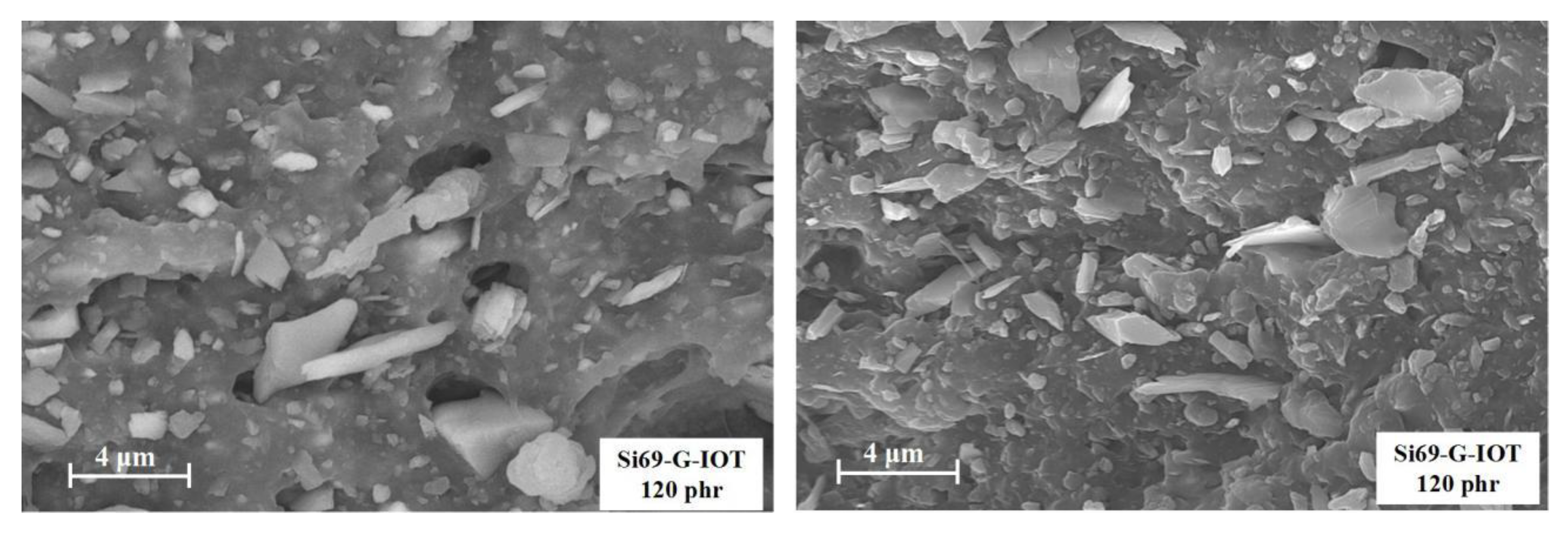
3.7. Morphology Characteristics of SBR Composites
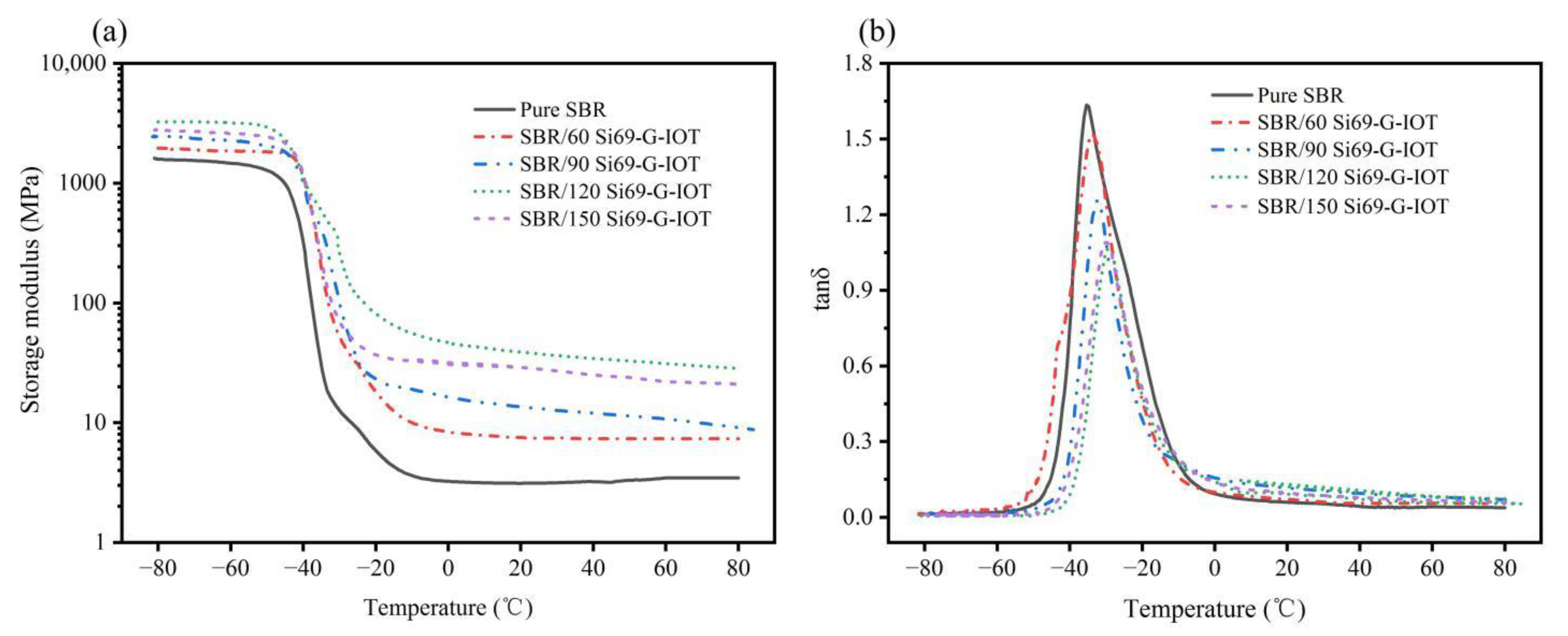
3.8. Dynamical Mechanical Analysis of SBR Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ricci, P.C.; Casula, R.; Gulleri, G.; Fumagalli, F.; Carbonaro, C.M.; Corpino, R. Mechanical stress in silicon nanosized architectures: Defects of SOD processed silica filler. J. Alloys Compd. 2014, 602, 157–162. [Google Scholar] [CrossRef]
- Zha, C.; Wang, W.; Lu, Y.; Zhang, L. Constructing Covalent Interface in Rubber/Clay Nanocomposite by Combining Structural Modification and Interlamellar Silylation of Montmorillonite. ACS Appl. Mater. Interfaces 2014, 6, 18769–18779. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Yang, Y.; Wang, F.; Liang, X.; Zhang, F.; Liang, J. Effect of Acid on Surface Properties of Modified Attapulgite and Performance of Styrene Butadiene Rubber Filled by Modified Attapulgite. Nanosci. Nanotechnol. Lett. 2014, 6, 231–237. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Zhang, K.; Liu, L.; Ji, L.; Liu, Q. Vulcanization, interfacial interaction, and dynamic mechanical properties of in-situ organic amino modified kaolinite/SBR nanocomposites based on latex compounding method. Appl. Clay Sci. 2020, 185, 105366. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Q.; Zhang, Y.; Cheng, H.; Lu, Y. Silane-grafted silica-covered kaolinite as filler of styrene butadiene rubber. Appl. Clay Sci. 2012, 65–66, 134–138. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Zhang, S.; Zhang, Y.; Zhang, Y.; Liang, P. Characterization of kaolinite/styrene butadiene rubber composite: Mechanical properties and thermal stability. Appl. Clay Sci. 2016, 124–125, 167–174. [Google Scholar] [CrossRef]
- Hayeemasae, N.; Ismail, H. Reinforcement of epoxidized natural rubber through the addition of sepiolite. Polym. Compos. 2019, 40, 924–931. [Google Scholar] [CrossRef]
- Carignani, E.; Cobani, E.; Martini, F.; Nardelli, F.; Borsacchi, S.; Calucci, L.; Di Credico, B.; Tadiello, L.; Giannini, L.; Geppi, M. Effect of sepiolite treatments on the oxidation of sepiolite/natural rubber nanocomposites prepared by latex compounding technique. Appl. Clay Sci. 2020, 189, 105528. [Google Scholar] [CrossRef]
- Zaini, N.A.M.; Ismail, H.; Rusli, A. Sepiolite hybridized commercial fillers, and their effects on curing process, mechanical properties, thermal stability, and flammability of ethylene propylene diene monomer rubber composites. Iran. Polym. J. 2018, 27, 663–675. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, F.; Liu, X.; Tang, M.; Zeng, Z.; Liang, J.; Guan, X.; Wang, J.; Mu, X. Surface modified palygorskite nanofibers and their applications as reinforcement phase in cis-polybutadiene rubber nanocomposites. Appl. Clay Sci. 2016, 132–133, 175–181. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, F.; Guo, H.; Yang, Y.; Du, Y.; Liang, J.; Zhang, F. Effect of coupling agent on surface free energy of organic modified attapulgite (OAT) powders and tensile strength of OAT/ethylene-propylene-diene monomer rubber nanocomposites. Powder Technol. 2015, 270, 92–97. [Google Scholar] [CrossRef]
- Chen, Y.L.; Du, J.Y.; Hou, H.B.; Zhou, M.; Zhang, H.L. Preparation and Characterization of the Lightweight Fired Brick with Low-silicon Iron Tailings. IOP Conf. Ser. Mater. Sci. Eng. 2018, 423, 012103. [Google Scholar] [CrossRef]
- Tang, L.; Liu, X.; Wang, X.; Liu, S.; Deng, H. Statistical analysis of tailings ponds in China. J. Geochem. Explor. 2020, 216, 106579. [Google Scholar] [CrossRef]
- Matschullat, J.; Borba, R.P.; Deschamps, E.; Figueiredo, B.R.; Gabrio, T.; Schwenk, M. Human and environmental contamination in the Iron Quadrangle, Brazil. Appl. Geochem. 2000, 15, 181–190. [Google Scholar] [CrossRef]
- Xu, D.-M.; Zhan, C.-L.; Liu, H.-X.; Lin, H.-Z. A critical review on environmental implications, recycling strategies, and ecological remediation for mine tailings. Environ. Sci. Pollut. Res. 2019, 26, 35657–35669. [Google Scholar] [CrossRef]
- Luo, L.; Li, K.; Fu, W.; Liu, C.; Yang, S. Preparation, characteristics and mechanisms of the composite sintered bricks produced from shale, sewage sludge, coal gangue powder and iron ore tailings. Constr. Build. Mater. 2020, 232, 117250. [Google Scholar] [CrossRef]
- Weishi, L.; Guoyuan, L.; Ya, X.; Qifei, H. The properties and formation mechanisms of eco-friendly brick building materials fabricated from low-silicon iron ore tailings. J. Clean. Prod. 2018, 204, 685–692. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, Y.; Kim, M.; Park, H. Preparation of high porosity bricks by utilizing red mud and mine tailing. J. Clean. Prod. 2019, 207, 490–497. [Google Scholar] [CrossRef]
- Mendes, B.C.; Pedroti, L.G.; Fontes, M.; Ribeiro, J.C.L.; Vieira, C.M.; Pacheco, A.A.; de Azevedo, A.R. Technical and environmental assessment of the incorporation of iron ore tailings in construction clay bricks. Constr. Build. Mater. 2019, 227, 116669. [Google Scholar] [CrossRef]
- Li, R.; Zhou, Y.; Li, C.; Li, S.; Huang, Z. Recycling of industrial waste iron tailings in porous bricks with low thermal conductivity. Constr. Build. Mater. 2019, 213, 43–50. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, H.; Liu, L.; Ji, R.; Wang, X. Preparation and characterization of permeable bricks from gangue and tailings. Constr. Build. Mater. 2017, 148, 484–491. [Google Scholar] [CrossRef]
- Yao, G.; Wang, Q.; Wang, Z.; Wang, J.; Lyu, X. Activation of hydration properties of iron ore tailings and their application as supplementary cementitious materials in cement. Powder Technol. 2020, 360, 863–871. [Google Scholar] [CrossRef]
- Young, G.; Yang, M. Preparation and characterization of Portland cement clinker from iron ore tailings. Constr. Build. Mater. 2019, 197, 152–156. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, F.; Qi, S.; Li, W.; Liu, R.; Li, G. Durability of concrete incorporated with siliceous iron tailings. Constr. Build. Mater. 2020, 242, 118147. [Google Scholar] [CrossRef]
- Shettima, A.U.; Hussin, M.W.; Ahmad, Y.; Mirza, J. Evaluation of iron ore tailings as replacement for fine aggregate in concrete. Constr. Build. Mater. 2016, 120, 72–79. [Google Scholar] [CrossRef]
- Yang, M.; Sun, J.; Dun, C.; Duan, Y.; Meng, Z. Cementitious activity optimization studies of iron tailings powder as a concrete admixture. Constr. Build. Mater. 2020, 265, 120760. [Google Scholar] [CrossRef]
- Shi, J.; He, F.; Ye, C.; Hu, L.; Xie, J.; Yang, H.; Liu, X. Preparation and characterization of CaO–Al2O3–SiO2 glass-ceramics from molybdenum tailings. Mater. Chem. Phys. 2017, 197, 57–64. [Google Scholar] [CrossRef]
- Li, L.; Jiang, T.; Chen, B.; Zhou, M.; Chen, C. Overall utilization of vanadium–titanium magnetite tailings to prepare lightweight foam ceramics. Process Saf. Environ. Prot. 2020, 139, 305–314. [Google Scholar] [CrossRef]
- Zheng, W.; Sun, H.; Peng, T.; Zeng, L. Novel Preparation of Foamed Glass-ceramics from Asbestos Tailings and Waste Glass by Self-expansion in High Temperature. J. Non-Cryst. Solids 2020, 529, 119767. [Google Scholar] [CrossRef]
- Chen, J.; Yan, B.; Li, H.; Li, P.; Guo, H. Vitrification of blast furnace slag and fluorite tailings for giving diopside-fluorapatite glass-ceramics. Mater. Lett. 2018, 218, 309–312. [Google Scholar] [CrossRef]
- Onitiri, M.; Adedayo, S. Compressive behaviour of polypropylene filled with iron ore tailings. J. Eng. Des. Technol. 2015, 13, 198–212. [Google Scholar] [CrossRef]
- Onitiri, M.A.; Akinlabi, E.T. Effects of Particle Size and Particle Loading on the Tensile Properties of Iron-Ore-Tailing-Filled Epoxy and Polypropylene Composites. Mech. Compos. Mater. 2017, 52, 817–828. [Google Scholar] [CrossRef]
- Piffer, V.; Soares, K.; Galdino, A. Evaluation of mechanical and thermal properties of PP/iron ore tailing composites. Compos. Part B Eng. 2021, 221, 109001. [Google Scholar] [CrossRef]
- Han, Y.; Hong, W.; Leann, E.F. Field-stiffening effect of magneto-rheological elastomers. Int. J. Solids Struct. 2013, 50, 2281–2288. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Kumar, V.; Lee, D.-J. Compressive properties of magnetorheological elastomer with different magnetic fields and types of filler. Polym. Adv. Technol. 2019, 30, 1106–1115. [Google Scholar] [CrossRef]
- Kumar, V.; Lee, D.-J. Iron particle and anisotropic effects on mechanical properties of magneto-sensitive elastomers. J. Magn. Magn. Mater. 2017, 441, 105–112. [Google Scholar] [CrossRef]
- Noriman, N.Z.; Ismail, H.; Rashid, A.A. Properties of styrene butadiene rubber/recycled acrylonitrile-butadiene rubber (SBR/NBRr) blends: Effect of the addition of trans-polyoctylene rubber. J. Appl. Polym. Sci. 2012, 126, E56–E63. [Google Scholar] [CrossRef]
- Austin, L. Introduction to the mathematical description of grinding as a rate process. Powder Technol. 1971, 5, 1–17. [Google Scholar] [CrossRef]
- Kapur, P.; Agrawal, P. Approximate solutions to the discretized batch grinding equation. Chem. Eng. Sci. 1970, 25, 1111–1113. [Google Scholar] [CrossRef]
- Berthiaux, H.; Varinot, C.; Dodds, J. Approximate calculation of breakage parameters from batch grinding tests. Chem. Eng. Sci. 1996, 51, 4509–4516. [Google Scholar] [CrossRef]
- Raman, V.; Abbas, A.; Zhu, W. Particle grinding by high-intensity ultrasound: Kinetic modeling and identification of breakage mechanisms. AIChE J. 2011, 57, 2025–2035. [Google Scholar] [CrossRef]
- Zhang, Q.; Tian, M.; Wu, Y.; Lin, G.; Zhang, L. Effect of particle size on the properties of Mg(OH)2-filled rubber composites. J. Appl. Polym. Sci. 2004, 94, 2341–2346. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, D.; Wang, C.; An, Q.; Qi, S. Effect of filler size distribution on the mechanical and physical properties of alumina-filled silicone rubber. Polym. Eng. Sci. 2008, 48, 1381–1388. [Google Scholar] [CrossRef]
- Gao, M.; Forssberg, E. Prediction of product size distributions for a stirred ball mill. Powder Technol. 1995, 84, 101–106. [Google Scholar] [CrossRef]
- Menacho, J. Some solutions for the kinetics of combined fracture and abrasion breakage. Powder Technol. 1986, 49, 87–95. [Google Scholar] [CrossRef]
- Austin, L.G. Some topics for research on fine grinding. In Lecture in Harrogate Meeting; International Food Policy Research Institute: Washington, DC, USA, 1992. [Google Scholar]
- Hennart, S.; Wildeboer, W.; van Hee, P.; Meesters, G. Identification of the grinding mechanisms and their origin in a stirred ball mill using population balances. Chem. Eng. Sci. 2009, 64, 4123–4130. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Monteiro, P.J.M.; Gartner, E.M. In situ imaging of ground granulated blast furnace slag hydration. J. Mater. Sci. 2006, 41, 7074–7081. [Google Scholar] [CrossRef]
- Gallias, J.; Kara-Ali, R.; Bigas, J. The effect of fine mineral admixtures on water requirement of cement pastes. Cem. Concr. Res. 2000, 30, 1543–1549. [Google Scholar] [CrossRef]
- Thongsang, S.; Sombatsompop, N. Effect of NaOH and Si69 treatments on the properties of fly ash/natural rubber composites. Polym. Compos. 2006, 27, 30–40. [Google Scholar] [CrossRef]
- Debnath, S.C.; Datta, R.N.; Noordermeer, J.W.M. Understanding the Chemistry of the Rubber/Silane Reaction for Silica Reinforcement, Using Model Olefins. Rubber Chem. Technol. 2003, 76, 1311–1328. [Google Scholar] [CrossRef]



| Compound | SiO2 | Fe2O3 | CaO | MgO | Al2O3 | K2O | Na2O | TiO2 |
|---|---|---|---|---|---|---|---|---|
| Concentration (wt%) | 39.0 | 19.1 | 14.2 | 11.2 | 9.87 | 1.53 | 1.01 | 1.65 |
| Samples | Ingredients (phr) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SBR | G-IOT | Si69-G-IOT | 4010NA | Zinc Oxide | Stearic Acid | M | TMTD | Sulfur | Aromatic Hydrocarbon Oil | |
| Pure SBR | 100 | 0 | 0 | 2 | 3 | 1 | 0.6 | 0.6 | 1.8 | 3 |
| SBR/30 G-IOT | 100 | 30 | 0 | 2 | 3 | 1 | 0.6 | 0.6 | 1.8 | 3 |
| SBR/60 G-IOT | 100 | 60 | 0 | 2 | 3 | 1 | 0.6 | 0.6 | 1.8 | 3 |
| SBR/90 G-IOT | 100 | 90 | 0 | 2 | 3 | 1 | 0.6 | 0.6 | 1.8 | 3 |
| SBR/120 G-IOT | 100 | 120 | 0 | 2 | 3 | 1 | 0.6 | 0.6 | 1.8 | 3 |
| SBR/150 G-IOT | 100 | 150 | 0 | 2 | 3 | 1 | 0.6 | 0.6 | 1.8 | 3 |
| SBR/30 Si69-G-IOT | 100 | 0 | 30 | 2 | 3 | 1 | 0.6 | 0.6 | 1.8 | 3 |
| SBR/60 Si69-G-IOT | 100 | 0 | 60 | 2 | 3 | 1 | 0.6 | 0.6 | 1.8 | 3 |
| SBR/90 Si69-G-IOT | 100 | 0 | 90 | 2 | 3 | 1 | 0.6 | 0.6 | 1.8 | 3 |
| SBR/120 Si69-G-IOT | 100 | 0 | 120 | 2 | 3 | 1 | 0.6 | 0.6 | 1.8 | 3 |
| SBR/150 Si69-G-IOT | 100 | 0 | 150 | 2 | 3 | 1 | 0.6 | 0.6 | 1.8 | 3 |
| Class i,j | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Size range/μm | >11.74 | 11.74–8.02 | 8.02–5.48 | 5.48–3.74 | 3.74–2.56 | 2.56–1.75 | 1.75–1.19 | 1.19–0.81 | 0.81–0.56 | 0.56–0.38 |
| Filler Loading | t02 (min) | t90 (min) | MH−ML (dN·m) | CRI (min−1) | |
|---|---|---|---|---|---|
| None | 2.17 | 10.10 | 11.67 | 12.61 | |
| 30 phr | G-IOT | 2.57 | 8.30 | 11.92 | 17.45 |
| Si69-G-IOT[M1] | 1.73 | 6.25 | 12.90 | 22.12 | |
| 60 phr | G-IOT | 2.15 | 6.27 | 12.05 | 24.27 |
| Si69-G-IOT | 11.50 | 5.10 | 14.61 | 27.78 | |
| 90 phr | G-IOT | 1.85 | 5.82 | 12.14 | 25.19 |
| Si69-G-IOT | 1.32 | 4.45 | 16.46 | 31.95 | |
| 120 phr | G-IOT | 1.51 | 5.27 | 13.52 | 26.60 |
| Si69-G-IOT | 1.35 | 4.10 | 19.67 | 36.36 | |
| 150 phr | G-IOT | 1.42 | 4.93 | 13.57 | 28.49 |
| Si69-G-IOT | 1.30 | 3.73 | 19.83 | 41.15 | |
| Samples | Storage Modulus (MPa) at Different Temperatures | Tg (°C) | tanδ | ||||
|---|---|---|---|---|---|---|---|
| −60 (°C) | −30 (°C) | 0 (°C) | 30 (°C) | 60 (°C) | |||
| SBR | 1469.05 | 12.32 | 3.24 | 3.15 | 3.46 | −35.38 | 1.63 |
| SBR/60 Si69-G-IOT | 1851.21 | 52.63 | 8.40 | 7.46 | 7.34 | −33.28 | 1.52 |
| SBR/90 Si69-G-IOT | 2262.04 | 100.37 | 16.45 | 12.67 | 10.72 | −32.25 | 1.25 |
| SBR/120 Si69-G-IOT | 3186.83 | 257.15 | 46.66 | 36.30 | 31.14 | −29.29 | 1.05 |
| SBR/150 Si69-G-IOT | 2591.94 | 69.94 | 30.68 | 27.32 | 22.63 | −29.93 | 1.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Tang, Q.; Zhao, W.; Su, Z.; Liang, C.; Duan, X.; Liang, J. Ultrafine Grinded and Silane Grafted Iron Ore Tailings as Reinforcing Filler of Styrene Butadiene Rubber. Materials 2022, 15, 1756. https://doi.org/10.3390/ma15051756
Liu Q, Tang Q, Zhao W, Su Z, Liang C, Duan X, Liang J. Ultrafine Grinded and Silane Grafted Iron Ore Tailings as Reinforcing Filler of Styrene Butadiene Rubber. Materials. 2022; 15(5):1756. https://doi.org/10.3390/ma15051756
Chicago/Turabian StyleLiu, Qian, Qingguo Tang, Weiwei Zhao, Zhiyuan Su, Cong Liang, Xinhui Duan, and Jinsheng Liang. 2022. "Ultrafine Grinded and Silane Grafted Iron Ore Tailings as Reinforcing Filler of Styrene Butadiene Rubber" Materials 15, no. 5: 1756. https://doi.org/10.3390/ma15051756
APA StyleLiu, Q., Tang, Q., Zhao, W., Su, Z., Liang, C., Duan, X., & Liang, J. (2022). Ultrafine Grinded and Silane Grafted Iron Ore Tailings as Reinforcing Filler of Styrene Butadiene Rubber. Materials, 15(5), 1756. https://doi.org/10.3390/ma15051756





