Development of an Oxide Layer on Al 6061 Using Plasma Arc Electrolytic Oxidation in Silicate-Based Electrolyte
Abstract
:1. Introduction
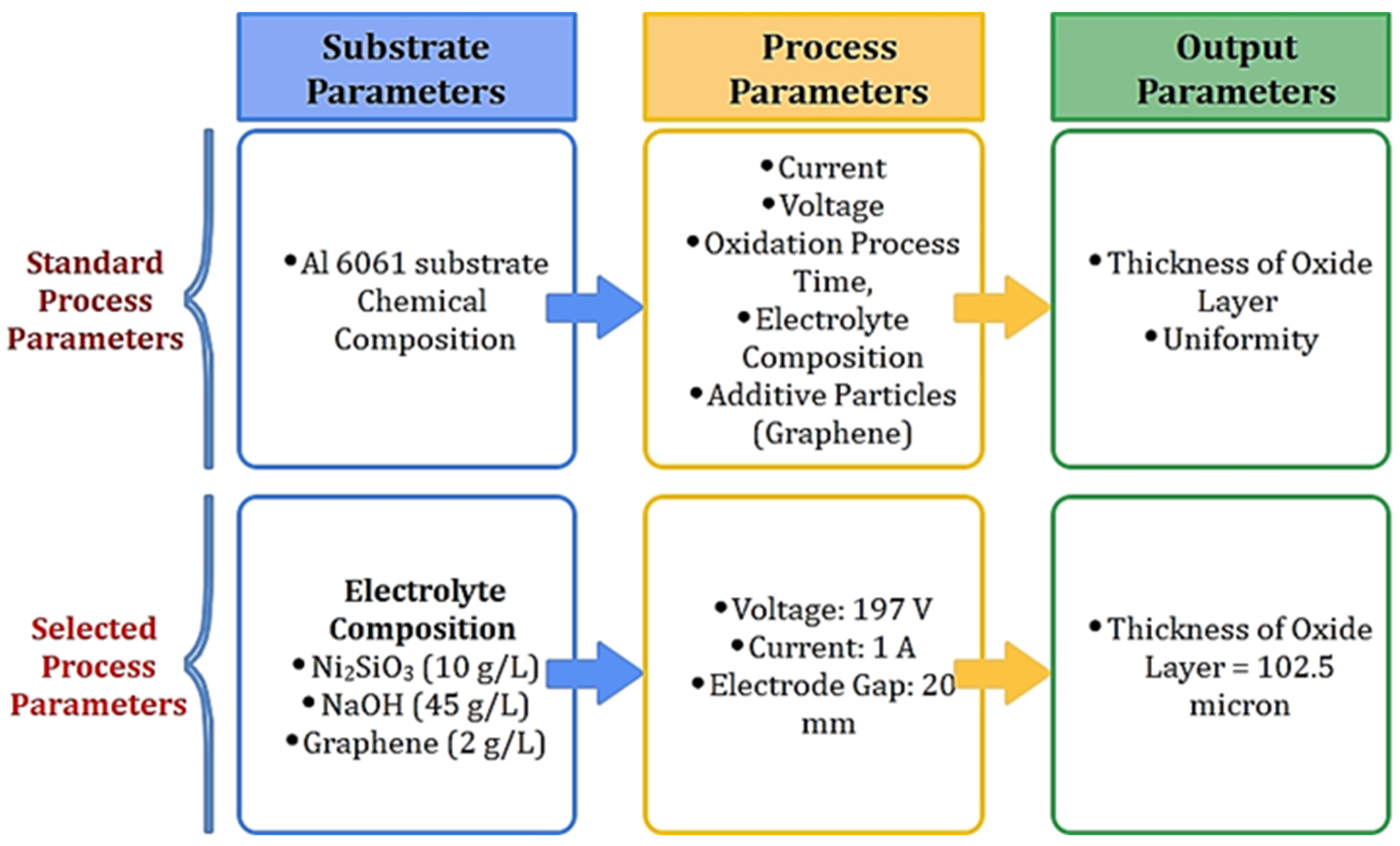
2. Materials and Methods
2.1. Mechanics of Oxidation Process
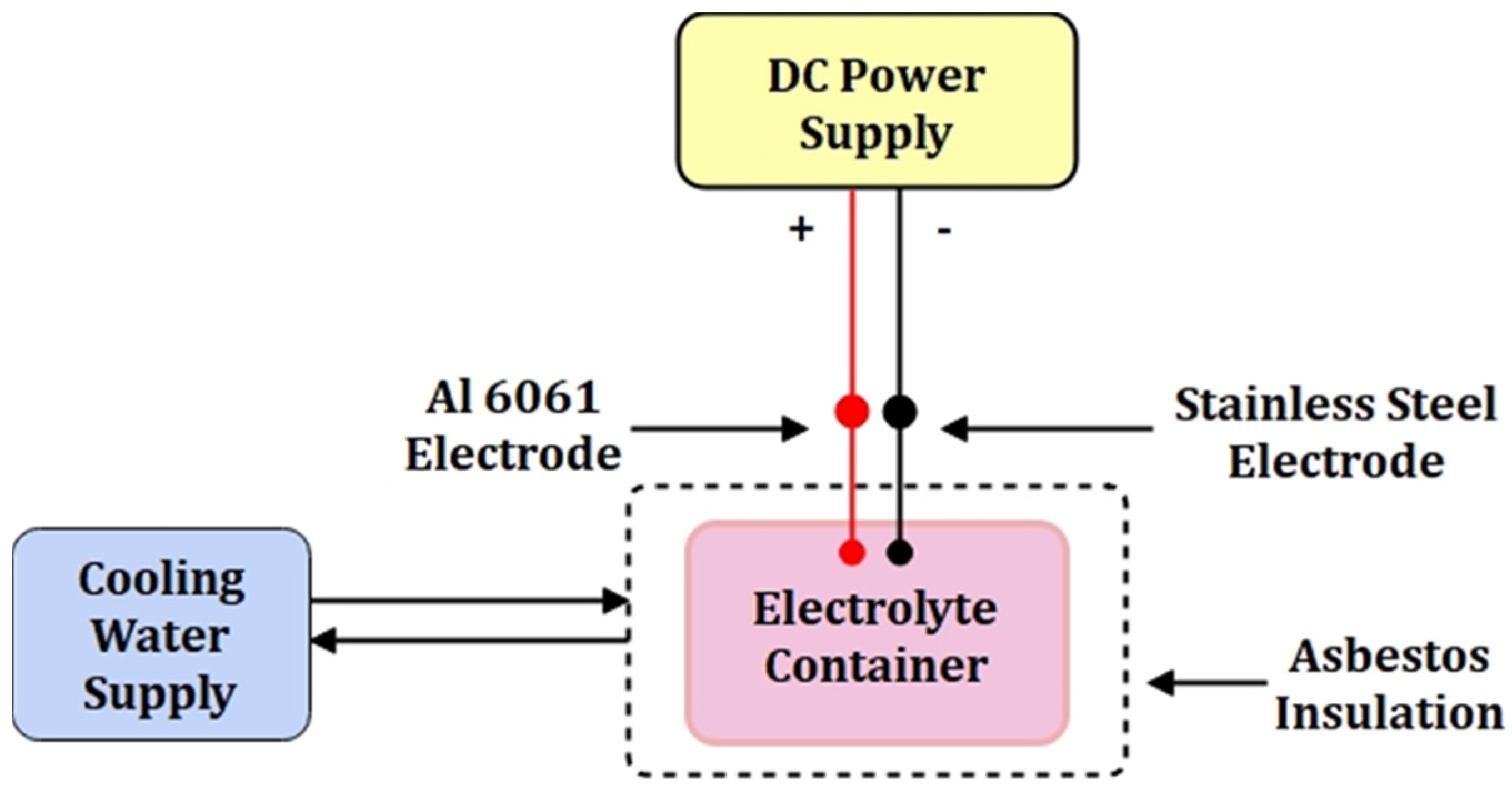
2.2. Configuration of the Experiment
3. Results and Discussion
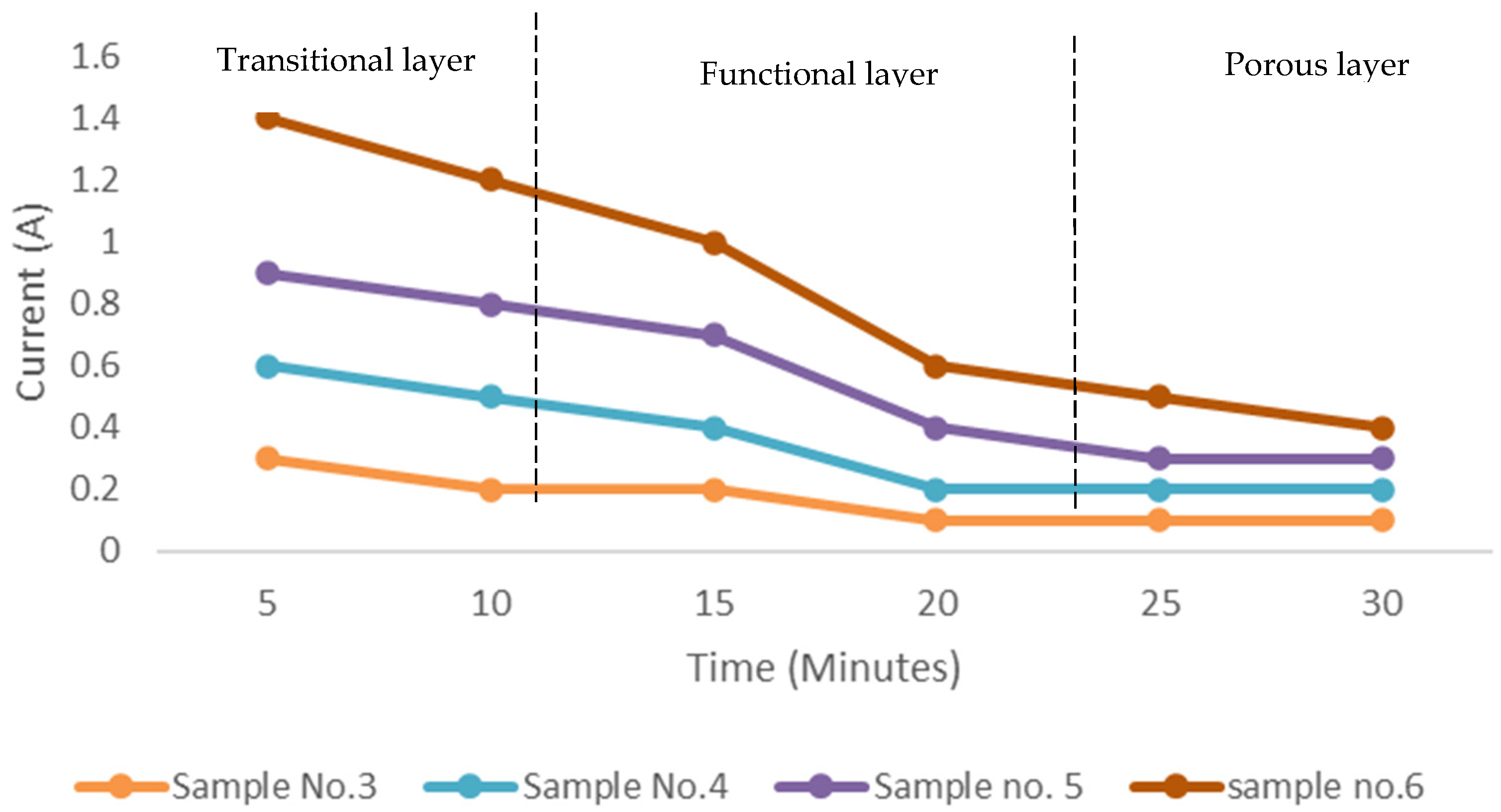
3.1. Formation of the Metal Oxide Layer
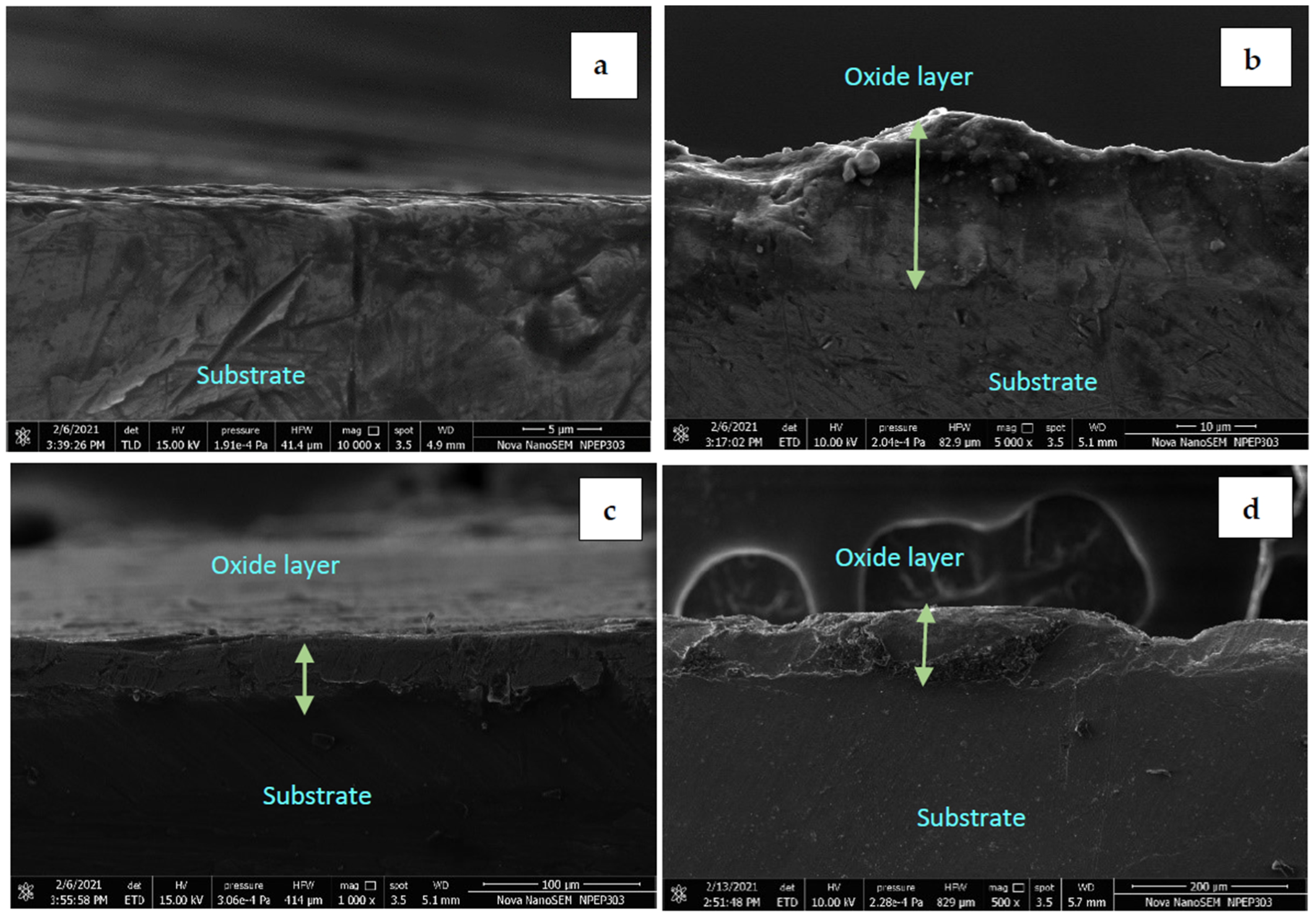
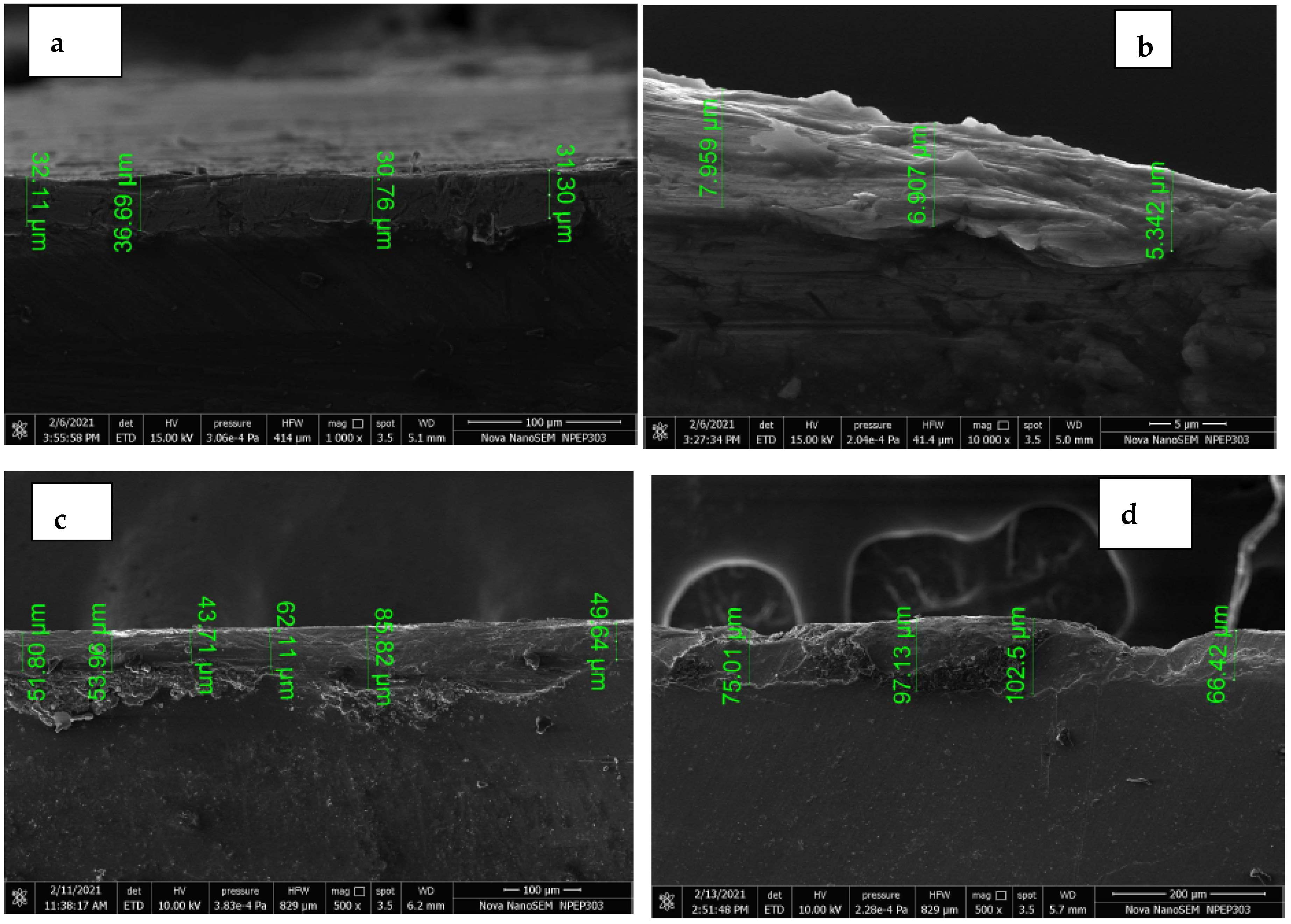
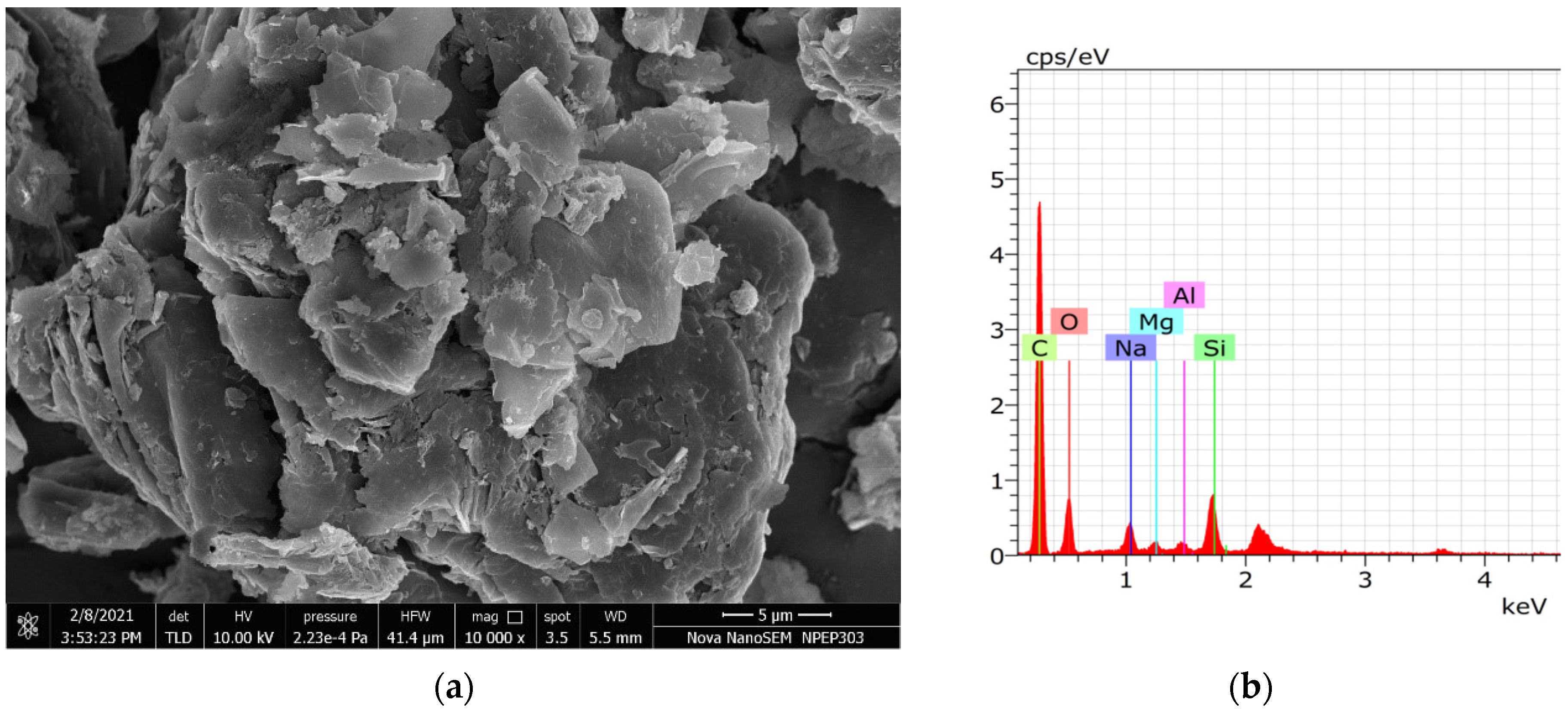
3.2. SEM Analysis
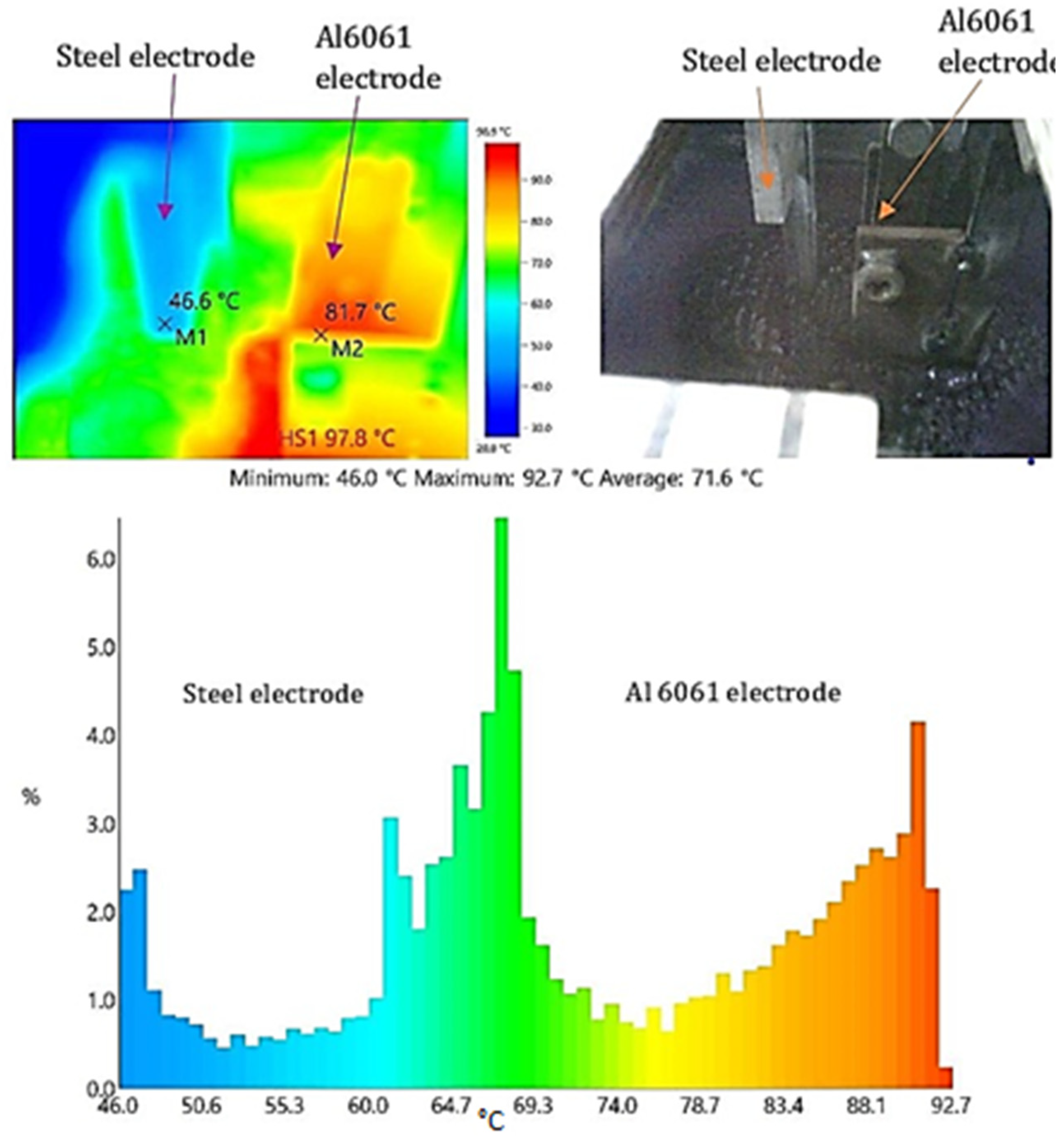
3.3. Effect of Temperature
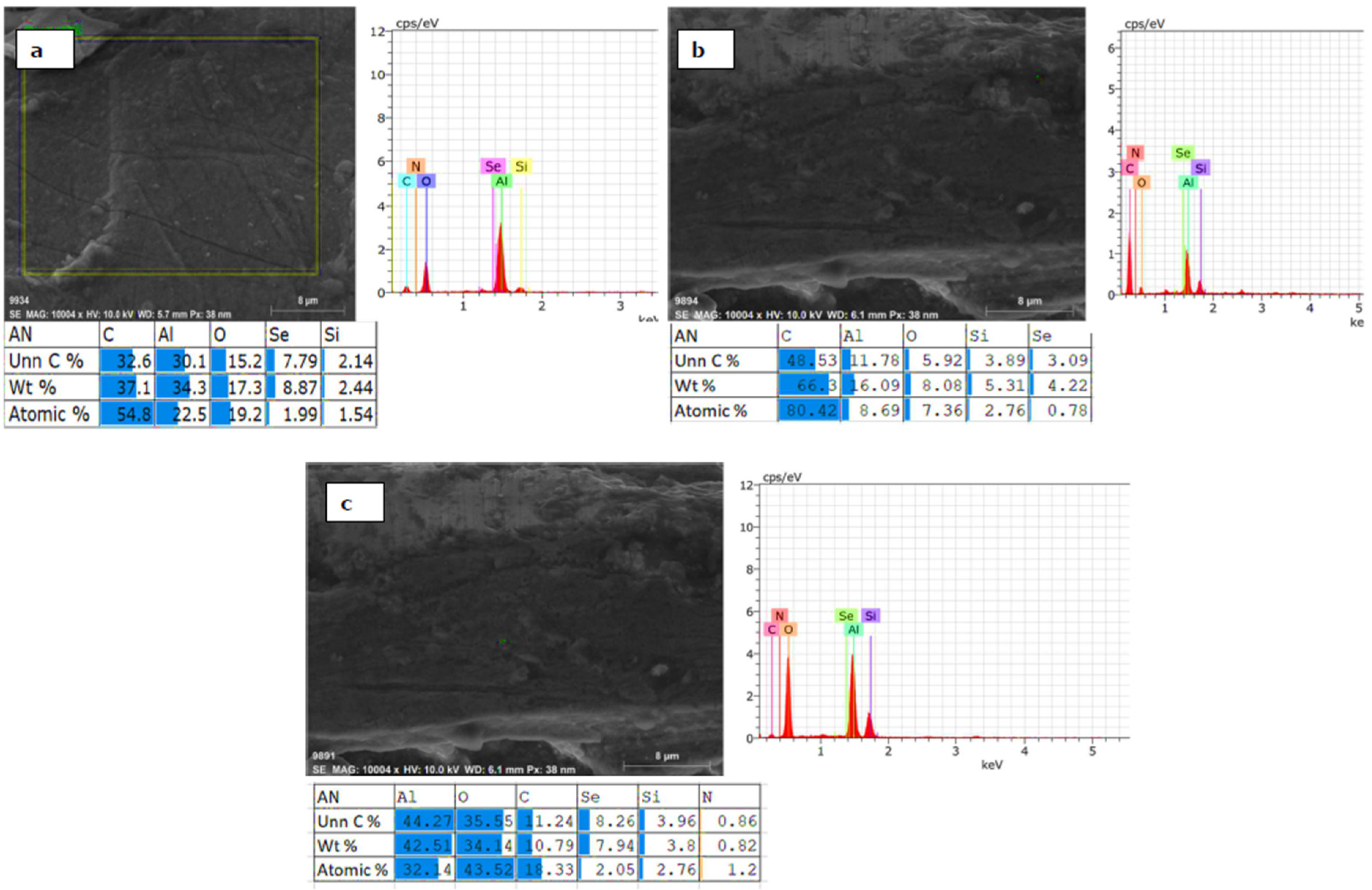
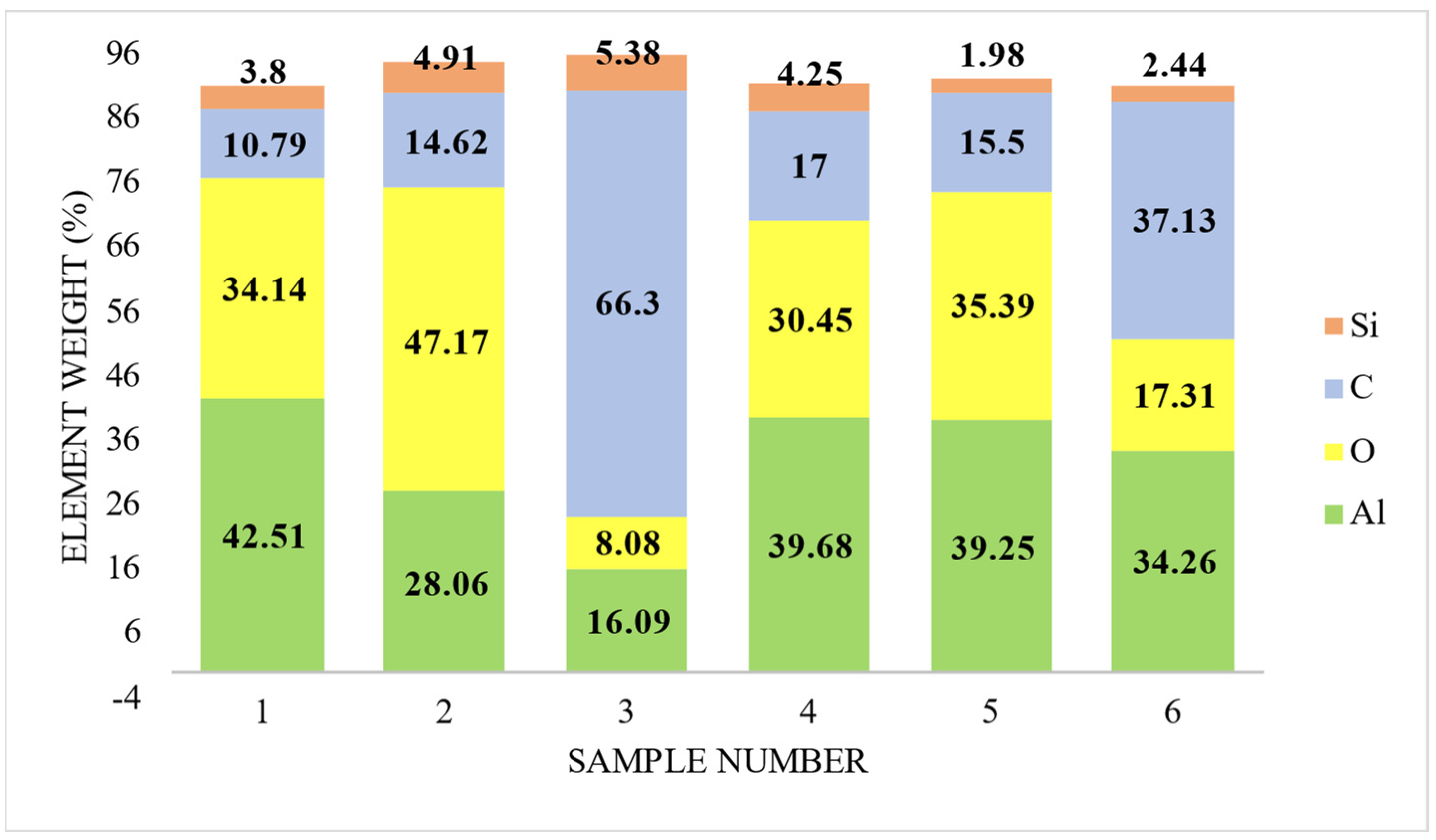
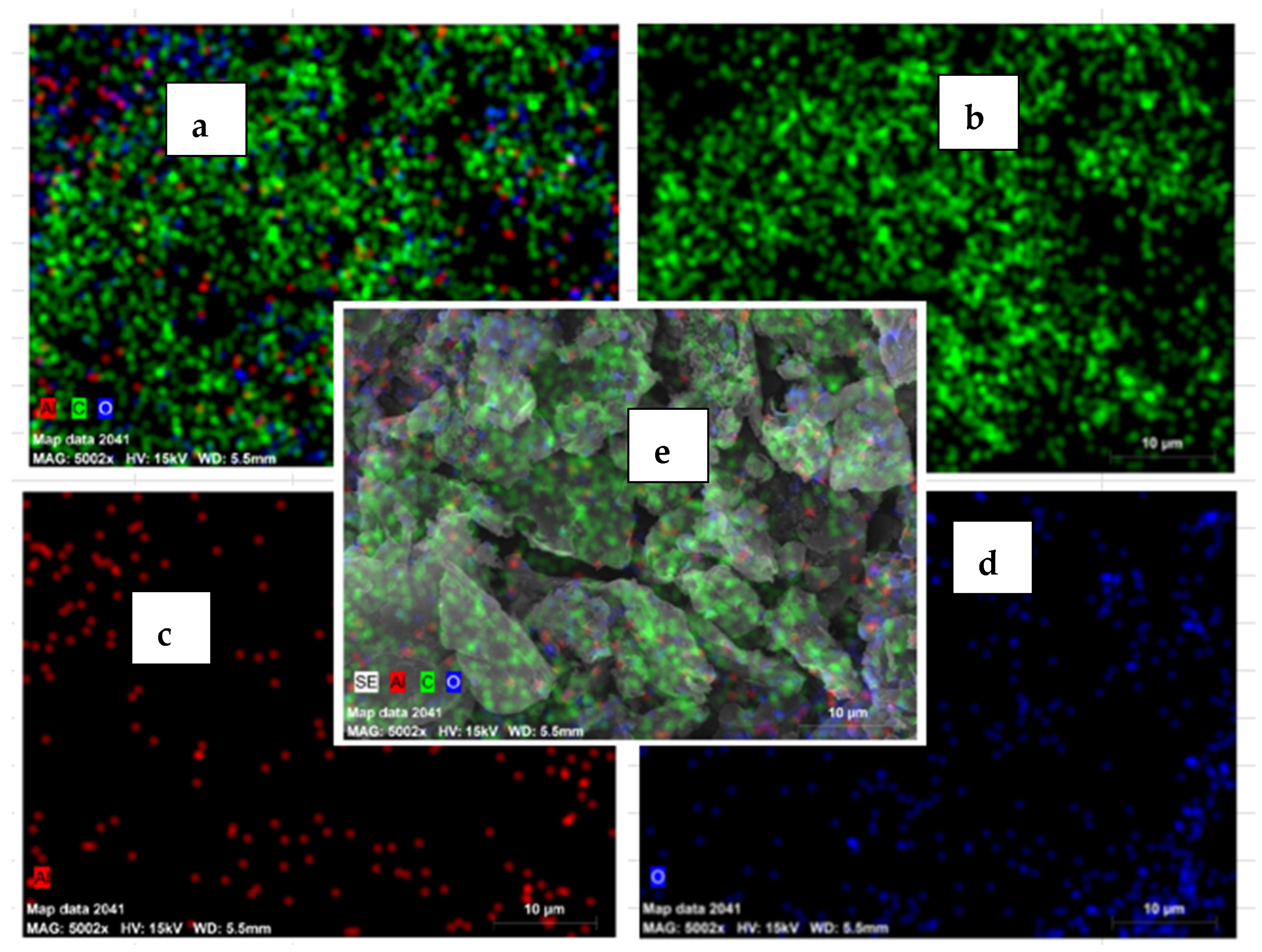
3.4. Element Distribution on Al 6061 Surface
3.5. EDS Analysis of Residue
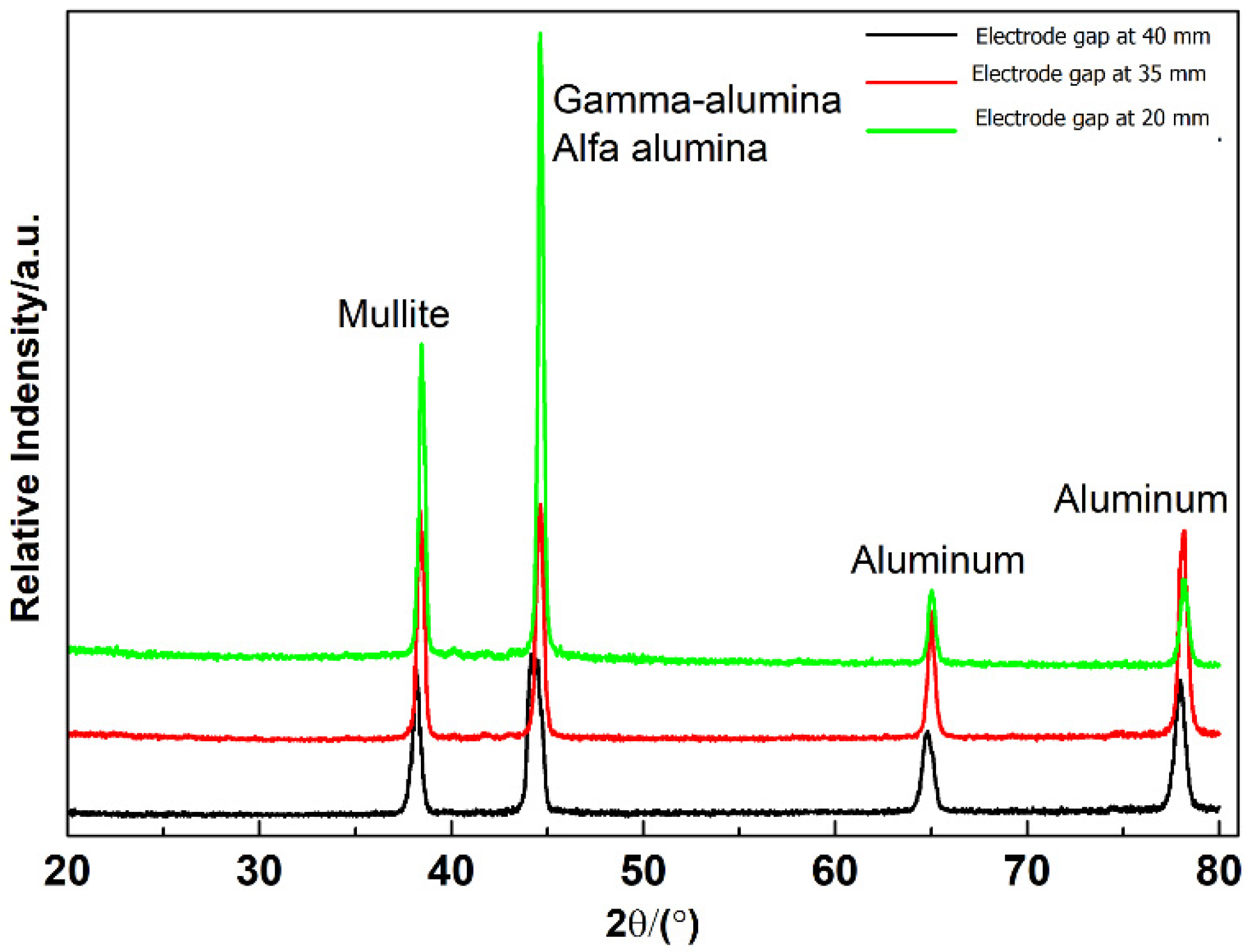
3.6. Phase Composition Analysis
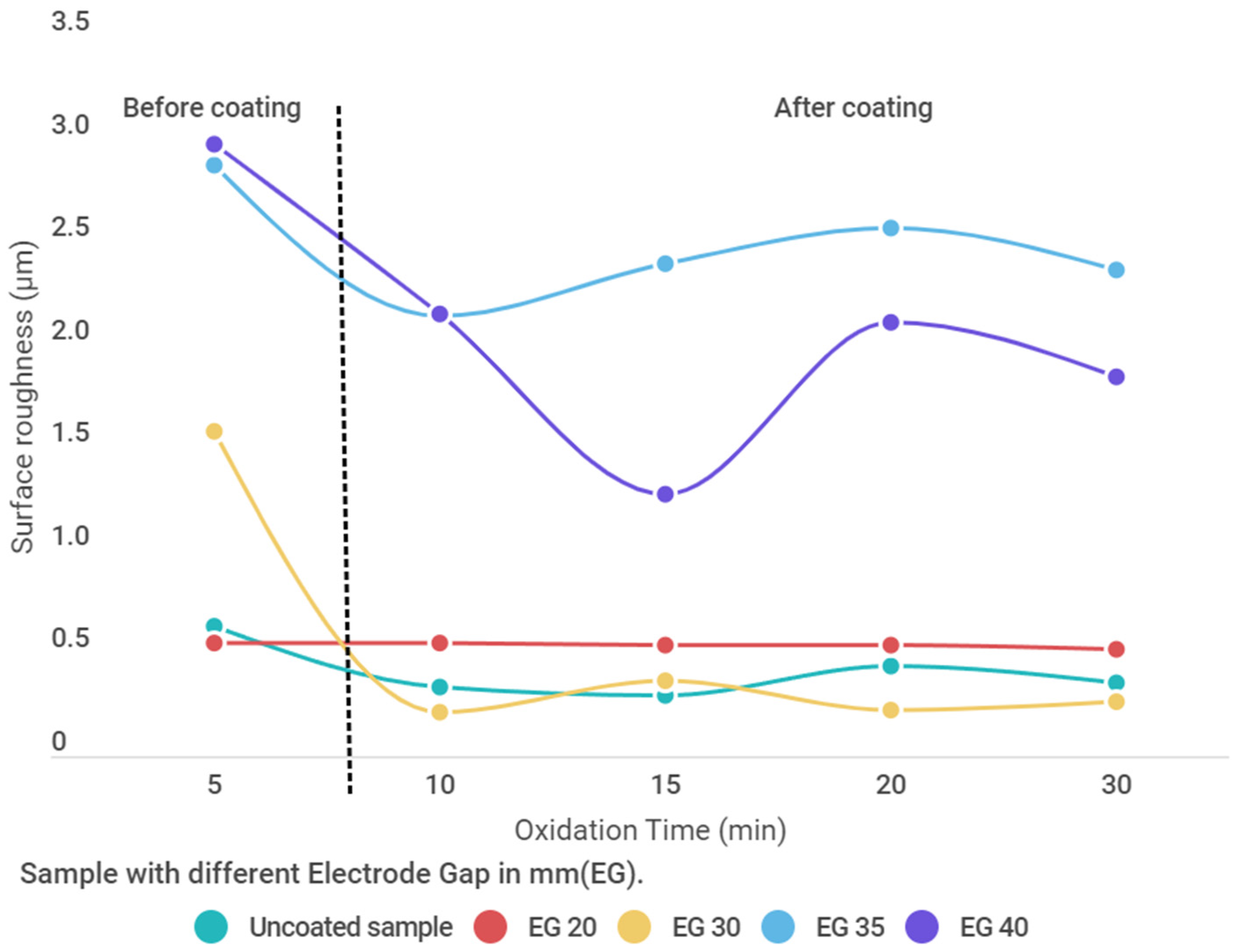
3.7. Surface Roughness Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aliofkhazraei, M.; Macdonald, D.; Matykina, E.; Parfenov, E.; Egorkin, V.; Curran, J.; Troughton, S.; Sinebryukhov, S.; Gnedenkov, S.; Lampke, T.; et al. Review of plasma electrolytic oxidation of titanium substrates: Mechanism, properties, applications and limitations. Appl. Surf. Sci. Adv. 2021, 5, 100121. [Google Scholar] [CrossRef]
- Clyne, T.W.; Troughton, S.C. A review of recent work on discharge characteristics during plasma electrolytic oxidation of various metals. Int. Mater. Rev. 2019, 64, 127–162. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Pu, Y.; Liao, H.; Lin, Y.; He, W. Corrosion and wear behavior of PEO coatings on D16T aluminum alloy with different concentrations of graphene. Coatings 2020, 10, 249. [Google Scholar] [CrossRef] [Green Version]
- Jadhav, P.; Bongale, A.; Kumar, S. The effects of processing parameters on the formation of oxide layers in aluminium alloys using plasma electrolytic oxidation technique. J. Mech. Behav. Mater. 2021, 30, 118–129. [Google Scholar] [CrossRef]
- Jadhav, P.; Bongale, A.; Kumar, S. A review of process characteristics of plasma electrolytic oxidation of aluminium alloy. J. Phys. Conf. Ser. 2021, 1854, 012030. [Google Scholar] [CrossRef]
- Usman, B.; Scenini, F.; Curioni, M. The effect of exposure conditions on performance evaluation of post-treated anodic oxides on an aerospace aluminium alloy: Comparison between salt spray and immersion testing. Surf. Coat. Technol. 2020, 399, 126157. [Google Scholar] [CrossRef]
- Hamzah, A.A.; Selvarajan, R.S.; Majlis, B.Y. Graphene for Biomedical Applications: A Review. Sains Malays. 2017, 46, 1125–1139. [Google Scholar] [CrossRef]
- Sowa, M.; Wala, M.; Blacha-Grzechnik, A.; Maciej, A.; Kazek-Kęsik, A.; Stolarczyk, A.; Simka, W. Corrosion Inhibitor-Modified Plasma Electrolytic Oxidation Coatings on 6061 Aluminum Alloy. Materials 2021, 14, 619. [Google Scholar] [CrossRef]
- Lu, C.; Shi, P.; Yang, J.; Jia, J.; Xie, E.; Sun, Y. Effects of surface texturing on the tribological behaviors of PEO/PTFE coating on aluminum alloy for heavy-load and long-performance applications. J. Mater. Res. Technol. 2020, 9, 12149–12156. [Google Scholar] [CrossRef]
- Sieradzka, M.; Ślusarczyk, C.; Biniaś, W.; Fryczkowski, R. The Role of the Oxidation and Reduction Parameters on the Properties of the Reduced Graphene Oxide. Coatings 2021, 11, 166. [Google Scholar] [CrossRef]
- Yang, W.; Xu, D.P.; Wang, J.L.; Jiang, B.L. Preparation of MAO coatings doped with graphene oxide. Surf. Eng. 2016, 33, 739–743. [Google Scholar] [CrossRef]
- Babaremu, K.; Joseph, O.; Akinlabi, E.; Jen, T.; Oladijo, O. Morphological investigation and mechanical behaviour of agrowaste reinforced aluminium alloy 8011 for service life improvement. Heliyon 2020, 6, e05506. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Nominé, A.; Ntomprougkidis, V.; Migot, S.; Bruyère, S.; Soldera, F.; Belmonte, T.; Henrion, G. Formation of a metastable nanostructured mullite during Plasma Electrolytic Oxidation of aluminium in “soft” regime condition. Mater. Des. 2019, 180, 107977. [Google Scholar] [CrossRef]
- Taylor, C.D.; Ke, H. Investigations of the intrinsic corrosion and hydrogen susceptibility of metals and alloys using density functional theory. Corros. Rev. 2021, 39, 177–209. [Google Scholar] [CrossRef]
- Tian, W.; Li, S.; Liu, J.; Yu, M.; DU, Y. Preparation of bimodal grain size 7075 aviation aluminum alloys and their corrosion properties. Chin. J. Aeronaut. 2017, 30, 1777–1788. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, F.; Zhang, Y.; Du, C. 1Influence of graphene oxide additive on the tribological and electrochemical corrosion properties of a PEO coating prepared on AZ31 magnesium alloy. Tribol. Int. 2020, 146, 106135. [Google Scholar] [CrossRef]
- Rogov, A.; Matthews, A.; Yerokhin, A. Role of cathodic current in plasma electrolytic oxidation of Al: A quantitative approach to in-situ evaluation of cathodically induced effects. Electrochim. Acta 2019, 317, 221–231. [Google Scholar] [CrossRef]
- Zhu, Q.J.; Bin Wang, B.; Zhao, X.; Bin Zhang, B. Robust Micro Arc Oxidation Coatings on 6061 Aluminum Alloys via Surface Thickening and Microvoid Reducing Approach. Solid State Phenom. 2018, 279, 148–152. [Google Scholar] [CrossRef]
- Tavares, M.D.M.; Vitoriano, J.D.O.; da Silva, R.C.; Franco, A.R.; de Souza, G.; da Costa, J.A.P.; Junior, C.A. Effect of duty cycle and treatment time on electrolytic plasma oxidation of commercially pure Al samples. J. Mater. Res. Technol. 2019, 8, 2141–2147. [Google Scholar] [CrossRef]
- Aliramezani, R.; Raeissi, K.; Santamaria, M.; Hakimizad, A. Characterization and properties of PEO coatings on 7075 Al alloy grown in alkaline silicate electrolyte containing KMnO 4 additive. Surf. Coat. Technol. 2017, 329, 250–261. [Google Scholar] [CrossRef]
- Jiang, B.L.; Wang, Y.M. Plasma Electrolytic Oxidation Treatment of Aluminium and Titanium Alloys. In Surface Engineering of Light Alloys Aluminium, Magnesium and Titanium Alloys; Woodhead Publishing: London, UK, 2010; pp. 110–154. [Google Scholar]
- Mui, T.S.M.; Silva, L.; Prysiazhnyi, V.; Kostov, K. Surface modification of aluminium alloys by atmospheric pressure plasma treatments for enhancement of their adhesion properties. Surf. Coat. Technol. 2017, 312, 32–36. [Google Scholar] [CrossRef]
- Zhao, T.; Munis, A.; Rehman, A.U.; Zheng, M. Corrosion behavior of aluminum in molten hydrated salt phase change materials for thermal energy storage. Mater. Res. Express 2020, 7, 015529. [Google Scholar] [CrossRef]
- Genzer, J.; Efimenko, K. Recent developments in superhydrophobic surfaces and their relevance to marine fouling: A review. Biofouling 2006, 22, 339–360. [Google Scholar] [CrossRef] [PubMed]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. Evaluation of Wear Performance of Plasma Electrolytic Oxidation Coatings on Aluminium. J. Manuf. Mater. Process. 2019, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Mann, R.; Hansal, S.; Hansal, W.E.G. Nanoparticle incorporation in plasma-electrolytic oxidation. Trans. IMF 2016, 94, 131–138. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Raaen, S.; Gaiaschi, S.; Chapon, P.; Matýsek, D.; Pietrzak, K.; Szymańska, M.; Dudek, Ł. Metal Ions Supported Porous Coatings by Using AC Plasma Electrolytic Oxidation Processing. Materials 2020, 13, 3838. [Google Scholar] [CrossRef]
- Tabish, M.; Yasin, G.; Anjum, M.J.; Malik, M.U.; Zhao, J.; Yang, Q.; Manzoor, S.; Murtaza, H.; Khan, W.Q. Reviewing the current status of layered double hydroxide-based smart nanocontainers for corrosion inhibiting applications. J. Mater. Res. Technol. 2020, 10, 390–421. [Google Scholar] [CrossRef]
- Hussein, R.O.; Northwood, D.O. Production of Anti-Corrosion Coatings on Light Alloys (Al, Mg, Ti) by Plasma-Electrolytic Oxidation (PEO). In Developments in Corrosion Protection; InTech: London, UK, 2014. [Google Scholar]
- Rokosz, K.; Hryniewicz, T.; Matýsek, D.; Raaen, S.; Valíček, J.; Harničárová, M. SEM, EDS and XPS Analysis of the Coatings Obtained on Titanium after Plasma Electrolytic Oxidation in Electrolytes Containing Copper Nitrate. Materials 2016, 9, 318. [Google Scholar] [CrossRef]
- Subbotina, V.; Sobol, O.; Belozerov, V.; Shnayder, V.; Smyrnov, O. An experimental analysis of the influence of electrolyte compositions, current density and duration of the micro—Arc oxidation process on the structural-phase state and properties of VT3-1 titanium alloy. East. Eur. J. Enterp. Technol. 2020, 5, 6–15. [Google Scholar] [CrossRef]
- Dudareva, N.; Ustimova, E.; Gallyamova, R. Corrosion Resistance of MAO Coatings on Al-Si Alloys. Solid State Phenom. 2020, 299, 749–754. [Google Scholar] [CrossRef]
- Yao, J.-T.; Wang, S.; Zhou, Y.; Dong, H. Effects of the Power Supply Mode and Loading Parameters on the Characteristics of Micro-Arc Oxidation Coatings on Magnesium Alloy. Metals 2020, 10, 1452. [Google Scholar] [CrossRef]
- Kombayev, K.K.; Doudkin, M.V.; Kim, A.I.; Młyńczak, M.; Rakhadilov, B.K. Surface Hardening of the Aluminium Alloy AL3 by Electrolytic–Plasma Treatment. Ser. Geol. Tech. Sci. 2019, 4, 222–229. [Google Scholar] [CrossRef]
- Haghighat-Shishavan, B.; Azari-Khosrowshahi, R.; Haghighat-Shishavan, S.; Nazarian-Samani, M.; Parvini-Ahmadi, N. Improving wear and corrosion properties of alumina coating on AA7075 aluminum by plasma electrolytic oxidation: Effects of graphite absorption. Appl. Surf. Sci. 2019, 481, 108–119. [Google Scholar] [CrossRef]
- Agureev, L.; Savushkina, S.; Ashmarin, A.; Borisov, A.; Apelfeld, A.; Anikin, K.; Tkachenko, N.; Gerasimov, M.; Shcherbakov, A.; Ignatenko, V.; et al. Study of Plasma Electrolytic Oxidation Coatings on Aluminum Composites. Metals 2018, 8, 459. [Google Scholar] [CrossRef] [Green Version]
- Yerokhin, A. Wear Resistant Coatings with a High Friction Coe ffi cient Produced by Plasma Electrolytic Oxidation of Al Alloys in Electrolytes with Basalt Mineral Powder Additions. Materials 2019, 12, 2738. [Google Scholar]
- Song, W.; Jiang, B.; Ji, D. Improving the Tribological Performance of MAO Coatings by Using a Stable Sol Electrolyte Mixed with Cellulose Additive. Materials 2019, 12, 4226. [Google Scholar] [CrossRef] [Green Version]


| Element | Percentage (wt.%) | Specification Aluminum 6061 |
|---|---|---|
| 0.28 | 0.15–0.4 | |
| 0.15 | 0.15 | |
| 1 | 0.8–1.2 | |
| 0.25 | 0.25 | |
| 0.2 | 0.04–0.35 | |
| 0.15 | 0.15 | |
| 0.6 | 0.4–0.8 | |
| 0.7 | 0.7 | |
| 96.67 | Remaining |
| Sr. No. | Parameter | Range |
|---|---|---|
| 1 | Voltage | 150–200 V DC |
| 2 | Current | 0–1 A DC |
| 3 | Time | 5 to 15 min |
| 4 | Temperature | 26–75 °C |
| 5 | Distance between two electrodes | 35 to 50 mm |
| 6 | Additive | Graphene (2 g/L) |
| 7 | Electrolyte composition | Na2SiO3 (10 g/L), NaOH (45 g/L) |
| Sample. No. | Electrolyte Composition | Voltage (V) | Time (min) | Electrode Gap (mm) |
|---|---|---|---|---|
| 1 | Na2SiO3 (10g/L), NaOH(45 g/L), | 150 | 15 | 35 |
| 2 | 150 | 5 | 35 | |
| 3 | Na2SiO3 (10 g/L), NaOH (45 g/L), Graphene (2 g/L) | 197 | 30 | 35 |
| 4 | 197 | 30 | 40 | |
| 5 | 197 | 30 | 30 | |
| 6 | 197 | 30 | 20 |
| Sr. No. | Distance between Electrodes in mm | The Thickness of Oxide Layer (µm) |
|---|---|---|
| 1 | 20 | 79.58 |
| 2 | 30 | 52.56 |
| 3 | 35 | 5.10 |
| 4 | 40 | 18.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jadhav, P.; Bongale, A.; Kumar, S.; Pimenov, D.Y.; Giasin, K.; Wojciechowski, S. Development of an Oxide Layer on Al 6061 Using Plasma Arc Electrolytic Oxidation in Silicate-Based Electrolyte. Materials 2022, 15, 1616. https://doi.org/10.3390/ma15041616
Jadhav P, Bongale A, Kumar S, Pimenov DY, Giasin K, Wojciechowski S. Development of an Oxide Layer on Al 6061 Using Plasma Arc Electrolytic Oxidation in Silicate-Based Electrolyte. Materials. 2022; 15(4):1616. https://doi.org/10.3390/ma15041616
Chicago/Turabian StyleJadhav, Priya, Arunkumar Bongale, Satish Kumar, Danil Yurievich Pimenov, Khaled Giasin, and Szymon Wojciechowski. 2022. "Development of an Oxide Layer on Al 6061 Using Plasma Arc Electrolytic Oxidation in Silicate-Based Electrolyte" Materials 15, no. 4: 1616. https://doi.org/10.3390/ma15041616
APA StyleJadhav, P., Bongale, A., Kumar, S., Pimenov, D. Y., Giasin, K., & Wojciechowski, S. (2022). Development of an Oxide Layer on Al 6061 Using Plasma Arc Electrolytic Oxidation in Silicate-Based Electrolyte. Materials, 15(4), 1616. https://doi.org/10.3390/ma15041616











