The Application of Polycaprolactone Scaffolds with Poly(ε-caprolactone)–Poly(ethylene glycol)–Poly(ε-caprolactone) Loaded on Kidney Cell Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Coating Materials
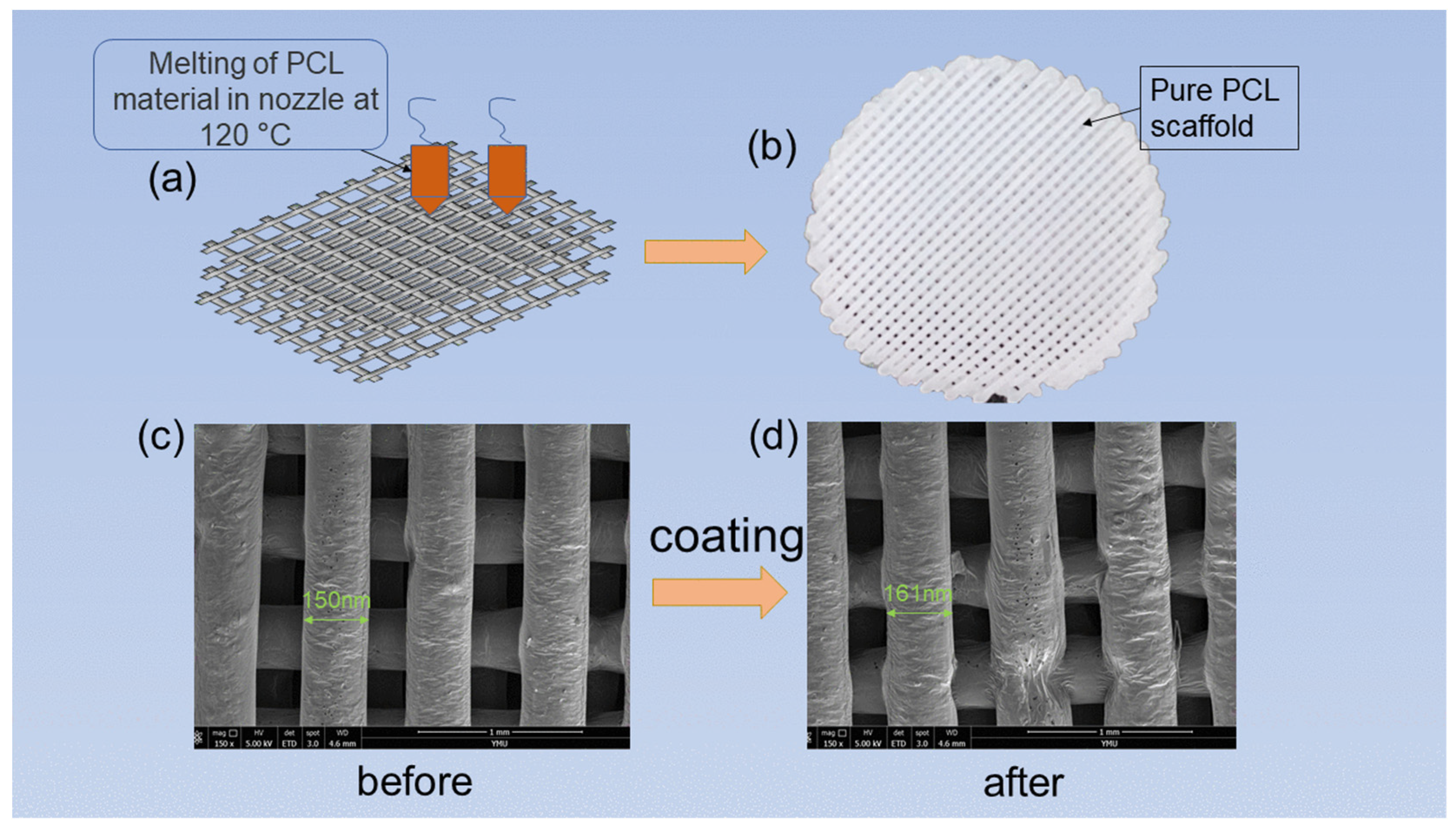
2.2. Preparation of 3D Cell Culture Scaffold
2.3. Characterization Methods
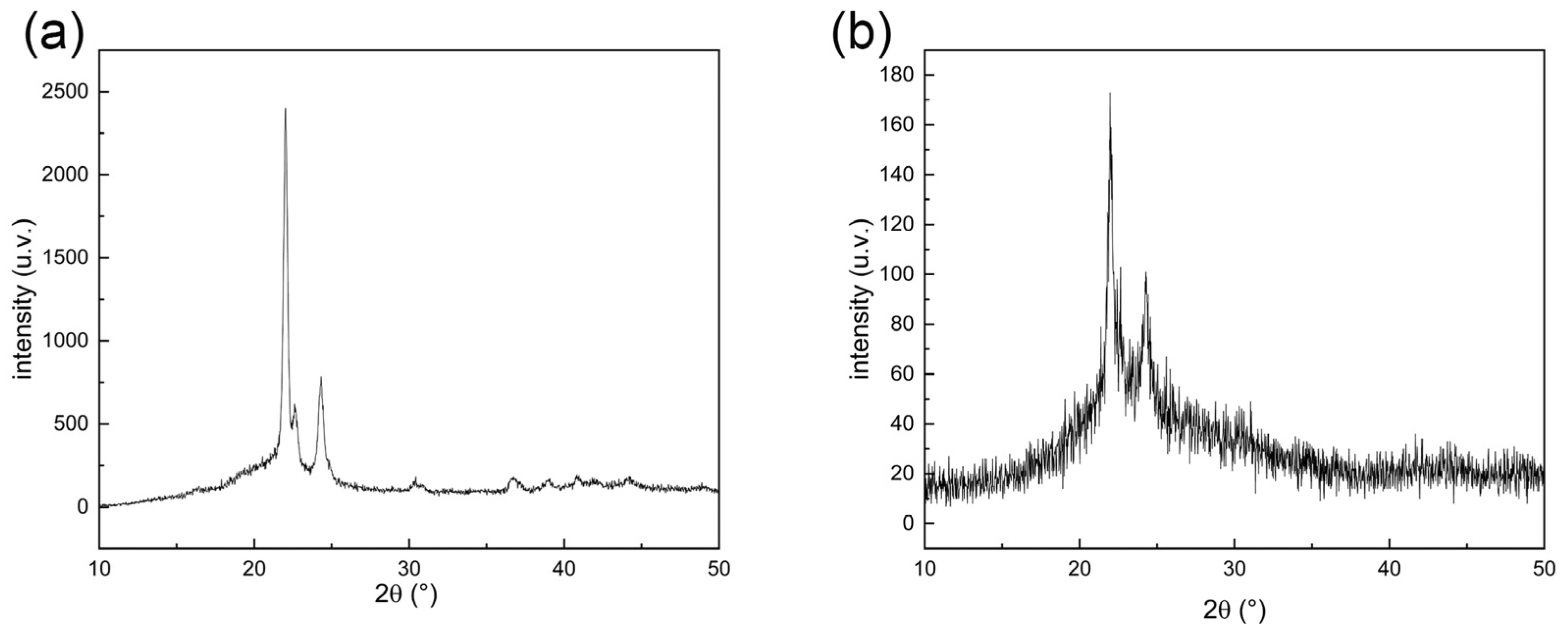
2.3.1. X-ray Diffraction Analysis
2.3.2. Morphology Analysis
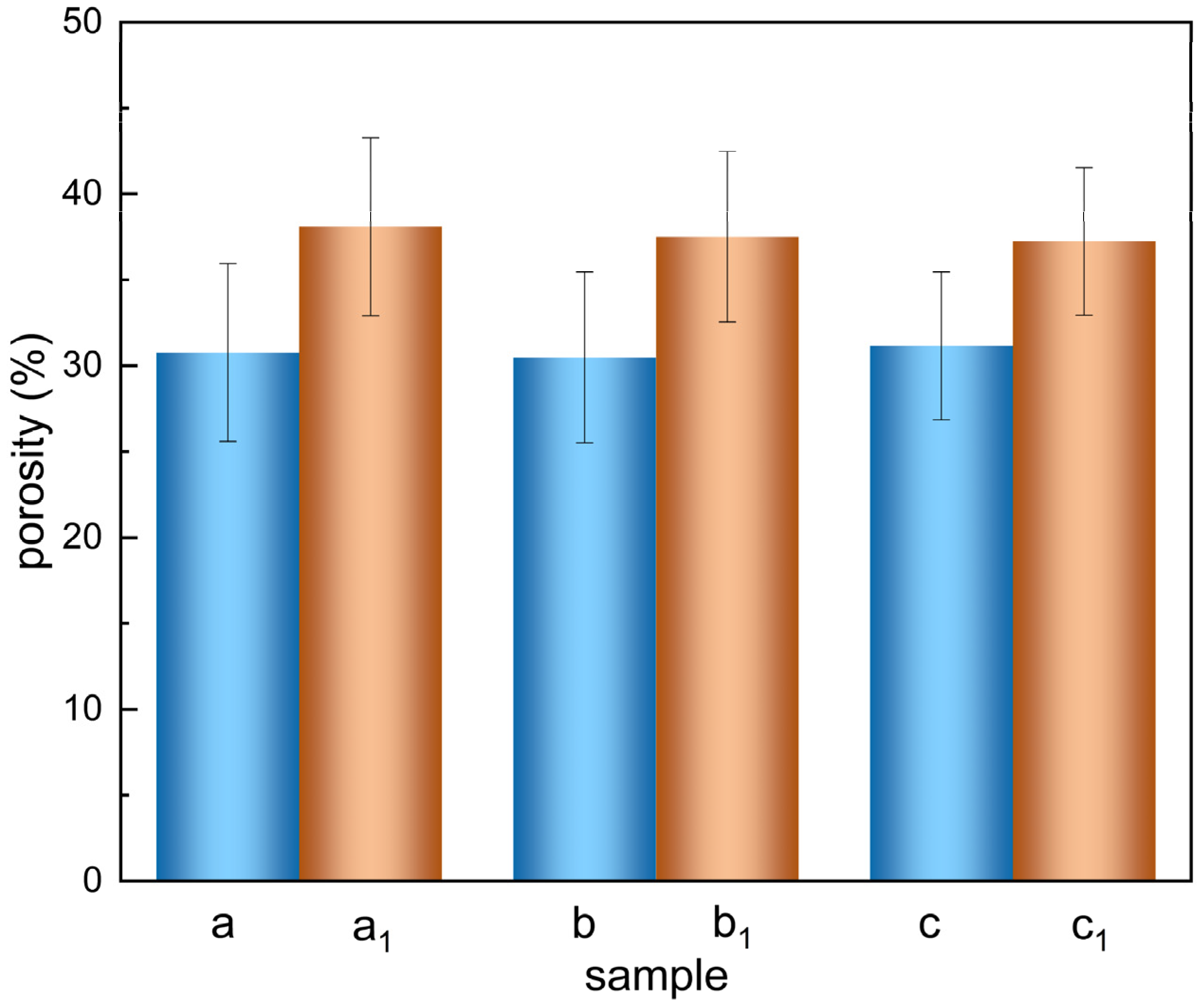
2.3.3. Porosity Determination
2.3.4. Water Contact Angle of the Material
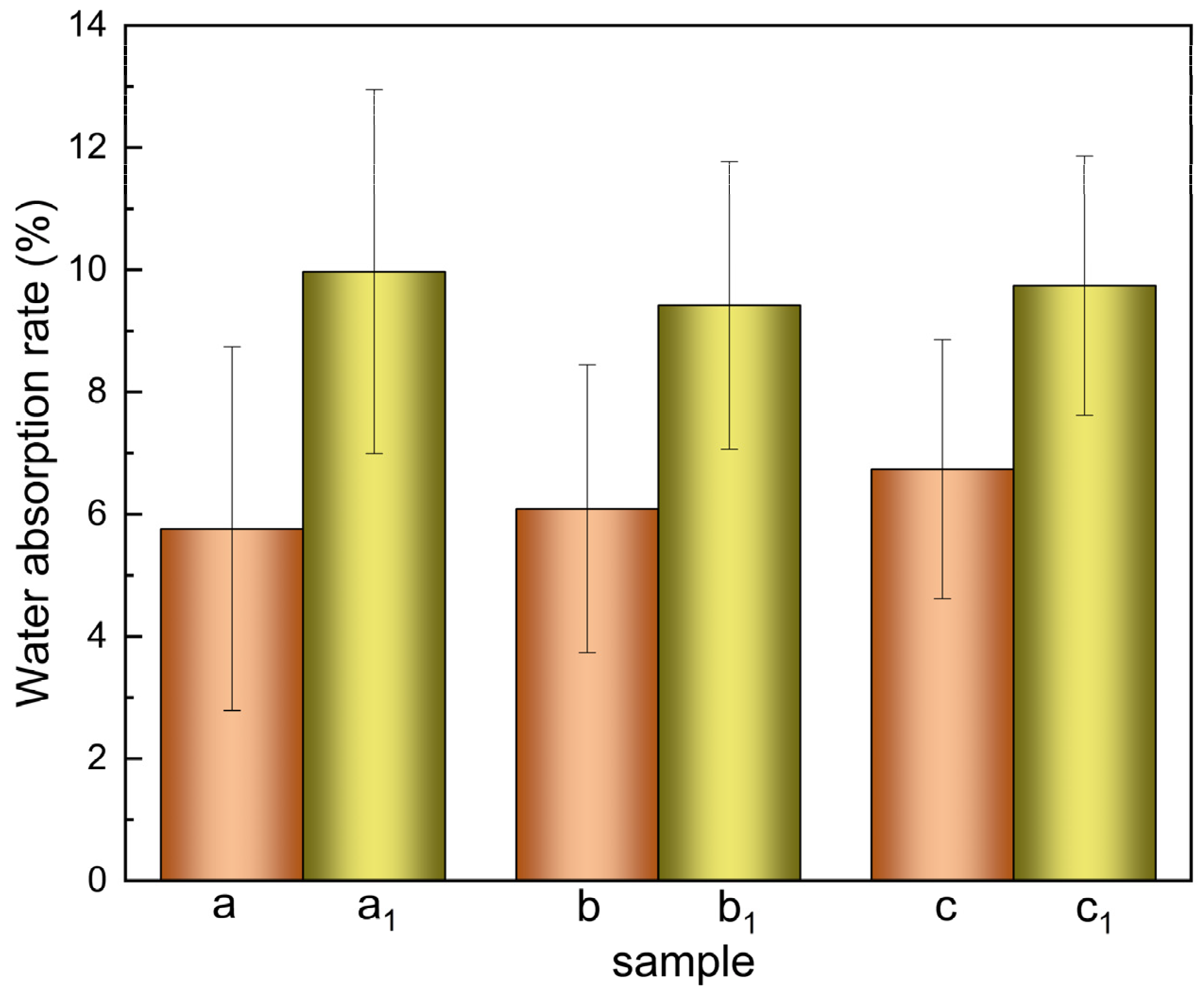
2.3.5. Determination of Water Absorption Rate
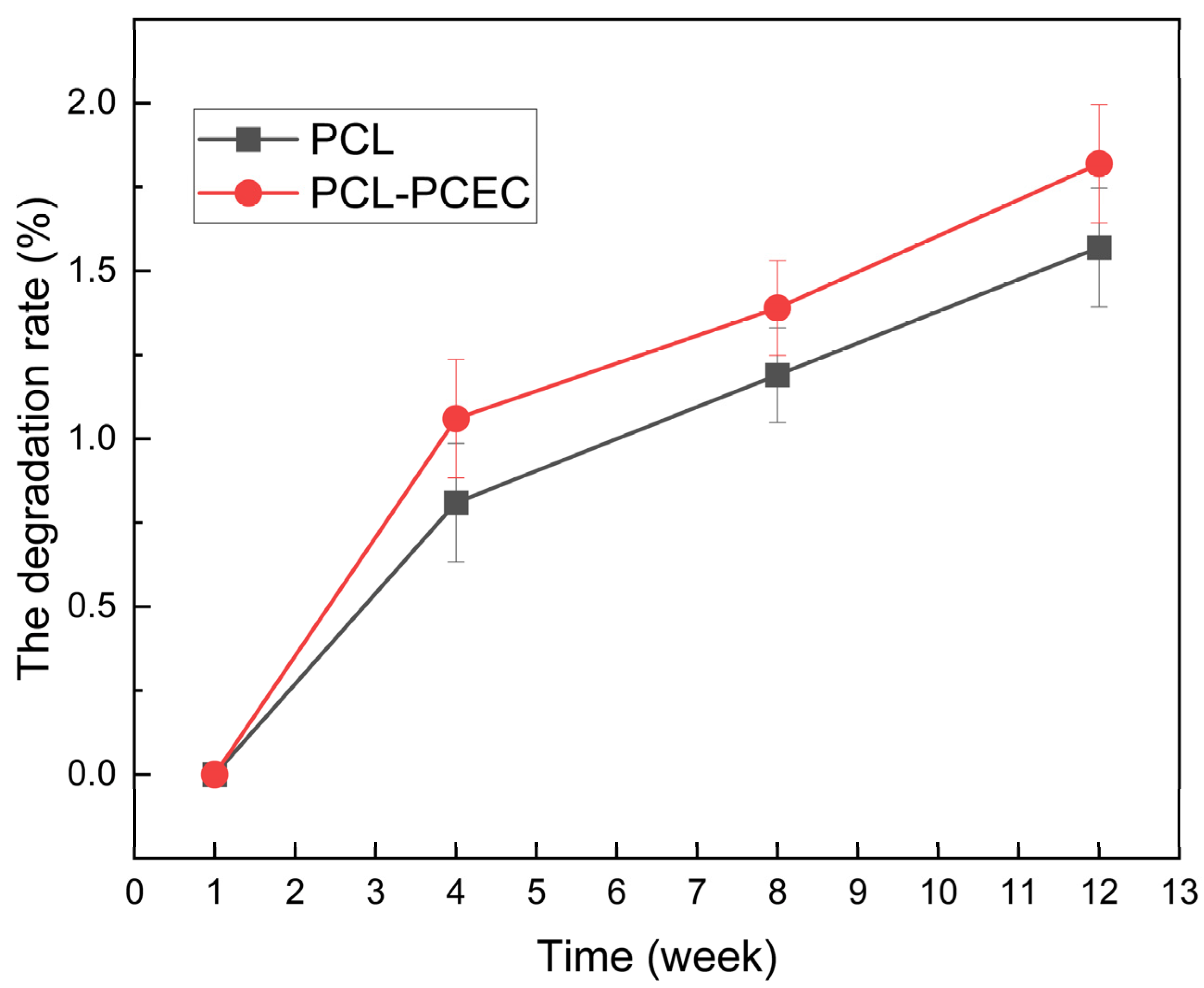
2.3.6. Determination of Degradation Rate
2.3.7. Cell 3D Inoculation and Culture Process
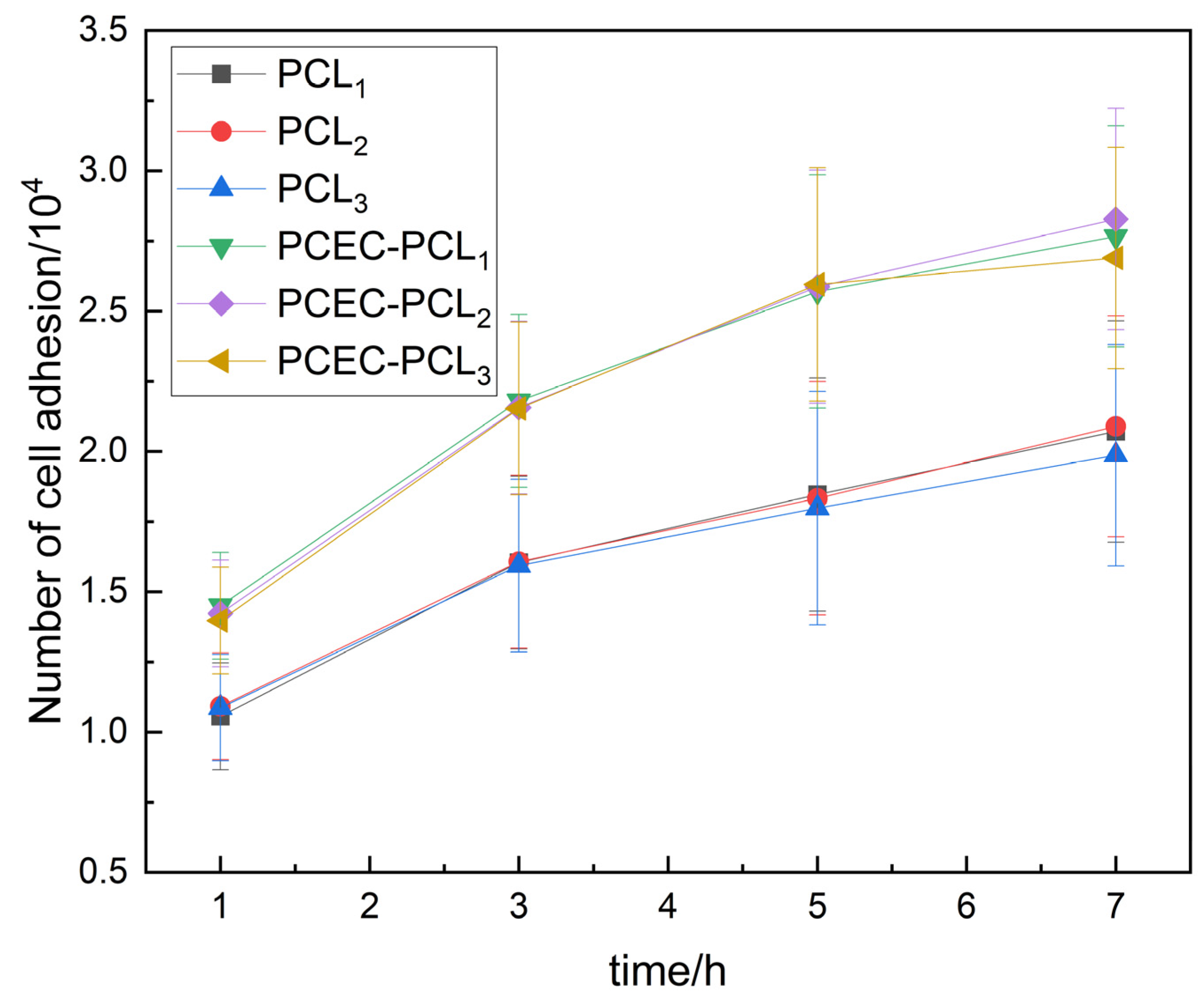
2.3.8. Live Cell Count
2.3.9. Cytotoxicity Test of Materials
Direct Contact Method
MTT (3-(4,5)-Dimethylthiahiazo (-z-y1)-3,5-Di-Phenytetrazoliumromide) Cytotoxicity Test
3. Results
3.1. Physical and Chemical Properties of Scaffold
3.1.1. XRD Characterization of Cell Culture Scaffolds
3.1.2. SEM of Scaffold Material
3.1.3. The Porosity of the Scaffolds
3.1.4. Hydrophilicity of the Scaffolds
3.1.5. Water Absorption Rate of the Scaffolds
3.1.6. Determination of Scaffold Degradation Rate
3.2. Cell Culture Results
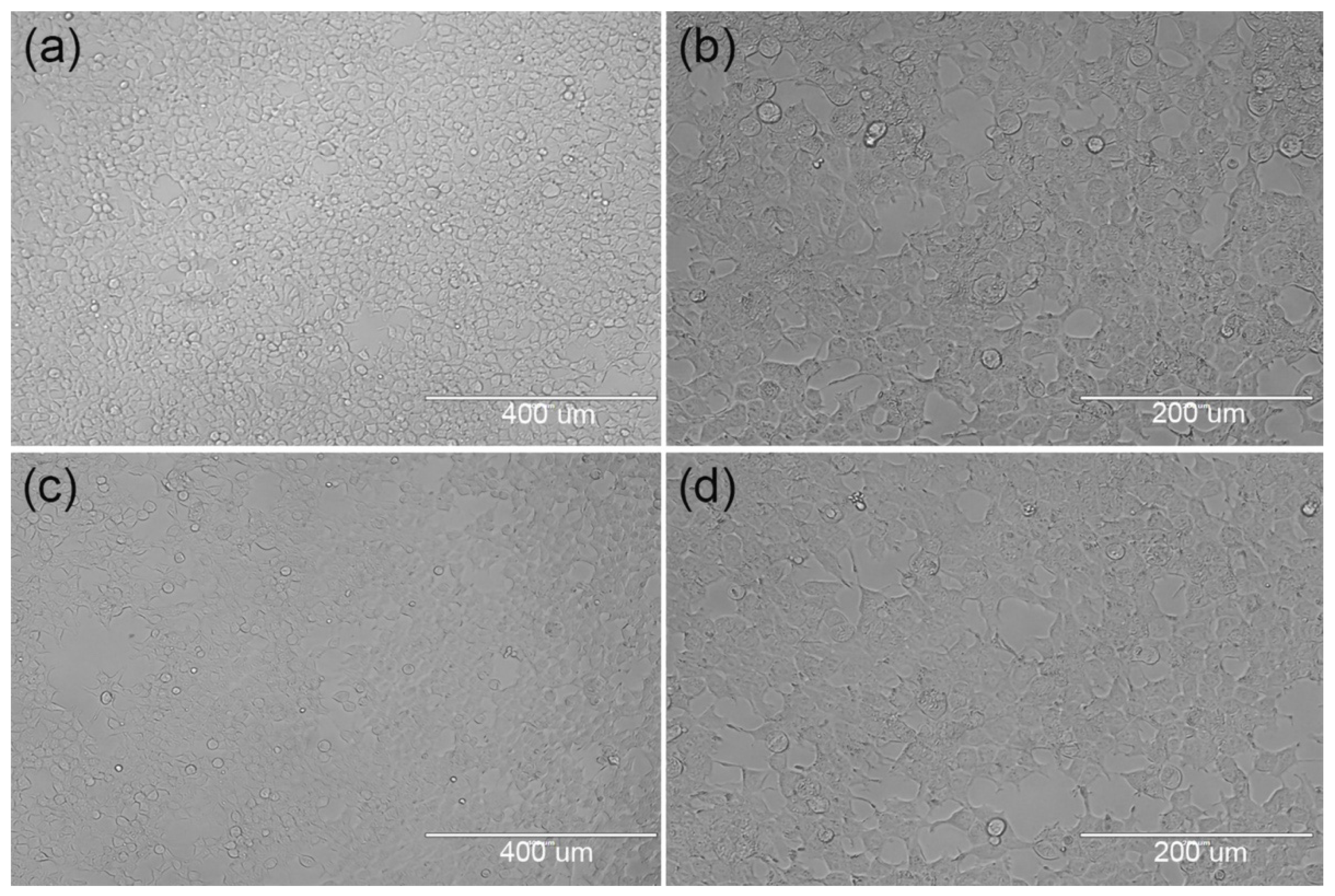
3.2.1. Initial Stage of Cell Inoculation
3.2.2. Cytotoxicity Test Results
Direct Contact Test Results
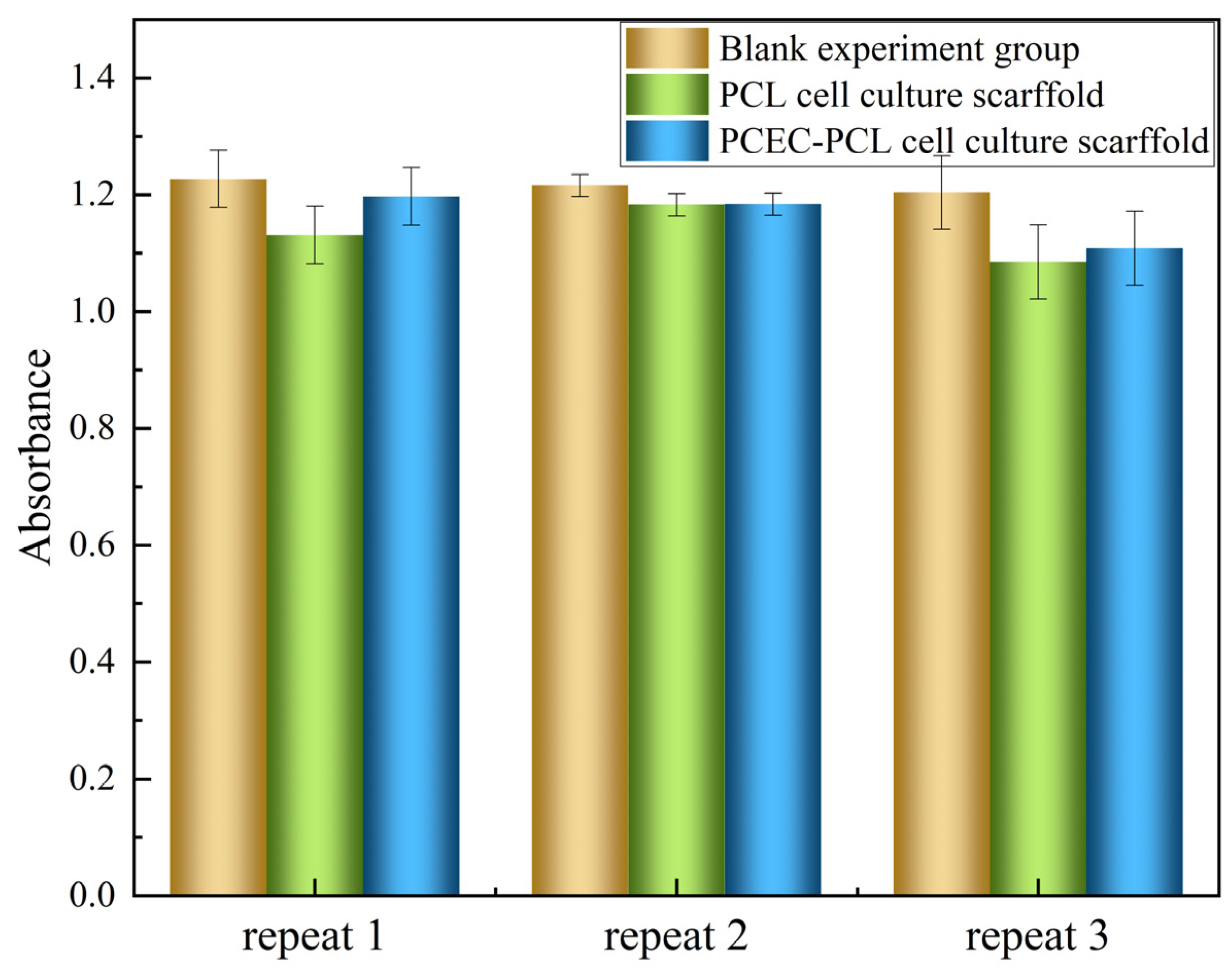
MTT Test Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mast, T.C.; Kierstead, L.; Gupta, S.B.; Nikas, A.A.; Kallas, E.G.; Novitsky, V.; Mbewe, B.; Pitisuttithum, P.; Schechter, M.; Vardas, E.; et al. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: Correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine 2010, 28, 950–957. [Google Scholar] [CrossRef]
- Wu, S.; Huang, J.; Zhang, Z.; Wu, J.; Zhang, J.; Hu, H.; Zhu, T.; Zhang, J.; Luo, L.; Fan, P.; et al. Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: Preliminary report of an open-label and randomised phase 1 clinical trial. Lancet Infect. Dis. 2021, 21, 1654–1664. [Google Scholar] [CrossRef]
- Zhu, F.-C.; Li, Y.-H.; Guan, X.-H.; Hou, L.-H.; Wang, W.-J.; Li, J.-X.; Wu, S.-P.; Wang, B.-S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- Tan, L.; Schirmer, K. Cell culture-based biosensing techniques for detecting toxicity in water. Curr. Opin. Biotechnol. 2017, 45, 59–68. [Google Scholar] [CrossRef]
- Ruiz-Alonso, S.; Lafuente-Merchan, M.; Ciriza, J.; Saenz-Del-Burgo, L.; Pedraz, J.L. Tendon tissue engineering: Cells, growth factors, scaffolds and production techniques. J. Control. Release 2021, 333, 448–486. [Google Scholar] [CrossRef]
- Xu, M.; Qin, M.; Cheng, Y.; Niu, X.; Kong, J.; Zhang, X.; Huang, D.; Wang, H. Alginate microgels as delivery vehicles for cell-based therapies in tissue engineering and regenerative medicine. Carbohydr. Polym. 2021, 266, 118128–118139. [Google Scholar] [CrossRef] [PubMed]
- Allafchian, A.; Jalali, S.A.H.; Mousavi, S.E.; Hosseini, S.S. Preparation of cell culture scaffolds using polycaprolactone/quince seed mucilage. Int. J. Biol. Macromol. 2020, 155, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Ham, J.; Lee, H.; Lee, K.; Koh, W.G. Integration of a fiber-based cell culture and biosensing system for monitoring of multiple protein markers secreted from stem cells. Biosens. Bioelectron. 2021, 193, 113531–113539. [Google Scholar] [CrossRef] [PubMed]
- Kou, F.; Zhu, B.; Zhou, W.; Lv, C.; Cheng, Y.; Wei, H. Targeted metabolomics in the cell culture media reveals increased uptake of branched amino acids by breast cancer cells. Anal. Biochem. 2021, 624, 114192. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Trujillo, S.; Rodríguez-Fariñas, N.; Jiménez-Moreno, M.; Rodríguez Martín-Doimeadios, R.D.C. Speciation of platinum nanoparticles in different cell culture media by HPLC-ICP-TQ-MS and complementary techniques: A contribution to toxicological assays. Anal. Chim. Acta 2021, 1182, 338935–338942. [Google Scholar] [CrossRef] [PubMed]
- Tohyama, S.; Fujita, J.; Fujita, C.; Yamaguchi, M.; Kanaami, S.; Ohno, R.; Sakamoto, K.; Kodama, M.; Kurokawa, J.; Kanazawa, H.; et al. Efficient Large-Scale 2D Culture System for Human Induced Pluripotent Stem Cells and Differentiated Cardiomyocytes. Stem Cell Rep. 2017, 9, 1406–1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Sun, Q.; Wang, Q.; Hu, C.; Chen, X.; Li, H.; Czajkowsky, D.M.; Shao, Z. Epithelial Cells in 2D and 3D Cultures Exhibit Large Differences in Higher-order Genomic Interactions. Genom. Proteom. Bioinform. 2021. [Google Scholar] [CrossRef] [PubMed]
- Collett, S.; Torresi, J.; Silveira, L.E.; Truong, V.K.; Christiansen, D.; Tran, B.M.; Vincan, E.; Ramsland, P.A.; Elbourne, A. Investigating virus-host cell interactions: Comparative binding forces between hepatitis C virus-like particles and host cell receptors in 2D and 3D cell culture models. J. Colloid Interface Sci. 2021, 592, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, Z.; Safari, F. Evaluating the in vitro therapeutic effects of human amniotic mesenchymal stromal cells on MiaPaca2 pancreatic cancer cells using 2D and 3D cell culture model. Tissue Cell 2021, 68, 101479–101485. [Google Scholar] [CrossRef]
- Duval, K.; Grover, H.; Han, L.H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Costa, E.C.; Moreira, A.F.; de Melo-Diogo, D.; Gaspar, V.M.; Carvalho, M.P.; Correia, I.J. 3D tumor spheroids: An overview on the tools and techniques used for their analysis. Biotechnol. Adv. 2016, 34, 1427–1441. [Google Scholar] [CrossRef]
- Matsusaki, M.; Case, C.P.; Akashi, M. Three-dimensional cell culture technique and pathophysiology. Adv. Drug Deliv. Rev. 2014, 74, 95–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, A.S.; Costa, E.C.; Nunes, A.S.; de Melo-Diogo, D.; Correia, I.J. Comparative study of the therapeutic effect of Doxorubicin and Resveratrol combination on 2D and 3D (spheroids) cell culture models. Int. J. Pharm. 2018, 551, 76–83. [Google Scholar] [CrossRef]
- Fontoura, J.C.; Viezzer, C.; Dos Santos, F.G.; Ligabue, R.A.; Weinlich, R.; Puga, R.D.; Antonow, D.; Severino, P.; Bonorino, C. Comparison of 2D and 3D cell culture models for cell growth, gene expression and drug resistance. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110264. [Google Scholar] [CrossRef]
- Nitta, S.; Hisasue, M.; Horiguchi, Y.; Yamada, Y.; Kikuchi, K.; Kubo, T.; Igarashi, H.; Neo, S. Three-dimensional spheroid culture of canine hepatocyte-like cells derived from bone marrow mesenchymal stem cells. Regen. Ther. 2020, 15, 210–215. [Google Scholar] [CrossRef]
- Periasamy, V.S.; Athinarayanan, J.; Alshatwi, A.A. Bio-inspired plant leaf skeleton based three dimensional scaffold for three dimensional cell culture. Sustain. Chem. Pharm. 2020, 18, 100321–100327. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, X.; Liu, H.; Yang, L.; Yuan, X.; Chen, Y.; Wu, W.; Luo, J.; Long, J.; Huang, M.; et al. 3D printed porous microgel for lung cancer cells culture in vitro. Mater. Des. 2021, 210, 11079–11088. [Google Scholar] [CrossRef]
- Liu, K.; Wiendels, M.; Yuan, H.; Ruan, C.; Kouwer, P.H.J. Cell-matrix reciprocity in 3D culture models with nonlinear elasticity. Bioact. Mater. 2021, 9, 316–331. [Google Scholar] [CrossRef]
- Diekjurgen, D.; Grainger, D.W. Polysaccharide matrices used in 3D in vitro cell culture systems. Biomaterials 2017, 141, 96–115. [Google Scholar] [CrossRef]
- Murphy, A.R.; Laslett, A.; O’Brien, C.M.; Cameron, N.R. Scaffolds for 3D in vitro culture of neural lineage cells. Acta Biomater. 2017, 54, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina-Santiago, C.; de Vicente, A.; Romero, D. Bacterial extracellular matrix as a natural source of biotechnologically multivalent materials. Comput. Struct. Biotechnol. J. 2021, 19, 2796–2805. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Mei, D.; Zhang, J.; Chen, D.; Li, J.; Wang, L.; Zhou, Y.; Zhu, S.; Guan, S. Improved corrosion resistance and cytocompatibility of Mg–Zn–Y–Nd alloy by the electrografted polycaprolactone coating. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127471–127478. [Google Scholar] [CrossRef]
- Sarkhosh-Inanlou, R.; Shafiei-Irannejad, V.; Azizi, S.; Jouyban, A.; Ezzati-Nazhad Dolatabadi, J.; Mobed, A.; Adel, B.; Soleymani, J.; Hamblin, M.R. Applications of scaffold-based advanced materials in biomedical sensing. TrAC Trends Anal. Chem. 2021, 143, 116342–116360. [Google Scholar] [CrossRef]
- Pierantozzi, D.; Scalzone, A.; Jindal, S.; Stīpniece, L.; Šalma-Ancāne, K.; Dalgarno, K.; Gentile, P.; Mancuso, E. 3D printed Sr-containing composite scaffolds: Effect of structural design and material formulation towards new strategies for bone tissue engineering. Compos. Sci. Technol. 2020, 191, 108069–108078. [Google Scholar] [CrossRef]
- Dong, Q.; Zhang, M.; Zhou, X.; Shao, Y.; Li, J.; Wang, L.; Chu, C.; Xue, F.; Yao, Q.; Bai, J. 3D-printed Mg-incorporated PCL-based scaffolds: A promising approach for bone healing. Mater. Sci. Eng. C 2021, 129, 112372–112388. [Google Scholar] [CrossRef]
- Cools, P.; Declercq, H.; Ghobeira, R.; Morent, R.; De Geyter, N. Acrylic acid plasma coatings for enhanced cell migration in PCL 3D additive manufactured scaffolds. Surf. Coat. Technol. 2018, 350, 925–935. [Google Scholar] [CrossRef]
- Kotcharat, P.; Chuysinuan, P.; Thanyacharoen, T.; Techasakul, S.; Ummartyotin, S. Development of bacterial cellulose and polycaprolactone (PCL) based composite for medical material. Sustain. Chem. Pharm. 2021, 20, 100404–100410. [Google Scholar] [CrossRef]
- Pandiyaraj, K.N.; Ghobeira, R.; Esbah Tabaei, P.S.; Cools, P.; De Geyter, N.; Morent, R.; Deshmukh, R.R. Non-thermal plasma jet-assisted development of phosphorus-containing functional coatings on 3D-printed PCL scaffolds intended for bone tissue engineering. J. Phys. Chem. Solids 2021, 154, 110025–110036. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Portoles-Gil, N.; Lopez-Periago, A.M.; Domingo, C.; Hosta-Rigau, L. Multi-layered polydopamine coatings for the immobilization of growth factors onto highly-interconnected and bimodal PCL/HA-based scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 117, 111245–111259. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Osgouei, M.; Li, Y.; Vahid, A.; Ataee, A.; Wen, C. High strength porous PLA gyroid scaffolds manufactured via fused deposition modeling for tissue-engineering applications. Smart Mater. Med. 2021, 2, 15–25. [Google Scholar] [CrossRef]
- Kovalcik, A.; Sangroniz, L.; Kalina, M.; Skopalova, K.; Humpolicek, P.; Omastova, M.; Mundigler, N.; Muller, A.J. Properties of scaffolds prepared by fused deposition modeling of poly(hydroxyalkanoates). Int. J. Biol. Macromol. 2020, 161, 364–376. [Google Scholar] [CrossRef]
- Dai, M.; Xu, X.; Song, J.; Fu, S.; Gou, M.; Luo, F.; Qian, Z. Preparation of camptothecin-loaded PCEC microspheres for the treatment of colorectal peritoneal carcinomatosis and tumor growth in mice. Cancer Lett 2011, 312, 189–196. [Google Scholar] [CrossRef]
- Gou, M.; Dai, M.; Li, X.; Yang, L.; Huang, M.; Wang, Y.; Kan, B.; Lu, Y.; Wei, Y.; Qian, Z. Preparation of mannan modified anionic PCL-PEG-PCL nanoparticles at one-step for bFGF antigen delivery to improve humoral immunity. Colloids Surf. B Biointerfaces 2008, 64, 135–139. [Google Scholar] [CrossRef]
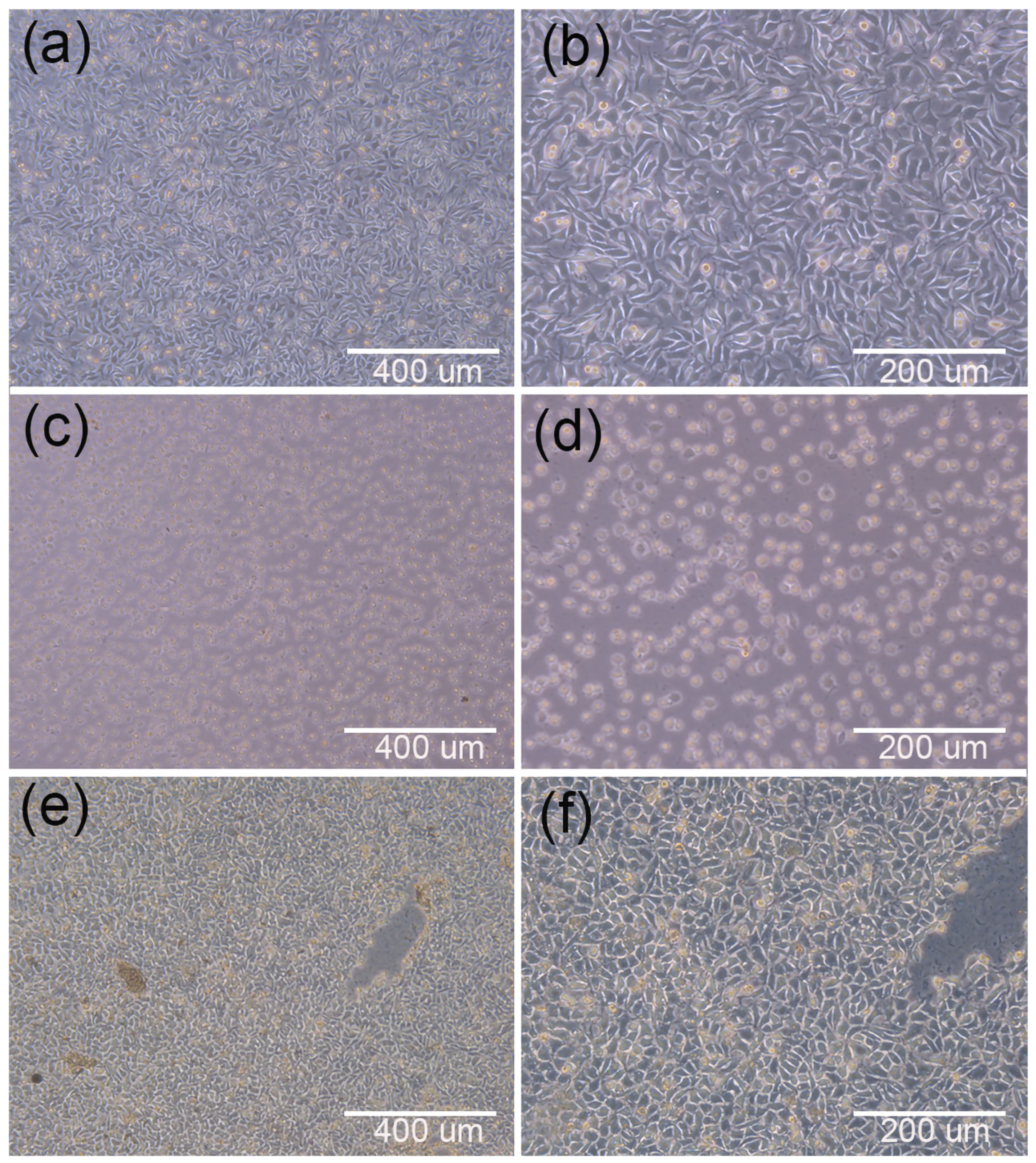
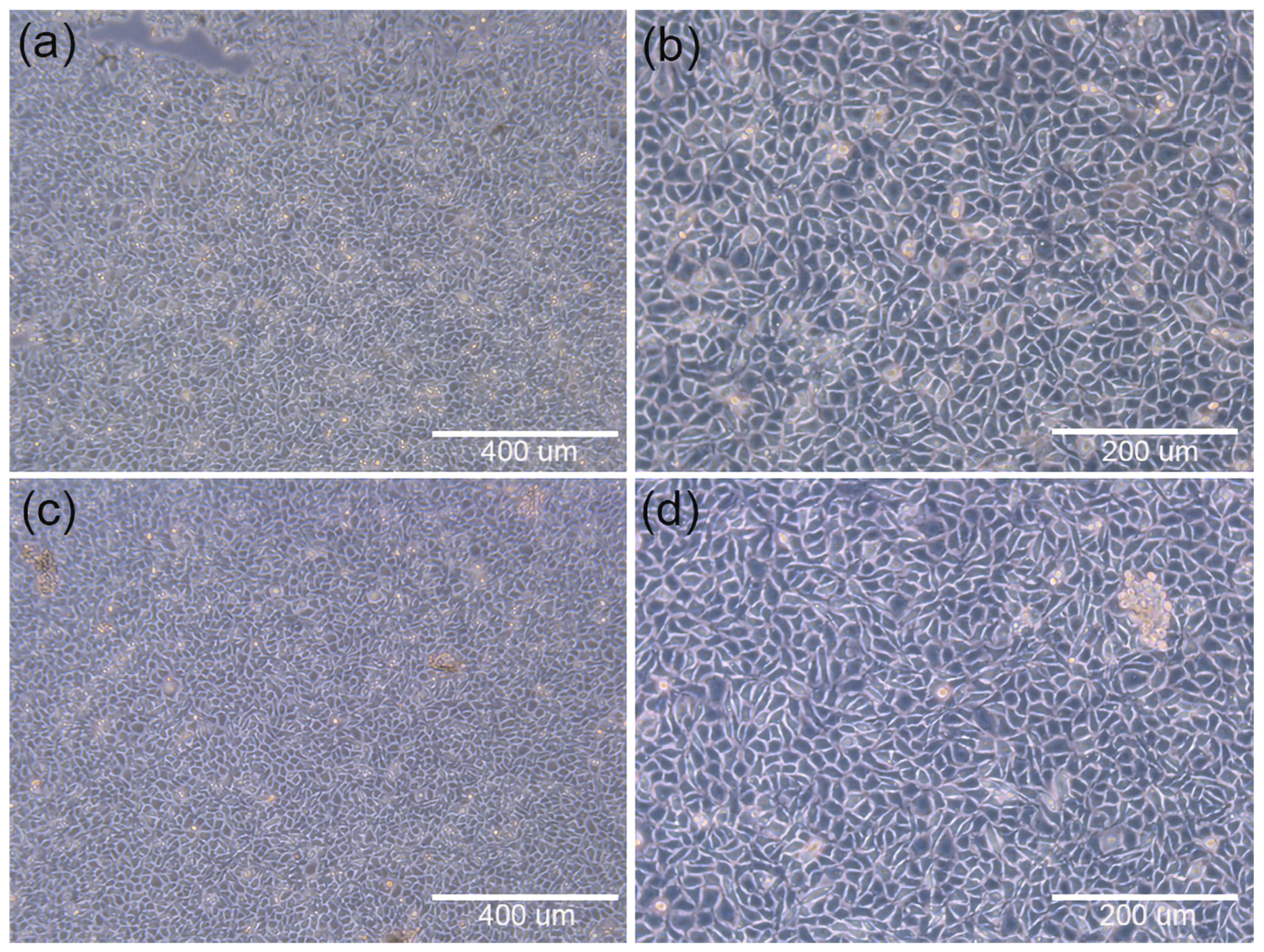
| Printing Parameter | Filling Rate (%) | Temperature (°C) | Speed (mm s−1) | Nozzle Diameter (mm−1) | Filling Spacing (μm) |
|---|---|---|---|---|---|
| / | 100 | 120 | 35.0 | 1.0 | 250–300 |
| Sample | Parallel Samples | Harvesting Cell Count | Proliferative Multiple | Results |
|---|---|---|---|---|
| PCL | 1 | 2.689 × 105 | 2.1 | Poor proliferation effect |
| 2 | 2.433 × 105 | 1.9 | ||
| 3 | 2.305 × 105 | 1.8 | ||
| PCEC-PCL | 1 | 6.855 × 105 | 5.3 | Good proliferation effect |
| 2 | 6.6 × 105 | 5.1 | ||
| 3 | 6.435 × 105 | 5.0 |
| Sample Number | Absorbance (OD = 570) | Relative Cell Survival (100%) | ||
|---|---|---|---|---|
| Repeat 1 | Repeat 2 | Repeat 3 | ||
| Blank experimental group | 1.227 | 1.216 | 1.204 | 100 |
| PCL cell culture scaffold | 1.131 | 1.183 | 1.085 | 93.1743 |
| PCEC–PCL cell culture scaffold | 1.197 | 1.184 | 1.108 | 90.5428 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Liu, X.; Chen, Z.; Jiang, L.; Yuan, M.; Yuan, M. The Application of Polycaprolactone Scaffolds with Poly(ε-caprolactone)–Poly(ethylene glycol)–Poly(ε-caprolactone) Loaded on Kidney Cell Culture. Materials 2022, 15, 1591. https://doi.org/10.3390/ma15041591
Sun J, Liu X, Chen Z, Jiang L, Yuan M, Yuan M. The Application of Polycaprolactone Scaffolds with Poly(ε-caprolactone)–Poly(ethylene glycol)–Poly(ε-caprolactone) Loaded on Kidney Cell Culture. Materials. 2022; 15(4):1591. https://doi.org/10.3390/ma15041591
Chicago/Turabian StyleSun, Junyu, Xinxin Liu, Zongrui Chen, Lin Jiang, Mingwei Yuan, and Minglong Yuan. 2022. "The Application of Polycaprolactone Scaffolds with Poly(ε-caprolactone)–Poly(ethylene glycol)–Poly(ε-caprolactone) Loaded on Kidney Cell Culture" Materials 15, no. 4: 1591. https://doi.org/10.3390/ma15041591
APA StyleSun, J., Liu, X., Chen, Z., Jiang, L., Yuan, M., & Yuan, M. (2022). The Application of Polycaprolactone Scaffolds with Poly(ε-caprolactone)–Poly(ethylene glycol)–Poly(ε-caprolactone) Loaded on Kidney Cell Culture. Materials, 15(4), 1591. https://doi.org/10.3390/ma15041591






