Effect of Expanded Graphite on the Reaction Sintering of Boron Carbide
Abstract
:1. Introduction
2. Materials and Methods
3. Results
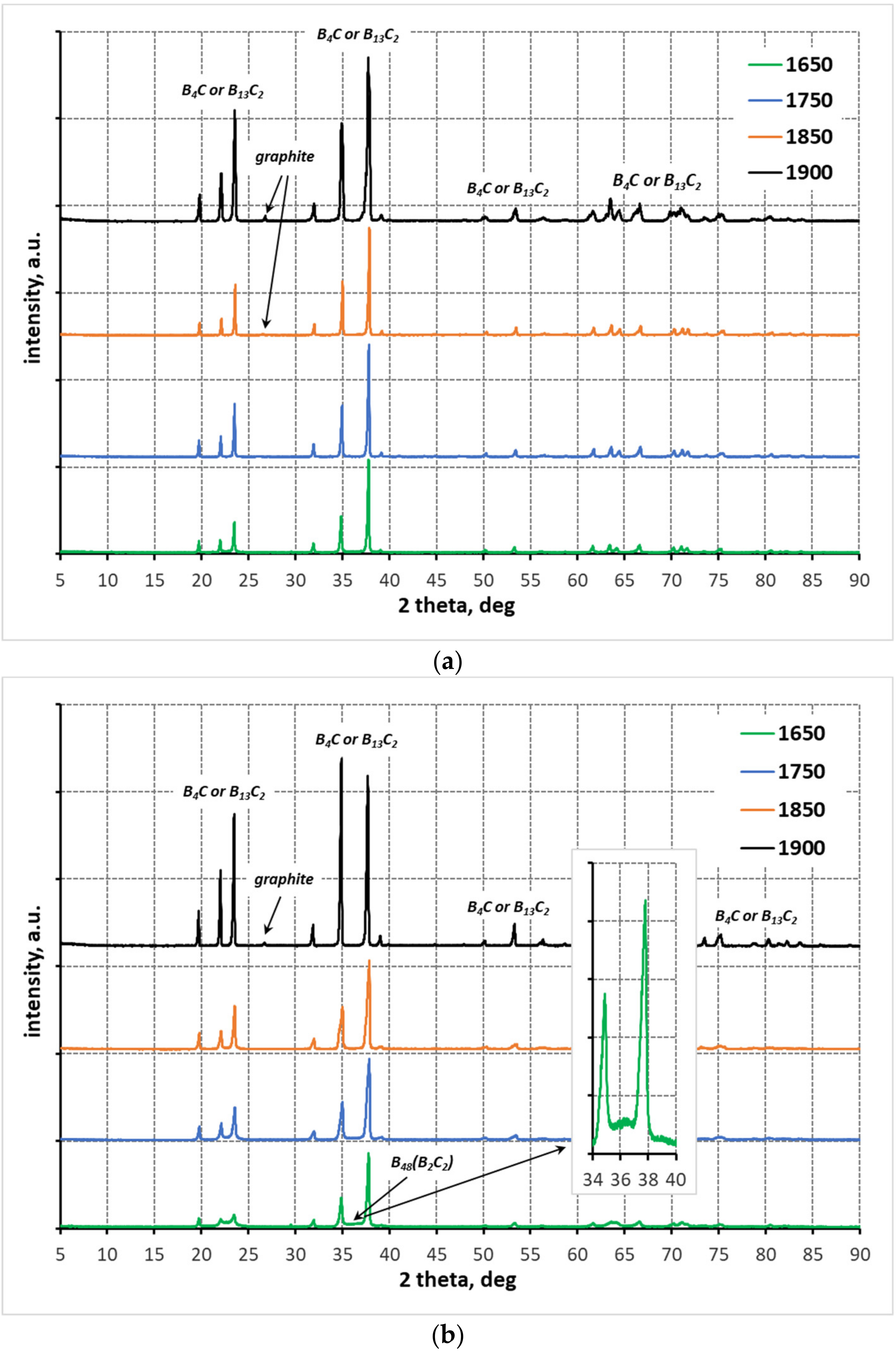
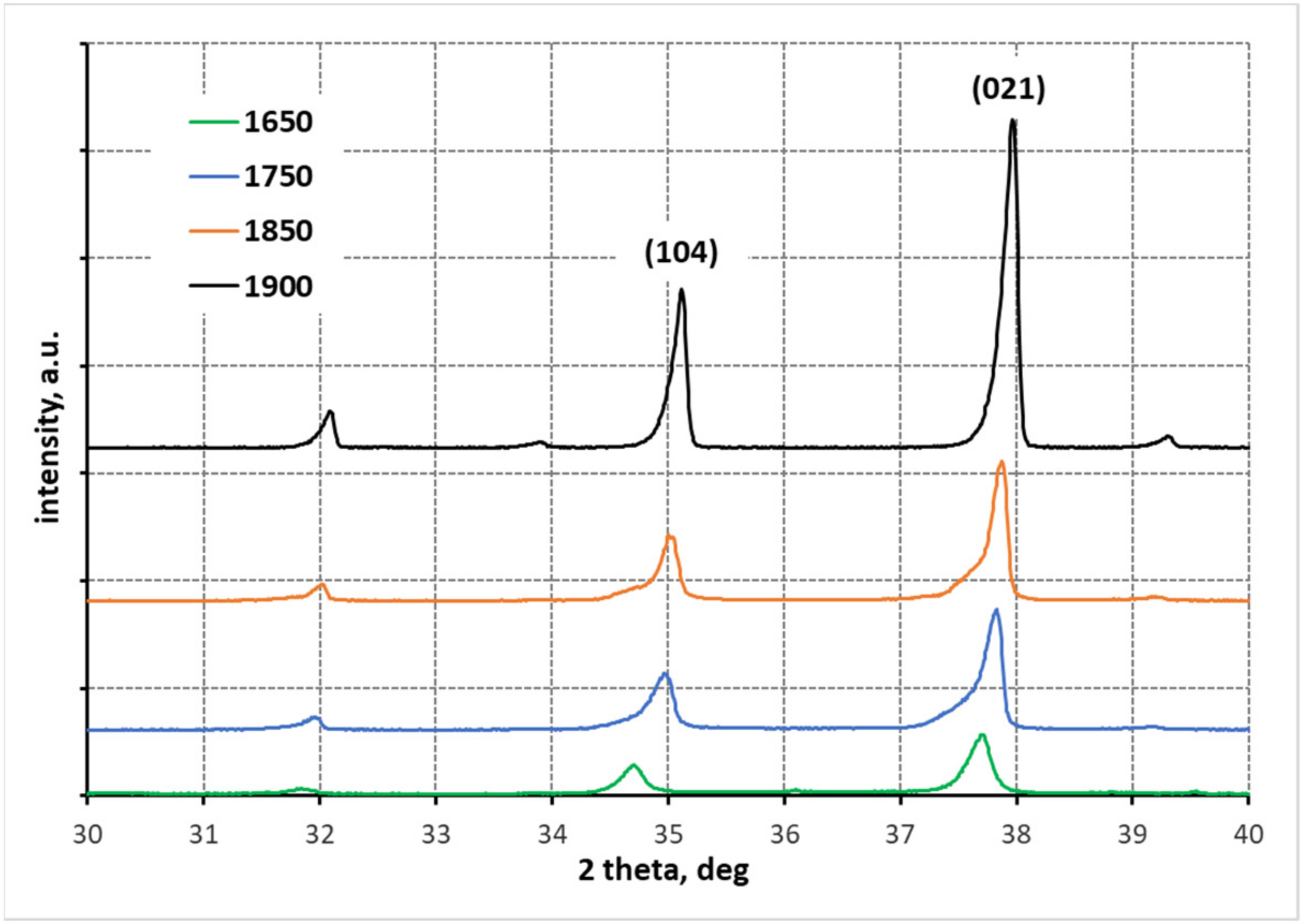
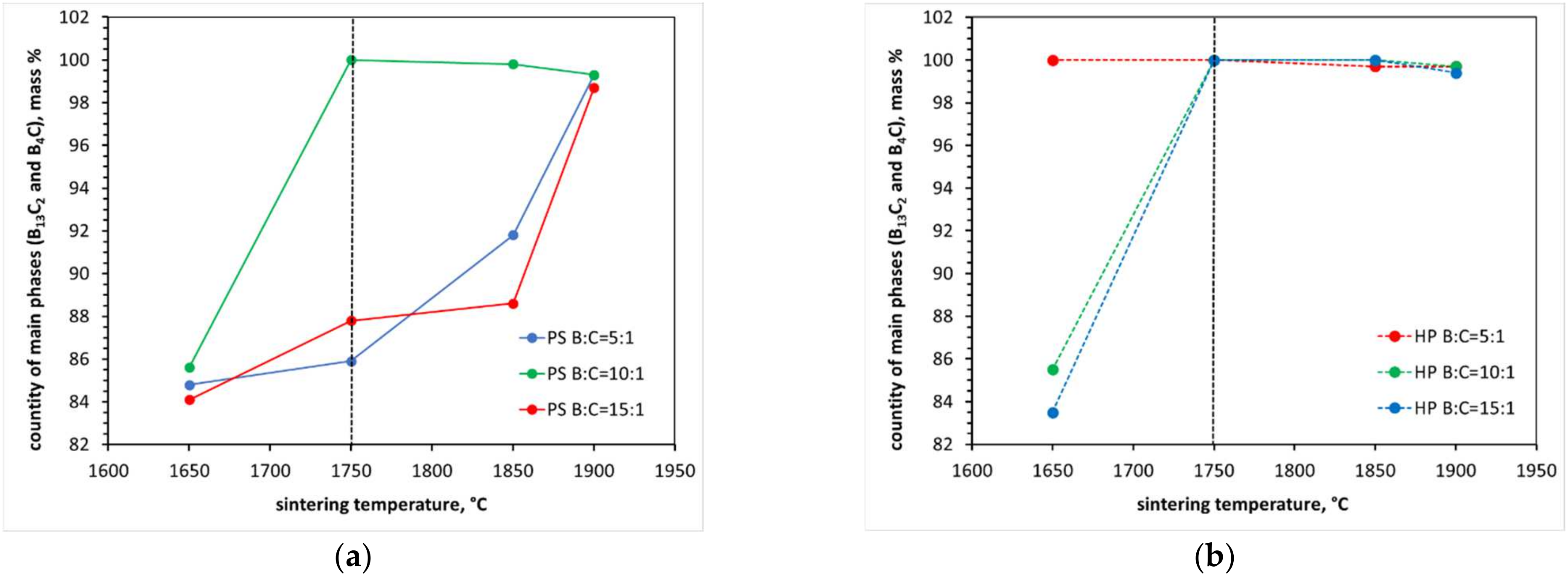
3.1. X-ray Diffraction Anallysis
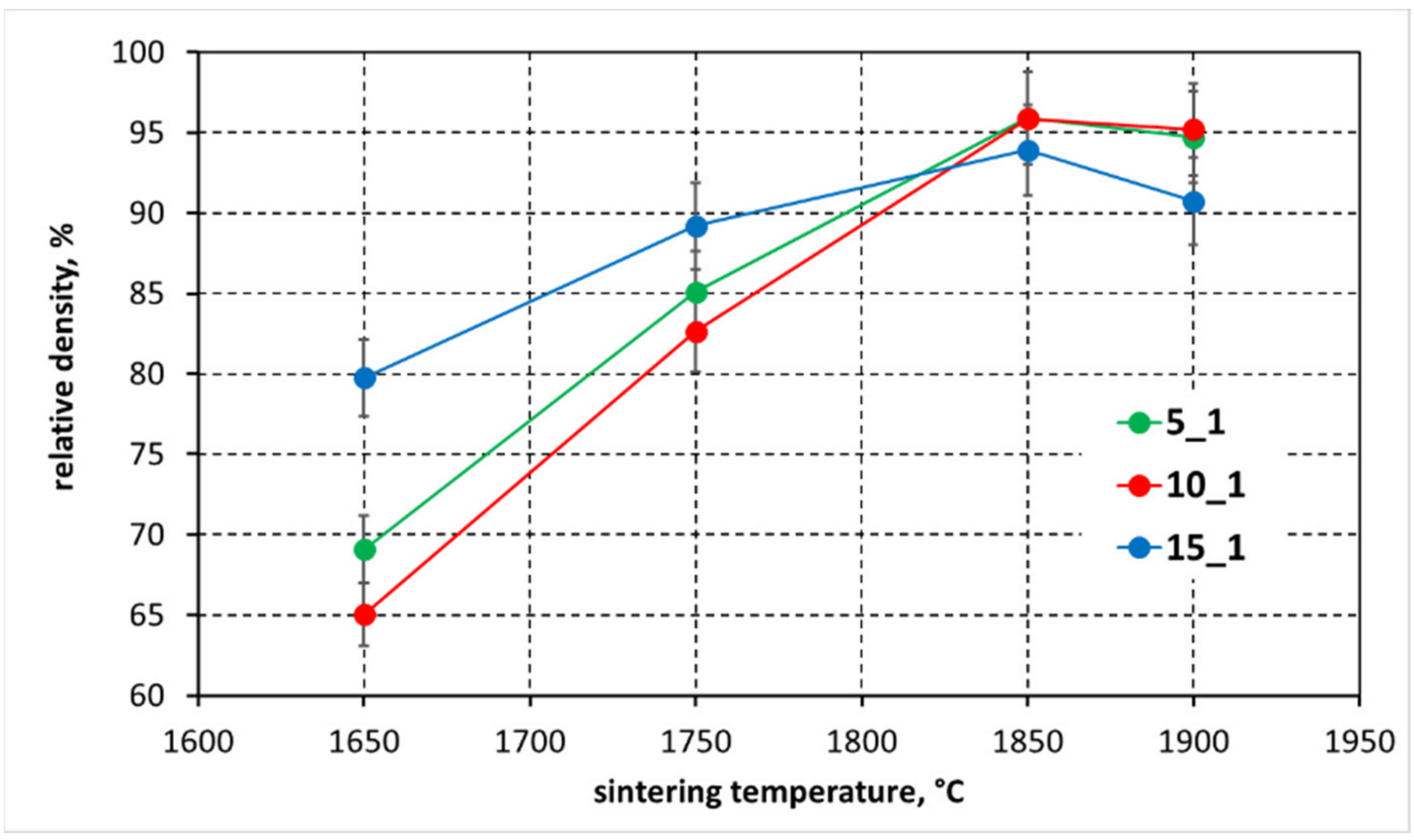
3.2. Density of Polycrystals
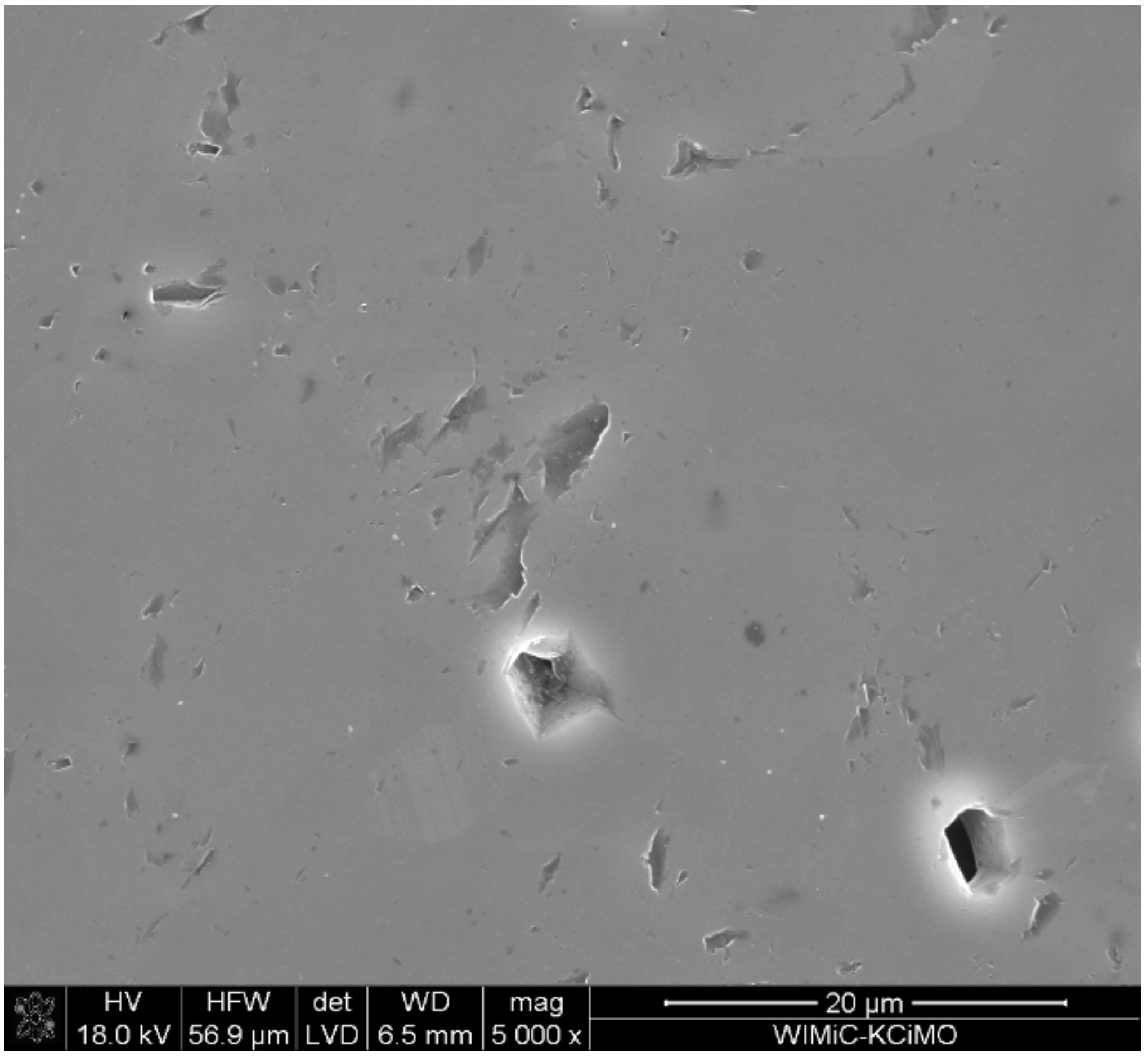
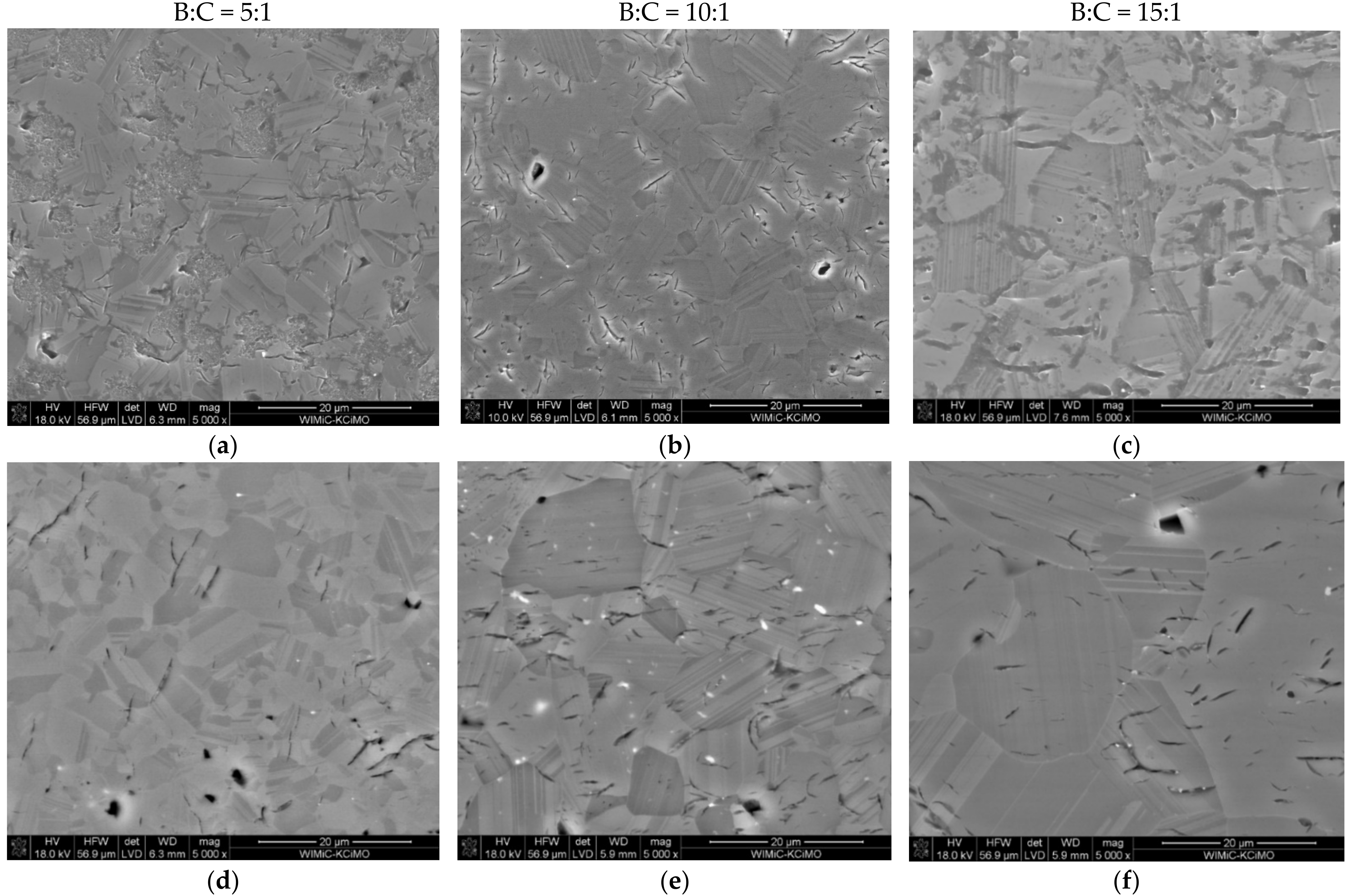
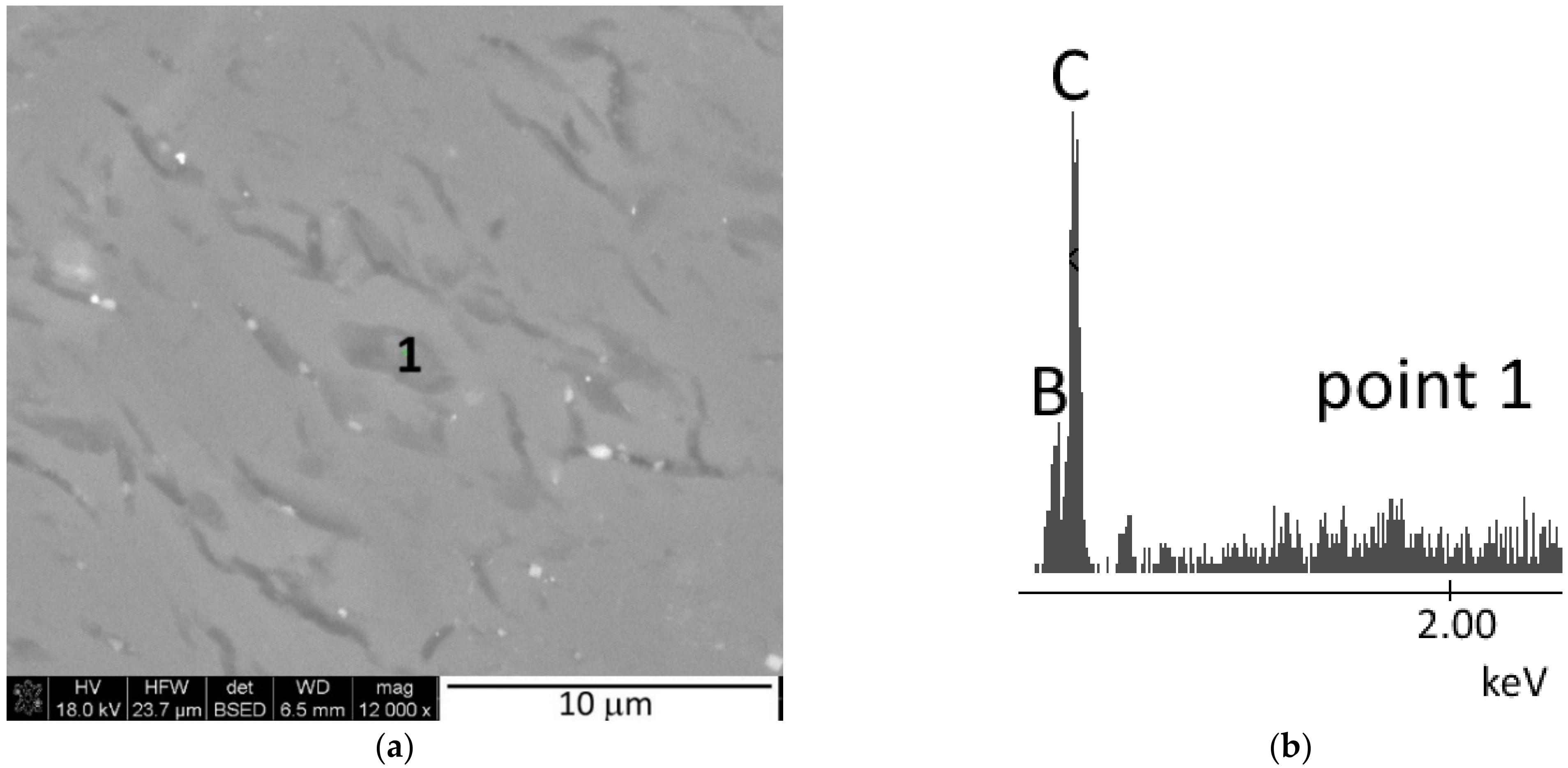
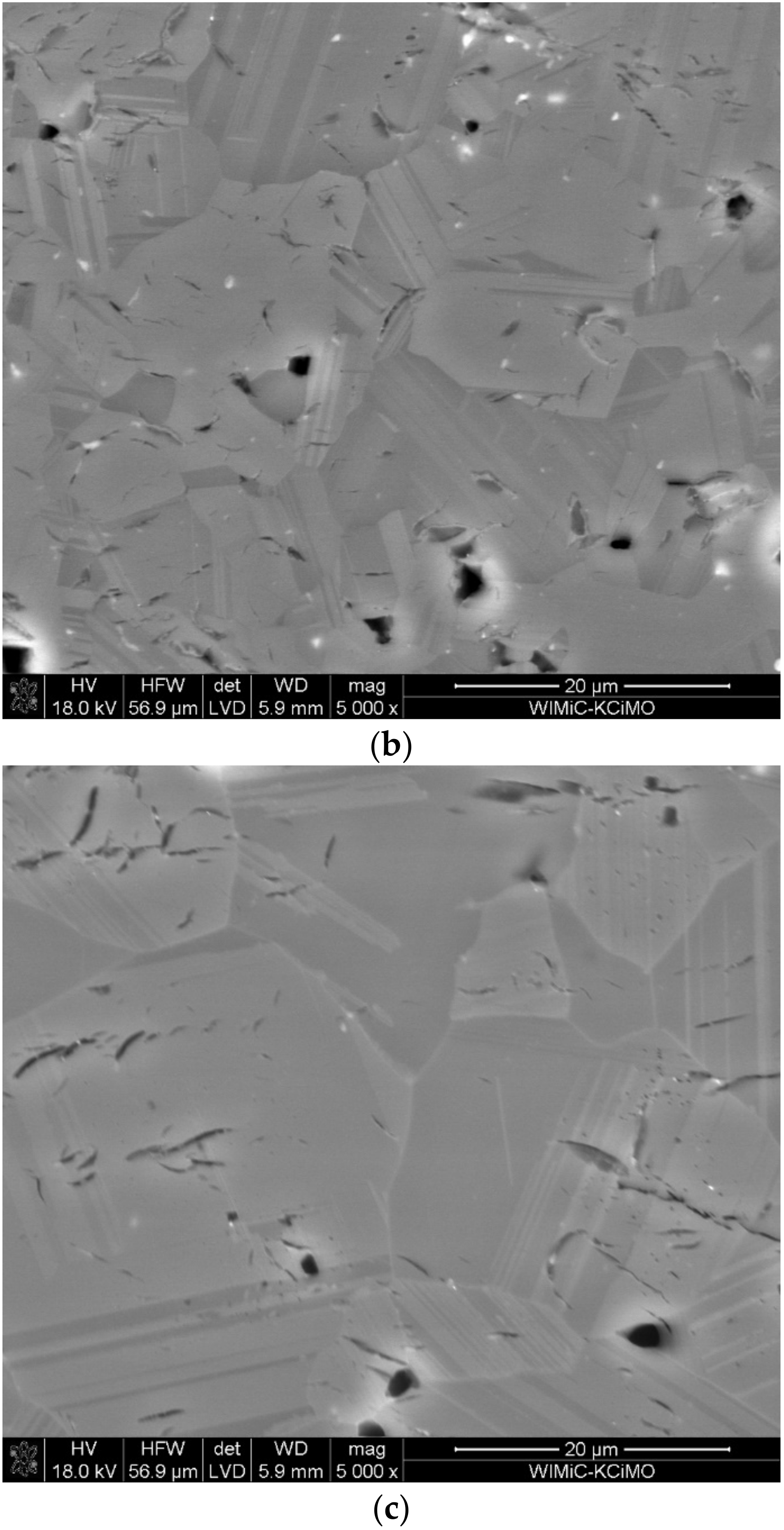
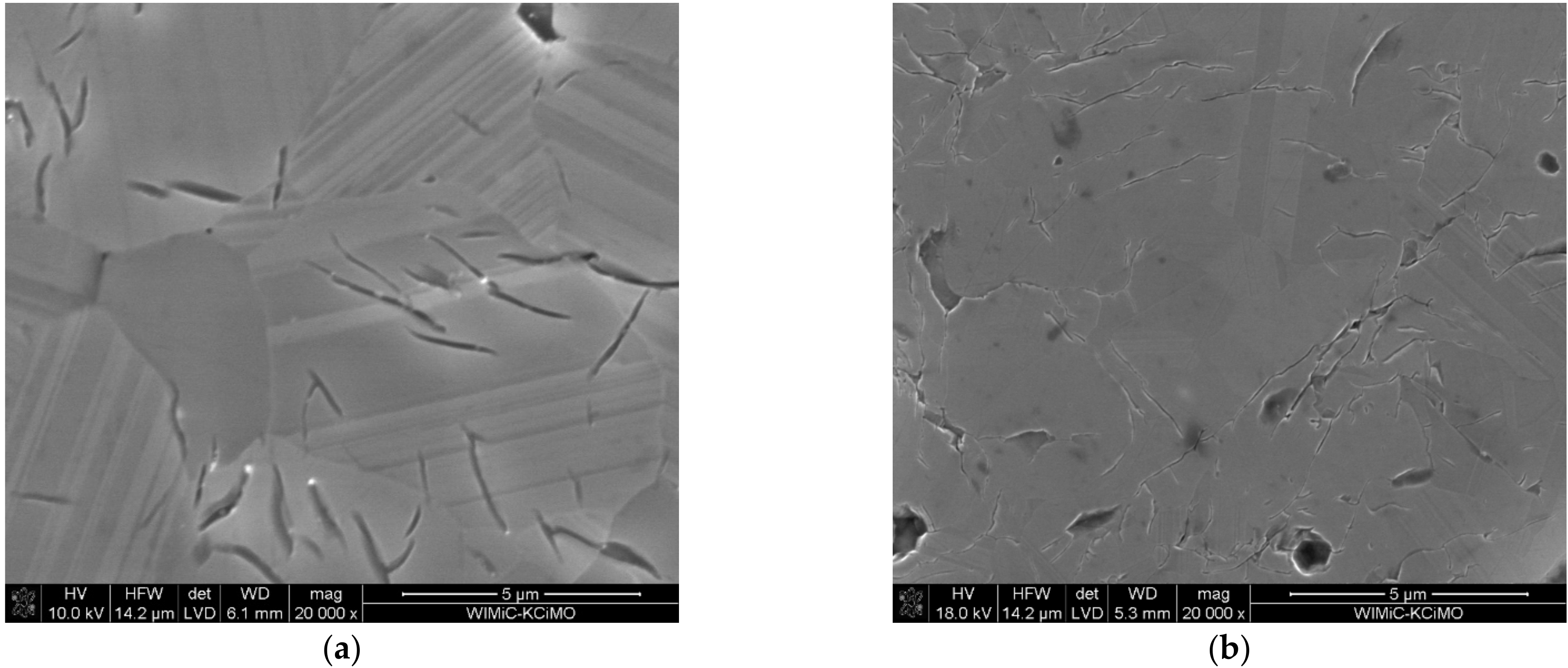
3.3. Microstructure Analysis
4. Discussion
5. Conclusions
- It has been shown that it is possible to combine elemental synthesis and sintering of boron carbide when expanded graphite, a porous and reactive form of carbon, is used as a carbon substrate;
- Dense polycrystals can be obtained from the mixtures of substrates with a significant excess of boron relative to carbon from B:C of 5:1 to B:C of 15:1;
- Reaction sintering was carried out on all mixtures in the temperature range of 1650–1900 °C. The best results considering polycrystalline density and synthesis reaction efficiency were obtained at 1850 °C. This is a significant reduction in sintering temperature, as compared to traditional techniques, of about 200–300 °C. The sinters obtained at this temperature, regardless of the starting composition, exhibit relative densities in the 90–96% TD range;
- At each of the applied reaction sintering temperatures, the obtained polycrystals consist only of boron carbides of different stoichiometry, dominated by boron carbides with stable structures (i.e., B13C2 and B4C). At the lowest reaction sintering temperature of 1650 °C, a boron-rich boron carbide with stoichiometry B50C2 can be identified. On the other hand, at the highest reaction sintering temperature, graphite precipitates appear in the polycrystals due to the maximum carbon saturation of the boron carbide structure;
- The micro-cracks, visible in the images of microstructures, are most likely formed due to the difficulty in the free discharge of the gaseous products of carbide synthesis. When the sample is in the graphite matrix, it is subjected to external pressure and begins to sinter. Separating the carbide synthesis from sintering reduces this effect but worsens the density of the materials produced.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pierson, O.H. Handbook of Refractory Carbides and Nitrides: Properties, Characteristics, Processing and Applications; Noyes Publications: New York, NY, USA, 1996. [Google Scholar]
- Suri, A.K.; Subramanian, C.; Sonber, J.K.; Murthy, T.S.R. Ch, Synthesis and consolidation of boron carbide: A review. Int. Mater. Rev. 2010, 55, 4–38. [Google Scholar] [CrossRef]
- Werheit, H. Boron carbide: Consistency of components, lattice parameters, fine structure and chemical composition makes the complex structure reasonable. Solid State Sci. 2016, 60, 45–54. [Google Scholar] [CrossRef]
- Schwetz, K.; Lipp, A. Boron carbide, boron nitride and metal borides. In Ullmann’s Encycl. Ind. Chem., 5th ed.; VCH: Weinheim, Germany, 1985; Volume A4, pp. 295–307. [Google Scholar]
- Thévenot, F. Boron carbide-A comprehensive review. J. Eur. Ceram. Soc. 1990, 6, 205–225. [Google Scholar] [CrossRef]
- Ekbom, L.; Amundin, C. Microstructural evaluations of sintered boron carbides with different compositions. Sci. Ceram. 1981, 11, 237–243. [Google Scholar]
- Bouchacourt, M.; Thevenot, F. Analytical investigations in the B-C system. J. Less. Common Met. 1981, 82, 219–226. [Google Scholar] [CrossRef]
- Bouchacourt, M.; Thevenot, F. The melting of boron carbide and the homogeneity range of the boron carbide phase. J. Less. Common Met. 1979, 67, 327–331. [Google Scholar] [CrossRef]
- Beauvy, M. Stoichiometric limits of carbon-rich boron carbide phases. J. Less. Common Met. 1983, 90, 169–175. [Google Scholar] [CrossRef]
- Mahagin, D.; Dahl, R. Nuclear applications of boron and the borides. In Boron and Refractory Borides; Matkovich, V., Ed.; Springer: Berlin, Germany, 1977; pp. 613–632. [Google Scholar]
- Birney, K.; Pitner, A.; Bourquin, R. Absorber materials for fast reactor control applications. Proc. Am. Nucl. Soc. Annu. Meet. 1977, 26, 173. [Google Scholar]
- Wilkins, M. Use of boron compounds in lightweight armor. In Boron and Refractory Borides; Matkovich, V., Ed.; Springer: Berlin, Germany, 1977; pp. 633–648. [Google Scholar]
- Kaufmann, C.; Cronin, D.; Worswick, M.; Pageau, G.; Beth, A. Influence of Material Properties on the Ballistic Performance of Ceramics for Personal Body Armour. Shock Vib. 2003, 10, 51–58. [Google Scholar] [CrossRef]
- Barth, R.; Soloway, A.; Fairchild, R. Boron neutron capture therapy of cancer. Cancer Res. 1990, 50, 1061–1070. [Google Scholar] [CrossRef]
- Hu, K.; Yang, Z.; Zhang, L.; Xie, L.; Wang, L.; Xu, H.; Josephson, L.; Liang, S.H.; Zhang, M.-R. Boron agents for neutron capture therapy. Coord. Chem. Rev. 2020, 405, 213139. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Iwagami, T. Boron Carbide Particle as a Boron Compound for Boron Neutron Capture Therapy. J. Nucl. Med. Radiat. Ther. 2014, 5, 177. [Google Scholar] [CrossRef]
- Mortensen, M.W.; Sørensen, P.G.; Björkdahl, O.; Jensen, M.R.; Gundersen, H.J.G.; Bjørnholm, T. Preparation and characterization of Boron carbide nanoparticles for use as a novel agent in T cell-guided boron neutron capture therapy. Appl. Radiat. Isot. 2006, 64, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Lezhava, D.; Darsavelidze, G.; Gabunia, D.; Tsagareishvili, O.; Antadze, M.; Gabunia, V. Influence of carbon content on physicomechanical characteristics of boron carbide. J. Solid State Chem. 2006, 179, 2934–2938. [Google Scholar] [CrossRef]
- Kuzenkova, M.; Kislyi, P.; Grabchuk, B.; Bodnaruk, N. The structure and properties of sintered boron carbide. J. Less. Common Met. 1979, 67, 217–223. [Google Scholar] [CrossRef]
- Henney, J.; Jones, J.W.S. Novel powder processing of sintered boron carbide. Key Eng. Mater. 2004, 264–268, 45–48. [Google Scholar]
- Suzuki, H.; Hase, T.; Maruyama, T. Effect of carbon on sintering of boron carbide. Yogyo Kyokai-Shi 1979, 87, 430–433. [Google Scholar] [CrossRef]
- Dole, L.; Prochazka, S.; Doremus, R. Microstructural coarsening during sintering of boron carbide. J. Am. Ceram. Soc. 1989, 72, 958–966. [Google Scholar] [CrossRef]
- Lee, H.; Speyer, R.F. Pressureless Sintering of Boron Carbide. J. Am. Ceram. Soc. 2003, 86, 1468–1473. [Google Scholar] [CrossRef]
- Schwetz, K.A.; Grellner, W. The influence of carbon on the microstructure and mechanical properties of sintered boron carbide. J. Less. Common Met. 1981, 82, 37–47. [Google Scholar] [CrossRef]
- Schwetz, K.A.; Sigl, L.S.; Pfau, L. Mechanical Properties of Injection Molded B4C–C Ceramics. J. Solid State Chem. 1997, 133, 68–76. [Google Scholar] [CrossRef]
- Singhal, S.; Singh, B. Sintering of boron carbide under high pressures and temperatures. Indian J. Eng. Mater. Sci. 2006, 13, 129–134. [Google Scholar]
- Cho, N.; Bao, Z.; Speyer, R.F. Density-and Hardness-optimized Pressureless Sintered and Post-hot Isostatic Pressed B4C. J. Mater. Res. 2005, 20, 2110–2116. [Google Scholar] [CrossRef]
- Katz, J.D.; Blake, R.D.; Petrovic, J.J.; Sheinberg, H. Microwave Sintering of Boron Carbide. MRS Proc. 1988, 124, 219. [Google Scholar] [CrossRef]
- Kuliiev, R.; Orlovskaya, N.; Hyer, H.; Sohn, Y.; Lugovy, M.; Ha, D.; Radovic, M.; Castle, E.G.; Reece, M.J.; Sasikumar, P.V.W.; et al. Spark Plasma Sintered B4C–Structural, Thermal, Electrical and Mechanical Properties. Materials 2020, 13, 1612. [Google Scholar] [CrossRef] [Green Version]
- Moskovskikh, D.O.; Paramonov, K.A.; Nepapushev, A.A.; Shkodich, N.F.; Mukasyan, A.S. Bulk boron carbide nanostructured ceramics by reactive spark plasma sintering. Ceram. Int. 2017, 43, 8190–8194. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Zhou, Z.; Wang, M.; Xie, S.; Zhao, Y.; Xu, Y.; Yuan, T.; Li, R. Multi-stage spark plasma sintering to study the densification mechanisms of boron carbide. Int. J. Refract. Met. Hard Mater. 2020, 93, 105351. [Google Scholar] [CrossRef]
- Kenny, J.; McDonald, N.; Binner, J.; Hong Chang, I.T.; Marinel, S. Low temperature synthesis and spark plasma sintering of a boron carbide with a low residual carbon content. J. Eur. Ceram. Soc. 2022, 42, 383–391. [Google Scholar] [CrossRef]
- Ji, W.; Rehman, S.S.; Wang, W.; Wang, H.; Wang, Y.; Zhang, J.; Zhang, F.; Fu, Z. Sintering boron carbide ceramics without grain growth by plastic deformation as the dominant densification mechanism. Sci. Rep. 2015, 5, 15827. [Google Scholar] [CrossRef] [Green Version]
- Kalandadze, G.I.; Shalamberidze, S.O.; Peikrishvili, A.B. Sintering of Boron and Boron Carbide. J. Solid State Chem. 2000, 154, 194–198. [Google Scholar] [CrossRef]
- Wang, C.B.; Zhang, S.; Shen, Q.; Zhang, L.M. Investigation on reactive sintering process of boron carbide ceramics by XRD. Mat. Sci. Technol. 2009, 25, 809–812. [Google Scholar] [CrossRef]
- Roszeitis, S.; Feng, B.; Martin, H.; Michaelis, A. Reactive sintering process and thermoelectric properties of boron rich boron carbides. J. Eur. Ceram. Soc. 2014, 34, 327–336. [Google Scholar] [CrossRef]
- Xie, K.Y.; Domnich, V.; Farbaniec, L.; Chen, B.; Kuwelkar, K.; Ma, L.; McCauley, J.W.; Haber, R.A.; Ramesh, K.T.; Chen, M.; et al. Microstructural characterization of boron-rich boron carbide. Acta. Mater. 2017, 136, 202–214. [Google Scholar] [CrossRef]
- An, Q.; Goddard, W.A.; Xie, K.Y.; Sim, G.; Hemker, K.J.; Munhollon, T.; Toksoy, M.F.; Haber, R.A. Superstrength through Nanotwinning. Nano Lett. 2016, 16, 7573–7579. [Google Scholar] [CrossRef]
- Rahaman, M. Sintering of Ceramics; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Guzman, I.Y. Reaction sintering and its practical application. Glas. Ceram. 1993, 50, 412–419. [Google Scholar] [CrossRef]
- German, R. Sintering Theory and Practice; John Wiley & Sons: New York, NY, USA, 1996. [Google Scholar]
- Expanded Graphite. Available online: https://www.gk-graphite.com/fileadmin/user_upload/Expandable_Graphite.pdf (accessed on 28 January 2022).
- Celzard, A.; Mareche, J.; Furdin, G. Modelling of exfoliated graphite. Prog. Mater. Sci. 2005, 50, 93–179. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Weng, W.; Wu, C. Exfoliation of graphite flake and its nanocomposites. Carbon 2003, 41, 619–621. [Google Scholar] [CrossRef]
- Prochazka, S.; Dole, S.; Hejna, C. Abnormal grain growth and microcracking in boron carbide. J. Am. Ceram. Soc. 1985, 68, 235–236. [Google Scholar] [CrossRef]
- Konovalikhin, S.V.; Kovalev, D.Y.; Ponomarev, V.I. Determination of the Thermal Expansion Coefficient of Boron Carbide B13C2. High Temp. 2018, 56, 668–672. [Google Scholar] [CrossRef]
- Gubernat, A.; Pichór, W.; Zientara, D.; Bućko, M.M.; Zych, Ł.; Kozień, D. Direct synthesis of fine boron carbide powders using expanded graphite. Ceram. Int. 2019, 45, 22104–22109. [Google Scholar] [CrossRef]





| B:C Ratio | 5:1 | 10:1 | 15:1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Phase Composition, Mass % | |||||||||
| Sintering Temperature, °C | B13C2 B4C | B48(B2C2) | C | B13C2 B4C | B48(B2C2) | C | B13C2 B4C | B48(B2C2) | C |
| 1650 | 100 | - | - | 85.5 | 14.5 | - | 83.5 | 16.5 | - |
| 1750 | 100 | - | - | 100 | - | - | 100 | - | - |
| 1850 | 99.7 | - | 0.3 | 100 | - | - | 100 | - | - |
| 1900 | 99.7 | - | 0.6 | 99.7 | - | 0.3 | 99.4 | - | 0.6 |
| Mass Ratio B:C | 5:1 | 10:1 | 15:1 | |||
|---|---|---|---|---|---|---|
| Temperature, °C | Apparent Density, g/cm3 | Relative Density, % | Apparent Density, g/cm3 | Relative Density, % | Apparent Density, g/cm3 | Relative Density, % |
| 1650 | 1.74 | 69.08 | 1.64 | 65.04 | 2.01 | 79.76 |
| 1750 | 2.14 | 85.11 | 2.08 | 82.63 | 2.25 | 89.21 |
| 1850 | 2.42 | 95.89 | 2.42 | 95.87 | 2.37 | 93.90 |
| 1900 | 2.39 | 94.71 | 2.40 | 95.19 | 2.18 | 90.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gubernat, A.; Kornaus, K.; Lach, R.; Zientara, D.; Dyl, P. Effect of Expanded Graphite on the Reaction Sintering of Boron Carbide. Materials 2022, 15, 1500. https://doi.org/10.3390/ma15041500
Gubernat A, Kornaus K, Lach R, Zientara D, Dyl P. Effect of Expanded Graphite on the Reaction Sintering of Boron Carbide. Materials. 2022; 15(4):1500. https://doi.org/10.3390/ma15041500
Chicago/Turabian StyleGubernat, Agnieszka, Kamil Kornaus, Radosław Lach, Dariusz Zientara, and Patryk Dyl. 2022. "Effect of Expanded Graphite on the Reaction Sintering of Boron Carbide" Materials 15, no. 4: 1500. https://doi.org/10.3390/ma15041500
APA StyleGubernat, A., Kornaus, K., Lach, R., Zientara, D., & Dyl, P. (2022). Effect of Expanded Graphite on the Reaction Sintering of Boron Carbide. Materials, 15(4), 1500. https://doi.org/10.3390/ma15041500






