Dentin Biomodification with Flavonoids and Calcium Phosphate Ion Clusters to Improve Dentin Bonding Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Formulation of Experimental Solutions
2.1.1. Preparation of Flavonoid Solutions
2.1.2. Preparation of a CPIC Solution
2.1.3. Preparation of Flavonoid and CPIC Combination Solutions
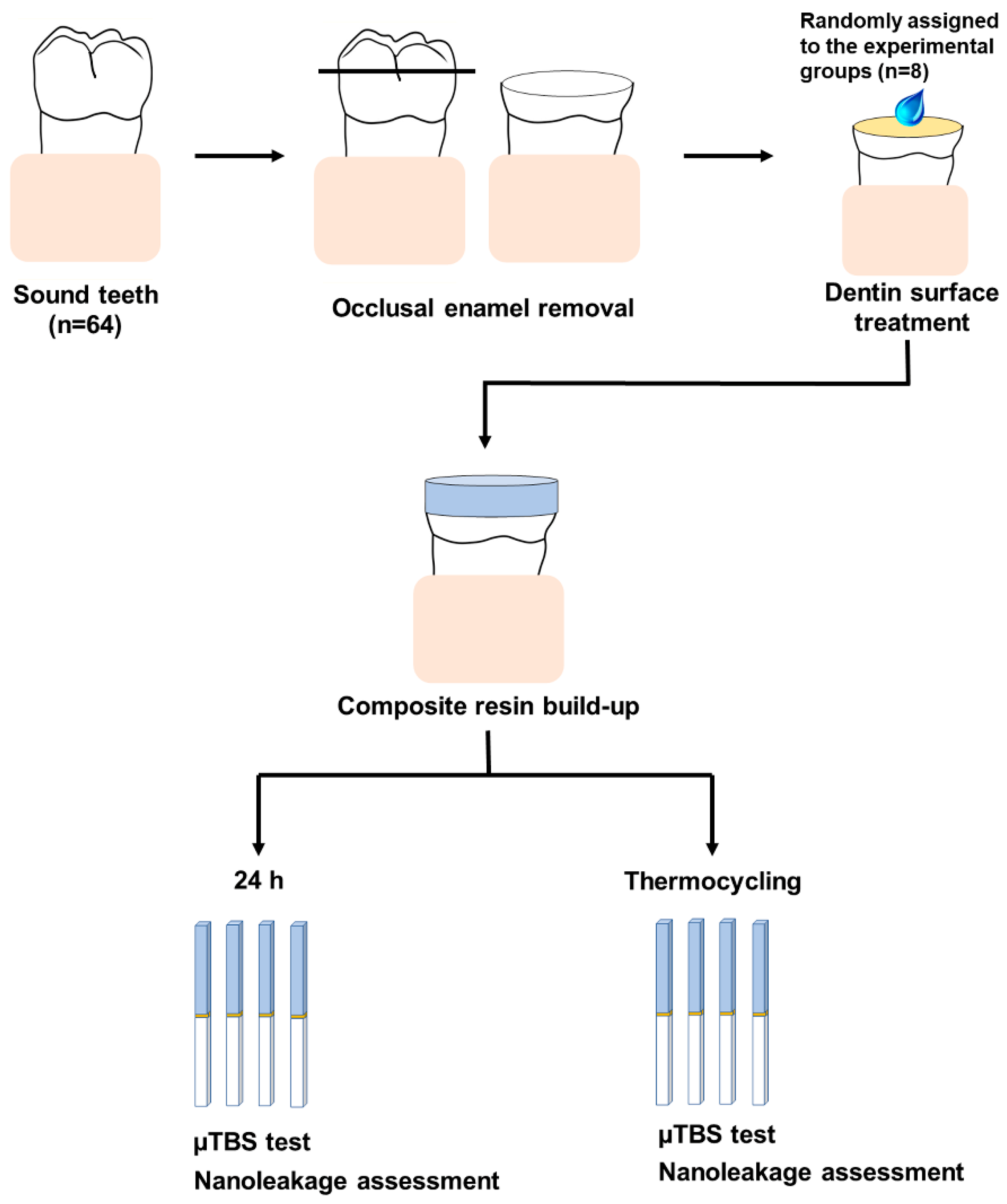
2.2. Dentin Specimen Preparation
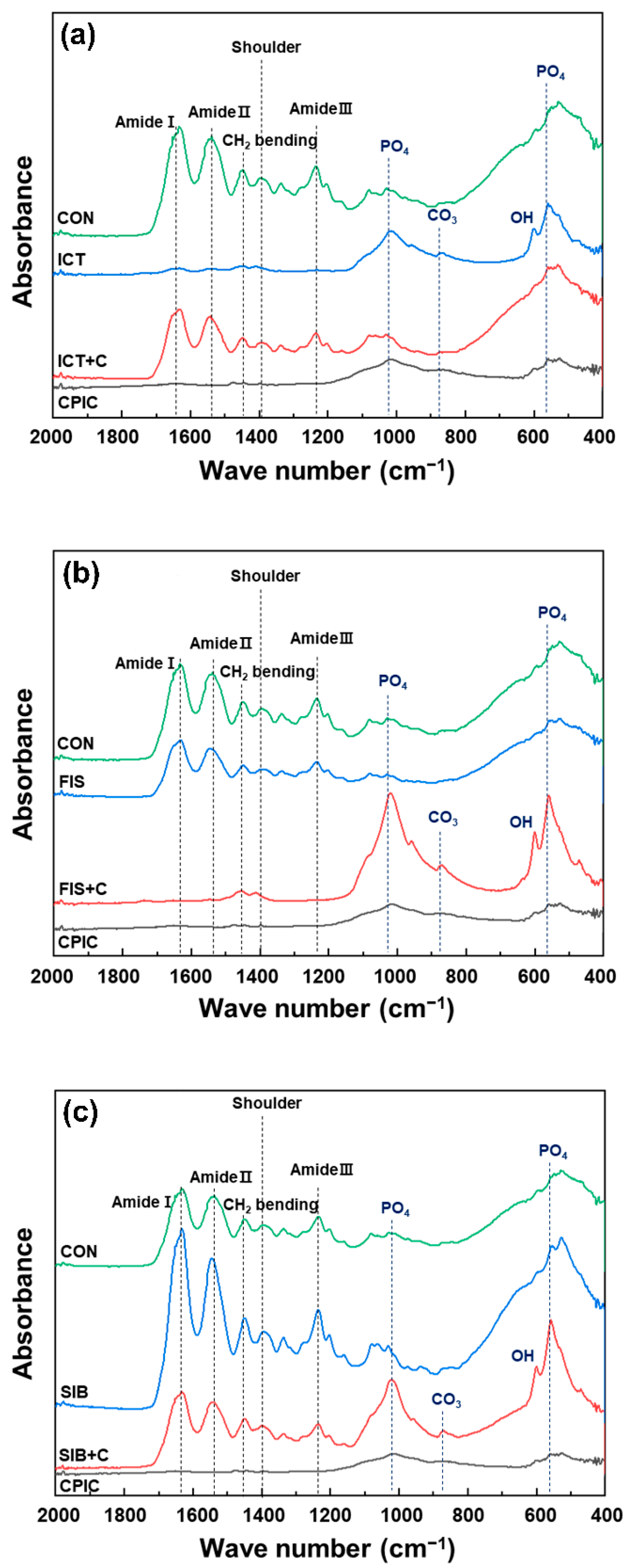
2.3. Fourier Transform Infrared Spectroscopy
2.4. Microtensile Bond Strength Testing
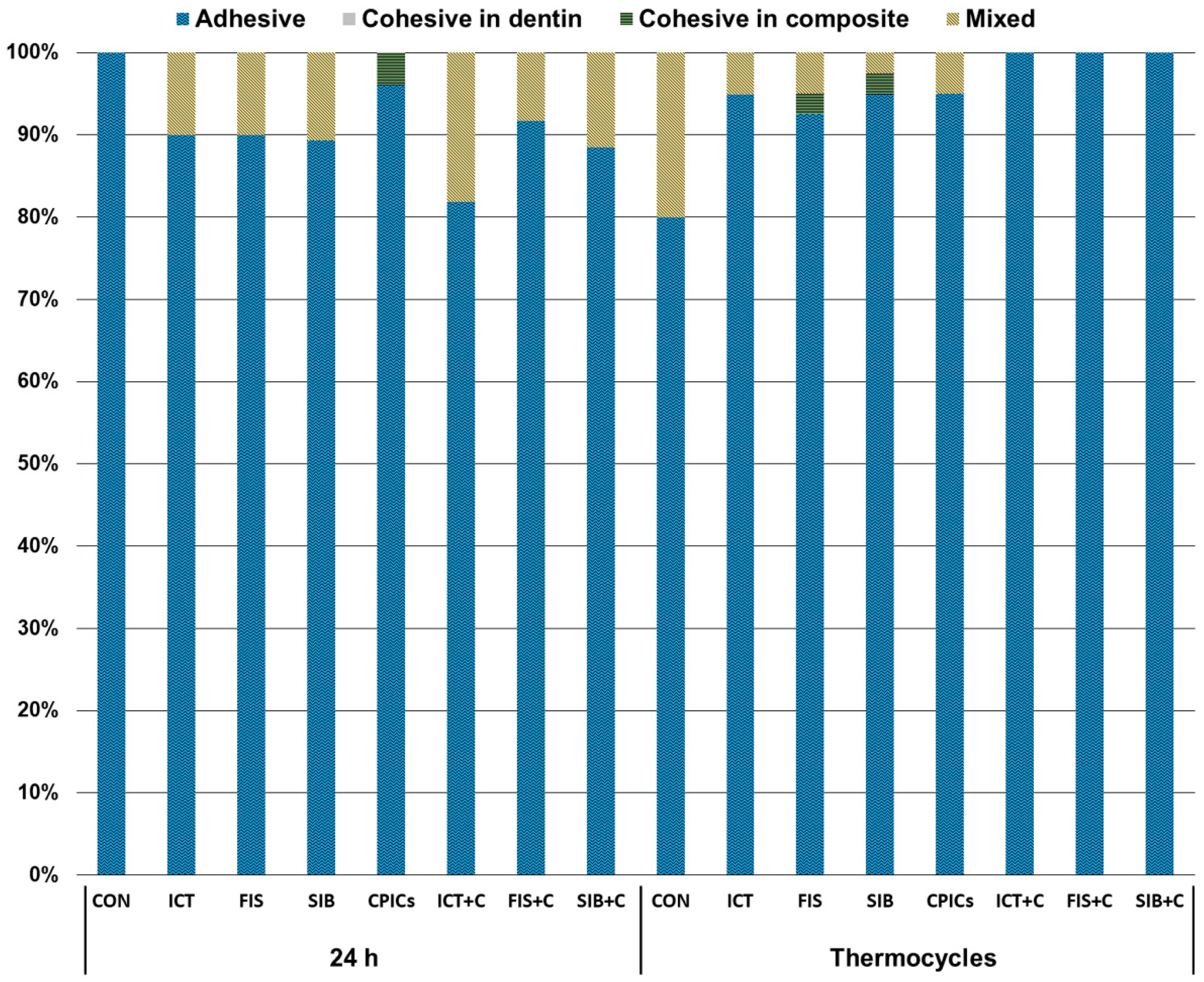
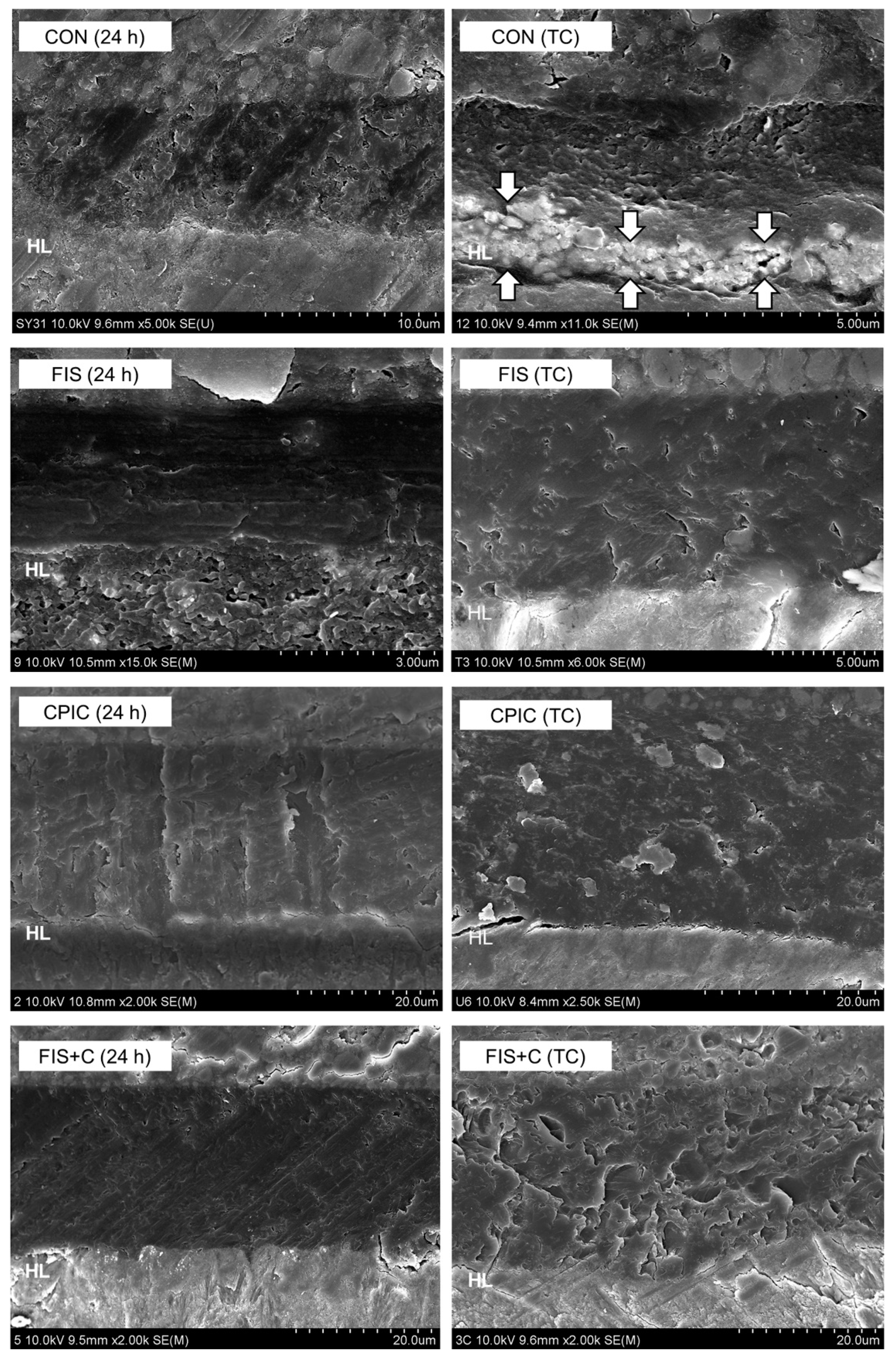
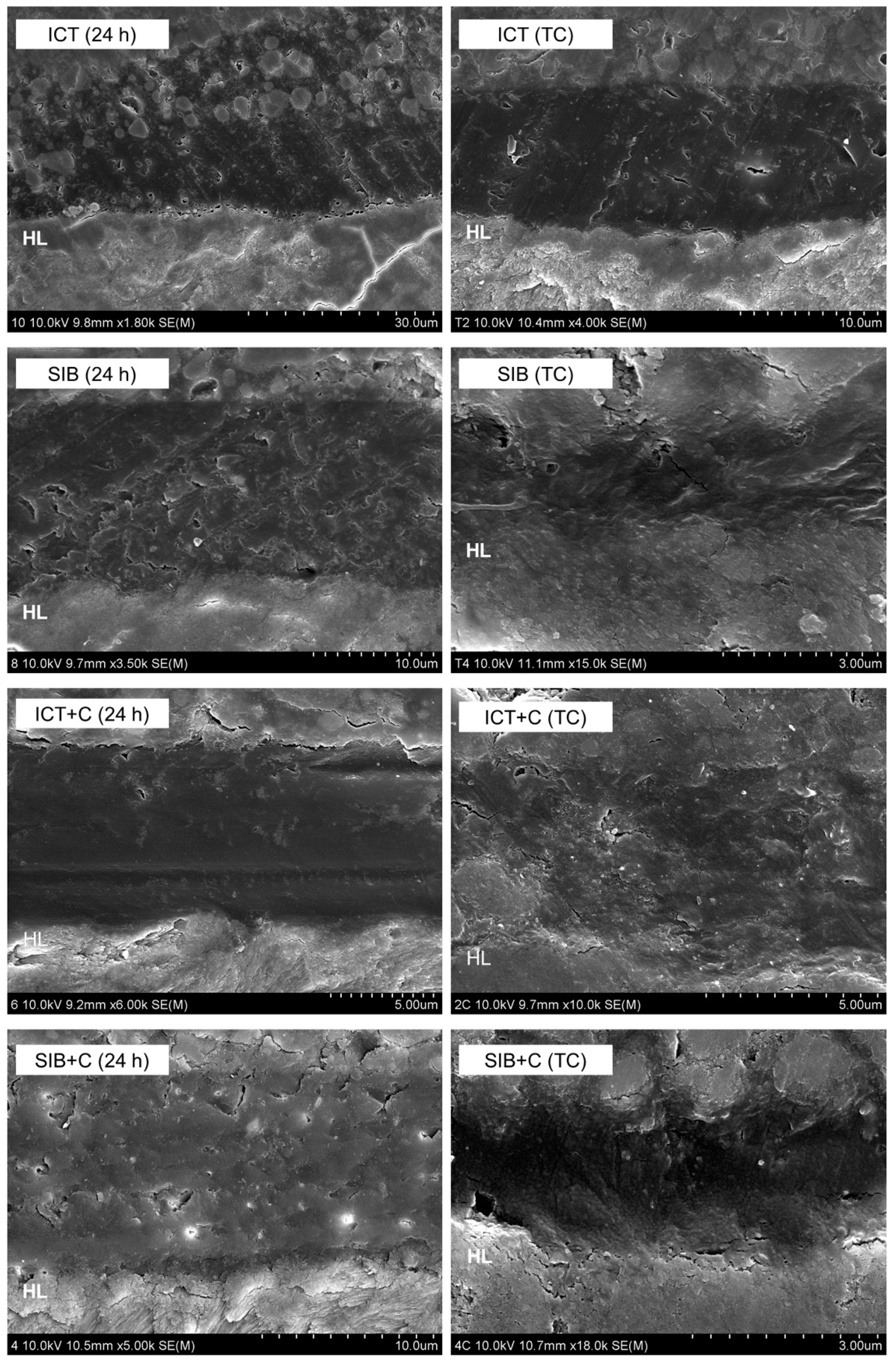
2.5. Nanoleakage Assessment
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Meerbeek, B.; Inokoshi, S.; Braem, M.; Lambrechts, P.; Vanherle, G. Morphological aspects of the resin-dentin interdiffusion zone with different dentin adhesive systems. J. Dent. Res. 1992, 71, 1530–1540. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.H.; Tay, F.R.; Breschi, L.; Tjäderhane, L.; Carvalho, R.M.; Carrilho, M.; Tezvergil-Mutluay, A. State of the art etch-and-rinse adhesives. Dent. Mater. 2011, 27, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, N.; Ashizawa, M.; Nakamura, M. Identification of a resin-dentin hybrid layer in vital human dentin created in vivo: Durable bonding to vital dentin. Quintessence Int. 1992, 23, 135–141. [Google Scholar]
- Hashimoto, M.; Ohno, H.; Kaga, M.; Endo, K.; Sano, H.; Oguchi, H. In vivo degradation of resin-dentin bonds in humans over 1 to 3 years. J. Dent. Res. 2000, 79, 1385–1391. [Google Scholar] [CrossRef]
- Sano, H.; Shono, T.; Takatsu, T.; Hosoda, H. Microporous dentin zone beneath resin-impregnated layer. Oper. Dent. 1994, 19, 59–64. [Google Scholar] [PubMed]
- Bourbia, M.; Finer, Y. Biochemical stability and interactions of dental resin composites and adhesives with host and bacteria in the oral cavity: A review. J. Can. Dent. Assoc. 2018, 84, 1–7. [Google Scholar]
- Tjäderhane, L.; Larjava, H.; Sorsa, T.; Uitto, V.J.; Larmas, M.; Salo, T. The activation and function of host matrix metalloproteinases in dentin matrix breakdown in caries lesions. J. Dent. Res. 1998, 77, 1622–1629. [Google Scholar] [CrossRef]
- Perdigão, J.; Reis, A.; Loguercio, A.D. Dentin adhesion and MMPs: A comprehensive review. J. Esthet. Restor. Dent. 2013, 25, 219–241. [Google Scholar] [CrossRef]
- Dos Santos, P.H.; Karol, S.; Bedran-Russo, A.K. Long-term nano-mechanical properties of biomodified dentin-resin interface components. J. Biomech. 2011, 44, 1691–1694. [Google Scholar] [CrossRef]
- Tjäderhane, L.; Nascimento, F.D.; Breschi, L.; Mazzoni, A.; Tersariol, I.L.; Geraldeli, S.; Tezvergil-Mutluay, A.; Carrilho, M.R.; Carvalho, R.M.; Tay, F.R.; et al. Optimizing dentin bond durability: Control of collagen degradation by matrix metalloproteinases and cysteine cathepsins. Dent. Mater. 2013, 29, 116–135. [Google Scholar] [CrossRef]
- Kim, H.; Choi, A.; Gong, M.K.; Park, H.R.; Kim, Y.I. Effect of remineralized collagen on dentin bond strength through calcium phosphate ion clusters or metastable calcium phosphate solution. Nanomaterials 2020, 10, 2203. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Hiraishi, N.; Nassar, M.; Yiu, C.; Otsuki, M.; Tagami, J. Effect of hesperidin incorporation into a self-etching primer on durability of dentin bond. Dent. Mater. 2014, 30, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Leme-Kraus, A.A.; Aydin, B.; Vidal, C.M.; Phansalkar, R.M.; Nam, J.W.; McAlpine, J.; Pauli, G.F.; Chen, S.; Bedran-Russo, A.K. Biostability of the proanthocyanidins-dentin complex and adhesion studies. J. Dent. Res. 2017, 96, 406–412. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Mu, C.; Shi, J.; Zhang, Q.; Shi, B.; Lin, W. Modification of collagen with a natural cross-linker, procyanidin. Int. J. Biol. Macromol. 2011, 48, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Dávila-Sánchez, A.; Gutierrez, M.F.; Bermudez, J.P.; Méndez-Bauer, M.L.; Hilgemberg, B.; Sauro, S.; Loguercio, A.D.; Arrais, C.A.G. Influence of flavonoids on long-term bonding stability on caries-affected dentin. Dent. Mater. 2020, 36, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Liu, R.; Xiao, Y.; Li, F.; Wang, D.; Hou, R.; Chen, J. Biomodification to dentin by a natural crosslinker improved the resin–dentin bonds. J. Dent. 2012, 40, 458–466. [Google Scholar] [CrossRef]
- Shao, C.; Jin, B.; Mu, Z.; Lu, H.; Zhao, Y.; Wu, Z.; Yan, L.; Zhang, Z.; Zhou, Y.; Pan, H.; et al. Repair of tooth enamel by a biomimetic mineralization frontier ensuring epitaxial growth. Sci. Adv. 2019, 5, eaaw9569. [Google Scholar] [CrossRef]
- Tay, F.R.; Pashley, D.H.; Yoshiyama, M. Two modes of nanoleakage expression in single-step adhesives. J. Dent. Res. 2002, 81, 472–476. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, X.; Li, S.; Liu, Y.; Keightley, A.; Wang, Y. Molecular weight and galloylation affect grape seed extract constituents’ ability to cross-link dentin collagen in clinically relevant time. Dent. Mater. 2015, 31, 814–821. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Chen, M.; Yao, X.; Xu, C.; Zhang, Y.; Wang, Y. Enhancement in dentin collagen’s biological stability after proanthocyanidins treatment in clinically relevant time periods. Dent. Mater. 2013, 29, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Mei, M.L.; Li, Q.; Xia, R.; Zhang, Z.; Chu, C.H. Self-cleaning and antibiofouling enamel surface by slippery liquid-infused technique. Sci. Rep. 2016, 6, 1–14. [Google Scholar]
- Berzina-Cimdina, L.; Borodajenko, N. Research of calcium phosphate using Fourier transform infrared spectroscopy. In Infrared Spectroscopy—Materials Science, Engineering and Technology; Theophile, T., Ed.; In Tech: Rijeka, Croatia, 2012; pp. 123–148. [Google Scholar]
- Carrilho, M.R.; Tay, F.R.; Donnelly, A.M.; Agee, K.A.; Tjäderhane, L.; Mazzoni, A.; Breschi, L.; Foulger, S.; Pashley, D.H. Host-derived loss of dentin matrix stiffness associated with solubilization of collagen. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Carrilho, M.R.; Geraldeli, S.; Tay, F.; de Goes, M.F.; Carvalho, R.M.; Tjäderhane, L.; Reis, A.F.; Hebling, J.; Mazzoni, A.; Breschi, L.; et al. In vivo preservation of the hybrid layer by chlorhexidine. J. Dent. Res. 2007, 86, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Breschi, L.; Martin, P.; Mazzoni, A.; Nato, F.; Carrilho, M.; Tjäderhane, L.; Visintini, E.; Cadenaro, M.; Tay, F.R.; De Stefano Dorigo, E.; et al. Use of a specific MMP-inhibitor (galardin) for preservation of hybrid layer. Dent. Mater 2010, 26, 571–578. [Google Scholar] [CrossRef]
- Li, H.; Li, T.; Li, X.; Zhang, Z.; Li, P.; Li, Z. Morphological effects of MMPs inhibitors on the dentin bonding. Int. J. Clin. Exp. Med. 2015, 8, 10793–10803. [Google Scholar]
- Scheffel, D.L.; Hebling, J.; Scheffel, R.H.; Agee, K.; Turco, G.; de Souza Costa, C.A.; Pashley, D. Inactivation of matrix-bound matrix metalloproteinases by cross-linking agents in acid-etched dentin. Oper. Dent. 2014, 39, 152–158. [Google Scholar] [CrossRef]
- De-Paula, D.M.; Lomonaco, D.; Ponte, A.M.P.; Cordeiro, K.E.; Moreira, M.M.; Mazzetto, S.E.; Feitosa, V.P. Influence of collagen cross-linkers addition in phosphoric acid on dentin biomodification and bonding of an etch-and-rinse adhesive. Dent. Mater. 2020, 36, e1–e8. [Google Scholar] [CrossRef]
- Wong, S.P.; Shen, P.; Lee, L.; Li, J.; Yong, E.L. Pharmacokinetics of prenylflavonoids and correlations with the dynamics of estrogen action in sera following ingestion of a standardized Epimedium extract. J. Pharm. Biomed. Anal. 2009, 50, 216–223. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Lou, Y.J. Proliferation-stimulating effects of icaritin and desmethylicaritin in MCF-7 cells. Eur. J. Pharmacol. 2004, 504, 147–153. [Google Scholar] [CrossRef]
- Yao, D.; Wang, X.; Xie, X.; Zhang, G.; Qin, L. Icaritin promotes osteogenic differentiation while inhibits osteoclastic differentiation in vitro. Bone 2010, 47, S426. [Google Scholar] [CrossRef]
- Long, J.; Zhou, Q.; Li, D.; Wang, X.; Cao, H.; Qin, L. Phytoestrogenic molecule icaritin prevents OVX-induced osteoporosis in mice. J. Orthop. Translat. 2014, 2, 224–225. [Google Scholar] [CrossRef][Green Version]
- Gupta, S.C.; Tyagi, A.K.; Deshmukh-Taskar, P.; Hinojosa, M.; Prasad, S.; Aggarwal, B.B. Downregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols. Arch. Biochem. Biophys. 2014, 559, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, N.; Zhao, J.; Wolf, D.M.; Agarwal, R. Inhibition of human carcinoma cell growth and DNA synthesis by silibinin, an active constituent of milk thistle: Comparison with silymarin. Cancer Lett. 1999, 147, 77–84. [Google Scholar] [CrossRef]
- Wang, X.F.; Wang, J. Icaritin suppresses the proliferation of human osteosarcoma cells in vitro by increasing apoptosis and decreasing MMP expression. Acta Pharmacol. Sin. 2014, 35, 531–539. [Google Scholar] [CrossRef]
- Park, J.H.; Jang, Y.J.; Choi, Y.J.; Jang, J.W.; Kim, J.H.; Rho, Y.K.; Kim, I.J.; Kim, H.J.; Leem, M.J.; Lee, S.T. Fisetin inhibits matrix metalloproteinases and reduces tumor cell invasiveness and endothelial cell tube formation. Nutr. Cancer 2013, 65, 1192–1199. [Google Scholar] [CrossRef]
- Oh, S.J.; Jung, S.P.; Han, J.; Kim, S.; Kim, J.S.; Nam, S.J.; Lee, J.E.; Kim, J.H. Silibinin inhibits TPA-induced cell migration and MMP-9 expression in thyroid and breast cancer cells. Oncol. Rep. 2013, 29, 1343–1348. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Li, L.; Sun, J.; Gu, X.; Xu, X.; Pan, H.; Tang, R. Remineralization of dentin collagen by meta-stabilized amorphous calcium phosphate. CrystEngComm 2013, 15, 6151–6158. [Google Scholar] [CrossRef]

| Substance | Molecular Mass | Number of Mols (6.5% mass) | Solubility |
|---|---|---|---|
| Icaritin (ICT) | 368.38 g/mol | 1.76 mM | 0.00821 mg/mL in water |
| Fisetin (FIS) | 286.24 g/mol | 2.27 mM | 5 mg/mL in ethanol |
| Silibinin (SIB) | 482.44 g/mol | 1.35 mM | <0.04 mg/mL in water |
| Component | Compound | Quantity % |
|---|---|---|
| Active compound | Flavonoid | 6.5% mass |
| Vehicle | Pure ethanol | 30% (3 mL) |
| Surfactant | Span® 20 (sorbitan monolaurate) | 1% (0.1 mL) |
| Aqueous medium | Distilled water | QS 10 mL |
| Groups | Dentin Treatment Solutions |
|---|---|
| CON | No application of experimental solution |
| ICT | Icaritin |
| FIS | Fisetin |
| SIB | Silibinin |
| CPIC | Calcium phosphate ion clusters |
| ICT + C | Icaritin + CPIC |
| FIS + C | Fisetin + CPIC |
| SIB + C | Silibinin + CPIC |
| Groups | 24 h | Thermocycling |
|---|---|---|
| CON | 21.66 (3.47) C | 19.18 (4.96) c,* |
| ICT | 24.40 (4.63) BC | 20.53 (3.09) bc,* |
| FIS | 26.81 (4.56) AB | 19.40 (4.84) c,* |
| SIB | 25.65 (4.41) BC | 22.04 (4.79) bc,* |
| CPIC | 25.97 (4.03) BC | 23.43 (3.37) ab,* |
| ICT + C | 30.63 (5.49) A | 26.74 (6.01) a,* |
| FIS + C | 25.63 (4.25) BC | 23.42 (4.10) ab |
| SIB + C | 24.76 (3.67) BC | 25.17 (4.08) a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paik, Y.; Kim, J.-H.; Yoo, K.-H.; Yoon, S.-Y.; Kim, Y.-I. Dentin Biomodification with Flavonoids and Calcium Phosphate Ion Clusters to Improve Dentin Bonding Stability. Materials 2022, 15, 1494. https://doi.org/10.3390/ma15041494
Paik Y, Kim J-H, Yoo K-H, Yoon S-Y, Kim Y-I. Dentin Biomodification with Flavonoids and Calcium Phosphate Ion Clusters to Improve Dentin Bonding Stability. Materials. 2022; 15(4):1494. https://doi.org/10.3390/ma15041494
Chicago/Turabian StylePaik, Youna, Jae-Hoon Kim, Kyung-Hyeon Yoo, Seog-Young Yoon, and Yong-Il Kim. 2022. "Dentin Biomodification with Flavonoids and Calcium Phosphate Ion Clusters to Improve Dentin Bonding Stability" Materials 15, no. 4: 1494. https://doi.org/10.3390/ma15041494
APA StylePaik, Y., Kim, J.-H., Yoo, K.-H., Yoon, S.-Y., & Kim, Y.-I. (2022). Dentin Biomodification with Flavonoids and Calcium Phosphate Ion Clusters to Improve Dentin Bonding Stability. Materials, 15(4), 1494. https://doi.org/10.3390/ma15041494






