Converting Sewage Water into H2 Fuel Gas Using Cu/CuO Nanoporous Photocatalytic Electrodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cu/CuO Nanomaterial Preparation
2.2. Characterization
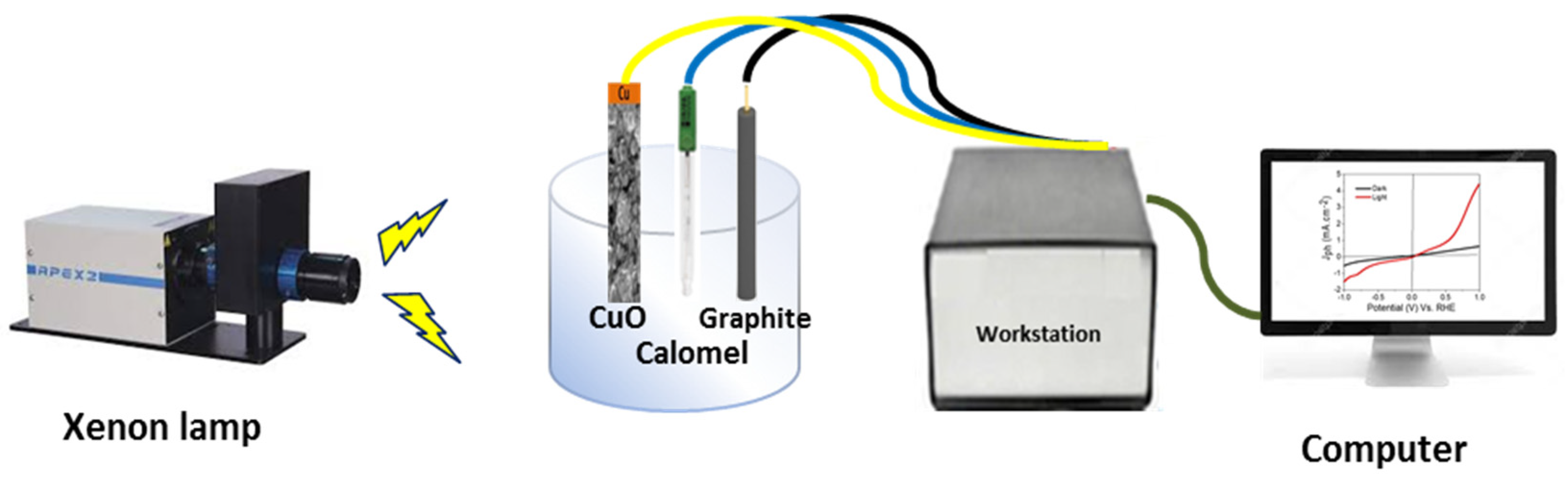
2.3. Electrochemical Measurements
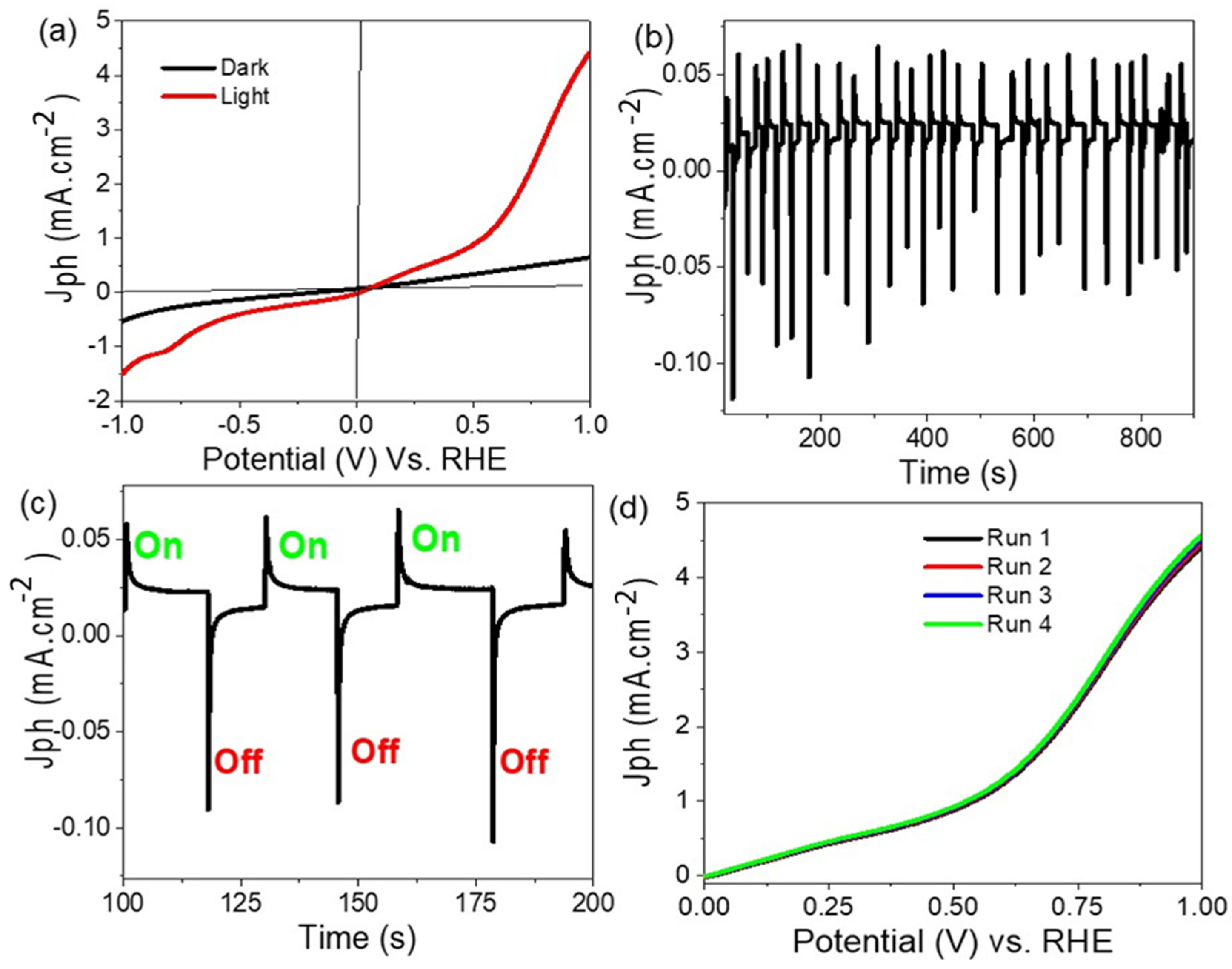
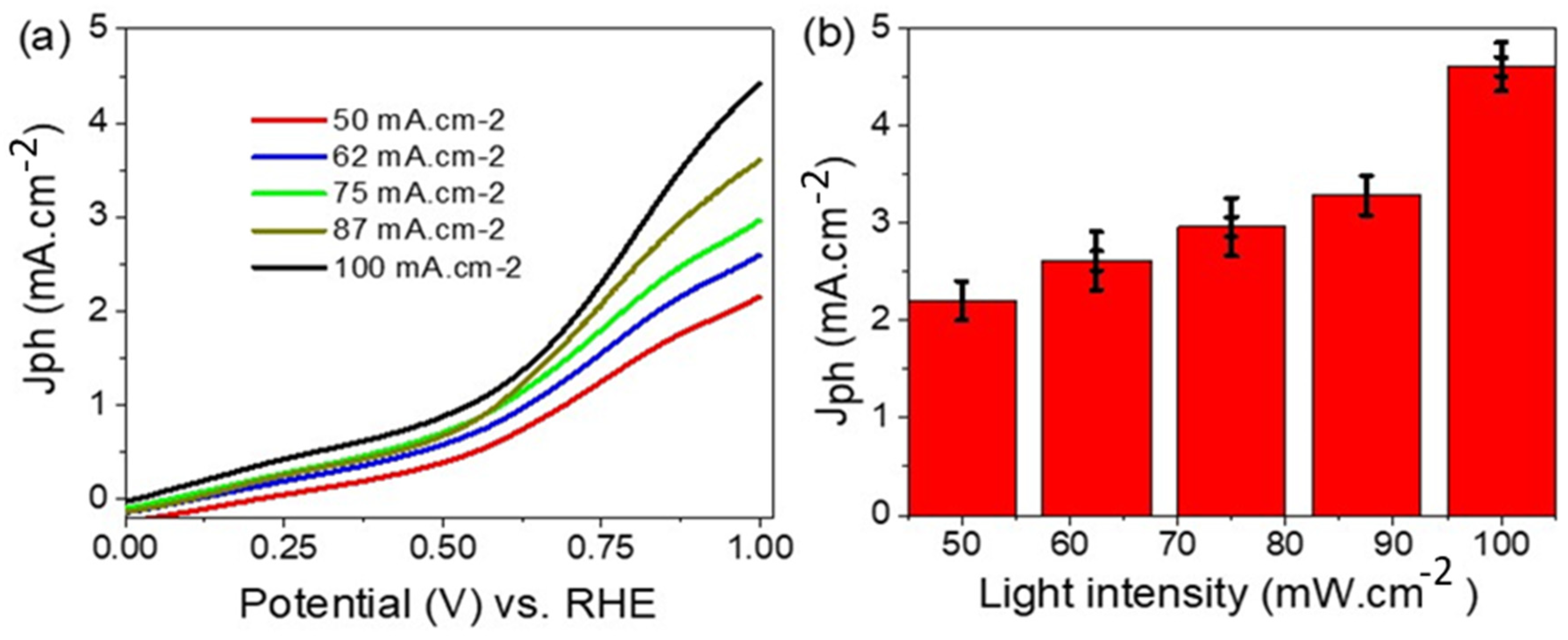
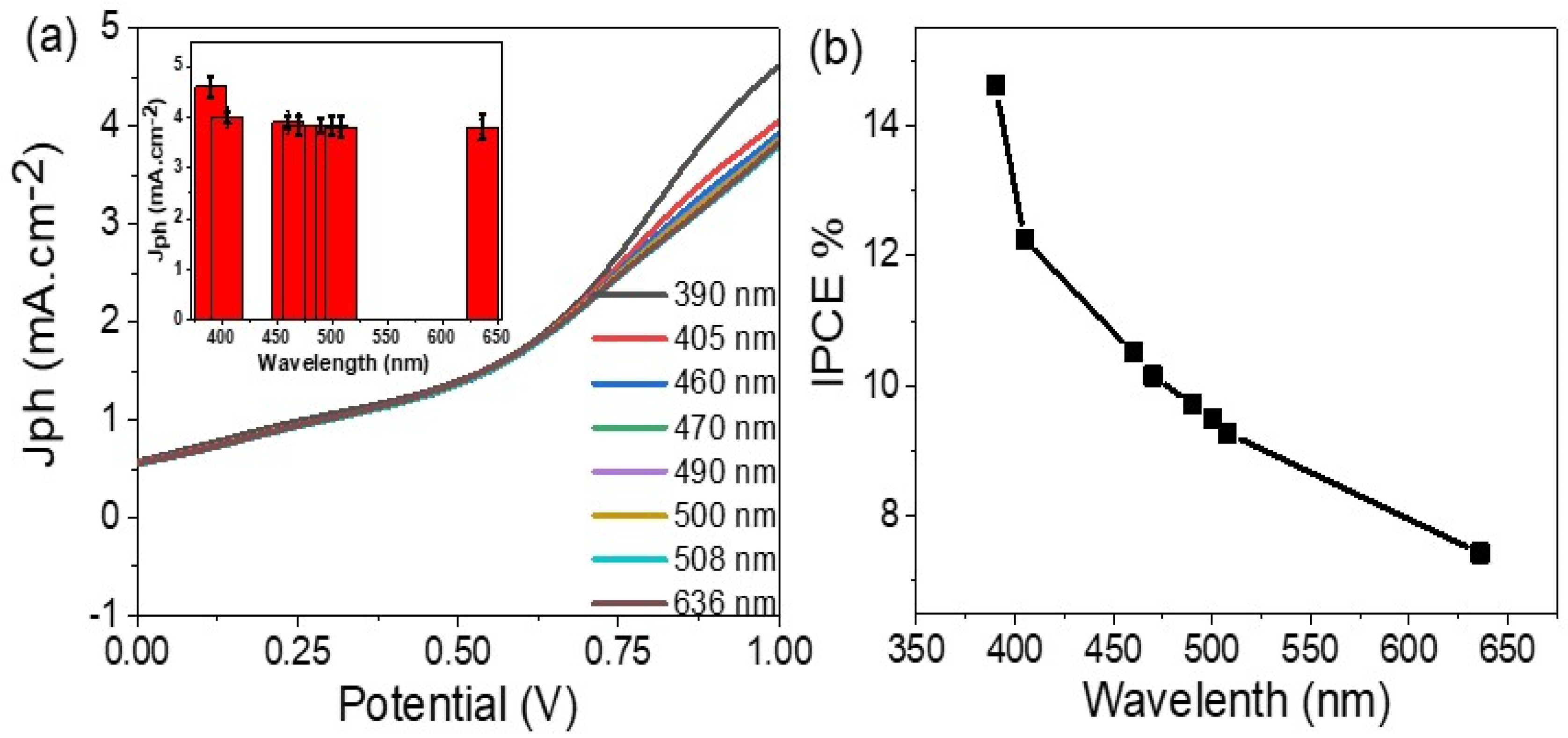
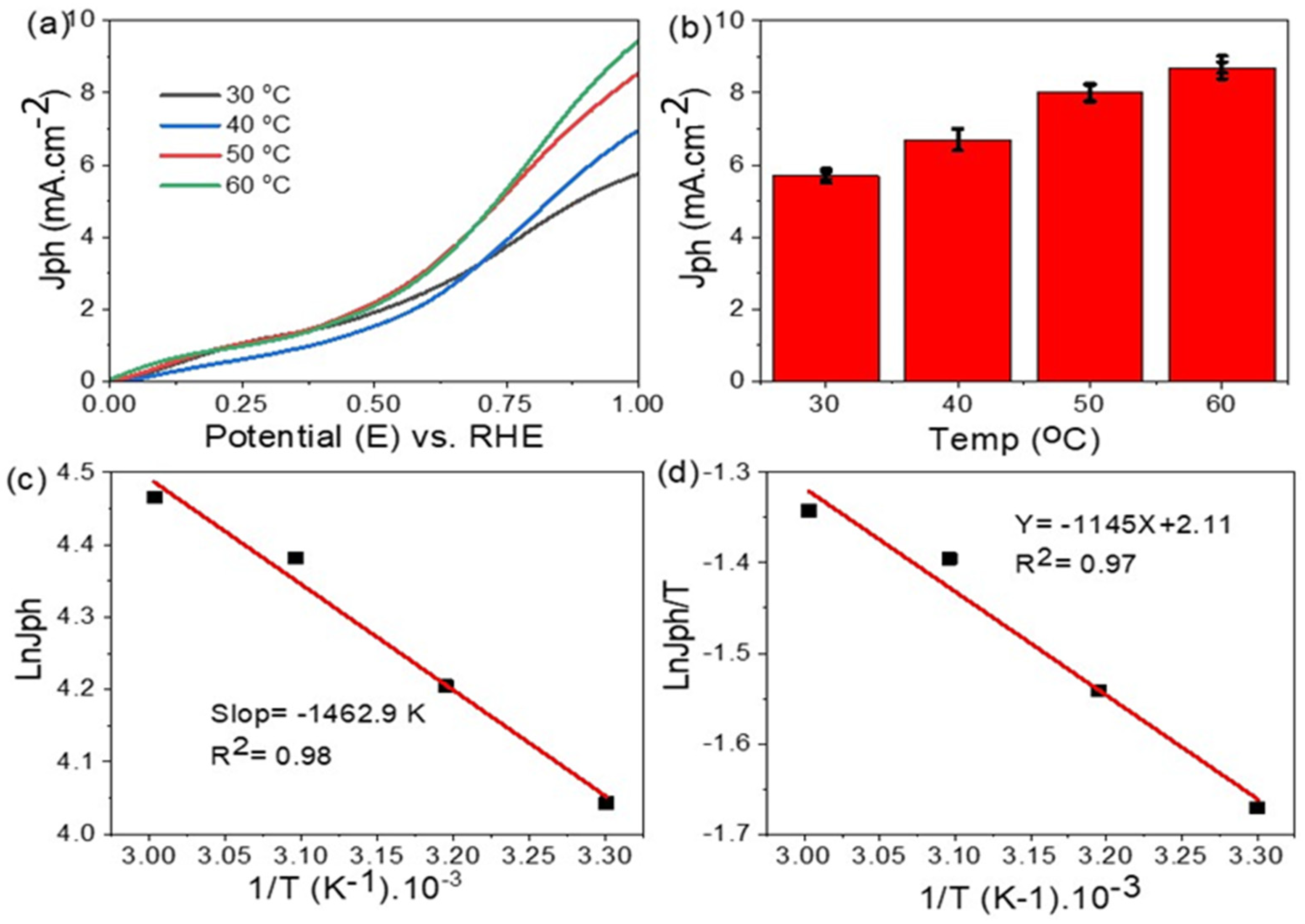
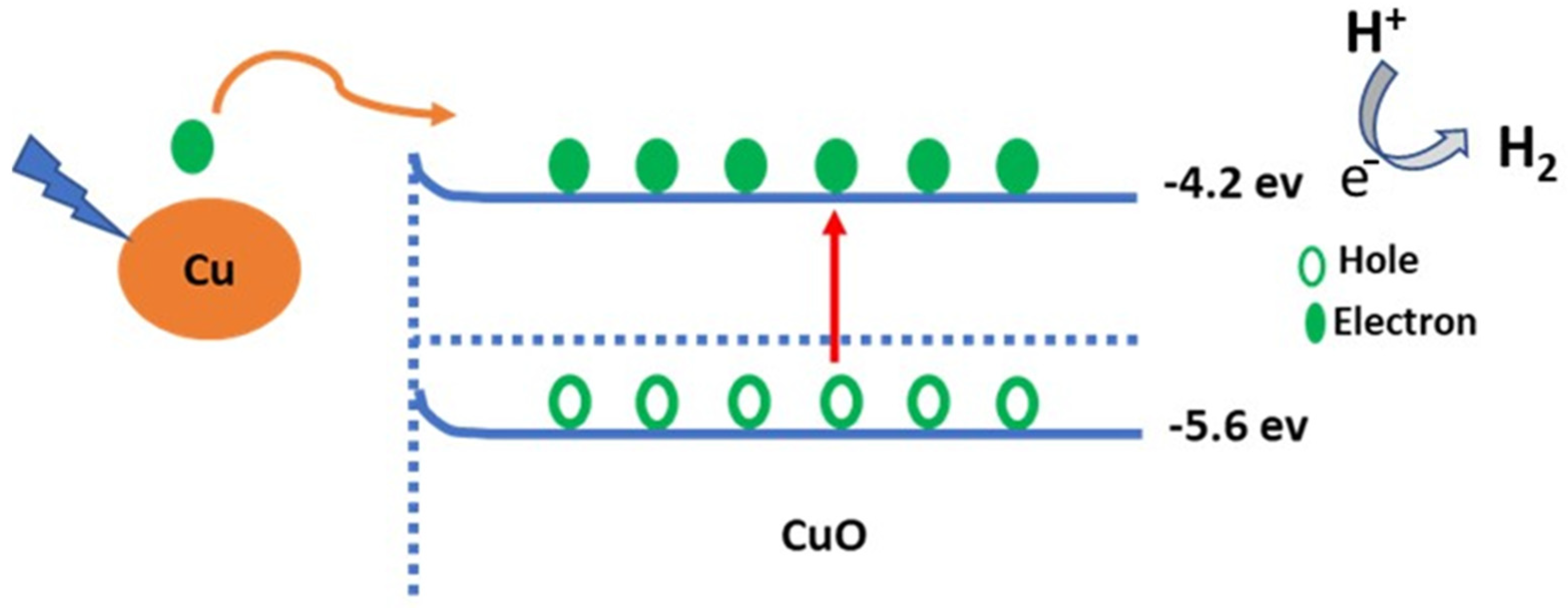
3. Results and Discussion
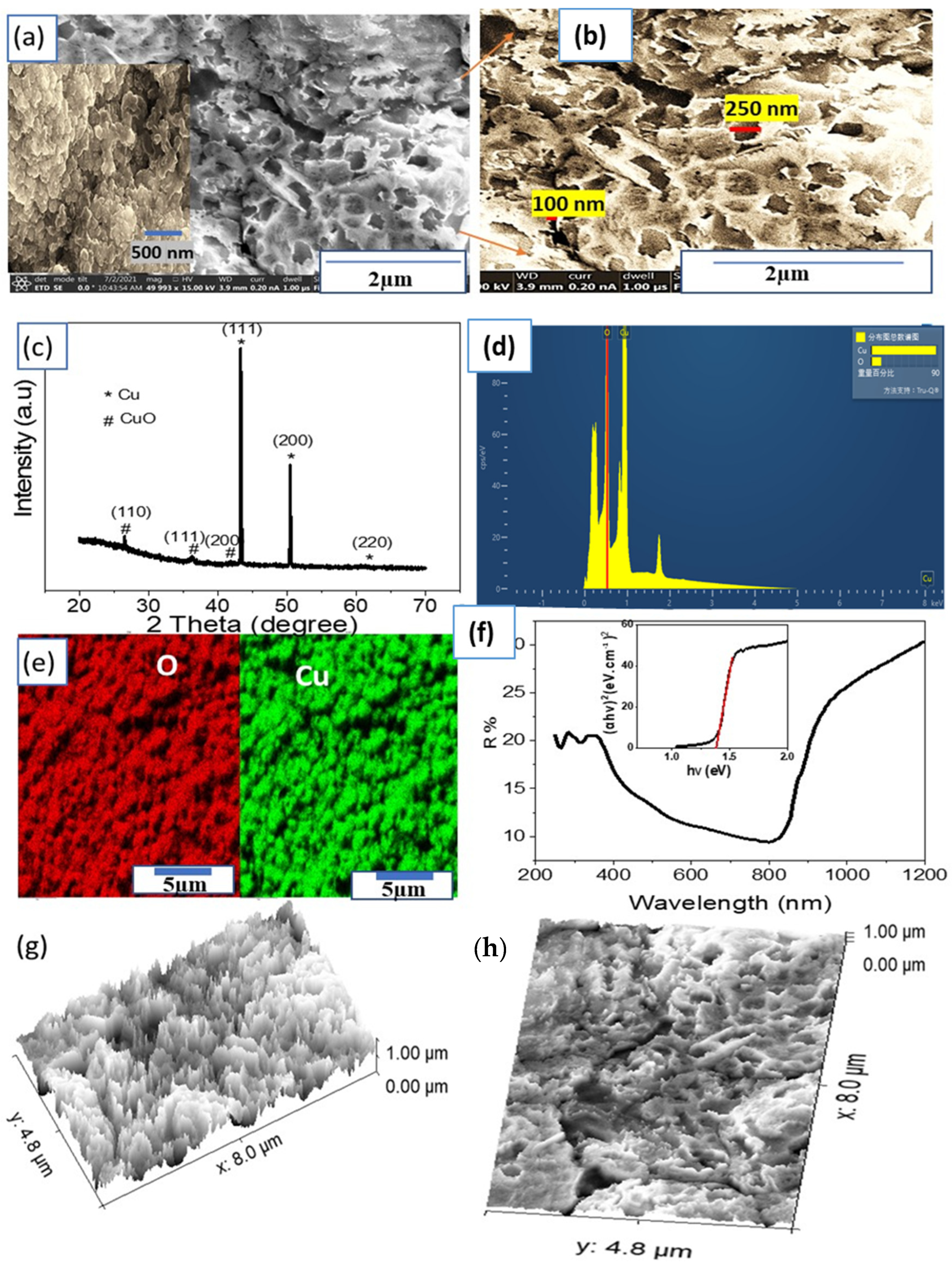
Morphological, Structural, and Optical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nishiyama, H.; Yamada, T.; Nakabayashi, M.; Maehara, Y.; Yamaguchi, M.; Kuromiya, Y.; Nagatsuma, Y.; Tokudome, H.; Akiyama, S.; Watanabe, T.; et al. Photocatalytic solar hydrogen production from water on a 100-m2 scale. Nature 2021, 598, 304–307. [Google Scholar] [CrossRef]
- Domen, K.; Hisatomi, T. Reaction systems for solar hydrogen production via water splitting with particulate semiconductor. Nat. Catal. 2019, 2, 387–399. [Google Scholar] [CrossRef]
- Pagliaro, M. Preparing for the future: Solar energy and bioeconomy in the United Arab Emirates. Energy Sci. Eng. 2019, 7, 1451–1457. [Google Scholar] [CrossRef] [Green Version]
- Takata, T. Photocatalytic water splitting with quantum efficiency of almost unity. Nature 2020, 581, 411–414. [Google Scholar] [CrossRef]
- Mohamed, F.; Rabia, M.; Shaban, M. Synthesis and characterization of biogenic iron oxides of different nanomorphologies from pomegranate peels for efficient solar hydrogen production. J. Mater. Res. Technol. 2020, 9, 4255–4271. [Google Scholar] [CrossRef]
- Shaban, M.; Ali, S.; Rabia, M. Design and application of nanoporous graphene oxide film for CO2, H2, and C2H2 gases sensing. J. Mater. Res. Technol. 2019, 8, 4510–4520. [Google Scholar] [CrossRef]
- Elsayed, A.M.; Rabia, M.; Shaban, M.; Aly, A.H.; Ahmed, A.M. Preparation of hexagonal nanoporous Al2O3/TiO2/TiN as a novel photodetector with high efficiency. Sci. Rep. 2021, 11, 17572. [Google Scholar]
- Kang, Z.; Cheng, Y.; Zheng, Z.; Cheng, F.; Chen, Z.; Li, L.; Tan, X.; Xiong, L.; Zhai, T.; Gao, Y. MoS2-Based photodetectors powered by asymmetric contact structure with large work function difference. Nano-Micro Lett. 2019, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Lee, W.W.; Yang, D.W.; Chang, W.J.; Kwon, S.S.; Park, W. Il anomalous photovoltaic response of graphene-on-GaN schottky photodiodes. ACS Appl. Mater. Interfaces 2018, 10, 14170–14174. [Google Scholar] [CrossRef] [PubMed]
- Abdelazeez, A.A.A.; El-Fatah, G.A.; Shaban, M.; Ahmed, A.M.; Rabia, M. ITO/Poly-3-Methylaniline/Au Electrode for Electrochemical Water Splitting and Dye Removal. ECS J. Solid State Sci. Technol. 2021, 10, 123009. [Google Scholar] [CrossRef]
- Rabia, M.; Mohamed, H.S.H.; Shaban, M.; Taha, S. Preparation of polyaniline/PbS core-shell nano/microcomposite and its application for photocatalytic H2 electrogeneration from H2O. Sci. Rep. 2018, 8, 1107. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, H.S.H.; Rabia, M.; Zhou, X.G.; Qin, X.S.; Khabiri, G.; Shaban, M.; Younus, H.A.; Taha, S.; Hu, Z.Y.; Liu, J.; et al. Phase-junction Ag/TiO2 nanocomposite as photocathode for H2 generation. J. Mater. Sci. Technol. 2021, 83, 179–187. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Almohammedi, A.; Shaban, M.; Mostafa, H.; Rabia, M. Nanoporous TiN/TiO2/Alumina Membrane for Photoelectrochemical Hydrogen Production from Sewage Water. Nanomaterials 2021, 11, 2617. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Yang, W.; Leng, B.; Niu, P.; Jiang, X.; Liu, B. High-performance flexible ultraviolet photodetectors based on AZO/ZnO/PVK/PEDOT:PSS heterostructures integrated on human hair. ACS Appl. Mater. Interfaces 2019, 11, 24459–24467. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.C.; Kim, K.H.; Hwang, M.S.; Park, J.S.; Lee, J.M.; So, J.P.; Choi, J.H.; Kwon, S.H.; Barrelet, C.J.; et al. Photon-triggered nanowire transistors. Nat. Nanotechnol. 2017, 12, 963–968. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Q.; Liu, B.; Yang, W.; Li, J.; Niu, P.; Jiang, X. Giant UV photoresponse of a GaN nanowire photodetector through effective Pt nanoparticle coupling. J. Mater. Chem. C 2017, 5, 4319–4326. [Google Scholar] [CrossRef]
- Li, M.; Yu, M.; Su, D.; Zhang, J.; Jiang, S.; Wu, J.; Wang, Q.; Liu, S. Ultrahigh responsivity UV photodetector based on Cu nanostructure/ZnO QD hybrid architectures. Small 2019, 15, 1901606. [Google Scholar] [CrossRef] [PubMed]
- Schultze, J.W.; Lohrengel, M.M. Stability, reactivity and breakdown of passive films. Problems of recent and future research. Electrochim. Acta 2000, 45, 2499–2513. [Google Scholar] [CrossRef]
- Basnet, P.; Zhao, Y. Tuning the CuxO nanorod composition for efficient visible light induced photocatalysis. Catal. Sci. Technol. 2016, 6, 2228–2238. [Google Scholar] [CrossRef]
- Chiang, C.Y.; Shin, Y.; Aroh, K.; Ehrman, S. Copper oxide photocathodes prepared by a solution based process. Int. J. Hydrogen Energy 2012, 37, 8232–8239. [Google Scholar] [CrossRef]
- Chiang, C.-Y.; Chang, M.-H.; Liu, H.-S.; Tai, C.Y.; Ehrman, S. Process Intensification in the production of photocatalysts for solar hydrogen generation. Ind. Eng. Chem. Res. 2012, 51, 5207–5215. [Google Scholar] [CrossRef]
- Ragupathi, V.; Raja, M.A.; Panigrahi, P.; Ganapathi Subramaniam, N. CuO/g-C3N4 nanocomposite as promising photocatalyst for photoelectrochemical water splitting. Optik 2020, 208, 164569. [Google Scholar] [CrossRef]
- Thi Quyen, V.; Jitae, K.; Thi Huong, P.; Thi Thu Ha, L.; My Thanh, D.; Minh Viet, N.; Quang Thang, P. Copper doped titanium dioxide as a low-cost visible light photocatalyst for water splitting. Sol. Energy 2021, 218, 150–156. [Google Scholar] [CrossRef]
- Chen, J.; Shen, S.; Guo, P.; Wang, M.; Wu, P.; Wang, X.; Guo, L. In-situ reduction synthesis of nano-sized Cu2O particles modifying g-C3N4 for enhanced photocatalytic hydrogen production. Appl. Catal. B Environ. 2014, 152–153, 335–341. [Google Scholar] [CrossRef]
- Teixeira, G.F.; Silva Junior, E.; Vilela, R.; Zaghete, M.A.; Colmati, F. Perovskite Structure Associated with Precious Metals: Influence on Heterogenous Catalytic Process. Catalysts 2019, 9, 721. [Google Scholar] [CrossRef] [Green Version]
- Mishra, M.; Chun, D.M. α-Fe2O3 as a photocatalytic material: A review. Appl. Catal. A Gen. 2015, 498, 126–141. [Google Scholar]
- Acar, C.; Dincer, I.; Naterer, G.F. Review of photocatalytic water-splitting methods for sustainable hydrogen production. Int. J. Energy Res. 2016, 40, 1449–1473. [Google Scholar]
- Liu, G.; Karuturi, S.K.; Chen, H.; Wang, D.; Ager, J.W.; Simonov, A.N.; Tricoli, A. Enhancement of the photoelectrochemical water splitting by perovskite BiFeO3 via interfacial engineering. Sol. Energy 2020, 202, 198–203. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, D.; Wen, L.; Xu, R.; Obergfell, M.; Mi, Y.; Zhan, Z.; Nasori, N.; Demsar, J.; Lei, Y. Manipulation of charge transfer and transport in plasmonic-ferroelectric hybrids for photoelectrochemical applications. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Davies, J.; du Preez, S.P.; Bessarabov, D.G. On-Demand Hydrogen Generation by the Hydrolysis of Ball-Milled Aluminum& ndash; Bismuth – Zinc Composites. Materials 2022, 15, 1197. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chou, S.-F.; Blaabjerg, F.; Davari, P. Overview of Power Electronic Converter Topologies Enabling Large-Scale Hydrogen Production via Water Electrolysis. Appl. Sci. 2022, 12, 1906. [Google Scholar] [CrossRef]
- Chiang, C.Y.; Aroh, K.; Franson, N.; Satsangi, V.R.; Dass, S.; Ehrman, S. Copper oxide nanoparticle made by flame spray pyrolysis for photoelectrochemical water splitting—Part II. Photoelectrochemical study. Int. J. Hydrogen Energy 2011, 36, 15519–15526. [Google Scholar] [CrossRef]
- Guo, X.; Diao, P.; Xu, D.; Huang, S.; Yang, Y.; Jin, T.; Wu, Q.; Xiang, M.; Zhang, M. CuO/Pd composite photocathodes for photoelectrochemical hydrogen evolution reaction. Int. J. Hydrogen Energy 2014, 39, 7686–7696. [Google Scholar] [CrossRef]
- Zúñiga, A.; Fonseca, L.; Souza, J.A.; Rivaldo-Gomez, C.; Pomar, C.D.; Criado, D. Anomalous ferromagnetic behavior and size effects in CuO nanowires. J. Magn. Magn. Mater. 2019, 471, 77–81. [Google Scholar] [CrossRef]
- Zhan, W.; Chen, Z.; Hu, J.; Chen, X. Vertical CuO nanowires array electrodes: Visible light sensitive photoelectrochemical biosensor of ethanol detection. Mater. Sci. Semicond. Process. 2018, 85, 90–97. [Google Scholar] [CrossRef]
- Mahmoodi, A.; Solaymani, S.; Amini, M.; Nezafat, N.B.; Ghoranneviss, M. Structural, morphological and antibacterial characterization of CuO nanowires. Silicon 2017, 10, 1427–1431. [Google Scholar] [CrossRef]
- Kim, J.-H.; Katoch, A.; Choi, S.-W.; Kim, S.S. Growth and sensing properties of networked p-CuO nanowires. Sensors Actuators B. Chem. 2015, 212, 190–195. [Google Scholar] [CrossRef]
- Shaban, M.; Rabia, M.; Fathallah, W.; El-Mawgoud, N.A.; Mahmoud, A.; Hussien, H.; Said, O. Preparation and characterization of polyaniline and Ag/polyaniline composite nanoporous particles and their antimicrobial activities. J. Polym. Environ. 2017, 26, 434–442. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Rabia, M.; Shaban, M.; Verpoort, F. Heulandite/polyaniline hybrid composite for efficient removal of acidic dye from water; kinetic, equilibrium studies and statistical optimization. Adv. Powder Technol. 2018, 29, 2501–2511. [Google Scholar] [CrossRef]
- Fadel, M.S.S.; Rabia, M.; Ezzat, S.; Mansour, N.; Saeed, E.; Sayyah, S.M. Effect of annealing temperature on VO2(M)/ITO film nanomaterials for thermochromic smart windows application and study its contact angle. J. Nanophotonics 2018, 12, 016009. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Rabia, M.; Shaban, M. The structure and photoelectrochemical activity of Cr-doped PbS thin films grown by chemical bath deposition. RSC Adv. 2020, 10, 14458–14470. [Google Scholar] [CrossRef]
- Ravichandran, A.T.; Dhanabalan, K.; Vasuhi, A.; Chandramohan, R.; Mantha, S. Morphology, bandgap, and grain size tailoring in Cu2O thin film by SILAR method. IEEE Trans. Nanotechnol. 2015, 14, 108–112. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Z. Study on light intensity in the process of photocatalytic degradation of indoor gaseous formaldehyde for saving energy. Energy Convers. Manag. 2007, 48, 882–889. [Google Scholar] [CrossRef]
- Baniasadi, E.; Dincer, I.; Naterer, G.F. Measured effects of light intensity and catalyst concentration on photocatalytic hydrogen and oxygen production with zinc sulfide suspensions. Int. J. Hydrogen Energy 2013, 38, 9158–9168. [Google Scholar] [CrossRef]
- Sayyah, S.M.; Shaban, M.; Rabia, M. A sensor of m-toluidine/m-cresol polymer film for detection of lead ions by potentiometric methods. Sens. Lett. 2016, 14, 522–529. [Google Scholar] [CrossRef]
- Sayyah, S.M.; Shaban, M.; Khaliel, A.B.; Rabia, M. Electropolymerization of M-Cresol on Platinum Electrode from Aqueous Acidic Solution. Int. J. Chem. 2014, 35, 2051–2732. [Google Scholar]
- Razek, S.A.; Popeil, M.R.; Wangoh, L.; Rana, J.; Suwandaratne, N.; Andrews, J.L.; Watson, D.F.; Banerjee, S.; Piper, L.F.J. Designing catalysts for water splitting based on electronic structure considerations. Electron. Struct. 2020, 2, 023001. [Google Scholar] [CrossRef]
- Freeman, E.; Kumar, S.; Thomas, S.R.; Pickering, H.; Fermin, D.J.; Eslava, S. PrFeO3 Photocathodes prepared through spray pyrolysis. ChemElectroChem 2020, 7, 1365–1372. [Google Scholar] [CrossRef]
- Rabia, M.; Shaban, M.; Jibali, B.M.; Abdelkhaliek, A.A. Effect of Annealing Temperature on the Photoactivity of ITO/VO2 (M)/Au Film Electrodes for Water Splitting. J. Nanosci. Nanotechnol. 2020, 20, 4120–4130. [Google Scholar] [CrossRef] [PubMed]
- Potashnikov, V.; Golub, A.; Brody, M.; Lugovoy, O. Decarbonizing Russia: Leapfrogging from Fossil Fuel to Hydrogen. Energies 2022, 15, 683. [Google Scholar] [CrossRef]
- Xiao, X.; Engelbrekt, C.; Zhang, M.; Li, Z.; Ulstrup, J.; Zhang, J.; Si, P. A straight forward approach to electrodeposit tungsten disulfide/poly(3,4-ethylenedioxythiophene) composites onto nanoporous gold for the hydrogen evolution reaction. Appl. Surf. Sci. 2017, 410, 308–314. [Google Scholar] [CrossRef]
- Modibane, K.D.; Waleng, N.J.; Ramohlola, K.E.; Maponya, T.C.; Monama, G.R.; Makgopa, K.; Hato, M.J. Poly(3-aminobenzoic acid) Decorated with Cobalt Zeolitic Benzimidazolate Framework for Electrochemical Production of Clean Hydrogen. Polymers 2020, 12, 1581. [Google Scholar] [CrossRef] [PubMed]
- Shaban, M.; Ahmed, A.M.; Abdel-Rahman, E.; Hamdy, H. Tunability and sensing properties of plasmonic/1d photonic crystal. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Sayyah, S.M.; Shaban, M.; Rabia, M. Electropolymerization of m-toluidin on platinum electrode from aqueous acidic solution and character of the obtained polymer. Adv. Polym. Technol. 2018, 37, 126–136. [Google Scholar] [CrossRef]
- Sayyah, E.S.M.; Shaban, M.; Rabia, M. A sensor of m-cresol nanopolymer/Pt-electrode film for detection of lead ions by potentiometric methods. Adv. Polym. Technol. 2018, 37, 1296–1304. [Google Scholar] [CrossRef]
- Arrhenius, S. Über die Dissociationswärme und den Einfluss der Temperatur auf den Dissociationsgrad der Elektrolyte. Z. Phys. Chem. 1889, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Awad, M.A.; Shaban, M.; Rabia, M. The efficiency of M (M = Li, Na, or Cs) doped CdS nanomaterials in optoelectronic applications. Int. J. Energy Res. 2022. [Google Scholar] [CrossRef]
- Rabia, M.; Mohamed, S.H.; Zhao, H.; Shaban, M.; Lei, Y.; Ahmed, A.M. TiO2/TiOxNY hollow mushrooms-like nanocomposite photoanode for hydrogen electrogeneration. J. Porous Mater. 2020, 27, 133–139. [Google Scholar] [CrossRef]
- Shaban, M.; Rabia, M.; Eldakrory, M.G.; Maree, R.M.; Ahmed, A.M. Efficient photoselectrochemical hydrogen production utilizing of APbI3 (A = Na, Cs, and Li) perovskites nanorods. Int. J. Energy Res. 2021, 45, 7436–7446. [Google Scholar] [CrossRef]
- Rabia, M.; Shaban, M.; Adel, A.; Abdel-Khaliek, A.A. Effect of plasmonic au nanoparticles on the photoactivity of polyaniline/indium tin oxide electrodes for water splitting. Environ. Prog. Sustain. Energy 2019, 38, 13171. [Google Scholar] [CrossRef]
- Eyring, H. The activated complex in chemical reactions. J. Chem. Phys. 1935, 3, 63–71. [Google Scholar] [CrossRef]
- Shaban, M.; Benghanem, M.; Almohammedi, A.; Rabia, M. Optimization of the Active Layer P3HT:PCBM for Organic Solar Cell. Coatings 2021, 11, 863. [Google Scholar] [CrossRef]
- Helmy, A.; Rabia, M.; Shaban, M.; Ashraf, A.M.; Ahmed, S.; Ahmed, A.M. Graphite/rolled graphene oxide/carbon nanotube photoelectrode for water splitting of exhaust car solution. Int. J. Energy Res. 2020, 44, 7687–7697. [Google Scholar] [CrossRef]
- Mohamed, H.S.H.; Rabia, M.; Shaban, M.; Taha, S. Controlled synthesis of CdS nanoflowers thin films for H2 electro-generation. Mater. Sci. Semicond. Process. 2020, 120, 105307. [Google Scholar] [CrossRef]
- Koya, A.N.; Zhu, X.; Ohannesian, N.; Yanik, A.A.; Alabastri, A.; Proietti Zaccaria, R.; Krahne, R.; Shih, W.C.; Garoli, D. Nanoporous metals: From plasmonic properties to applications in enhanced spectroscopy and photocatalysis. ACS Nano 2021, 15, 6038–6060. [Google Scholar] [CrossRef] [PubMed]
- Podder, S.; Pal, A.R. Plasmonic visible-NIR photodetector based on hot electrons extracted from nanostructured titanium nitride. J. Appl. Phys. 2019, 126, 083108. [Google Scholar] [CrossRef]
- Hussain, A.A.; Sharma, B.; Barman, T.; Pal, A.R. Self-powered broadband photodetector using plasmonic titanium nitride. ACS Appl. Mater. Interfaces 2016, 8, 4258–4265. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.A.; Pal, A.R.; Patil, D.S. An efficient fast response and high-gain solar-blind flexible ultraviolet photodetector employing hybrid geometry. Appl. Phys. Lett. 2014, 104, 193301. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, G.; Mi, J.; Wu, Z. Fabrication of visible-light-driven one-dimensional anatase TiO2/Ag heterojunction plasmonic photocatalyst. Catal. Commun. 2012, 24, 48–51. [Google Scholar] [CrossRef]
- Cao, Q.; Yu, J.; Cao, Y.; Delaunay, J.J.; Che, R. Unusual effects of vacuum annealing on large-area Ag3PO4 microcrystalline film photoanode boosting cocatalyst- and scavenger-free water splitting. J. Mater. 2021, 7, 929–939. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Yuan, K.; Yang, J.G.; Ma, H.P.; Wang, T.; Ji, X.M.; Feng, J.J.; Devi, A.; Lu, H.L. Fabrication of heterostructured p-CuO/n-SnO2 core-shell nanowires for enhanced sensitive and selective formaldehyde detection. Sens. Actuators B Chem. 2019, 290, 233–241. [Google Scholar] [CrossRef]
- Cao, Q.; Hao, S.; Wu, Y.; Pei, K.; You, W.; Che, R. Interfacial charge redistribution in interconnected network of Ni2P–Co2P boosting electrocatalytic hydrogen evolution in both acidic and alkaline conditions. Chem. Eng. J. 2021, 424, 130444. [Google Scholar] [CrossRef]
- Sayyah, S.M.; Shaban, M.; Rabia, M. M-toluidine polymer film coated platinum electrode as a pH sensor by potentiometric methods. Sens. Lett. 2015, 13, 961–966. [Google Scholar] [CrossRef]
- Sayyah, S.M.; Shaban, M.; Rabia, M. A High-sensitivity potentiometric mercuric ion sensor based on m-toluidine films. IEEE Sens. J. 2016, 16, 1541–1548. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, M.; Sun, R.; Long, G.; Liu, Y.; Zhao, W. Enhanced hydrogen evolution from CuOx-C/TiO2 with multiple electron transport pathways. PLoS ONE 2019, 14, 0215339. [Google Scholar] [CrossRef]
- Li, J.; Jin, X.; Li, R.; Zhao, Y.; Wang, X.; Liu, X.; Jiao, H. Copper oxide nanowires for efficient photoelectrochemical water splitting. Appl. Catal. B Environ. 2019, 240, 1–8. [Google Scholar] [CrossRef]
- Baran Aydin, E. Fabrication and characterization of CuO nanostructures: Applications in electrocatalytic hydrogen production. Çukurova Univ. J. Fac. Eng. Archit. 2020, 35, 127–138. [Google Scholar]
- Masudy-Panah, S.; Moakhar, R.S.; Chua, C.S.; Tan, H.R.; Wong, T.I.; Chi, D.; Dalapati, G.K. Nanocrystal Engineering of sputter-grown CuO photocathode for visible-light-driven electrochemical water splitting. ACS Appl. Mater. Interfaces 2016, 8, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, H.; Isobe, K.; Kozuka, H. Preparation of porous CuO films from Cu(NO3)2 aqueous solutions containing poly(vinylpyrrolidone) and their photocathodic properties. RSC Adv. 2017, 7, 18014–18018. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Al Otaibi, B.; Benetti, D.; Li, S.; Zhao, H.; Mi, Z.; Vomiero, A.; Rosei, F. Near-infrared colloidal quantum dots for efficient and durable photoelectrochemical solar-driven hydrogen production. Adv. Sci. 2016, 3, 1500345. [Google Scholar] [CrossRef] [Green Version]
- Ebaid, M.; Kang, J.-H.; Ryu, S.-W. Controlled synthesis of GaN-based nanowires for photoelectrochemical water splitting applications. Semicond. Sci. Technol. 2016, 32, 013001. [Google Scholar] [CrossRef]
- Li, Z.; Feng, S.; Liu, S.; Li, X.; Wang, L.; Lu, W. A three-dimensional interconnected hierarchical FeOOH/TiO2/ZnO nanostructural photoanode for enhancing the performance of photoelectrochemical water oxidation. Nanoscale 2015, 7, 19178–19183. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.D.; Ashford, D.L.; Lapides, A.M.; Sheridan, M.V.; Wee, K.-R.; Meyer, T.J. Light-Driven water splitting with a molecular electroassembly-based core/shell photoanode. J. Phys. Chem. Lett. 2015, 6, 3213–3217. [Google Scholar] [CrossRef]
- Shaban, M.; Rabia, M.; El-Sayed, A.M.A.; Ahmed, A.; Sayed, S. Photocatalytic properties of PbS/graphene oxide/polyaniline electrode for hydrogen generation. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Naldoni, A.; Guler, U.; Wang, Z.; Marelli, M.; Malara, F.; Meng, X.; Besteiro, L.V.; Govorov, A.O.; Kildishev, A.V.; Boltasseva, A.; et al. Broadband hot-electron collection for solar water splitting with plasmonic titanium nitride. Adv. Opt. Mater. 2017, 5, 1601031. [Google Scholar] [CrossRef] [Green Version]
| Material or Element | Concentration (mg/L) |
|---|---|
| Phenols | 0.015 |
| F− | 1.0 |
| Al3+ | 3.0 |
| NH3 | 5.0 |
| Hg2+ | 0.005 |
| Pb2+ | 0.5 |
| Cd3+ | 0.05 |
| As3+ | 0.05 |
| Cr3+ | 1.0 |
| Cu2+ | 1.5 |
| Ni3+ | 0.1 |
| Fe3+ | 1.5 |
| Mn2+ | 1.0 |
| Zn2+ | 5.0 |
| Ag+ | 0.1 |
| Ba3+ | 2.0 |
| Co2+ | 2.0 |
| Other cations | 0.1 |
| Pesticides | 0.2 |
| CN−1 | 0.1 |
| Industrial washing | 0.5 |
| Coli groups | 4000/100 cm3 |
| Photoelectrode | Electrolyte | Jph (mA/cm2) | Applied Voltage (V) | IPCE% (390 nm) | Light Source |
|---|---|---|---|---|---|
| g-C3N4-CuO [23] | NaOH | 0.01 | 1.6 | - | 300 W xenon lamp |
| CuO-C/TiO2 [76] | glycerol | 0.001 | −0.5 | - | 300 W xenon lamp |
| CuO nanowire [77] | Na2SO4 | 1.5 | −0.5 | - | simulated AM1.5 illumination |
| CuO nanostructure [78] | KOH | 1 | −1.2 | - | White light |
| CuO thin films [79] | Na2SO4 | 2.5 | 0 | 3.1 | Solar simulator 1.5 global (AM 1.5G) |
| CuO nanocrystals [80] | Na2SO4 | 1.1 | −0.5 | 8.7 | Xenon lamp light |
| TiO2/CdS/PbS [81] | Na2S/Na2S2O3 | 2 | 0.2 | 4 | AM 1.5G illumination |
| GaN [82] | HBr | 0.6 | +1 | 8 | Sunlight |
| ZnO/TiO2/FeOOH [83] | Na2S2O3 | 1.59 | 0.8 | - | A 150 W xenon lamp |
| SnO2/TiO2 [84] | Na2S2O3 | 0.4 | 0.6 | - | 1 Sun (100 mW cm−2) |
| Au/PbS/Ro-GO/PANI [85] | Na2S2O3 | 1.1 | +1 | 10 | 400 W xenon lamp |
| TiN-TiO2 [86] | NaOH | 3.0 × 10−4 | 0.2 | 0.03 | Solar simulator (150 mW cm−2) |
| BiFeO3 [29] | NaOH | 0.1 | 1.6 | 0.21 | 1 sun (AM 1.5G solar spectrum) |
| ITO/VO2 [50] | Na2S2O3 | 1.5 | +1 | 4 | 400 W metal halid |
| PrFeO [49] | Na2SO4 | 0.130 | −0.6 | - | Simulated sunlight |
| Poly(3-aminobenzoic acid) frame [53] | H2SO4 | 1.2 | 1.6 | - | 150 W xenon lamp |
| Cu/CuO (Present work) | Sewage water | 4.7 | +1 | 14.6 | Simulated sunlight |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadia, N.M.A.; Abdelazeez, A.A.A.; Alzaid, M.; Shaban, M.; Mohamed, S.H.; Hoex, B.; Hajjiah, A.; Rabia, M. Converting Sewage Water into H2 Fuel Gas Using Cu/CuO Nanoporous Photocatalytic Electrodes. Materials 2022, 15, 1489. https://doi.org/10.3390/ma15041489
Hadia NMA, Abdelazeez AAA, Alzaid M, Shaban M, Mohamed SH, Hoex B, Hajjiah A, Rabia M. Converting Sewage Water into H2 Fuel Gas Using Cu/CuO Nanoporous Photocatalytic Electrodes. Materials. 2022; 15(4):1489. https://doi.org/10.3390/ma15041489
Chicago/Turabian StyleHadia, N. M. A., Ahmed Adel A. Abdelazeez, Meshal Alzaid, Mohamed Shaban, S. H. Mohamed, Bram Hoex, Ali Hajjiah, and Mohamed Rabia. 2022. "Converting Sewage Water into H2 Fuel Gas Using Cu/CuO Nanoporous Photocatalytic Electrodes" Materials 15, no. 4: 1489. https://doi.org/10.3390/ma15041489
APA StyleHadia, N. M. A., Abdelazeez, A. A. A., Alzaid, M., Shaban, M., Mohamed, S. H., Hoex, B., Hajjiah, A., & Rabia, M. (2022). Converting Sewage Water into H2 Fuel Gas Using Cu/CuO Nanoporous Photocatalytic Electrodes. Materials, 15(4), 1489. https://doi.org/10.3390/ma15041489







