Local Coordination Environment of 3d and 4d Transition Metal Ions in LiCl-KCl Eutectic Mixture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Experimental Methods
2.3. Computational Methods
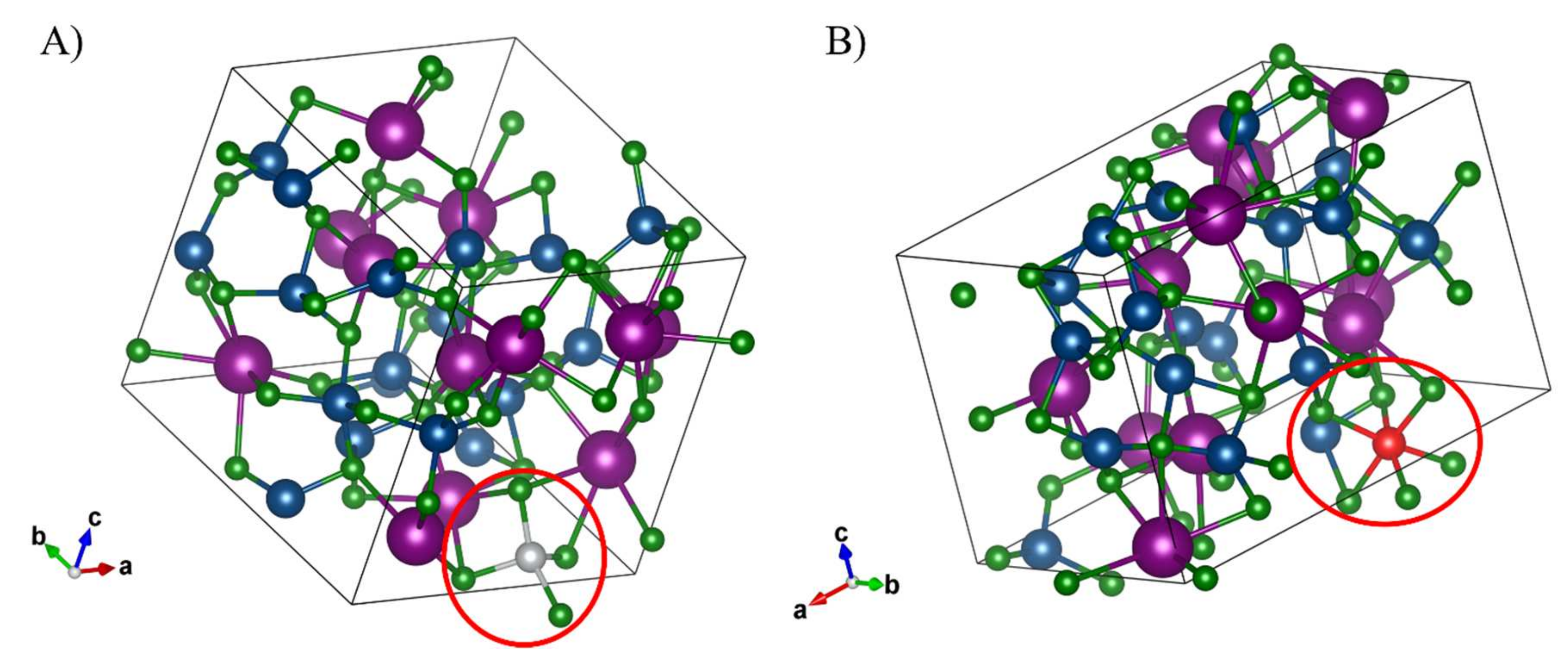
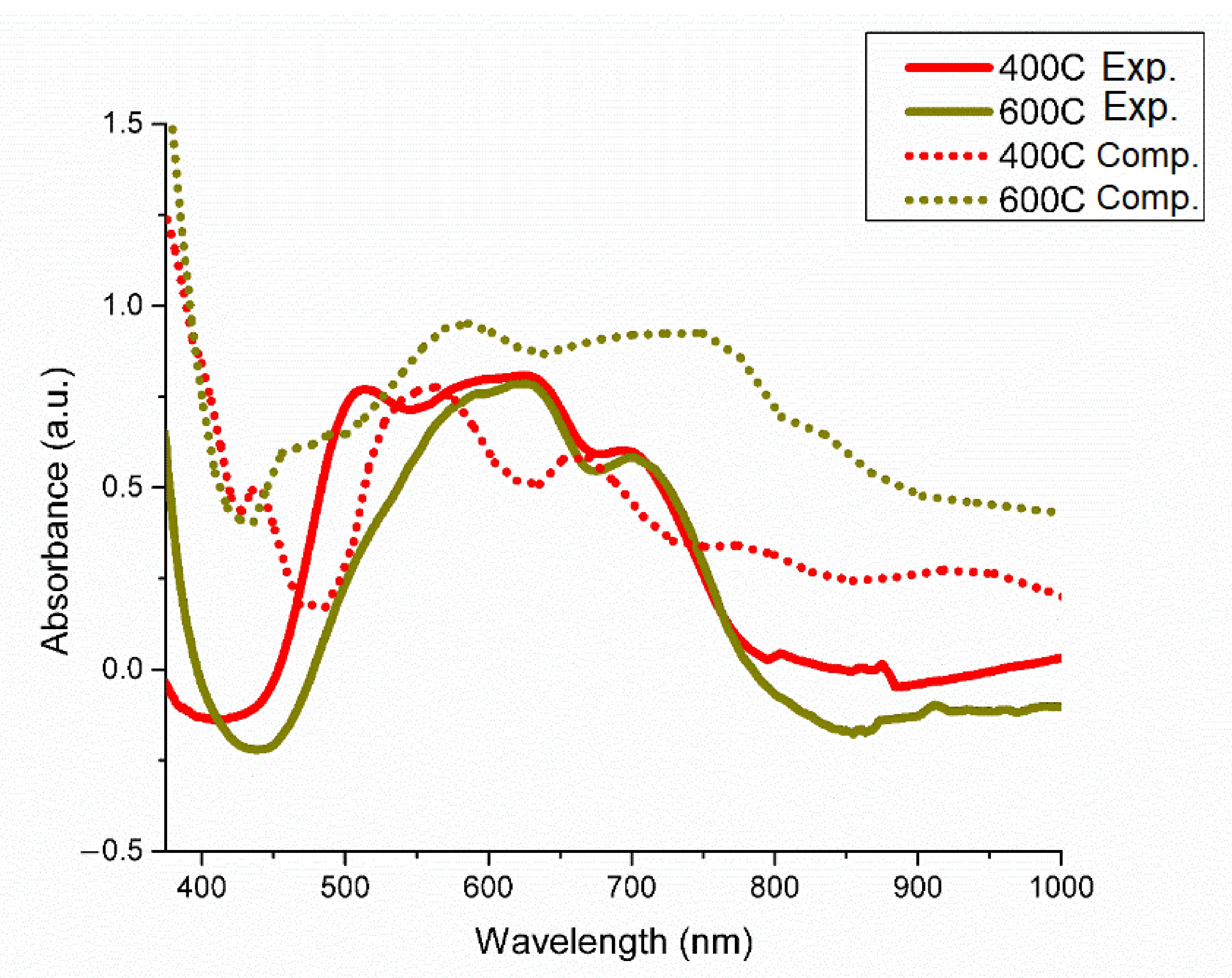
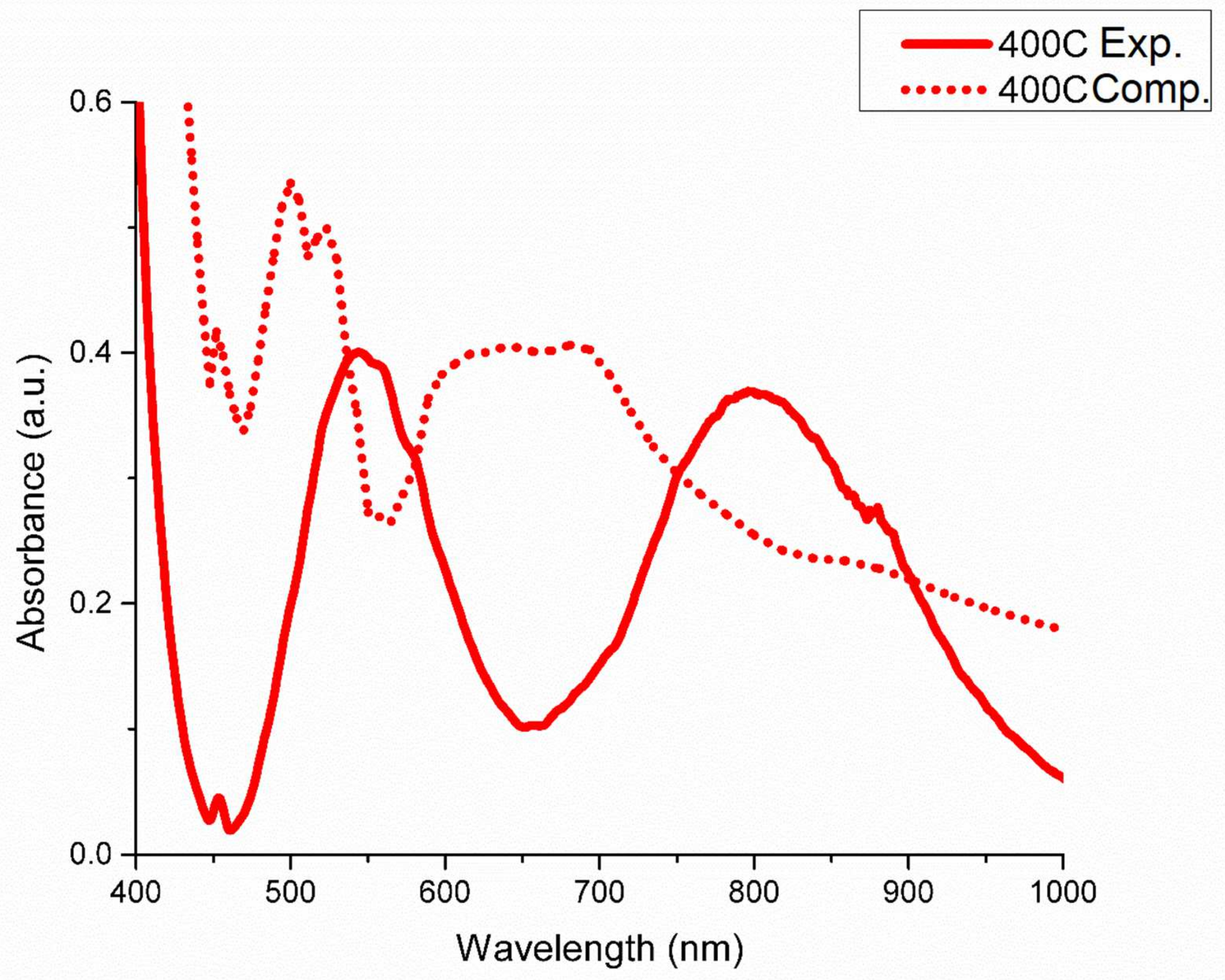
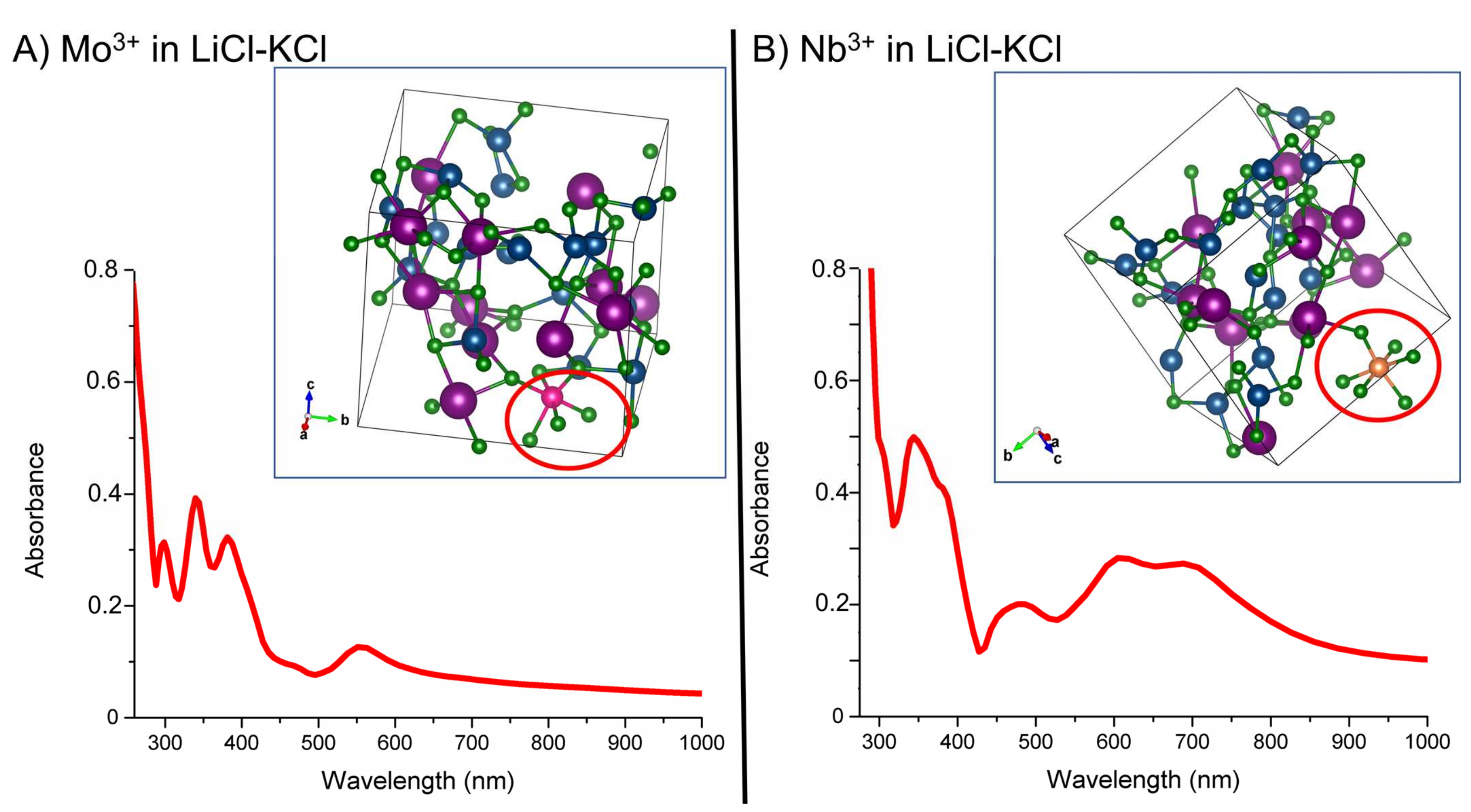
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Volkov, S.V. Coordination Compounds in Melts. In Molten Salts: From Fundamentals to Applications; Springer: Dordrecht, The Netherlands, 2012; pp. 357–373. [Google Scholar]
- Sridharan, K.; Allen, T.R. Corrosion in Molten Salts. In Molten Salts Chemistry; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 241–267. [Google Scholar]
- Li, S.X. Anodic Process of Electrorefining Spent Nuclear Fuel in Molten LiCl-KCl-UCl3/Cd System. ECS Proc. Vol. 2002, 2002, 541. [Google Scholar] [CrossRef]
- Zhang, J. Electrochemistry of actinides and fission products in molten salts—Data review. J. Nucl. Mater. 2014, 447, 271–284. [Google Scholar] [CrossRef]
- Barbanel, Y.A.; Kolin, V.V.; Kotlin, V.P.; Lumpov, A.A. Coordination chemistry of actinides in molten salts. J. Radioanal. Nucl. Chem. Artic. 1990, 143, 167–179. [Google Scholar] [CrossRef]
- Harrison, T.J.; Holcomb, D.E.; Flanagan, G.F.; Patton, B.W.; Gehin, J.C.; Howard, R.L. Fast Spectrum Molten Salt Reactor Options; ORNL: Oak Ridge, TN, USA, 2011. Available online: https://info.ornl.gov/sites/publications/Files/Pub29596.pdf (accessed on 22 November 2021).
- Fujii, T.; Nagai, T.; Sato, N.; Shirai, O.; Yamana, H. Electronic absorption spectra of lanthanides in a molten chloride: II. Absorption characteristics of neodymium(III) in various molten chlorides. J. Alloys Compd. 2005, 393, L1–L5. [Google Scholar] [CrossRef]
- Yatsimirskii, K.B. Spectroscopic studies on coordination compounds formed in molten salts. In Non-Aqueous Solutions–5; Pergamon: Leeds, UK, 1977. [Google Scholar] [CrossRef]
- Phillips, W.C.; Gakhar, R.; Horne, G.P.; Layne, B.; Iwamatsu, K.; Ramos-Ballesteros, A.; Shaltry, M.R.; LaVerne, J.A.; Pimblott, S.M.; Wishart, J.F. Design and performance of high- temperature furnace and cell holder for in situ spectroscopic, electrochemical, and radiolytic investigations of molten salts Design and performance of high-temperature furnace and cell holder for in situ spectroscopic. Rev. Sci. Instrum. 2020, 91, 083105. [Google Scholar] [CrossRef]
- Smith, G.P.; James, D.W.; Boston, C.R. Optical Spectra of Tl+, Pb2+, and Bi3+ in the Molten Lithium Chloride—Potassium Chloride Eutectic. J. Chem. Phys. 1965, 42, 2249–2250. [Google Scholar] [CrossRef]
- Brynestad, J.; Boston, C.R.; Smith, G.P. Electronic spectra and coordination of nickel centers in liquid lithium chloride-potassium chloride mixtures. J. Chem. Phys. 1967, 47, 3179–3189. [Google Scholar] [CrossRef]
- Smith, W.E.; Brynestad, J.; Smith, G.P.; Smith, W.E.; Brynestad, O.; Smitht, G.P. Spectroscopic Behavior and Coordination of Nickel(II) in Liquid Mixtures of Zinc and Cesium Chlorides Spectroscopic Behavior and Coordination of Nickel(II) in Liquid Mixtures of Zinc and Cesium Chlorides. J. Chem. Phys. 1970, 52, 3890. [Google Scholar] [CrossRef]
- Gruen, D.M.; Division, C. Octahedral and tetrahedral co-ordination states of cobalt (II) in molten zinc chloride—aluminium chloride mixtures. J. Inorg. Nucl. Chem. 1967, 29, 2243–2247. [Google Scholar]
- Gruen, D.M.; McBeth, R.L. The coordination chemistry of 3d transition metal ions in fused salt solutions. Pure Appl. Chem. 1963, 6, 23–48. [Google Scholar] [CrossRef]
- Brookes, H.C.; Flengas, S.N. Spectroscopy of chromium compounds in molten salts. Can. J. Chem. 1970, 48, 55–58. [Google Scholar] [CrossRef]
- Jørgensen, C.K. Ligand field bands of four-coordinated paramagnetic nickel(ii) complexes. Mol. Phys. 1958, 1, 410–412. [Google Scholar] [CrossRef]
- Cho, Y.H.; Kim, T.J.; Park, Y.J.; Im, H.J.; Song, K. Electronic absorption spectra of Sm(II) and Yb(II) ions in a LiCl-KCl eutectic melt at 450 °C. J. Lumin. 2010, 130, 280–282. [Google Scholar] [CrossRef]
- Banks, C.V.; Heusinkveld, M.R.; O’laughlin, J.W. Absorption Spectra of the Lanthanides in Fused Lithium Chloride—Potassium Chloride Eutectic. Anal. Chem. 1961, 33, 1235–1240. [Google Scholar] [CrossRef]
- Kim, B.Y.; Yun, J.I. Optical absorption and fluorescence properties of trivalent lanthanide chlorides in high temperature molten LiCl-KCl eutectic. J. Lumin. 2016, 178, 331–339. [Google Scholar] [CrossRef]
- Yang, L.; Cohen, M.L.; Louie, S.G. Excitonic effects in the optical spectra of graphene nanoribbons. Nano Lett. 2007, 7, 3112–3115. [Google Scholar] [CrossRef] [Green Version]
- Marini, A.; Del Sole, R. Dynamical excitonic effects in metals and semiconductors. Phys. Rev. Lett. 2003, 91, 176402. [Google Scholar] [CrossRef] [Green Version]
- Kunz, B.; Flynn, C.P. Excitonic effects in the interband spectra of metals. Phys. Rev. Lett. 1983, 50, 1524–1527. [Google Scholar] [CrossRef]
- Wang, H. Excitonic effects and optical spectra of graphene nanoflakes. J. Appl. Phys. 2017, 122, 084301. [Google Scholar] [CrossRef]
- Kresse, G. Ab initio molecular dynamics for liquid metals. J. Non. Cryst. Solids 1995, 192–193, 222–229. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metalamorphous- semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B—Condens. Matter Mater. Phys. 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Ernzerhof, M.; Perdew, J.P.; Burke, K. Coupling-constant dependence of atomization energies. Int. J. Quantum Chem. 1997, 64, 285–295. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Gajdoš, M.; Hummer, K.; Kresse, G.; Furthmüller, J.; Bechstedt, F. Linear optical properties in the projector-augmented wave methodology. Phys. Rev. B—Condens. Matter Mater. Phys. 2006, 73, 045112. [Google Scholar] [CrossRef] [Green Version]
- Wang, V.; Xu, N.; Liu, J.-C.; Tang, G.; Geng, W.T. VASPKIT: A Pre-and Post-Processing Program for VASP code. Comp. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.K.; Huang, J.; Mausz, J.; Gakhar, R.; Roy, S.; Vila, F.; Topsakal, M.; Phillips, W.C.; Layne, B.; Frenkel, A.I.; et al. Connections between the Speciation and Solubility of Ni(II) and Co(II) in Molten ZnCl2. J. Phys. Chem. B 2020, 124, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.; Sundheim, B.R. Absorption spectra in molten salt solutions. J. Chem. Phys. 1958, 29, 1029–1032. [Google Scholar] [CrossRef]
- Sundheim, R.; Harrington, G. Absorption spectrum of NiCl2 in molten LiCl/KCl. J. Chem. Phys. 1959, 31, 700–701. [Google Scholar] [CrossRef]
- Gabriel, J.; Vincent, D.; Bouteillon, J.; Poignet, J.; Volkovich, V.; Griffiths, T. Molybdenum chemistry in molten LiCl–KCl eutectic: An electrochemical and absorption spectroscopy study of the concentration dependent stability of solutions of K3MoCl6. Electrochim. Acta 1999, 44, 4619–4629. [Google Scholar] [CrossRef]
- Brevnova, N.P.; Polovov, I.B.; Chernyshov, M.V.; Volkovich, V.A.; Vasin, B.D.; Griffiths, T.R. Electronic Absorption Spectra of Niobium Species in Halide Melts. ECS Trans. 2013, 50, 325–338. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuller, J.; Phillips, W.; An, Q.; Gakhar, R. Local Coordination Environment of 3d and 4d Transition Metal Ions in LiCl-KCl Eutectic Mixture. Materials 2022, 15, 1478. https://doi.org/10.3390/ma15041478
Fuller J, Phillips W, An Q, Gakhar R. Local Coordination Environment of 3d and 4d Transition Metal Ions in LiCl-KCl Eutectic Mixture. Materials. 2022; 15(4):1478. https://doi.org/10.3390/ma15041478
Chicago/Turabian StyleFuller, Jon, William Phillips, Qi An, and Ruchi Gakhar. 2022. "Local Coordination Environment of 3d and 4d Transition Metal Ions in LiCl-KCl Eutectic Mixture" Materials 15, no. 4: 1478. https://doi.org/10.3390/ma15041478
APA StyleFuller, J., Phillips, W., An, Q., & Gakhar, R. (2022). Local Coordination Environment of 3d and 4d Transition Metal Ions in LiCl-KCl Eutectic Mixture. Materials, 15(4), 1478. https://doi.org/10.3390/ma15041478





