Humus Acids in the Digested Sludge and Their Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
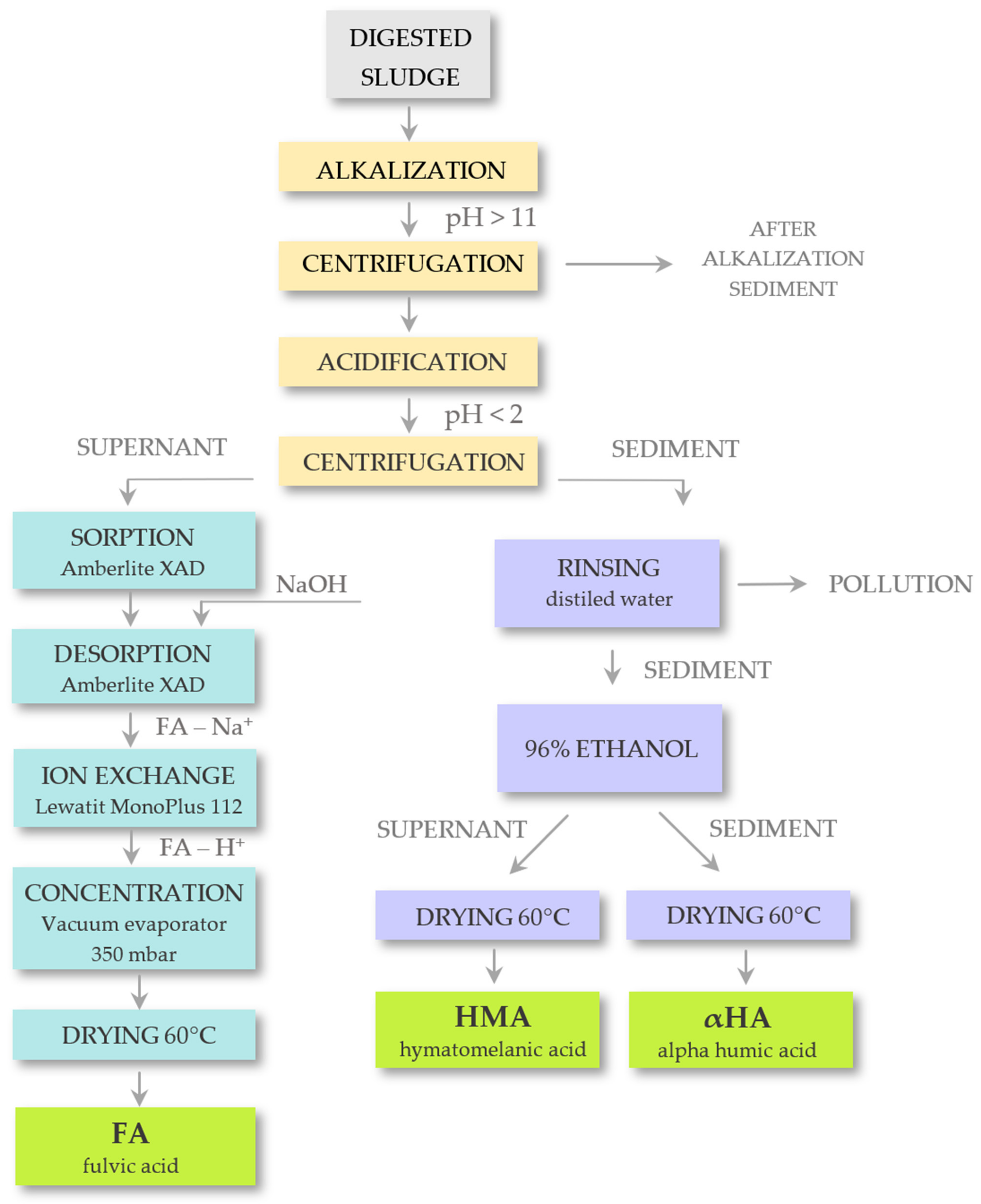
2.2. Procedure of HAs Extraction and Analytic
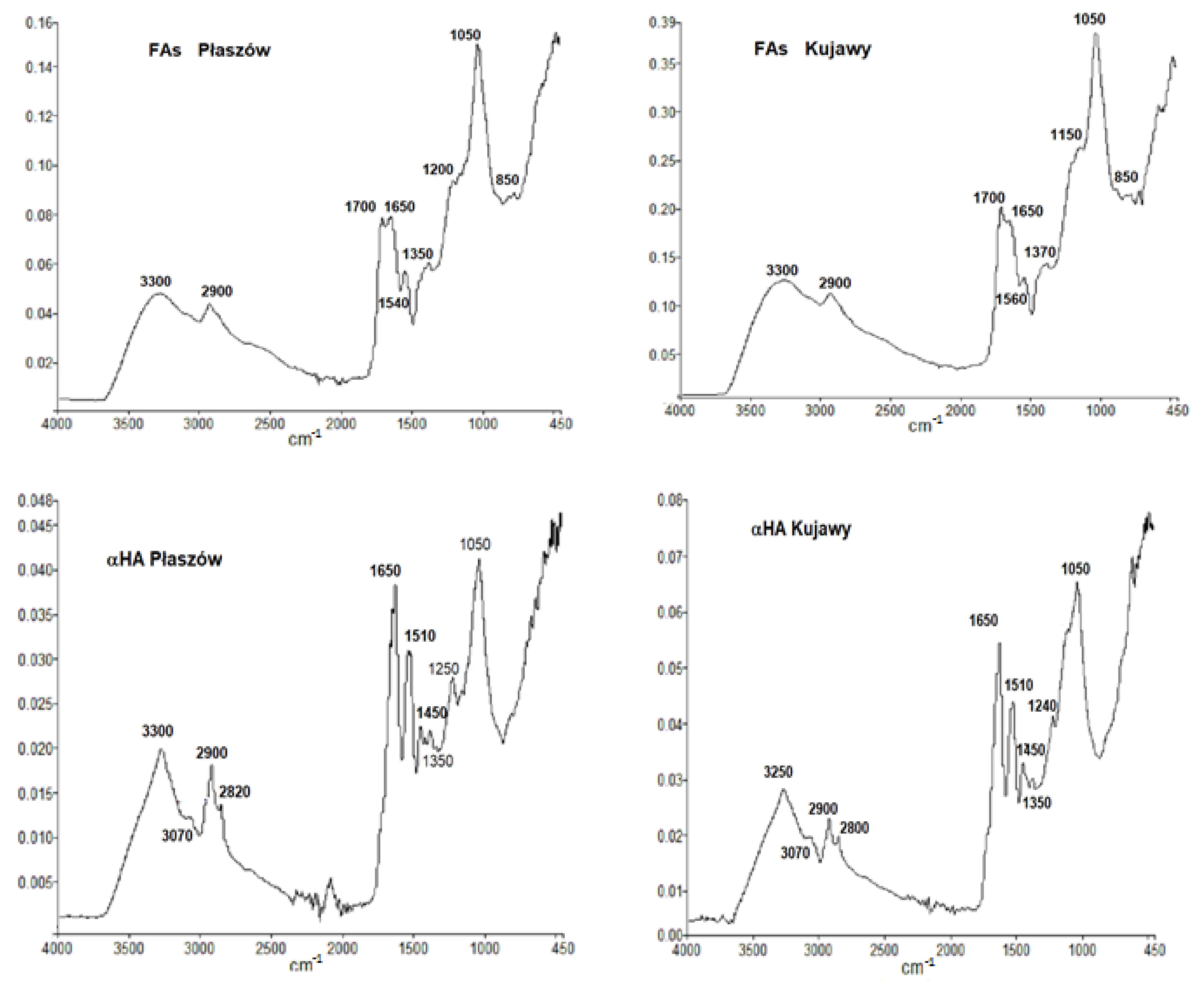
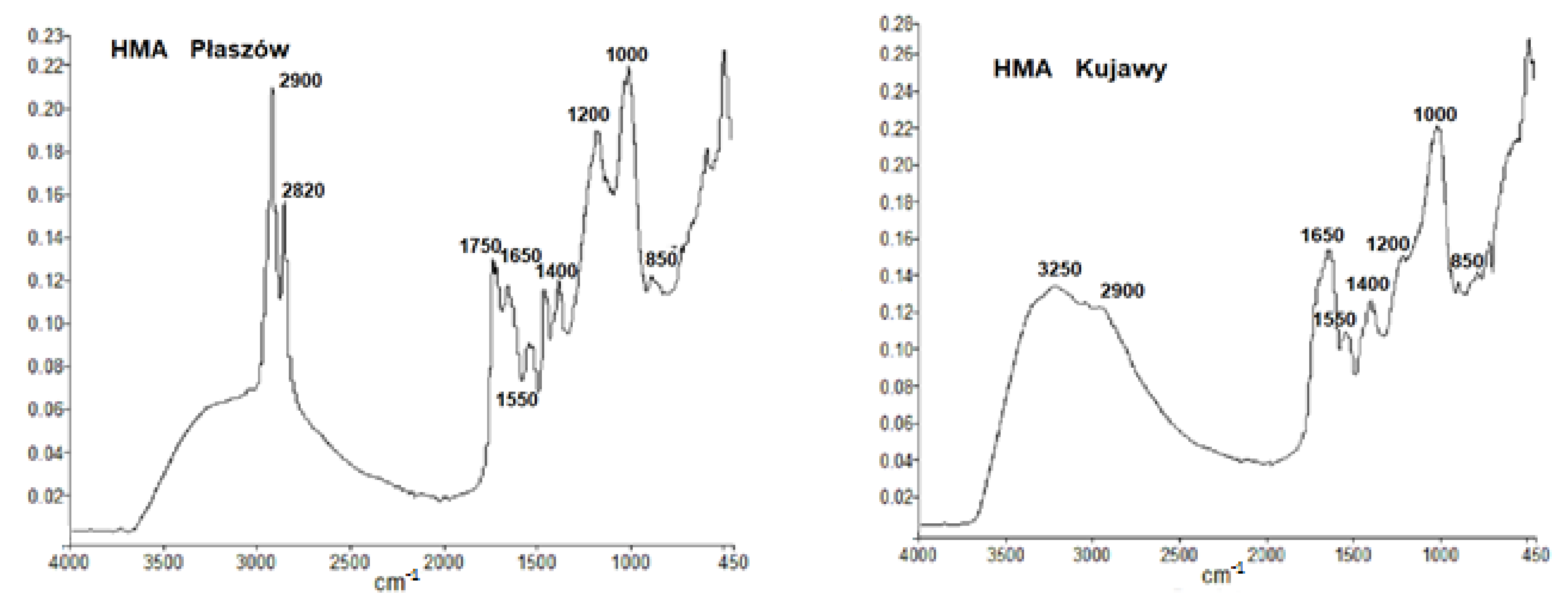
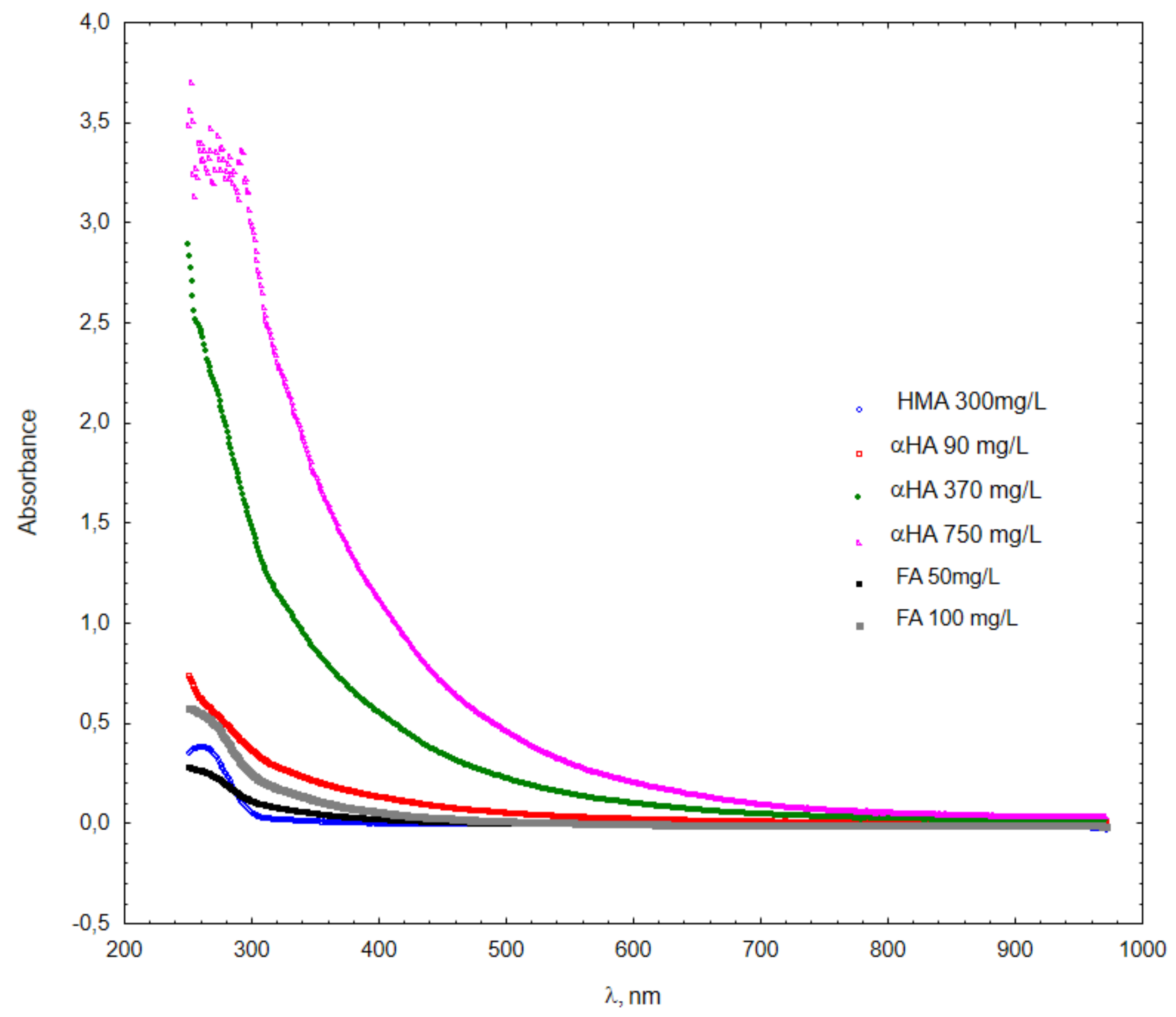
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Machado, W.; Franchini, J.C.; de Fátima Guimarães, M.; Filho, J.T. Spectroscopic characterization of humic and fulvic acids in soil aggregates. Heliyon 2020, 6, e04078. [Google Scholar] [CrossRef]
- Chua, S.F.; Nouri, A.; Ang, W.L.; Mahmoudi, E.; Ba-Abbad, M. The emergence of multifunctional adsorbents and their role in environmental remediation. J. Environ. Chem. Eng. 2021, 9, 104793. [Google Scholar] [CrossRef]
- Satisha, G.C.; Devarajan, L. Humic substances and their complexation with phosphorus and calcium during composting of pressmud and other biodegradables. Commun. Soil Sci. Plant Anal. 2005, 36, 805–818. [Google Scholar] [CrossRef]
- Gong, G.Q.; Zhao, Y.F.; Zhang, Y.J.; Deng, B.; Liu, W.X.; Wang, M.; Yuan, X.; Xu, L.W. Establishment of a molecular structure model for classified products of coal-based fulvic acid. Fuel 2020, 267, 117210. [Google Scholar] [CrossRef]
- Tan, W.; Jia, Y.; Huang, C.; Zhang, H.; Li, D.; Zhao, X.; Wang, G.; Jiang, J.; Xi, B. Increased suppression of methane production by humic substances in response to warming in anoxic environments. J. Environ. Manag. 2018, 206, 602–606. [Google Scholar] [CrossRef]
- Zashikhina, A.V.; Sviridova, M.L. Gold Leaching with Humic Substances. J. Min. Sci. 2019, 55, 652–657. [Google Scholar] [CrossRef]
- Khan, R.; Jain, P.; Aqil, M.; Agarwal, S.P.; Mirza, M.A.; Iqbal, Z. Pharmacokinetic evaluation of fulvic acid-ketoconazole complexes: A validation and line extension study. J. Drug Deliv. Sci. Technol. 2020, 55, 101469. [Google Scholar] [CrossRef]
- Mou, J.; Li, C.; Wang, G.; Shi, W. Complexation and sorption studies of Co(II) with [gamma]-alumina-bound fulvic acid: Effect of pH, ionic strength, fulvic acid and alumina concentration. J. Radioanal. Nucl. Chem. 2012, 292, 1099–1104. [Google Scholar] [CrossRef]
- Efimova, I.V.; Khil’ko, S.L.; Smirnova, O.V.; Berezhnoi, V.S.; Rybachenko, V.I. Antioxidant properties of hymatomelanic acids from brown coal. Solid Fuel Chem. 2013, 47, 193–196. [Google Scholar] [CrossRef]
- Kapoore, R.V.; Wood, E.E.; Llewellyn, C.A. Algae biostimulants: A critical look at microalgal biostimulants for sustainable agricultural practices. Biotechnol. Adv. 2021, 49, 107754. [Google Scholar] [CrossRef]
- Xu, D.; Deng, Y.; Xi, P.; Yu, G.; Wang, Q.; Zeng, Q.; Jiang, Z.; Gao, L. Fulvic acid-induced disease resistance to Botrytis cinerea in table grapes may be mediated by regulating phenylpropanoid metabolism. Food Chem. 2019, 286, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.F.; Wang, L.J.; Zhang, W.J.; Putnis, C.V. Molecular Understanding of Humic Acid-Limited Phosphate Precipitation and Transformation. Environ. Sci. Technol. 2020, 54, 207–215. [Google Scholar] [CrossRef]
- Fizer, M.; Sidey, V.; Milyovich, S.; Fizer, O. DFT study of fulvic acid binding with bivalent metals: Cd, Cu, Mg, Ni, Pb, Zn. J. Mol. Graph. 2020, 102, 107800. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhu, H.; Zhang, M.; Zhang, H.; Xiang, C.; Li, B. GC-MS Analysis of Membrane-Graded Fulvic Acid and Its Activity on Promoting Wheat Seed Germination. Molecules 2016, 21, 1363. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Baky, Y.R.; Abouziena, H.F.; Amin, A.A.; El-Sh, M.R.; El-Sttar, A.M.A. Improve quality and productivity of some faba bean cultivars with foliar application of fulvic acid. Bull. Natl. Res. Cent. 2019, 43, 2. [Google Scholar] [CrossRef]
- Gong, G.; Yuan, X.; Zhang, Y.; Li, Y.; Liu, W.; Wang, M.; Zhao, Y.; Xu, L. Characterization of coal-based fulvic acid and the construction of a fulvic acid molecular model. RSC Adv. 2020, 10, 5468–5477. [Google Scholar] [CrossRef] [Green Version]
- Morales, O.Y.; Navarrete, J.M.; Gracia, I.; Rivera, M.; Sánchez, F. Fulvic acids, fertilizers first and then stimulants of vital functions in vertebrates. J. Radioanal. Nucl. Chem. 2014, 299, 839–841. [Google Scholar] [CrossRef]
- Jayasooriya, R.; Dilshara, M.G.; Kang, C.H.; Lee, S.; Choi, Y.H.; Jeong, Y.K.; Kim, G.Y. Fulvic acid promotes extracellular anti-cancer mediators from RAW 264.7 cells, causing to cancer cell death in vitro. Int. Immunopharmacol. 2016, 36, 241–248. [Google Scholar] [CrossRef]
- Tan, K.H. Humic Matter in Soil and the Environment: Principles and Controversies, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Peng, A.; Xu, Y.; Wang, Z.J. The effect of fulvic acid on the dose effect of selenite on the growth of wheat. Biol. Trace Elem. Res. 2001, 83, 275–279. [Google Scholar] [CrossRef]
- Boult, S.; Jugdaohsingh, R.; White, K.; Smith, B.; Powell, J. Evidence that polysaccharide and humic and fulvic acids are co-extracted during analysis but have different roles in the chemistry of natural waters. J. Appl. Geochem. 2001, 16, 1261–1267. [Google Scholar] [CrossRef]
- Rodriguez, F.J.; García-Valverde, M. Influence of preozonation on the adsorptivity of humic substances onto activated carbon. Environ. Sci. Pollut. Res. 2016, 23, 21980–21988. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Antonietti, M. The sleeping giant: A polymer View on humic matter in synthesis and applications. Prog. Polym. Sci. 2020, 100, 101182. [Google Scholar] [CrossRef]
- Hassan, M.K.; McInroy, J.A.; Kloepper, J.W. The Interactions of Rhizodeposits with Plant Growth-Promoting Rhizobacteria in the Rhizosphere: A Review. Agriculture 2019, 9, 142. [Google Scholar] [CrossRef] [Green Version]
- Jaeger, F.; Shchegolikhina, A.; van As, H.; Schaumann, G.E. Proton NMR Relaxometry as a Useful Tool to Evaluate Swelling Processes in Peat Soils. J. Magn. Reson. 2010, 3, 27–45. [Google Scholar] [CrossRef]
- Yang, Y.; Li, H.; Li, J. Variation in humic and fulvic acids during thermal sludge treatment assessed by size fractionation, elementary analysis, and spectroscopic methods. Front. Sci. Eng. 2014, 8, 854–862. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Li, C. Evolution of humic substances during anaerobic sludge digestion. Environ. Eng. Manag. J. 2017, 16, 1577–1582. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Zou, S.; Li, C. Extracting humic acids from digested sludge by alkaline treatment and ultrafiltration. J. Mater. Cycles Waste Manag. 2014, 16, 93–100. [Google Scholar] [CrossRef]
- Anielak, A.M.; Świderska, R. The influence of the adsorbents’ electrokinetic potential on the adsorption process of humic substances. Environ. Prot. Eng. 2001, 27, 23–34. [Google Scholar]
- Łomińska-Płatek, D.; Anielak, A.M. Quantitative balance and analysis of fulvic acids changes in the process of municipal sewage treatment. Water Resour. Ind. 2021, 26, 100155. [Google Scholar] [CrossRef]
- Orliński, T.; Anielak, A.M. Characteristics of fulvic acids generated in communes waste landfills. Arch. Environ. Prot. 2021, 47, 41–52. [Google Scholar]
- Xiaoli, C.; Shimaoka, T.; Qiang, G.; Youcai, Z. Characterization of humic and fulvic acids extracted from landfill by elemental composition, 13C CP/MAS NMR and TMAH-Py-GC/MS. Waste Manag. 2008, 28, 896–903. [Google Scholar] [CrossRef]
- Xiaoli, C.; Guixiang, L.; Xin, Z.; Yongxia, H.; Youcai, Z. Fluorescence excitation–emission matrix combined with regional integration analysis to characterize the composition and transformation of humic and fulvic acids from landfill at different stabilization stages. Waste Manag. 2012, 32, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Usmani, Z.; Sharma, M.; Awasthi, A.K.; Sivakumar, N.; Lukk, T.; Pecoraro, L.; Gupta, V.K. Bioprocessing of waste biomass for sustainable product development and minimizing environmental impact. Bioresour Technol. 2021, 322, 124548. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, F.; Xing, B.; Meng, W.; Shi, G.; Ma, Y.; Giesy, J.P. Isolation and Characterization of Chinese Standard Fulvic Acid Sub-fractions Separated from Forest Soil by Stepwise Elution with Pyrophosphate Buffer. Sci. Rep. 2015, 5, 8723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trubetskaya, O.E.; Trubetskoj, O.A.; Voyard, G.; Richard, C. Determination of hydrophobicity and optical properties of soil humic acids isolated by different methods. J. Geochem. Explor. 2013, 132, 84–89. [Google Scholar] [CrossRef]
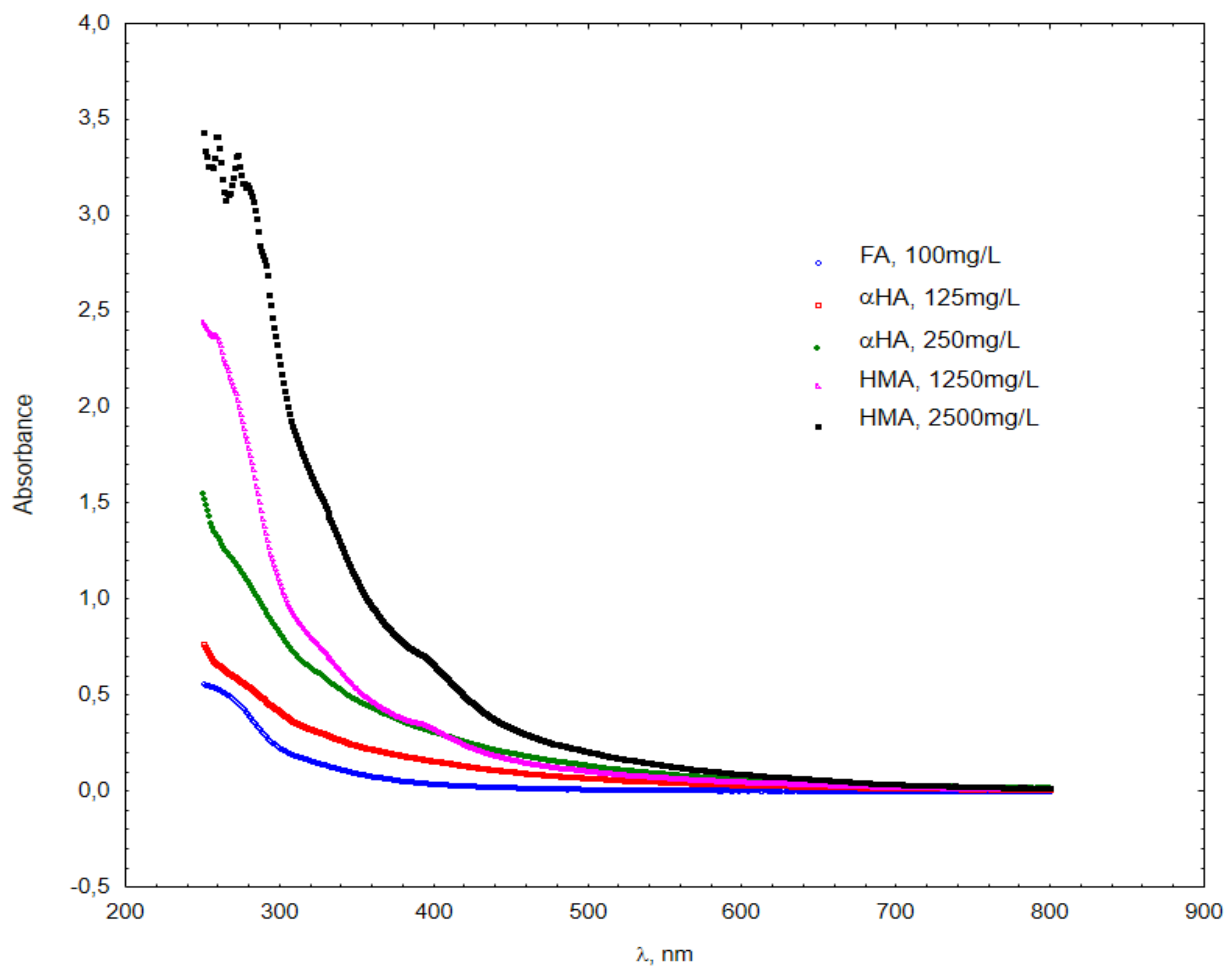
| KUJAWY Sludge | Ba | Mn | TOC | K | Ag | Mg | Ca | As | Cr | Cd | Cu | Ni | Pb | Hg | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/kg DM | mg/kg DM | mg/kg DM | mg/kg DM | mg/kg DM | % DM | % DM | mg/kg DM | mg/kg DM | mg/kg DM | mg/kg DM | mg/kg DM | mg/kg DM | mg/kg DM | mg/kg DM | |
| Sample 1 | 228 | 197 | 57,428 | 10,599 | <10 | 0.315 | 6.17 | <10 | 309 | <10 | 233 | 33.4 | 15.8 | 0.845 | 1151 |
| Sample 2 | 235 | 208 | 62,841 | 10,467 | <10 | 2.260 | 6.05 | <10 | 323 | <10 | 241 | 34.9 | 16.8 | 0.908 | 1194 |
| Sample 3 | 233 | 205 | 55,887 | 9981 | <10 | 1.920 | 6.88 | <10 | 304 | <10 | 237 | 35.0 | 18.4 | 1.010 | 1200 |
| Sample 4 | 232 | 201 | 60,752 | 10,348 | <10 | 1.490 | 6.30 | <10 | 310 | <10 | 220 | 34.4 | 16.6 | 0.624 | 1184 |
| Avg. | 232 | 203 | 59,227 | 10,349 | <10 | 1.496 | 6.35 | <10 | 312 | <10 | 233 | 34.4 | 16.9 | 0.847 | 1182 |
| PŁASZÓW Sludge | Ba | Mn | TOC | K | Ag | Mg | Ca | As | Cr | Cd | Cu | Ni | Pb | Hg | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/kg DM | mg/kg DM | mg/kg DM | mg/kg DM | mg/kg DM | % DM | % DM | mg/kg DM | mg/kg DM | mg/kg DM | mg/kg DM | mg/kg DM | mg/kg DM | mg/kg DM | mg/kg DM | |
| Sample 1 | 245 | 232 | 54,592 | 10,016 | <10 | 1.84 | 5.05 | <10 | 161 | <10 | 276 | 69.6 | 34.1 | 0.699 | 1274 |
| Sample 2 | 244 | 237 | 59,892 | 11,596 | <10 | 2.05 | 4.90 | <10 | 159 | <10 | 273 | 68.3 | 34.2 | 0.457 | 1264 |
| Sample 3 | 238 | 221 | 60,393 | 17,936 | <10 | 3.40 | 3.44 | <10 | 154 | <10 | 274 | 67.5 | 32.5 | 0.466 | 1300 |
| Avg. | 242 | 230 | 58,292 | 13,183 | <10 | 2.43 | 4.46 | <10 | 158 | <10 | 274 | 68.47 | 33.6 | 0.541 | 1279 |
| Samples | Composition Characteristic Humus Acids | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Elementals Content Ash Free (%) | Atomic Ration (-) | Ash Contents (%) | |||||||
| Kujawy | C | O | H | N | O/C | H/C | C/N | O/H | |
| FA | 45.69 | 43.42 | 6.53 | 4.36 | 0.71 | 1.71 | 12.29 | 0.42 | 17.29 |
| αHA | 54.70 | 29.97 | 7.27 | 8.06 | 0.41 | 1.59 | 7.91 | 0.26 | 3.50 |
| HMA | 26.65 | 56.08 | 6.92 | 10.35 | 1.58 | 3.12 | 3.00 | 0.51 | 51.90 |
| Płaszów | |||||||||
| FA | 44.83 | 43.47 | 6.47 | 5.23 | 0.73 | 1.73 | 10.08 | 0.42 | 14.66 |
| αHA | 48.06 | 36.46 | 7.13 | 8.35 | 0.57 | 1.79 | 6.68 | 0.32 | 7.32 |
| HMA | 45.42 | 34.08 | 9.49 | 11.01 | 0.56 | 2.50 | 4.80 | 0.22 | 41.32 |
| Ion | Kujawy | Płaszów | ||||
|---|---|---|---|---|---|---|
| FA mg/kgDM | αHA mg/kgDM | HMA mg/kgDM | FA mg/kgDM | αHA mg/kgDM | HMA mg/kgDM | |
| Cl | - | - | - | 60,800 | 31,900 | 90,800 |
| Na | 285 | 20 | 4216 | 18,400 | 16,100 | 4400 |
| P | - | - | - | 16,300 | 8500 | 13,100 |
| Fe | 589 | 5264 | 1494 | 4300 | 10,500 | 900 |
| Ca | 436 | 127 | 2477 | 800 | 6000 | 378 |
| Br | - | - | - | 500 | - | - |
| Al | 1151 | 1293 | 1083 | 400 | 5 700 | 423 |
| K | 252 | 145 | 16,841 | 84 | 2500 | 2700 |
| Zn | 78 | 428 | 956 | 79 | 400 | 1900 |
| Mn | 9 | 51 | 24 | 39 | 3 | 12 |
| Cr | 22 | 70 | 18 | 21 | 31 | 11 |
| Mg | 45 | 87 | 336 | 5 | 161 | 664 |
| Ba | 8 | 1 | 13 | 4 | 17 | - |
| Sr | - | - | - | 2 | 13 | - |
| Si | - | - | - | - | 2100 | - |
| Cu | 27 | 281 | 47 | - | 242 | 800 |
| Ni | 20 | - | 21 | - | - | 81 |
| Ag | - | 20 | - | - | - | 6 |
| Humus Acids | Płaszów (g/kg DM) | Kujawy (g/kg DM) |
|---|---|---|
| FA | 5.07 | 5.29 |
| αHA | 74.72 | 59.22 |
| HMA | 43.66 | 20.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anielak, A.M.; Kłeczek, A. Humus Acids in the Digested Sludge and Their Properties. Materials 2022, 15, 1475. https://doi.org/10.3390/ma15041475
Anielak AM, Kłeczek A. Humus Acids in the Digested Sludge and Their Properties. Materials. 2022; 15(4):1475. https://doi.org/10.3390/ma15041475
Chicago/Turabian StyleAnielak, Anna M., and Aneta Kłeczek. 2022. "Humus Acids in the Digested Sludge and Their Properties" Materials 15, no. 4: 1475. https://doi.org/10.3390/ma15041475
APA StyleAnielak, A. M., & Kłeczek, A. (2022). Humus Acids in the Digested Sludge and Their Properties. Materials, 15(4), 1475. https://doi.org/10.3390/ma15041475






