Prospects of Using Pseudobrookite as an Iron-Bearing Mineral for the Alkaline Electrolytic Production of Iron
Abstract
:1. Introduction
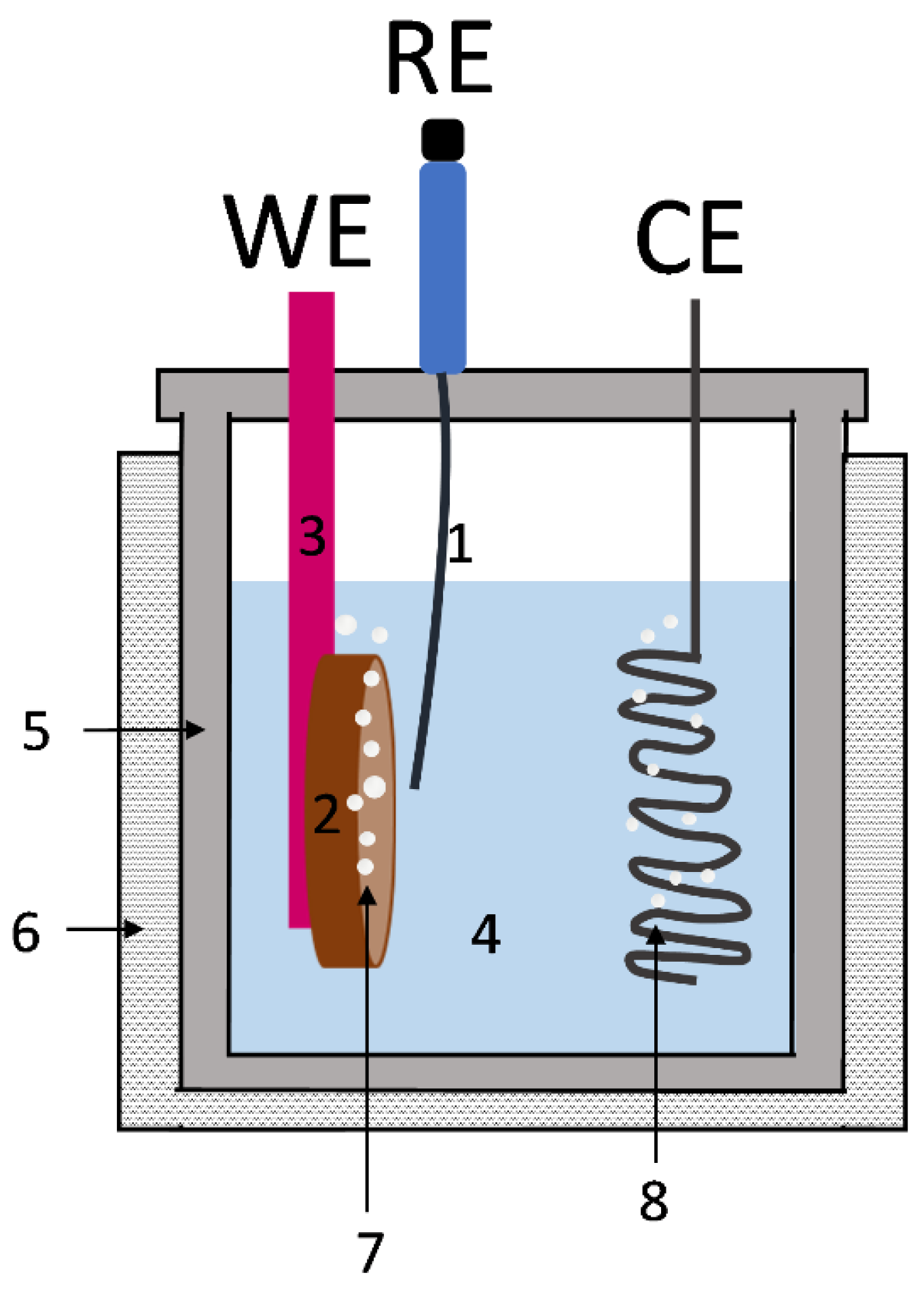
2. Materials and Methods
3. Results and Discussion
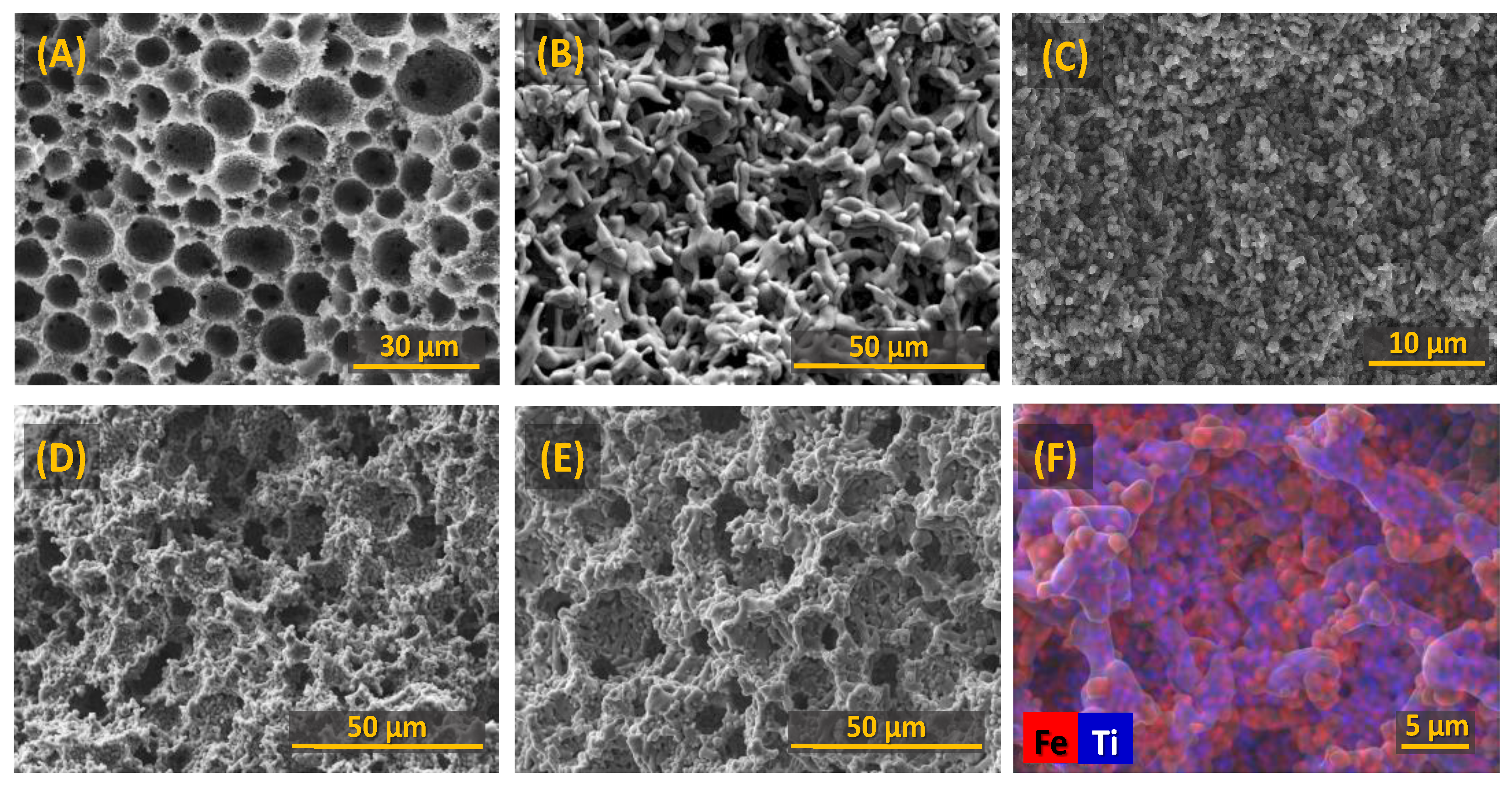
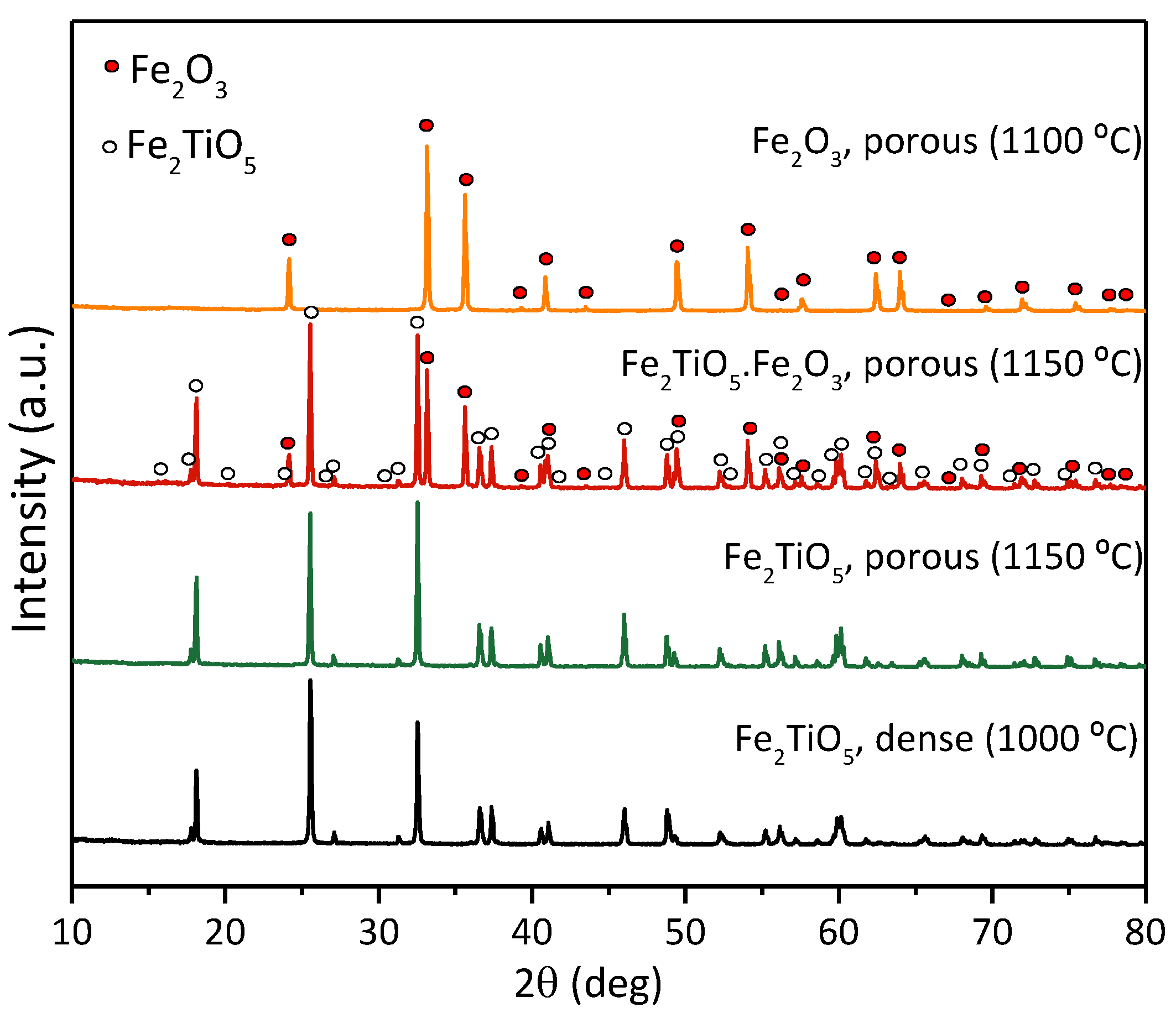
3.1. Structural and Microstructural Features of the Ceramic Cathodes
3.2. Electrochemical Reduction of the Fe-Ti-Bearing Ceramic Cathodes
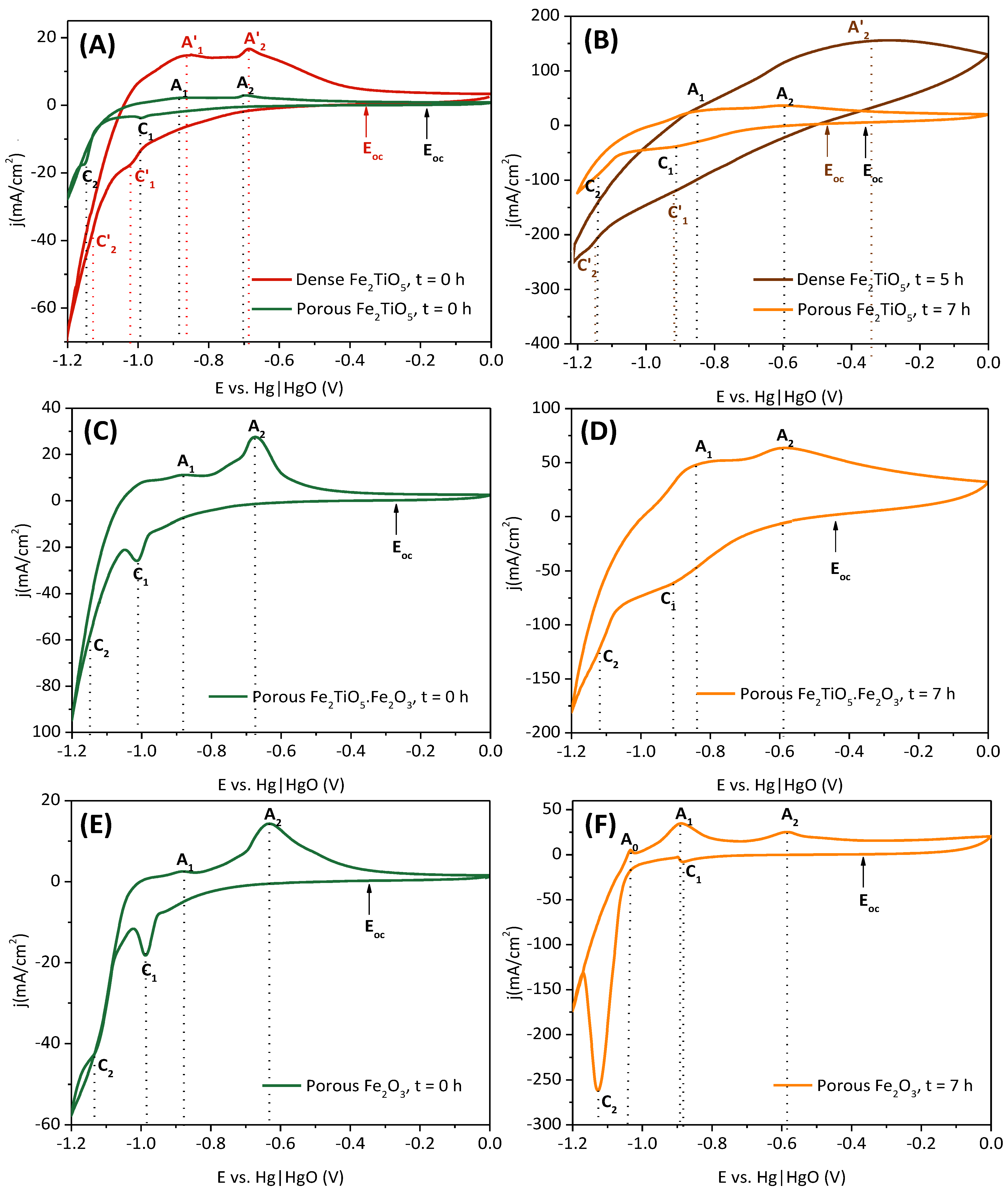
3.2.1. Studies of the Relevant Redox Processes by Cyclic Voltammetry
3.2.2. Chronoamperometric Studies of the Dense Fe2TiO5 Ceramic Cathodes
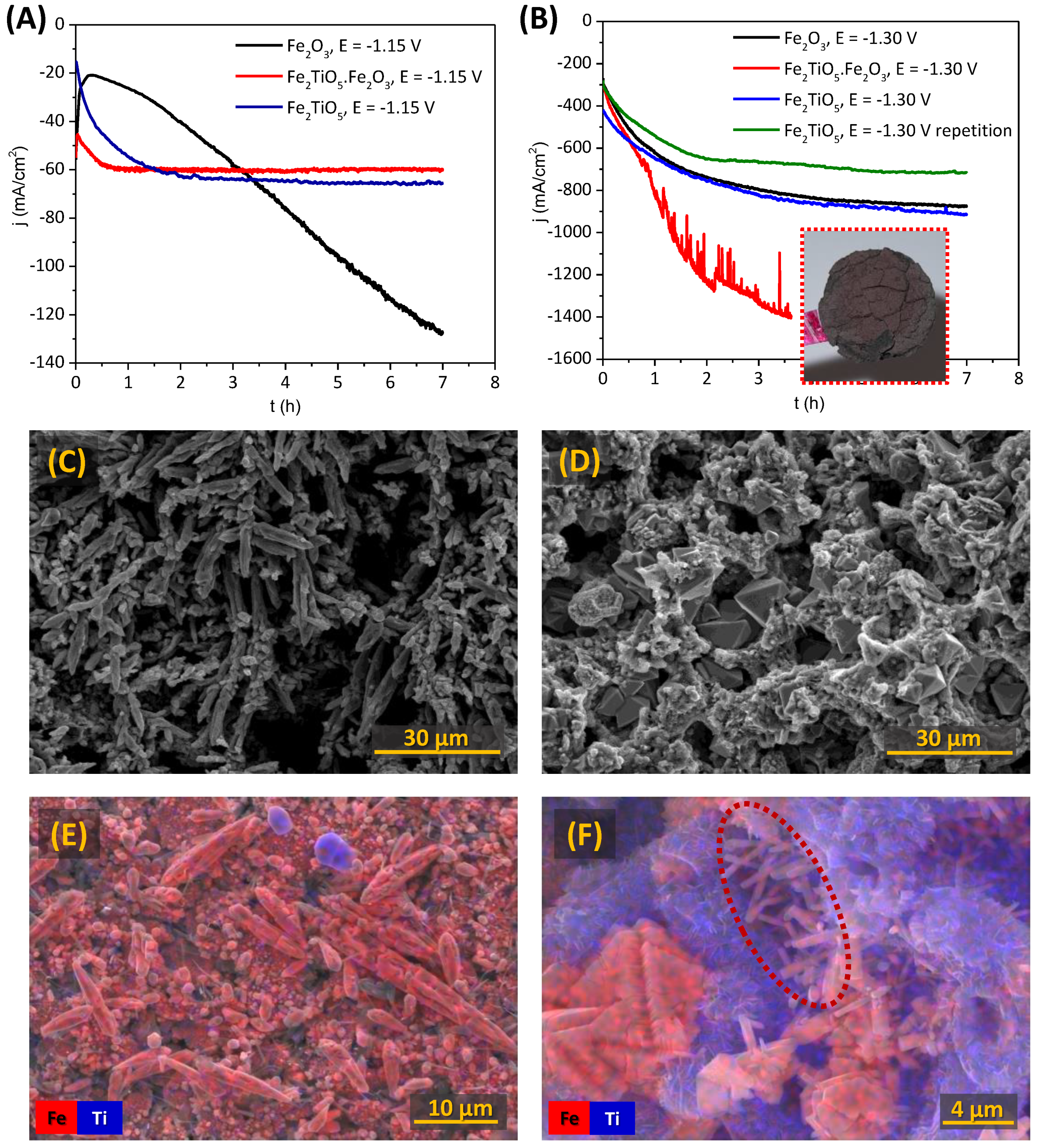
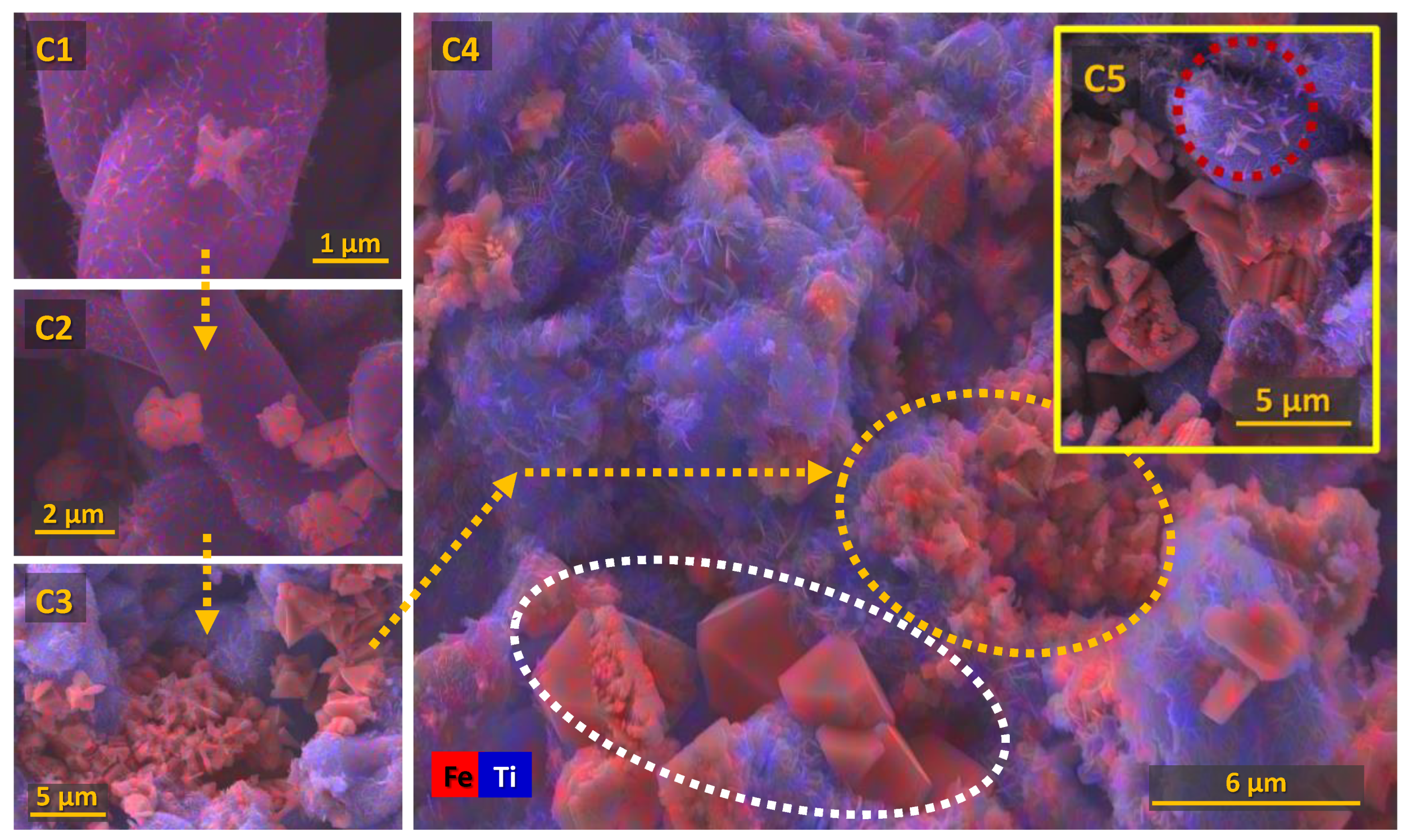
3.2.3. Chronoamperometric Studies of the Porous Cathodes
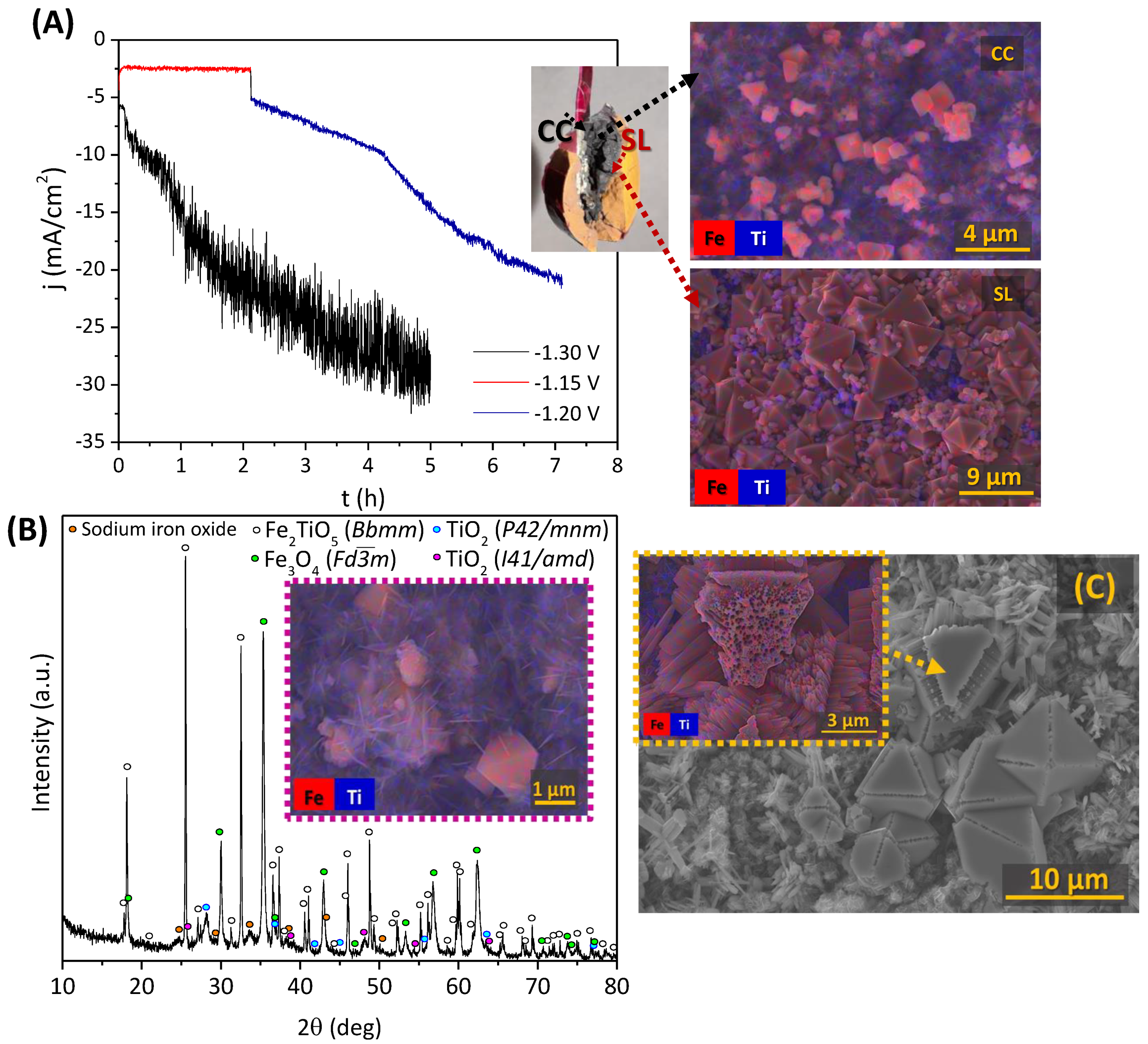
3.2.4. Galvanostatic Electroreduction
3.3. Electrochemical Reduction of Alkaline Fe2TiO5 Suspensions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mousa, E.A. Modern blast furnace ironmaking technology: Potentials to meet the demand of high hot metal production and lower energy consumption. Metall. Mater. Eng. 2019, 25, 69–104. [Google Scholar] [CrossRef] [Green Version]
- World Steel Association. World Steel in Figures, 2020 (Report); World Steel Association: Brussels, Belgium, 2020. [Google Scholar]
- Wiencke, J.; Lavelaine, H.; Panteix, P.J.; Petitjean, C.; Rapin, C. The Impact of Iron Oxide Concentration on the Performance of Molten Oxide Electrolytes for the Production of Liquid Iron Metal. Metall. Mater. Trans. B 2020, 51B, 365–376. [Google Scholar] [CrossRef] [Green Version]
- Wiencke, J.; Lavelaine, H.; Panteix, P.J.; Petitjean, C.; Rapin, C. Electrolysis of iron in a molten oxide electrolyte. J. Appl. Electrochem. 2018, 48, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Allanore, A.; Lavelaine, H.; Valentin, G.; Birat, J.P.; Lapicque, F. Electrodeposition of metal iron from dissolved species in alkaline media. J. Electrochem. Soc. 2007, 154, E187–E193. [Google Scholar] [CrossRef]
- Allanore, A.; Lavelaine, H.; Valentin, G.; Birat, J.P.; Lapicque, F. Iron Metal Production by Bulk Electrolysis of Iron Ore Particles in Aqueous Media. J. Electrochem. Soc. 2008, 155, E125–E129. [Google Scholar] [CrossRef]
- Allanore, A.; Lavelaine, H.; Valentin, G.; Birat, J.P.; Delcroix, P.; Lapicque, F. Observation and modeling of the reduction of hematite particles to metal in alkaline solution by electrolysis. Electrochim. Acta 2010, 55, 4007–4013. [Google Scholar] [CrossRef]
- Ivanova, Y.; Monteiro, J.; Teixeira, L.; Vitorino, N.; Kovalevsky, A.; Frade, J. Designed porous microstructures for electrochemical reduction of bulk hematite ceramics. Mater. Des. 2017, 122, 307–314. [Google Scholar] [CrossRef]
- Monteiro, J.; Ivanova, Y.; Kovalevsky, A.; Ivanou, D.; Frade, J. Reduction of magnetite to metallic iron in strong alkaline medium. Electrochim. Acta 2016, 193, 284–292. [Google Scholar] [CrossRef]
- Allanore, A.; Lavelaine, H.; Birat, J.P.; Valentin, G.; Lapicque, F. Experimental investigation of cell design for the electrolysis of iron oxide suspensions in alkaline electrolyte. J. Appl. Electrochem. 2010, 40, 1957–1966. [Google Scholar] [CrossRef]
- Cavaliere, P. Clean Ironmaking and Steelmaking Processes, 1st ed.; Springer International Publishing: Lecce, Italy, 2019; pp. 555–575. [Google Scholar]
- Izaki, M. Electrodeposition of iron and iron alloys. In Modern Electroplating, 5th ed.; Schlesinger, M., Paunovic, M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 309–326. [Google Scholar]
- Ivanova, Y.; Monteiro, J.; Horovistiz, A.; Ivanou, D.; Mata, D.; Silva, R.; Frade, J. Electrochemical deposition of Fe and Fe/CNTs composites from strongly alkaline hematite suspensions. J. Appl. Electrochem. 2015, 45, 515–522. [Google Scholar] [CrossRef]
- Feynerol, V.; Lavelaine, H.; Marlier, P.; Pons, M.N.; Lapicque, F. Reactivity of suspended iron oxide particles in low temperature alkaline electrolysis. J. Appl. Electrochem. 2017, 47, 1339–1350. [Google Scholar] [CrossRef]
- Zou, X.; Gu, S.; Lu, X.; Xie, X.; Lu, C.; Zhou, Z.; Ding, W. Electroreduction of Iron (III) Oxide Pellets to Iron in Alkaline Media: A Typical Shrinking-Core Reaction Process. Metall. Mater. Trans. B 2015, 46B, 1262–1274. [Google Scholar] [CrossRef] [Green Version]
- Maihatchi, A.; Pons, M.-N.; Ricoux, Q.; Goettmann, F.; Lapicque, F. Electrolytic iron production from alkaline suspensions of solid oxides: Compared cases of hematite, iron ore and iron-rich Bayer process residues. J. Electrochem. Sci. Eng. 2020, 10, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Koutsoupa, S.; Koutalidi, S.; Bourbos, E.; Balomenos, E.; Panias, D. Electrolytic iron production from alkaline bauxite residue slurries at low temperatures. Johns. Matthey Technol. Rev. 2021, 65, 366–374. [Google Scholar] [CrossRef]
- Lopes, D.V.; Ivanova, Y.A.; Kovalevsky, A.V.; Sarabando, A.R.; Frade, J.R.; Quina, M.J. Electrochemical reduction of hematite-based ceramics in alkaline medium: Challenges in electrode design. Electrochim. Acta 2019, 327, 135060. [Google Scholar] [CrossRef]
- Lopes, D.V.; Kovalevsky, A.; Quina, M.; Frade, J. Electrochemical deposition of zero-valent iron from alkaline ceramic suspensions of Fe2−xAlxO3 for iron valorisation. J. Electrochem. Soc. 2020, 167, 102508. [Google Scholar] [CrossRef]
- Lopes, D.V.; Lisenkov, A.D.; Sergiienko, S.A.; Constantinescu, G.; Sarabando, A.; Quina, M.J.; Frade, J.R.; Kovalevsky, A.V. Alkaline Electrochemical Reduction of a Magnesium Ferrospinel into Metallic Iron for the Valorisation of Magnetite-Based Metallurgical Waste. J. Electrochem. Soc. 2021, 168, 073504. [Google Scholar] [CrossRef]
- Bjareborn, O.; Arif, T.; Monaghan, B.; Bumby, C.W. Fate of titanium in alkaline electro-reduction of sintered titanomagnetite. Mater. Res. Express 2020, 7, 106508. [Google Scholar] [CrossRef]
- Ruivo, L.C.M.; Pio, D.T.; Yaremchenko, A.A.; Tarelho, L.A.C.; Frade, J.R.; Kantarelis, E.; Engvall, K. Iron-based catalyst (Fe2−xNixTiO5) for tar decomposition in biomass gasification. Fuel 2021, 300, 120859. [Google Scholar] [CrossRef]
- Zhao, Q.; Feng, G.; Jiang, F.; Lan, S.; Chen, J.; Liu, M.; Huang, Z.; Liu, J.; Hu, Q.; Jiang, W. Comparison of Fe2TiO5/C photocatalysts synthesized via a nonhydrolytic sol–gel method and solid-state reaction method. RSC Adv. 2020, 10, 43762–43772. [Google Scholar] [CrossRef]
- Vasiljevic, Z.Z.; Dojcinovic, M.P.; Vujancevic, J.D.; Jankovic-Castvan, I.; Ognjanovic, M.; Tadic, N.B.; Stojadinovic, S.; Brankovic, G.O.; Nikolic, M. V Photocatalytic degradation of methylene blue under natural sunlight using iron titanate nanoparticles prepared by a modified sol—gel method. R. Soc. Open Sci. 2020, 7, 200708. [Google Scholar] [CrossRef] [PubMed]
- Fernando, N.; Swaminathan, J.; Hernandez, F.; Priyadarshana, G.; Sandaruwan, C.; Yang, W.; Karunaratne, V.; Wang, Z.; Amaratunga, G.; Kottegoda, N.; et al. Pseudobrookite based heterostructures for efficient electrocatalytic hydrogen evolution. Mater. Rep. Energy 2021, 1, 100020. [Google Scholar] [CrossRef]
- Miskovic, G.; Lukovic, M.D.; Nikolic, M.V.; Vasiljevic, Z.Z.; Nicolics, J.; Aleksic, O.S. Analysis of electronic properties of pseudobrookite thick films with possible application for NO gas sensing. In Proceedings of the 39th International Spring Seminar on Electronics Technology, Pilsen, Czech Republic, 18–22 May 2016; pp. 386–391. [Google Scholar]
- Dondi, M.; Matteucci, F.; Cruciani, G.; Gasparotto, G.; Tobaldi, D.M. Pseudobrookite ceramic pigments: Crystal structural, optical and technological properties. Solid State Sci. 2007, 9, 362–369. [Google Scholar] [CrossRef] [Green Version]
- Min, K.; Park, K.; Lim, A.; Kim, J.; Kim, D. Synthesis of pseudobrookite-type Fe2TiO5 nanoparticles and their Li-ion electroactivity. Ceram. Int. 2012, 38, 6009–6013. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Sekulic, D.L.; Vasiljevic, Z.Z.; Lukovic, M.D.; Pavlovic, V.B.; Aleksic, O.S. Dielectric properties, complex impedance and electrical conductivity of Fe2TiO5 nanopowder compacts and bulk samples at elevated temperatures. J. Mater. Sci. Mater. Electron. 2017, 28, 4796–4806. [Google Scholar] [CrossRef] [Green Version]
- Lopes, D.V.; Kovalevsky, A.V.; Quina, M.J.; Frade, J.R. Processing of highly-porous cellular iron oxide-based ceramics by emulsification of ceramic suspensions. Ceram. Int. 2018, 44, 20354–20360. [Google Scholar] [CrossRef]
- Hughes, S.W. Archimedes revisited: A faster, better, cheaper method of accurately measuring the volume of small objects. Phys. Educ. 2005, 40, 468–474. [Google Scholar] [CrossRef] [Green Version]
- Cleveland, J.; Bradt, R. Grain Size/Microcracking Relations for Pseudobrookite Oxides. J. Am. Ceram. Soc. 1978, 61, 478–481. [Google Scholar] [CrossRef]
- Chen, C.; Giovannelli, F.; Duclère, J.R.; Delorme, F. Thermoelectric properties of Fe2(Ti1−xNbx)O5 pseudobrookite ceramics with low thermal conductivity. J. Eur. Ceram. Soc. 2017, 37, 4681–4685. [Google Scholar] [CrossRef]
- Morin, F. Electrical properties of αFe2O3 and α-Fe2O3 containing Titanium. Phys. Rev. 1951, 83, 1005–1010. [Google Scholar] [CrossRef]
- Guskos, N.; Papadopoulos, G.J.; Likodimos, V.; Patapis, S.; Yarmis, D.; Przepiera, A.; Przepiera, K.; Majszczyk, J.; Typek, J.; Wabia, M.; et al. Photoacoustic, EPR and electrical conductivity investigations of three synthetic mineral pigments: Hematite, goethite and magnetite. Mater. Res. Bull. 2002, 37, 1051–1061. [Google Scholar] [CrossRef]
- Lee, K.K.; Deng, S.; Fan, H.M.; Mhaisalkar, S.; Tan, H.R.; Tok, E.S.; Loh, K.P.; Chin, W.S.; Sow, C.H. α-Fe2O3 nanotubes-reduced graphene oxide composites as synergistic electrochemical capacitor materials. Nanoscale 2012, 4, 2958–2961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huda, M.N.; Walsh, A.; Yan, Y.; Wei, S.-H.; Al-Jassim, M.M. Electronic, structural, and magnetic effects of 3d transition metals in hematite. J. Appl. Phys. 2010, 107, 123712. [Google Scholar] [CrossRef] [Green Version]
- Nikolic, M.V.; Sekulic, D.L.; Nikolic, N.; Slankamenac, M.P.; Aleksic, O.S.; Danninger, H.; Halwax, E.; Pavlovic, V.B.; Nikolic, P.M. Structural and electrical properties of Ti doped α-Fe2O3. Sci. Sinter. 2013, 45, 281–292. [Google Scholar] [CrossRef] [Green Version]
- Nikolic, M.V.; Lukovic, M.D.; Vasiljevic, Z.Z.; Labus, N.J.; Aleksic, O.S. Humidity sensing potential of Fe2TiO5—pseudobrookite. J. Mater. Sci. Mater. Electron. 2018, 29, 9227–9238. [Google Scholar] [CrossRef] [Green Version]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions, 2nd ed.; Cebelcor: Brussels, Belgium, 1974; p. 648. [Google Scholar]
- Verink, J.E. Simplified Procedure for Constructing Pourbaix Diagrams. In Uhlig’s Corrosion Handbook, 2nd ed.; Revie, R., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000; pp. 111–124. [Google Scholar]
- Le, H.; Ghali, E. Interpretation des diagrammes E-pH du systeme Fe-H2O en relation avec la fragilisation caustique des aciers. J. Appl. Electrochem. 1993, 23, 72–77. [Google Scholar] [CrossRef]
- Armstrong, R.; Baurhoo, I. The dissolution of iron in concentrated alkali. J. Electroanal. Chem. Interfacial Electrochem. 1972, 40, 325–338. [Google Scholar] [CrossRef]
- Kitamura, H.; Zhao, L.; Hang, B.T.; Okada, S.; Yamaki, J. Effect of Charge Current Density on Electrochemical Performance of Fe/C Electrodes in Alkaline Solutions. J. Electrochem. Soc. 2012, 159, A720–A724. [Google Scholar] [CrossRef]
- Xiao, W.; Jin, X.; Deng, Y.; Wang, D.; Chen, G.Z. Three-phase interlines electrochemically driven into insulator compounds: A penetration model and its verification by electroreduction of solid AgCl. Chem. A Eur. J. 2007, 13, 604–612. [Google Scholar] [CrossRef]
- Allanore, A.; Feng, J.; Lavelaine, H.; Ogle, K. The Adsorption of Hematite Particles on Steel in Strongly Alkaline Electrolyte. J. Electrochem. Soc. 2010, 157, E24–E30. [Google Scholar] [CrossRef]
- Siebentritt, M.; Volovitch, P.; Ogle, K.; Lefèvre, G. Adsorption and electroreduction of hematite particles on steel in strong alkaline media. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 440, 197–201. [Google Scholar] [CrossRef]
- LeDuc, J.A.M.; Loftfield, R.E.; Vaaler, L.E. Electrolytic Iron Powder from a Caustic Soda Solution. J. Electrochem. Soc. 1959, 106, 659–667. [Google Scholar] [CrossRef]
- Xue, T.; Wang, L.; Qi, T.; Chu, J.; Qu, J.; Liu, C. Decomposition kinetics of titanium slag in sodium hydroxide system. Hydrometallurgy 2009, 95, 22–27. [Google Scholar] [CrossRef]
- Shukaev, I.L.; Butova, V.V.; Chernenko, S.V.; Pospelov, A.A.; Shapovalov, V.V.; Guda, A.A.; Aboraia, A.M.; Zahran, H.Y.; Yahia, I.S.; Soldatov, A.V. New orthorhombic sodium iron(+2) titanate. Ceram. Int. 2020, 46, 4416–4422. [Google Scholar] [CrossRef]



| Composition | Before Reduction | After Reduction | ||
|---|---|---|---|---|
| Cathodic Peaks/Shoulders (V) | Anodic Peaks/Shoulders (V) | Cathodic Peaks/Shoulders (V) | Anodic Peaks/Shoulders (V) | |
| Dense Fe2TiO5 | C′1 = −1.04 C′2 = −1.14 | A′1 = −0.87 A′2 = −0.69 | C′1 = −0.92 C’2 = −1.15 | - A′2 = −0.35 |
| Porous Fe2TiO5 | C1 = −0.99 C2 = −1.15 | A1 = −0.88 A2 = −0.68 | C1 = −0.91 C2 = −1.14 | A1 = −0.85 A2 = −0.59 |
| Porous Fe2TiO5·Fe2O3 | C1 = −1.01 C2 = −1.15 | A1 = −0.88 A2 = −0.67 | C1 = −0.91 C2 = −1.13 | A1 = −0.84 A2 = −0.59 |
| Porous Fe2O3 | - C1 = −0.98 C2 = −1.13 | - A1 = −0.87 A2 = −0.63 | - C1 = −0.88 C2 = −1.12 | A0 = −1.04 A1 = −0.89 A2 = −0.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, D.V.; Lisenkov, A.D.; Ruivo, L.C.M.; Yaremchenko, A.A.; Frade, J.R.; Kovalevsky, A.V. Prospects of Using Pseudobrookite as an Iron-Bearing Mineral for the Alkaline Electrolytic Production of Iron. Materials 2022, 15, 1440. https://doi.org/10.3390/ma15041440
Lopes DV, Lisenkov AD, Ruivo LCM, Yaremchenko AA, Frade JR, Kovalevsky AV. Prospects of Using Pseudobrookite as an Iron-Bearing Mineral for the Alkaline Electrolytic Production of Iron. Materials. 2022; 15(4):1440. https://doi.org/10.3390/ma15041440
Chicago/Turabian StyleLopes, Daniela V., Aleksey D. Lisenkov, Luís C. M. Ruivo, Aleksey A. Yaremchenko, Jorge R. Frade, and Andrei V. Kovalevsky. 2022. "Prospects of Using Pseudobrookite as an Iron-Bearing Mineral for the Alkaline Electrolytic Production of Iron" Materials 15, no. 4: 1440. https://doi.org/10.3390/ma15041440
APA StyleLopes, D. V., Lisenkov, A. D., Ruivo, L. C. M., Yaremchenko, A. A., Frade, J. R., & Kovalevsky, A. V. (2022). Prospects of Using Pseudobrookite as an Iron-Bearing Mineral for the Alkaline Electrolytic Production of Iron. Materials, 15(4), 1440. https://doi.org/10.3390/ma15041440









