Facile Synthesis of Unsupported Pd Aerogel for High Performance Formic Acid Microfluidic Fuel Cell
Abstract
:1. Introduction
2. Experimental
2.1. Pd Aerogels Chemical Synthesis
2.2. Physicochemical Characterization
2.3. Electrochemical Measurements
2.3.1. Electrocatalytic Activity in Half-Cell Configuration
2.3.2. FAO Performance
2.3.3. Stability Performance
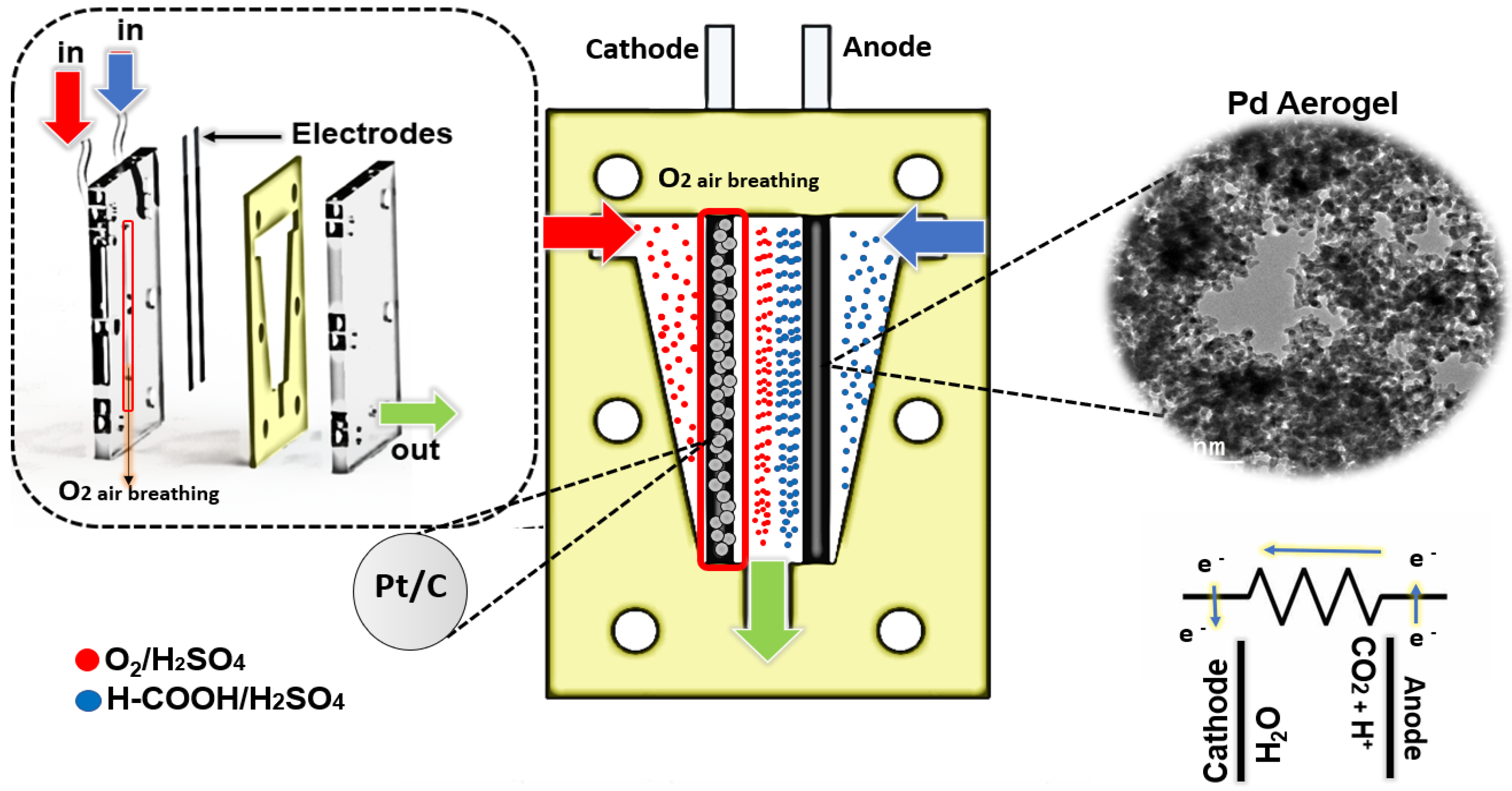
2.3.4. Evaluation of the Microfluidic Fuel Cell System
3. Results and Discussion
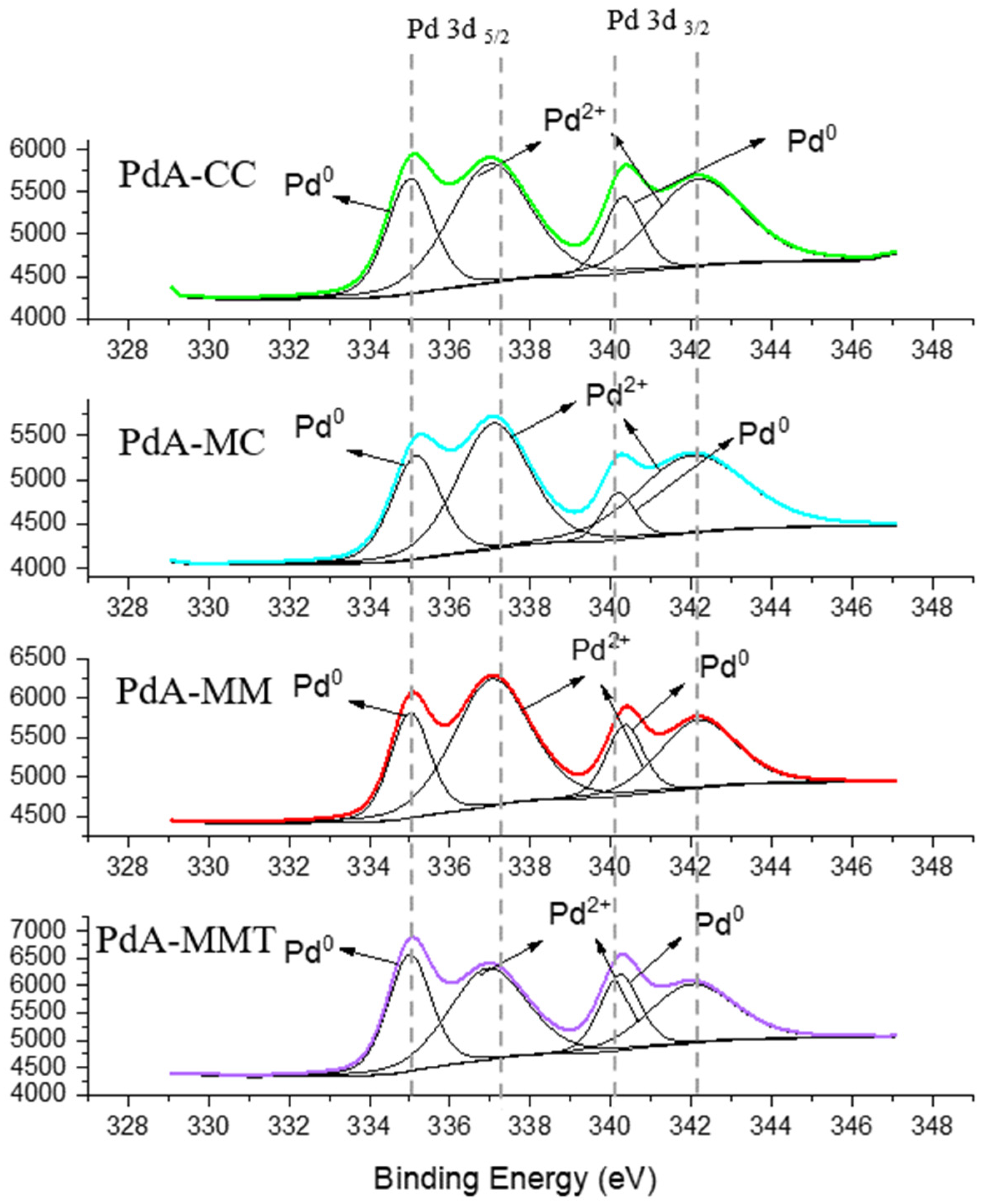
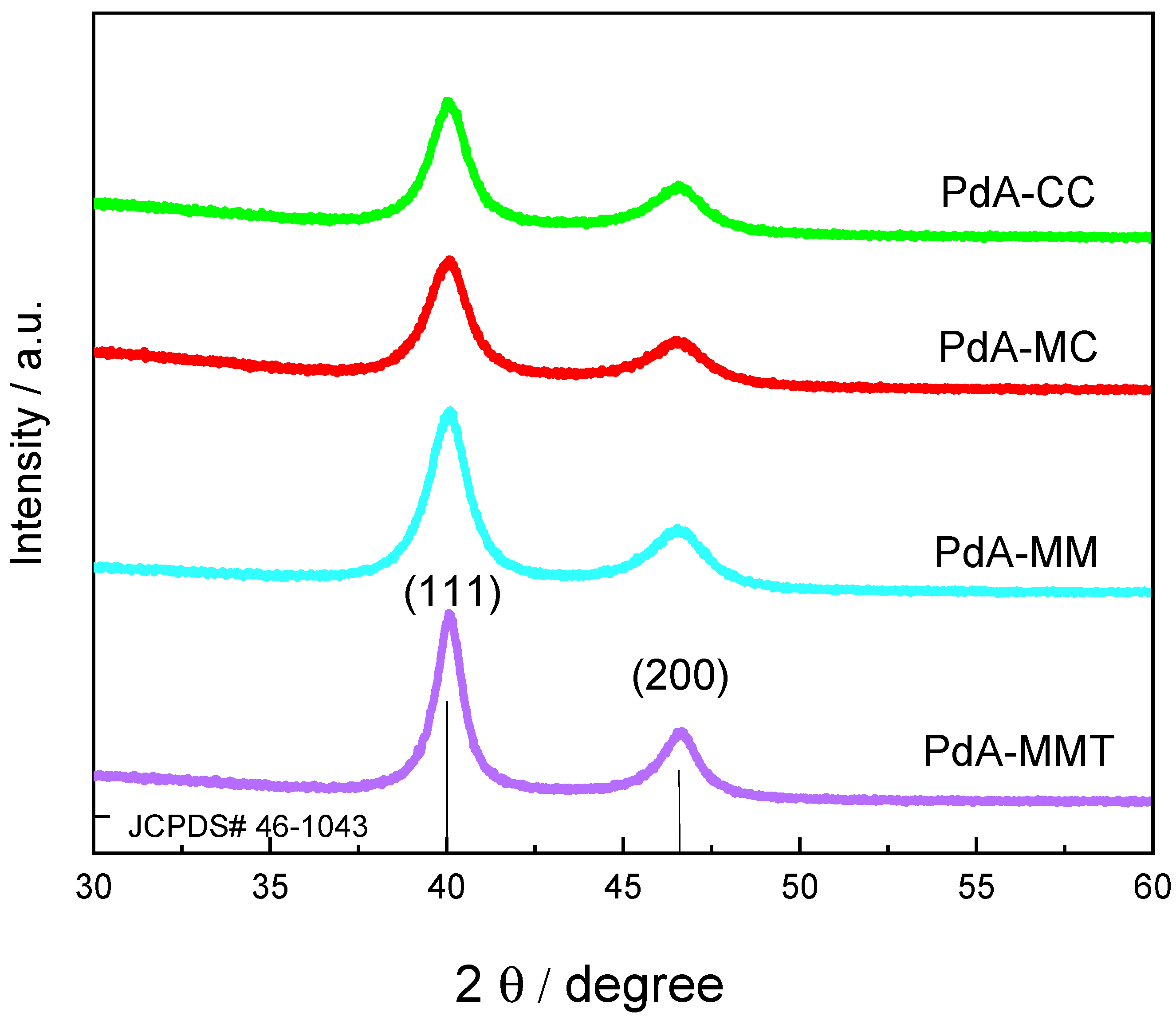
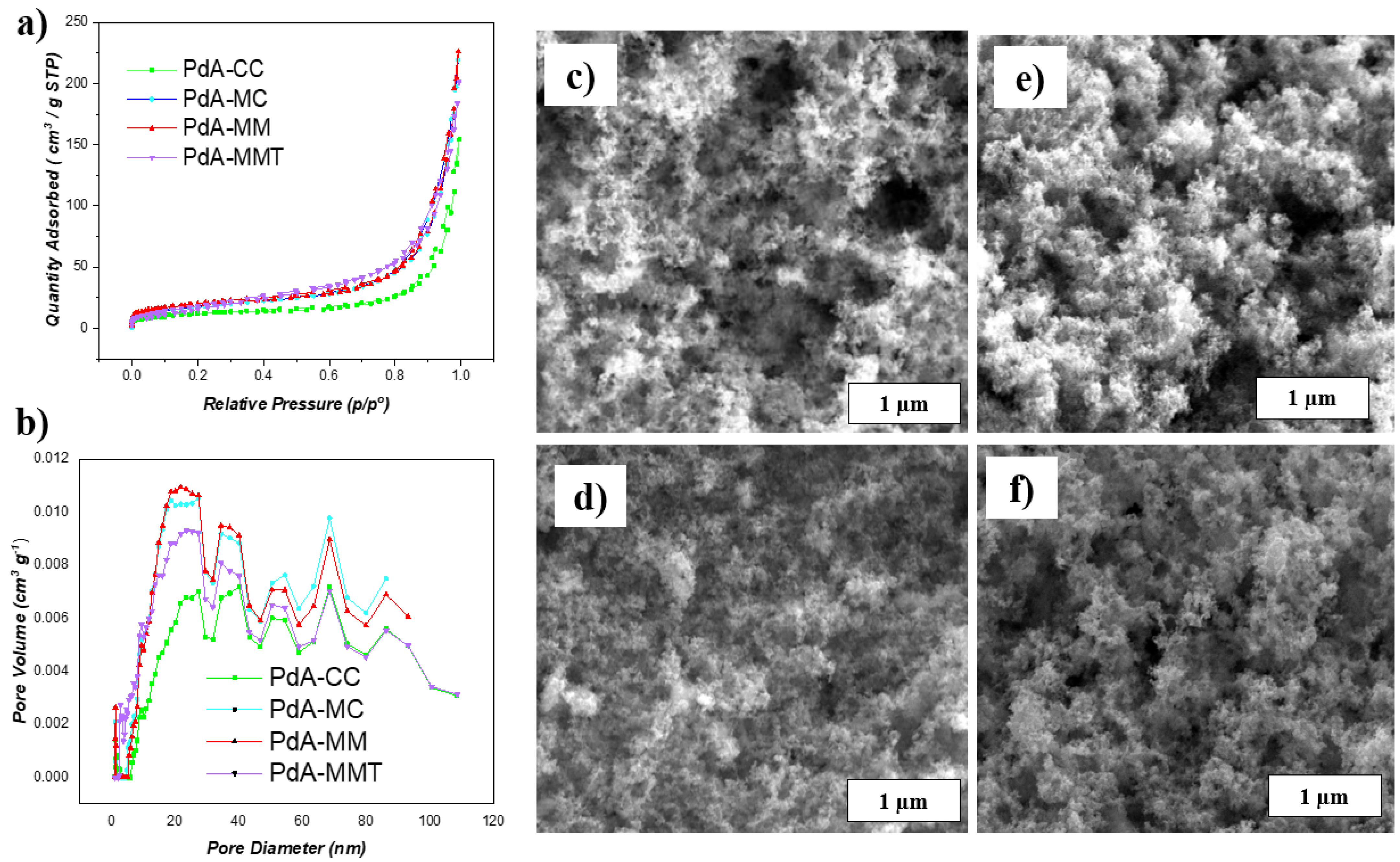
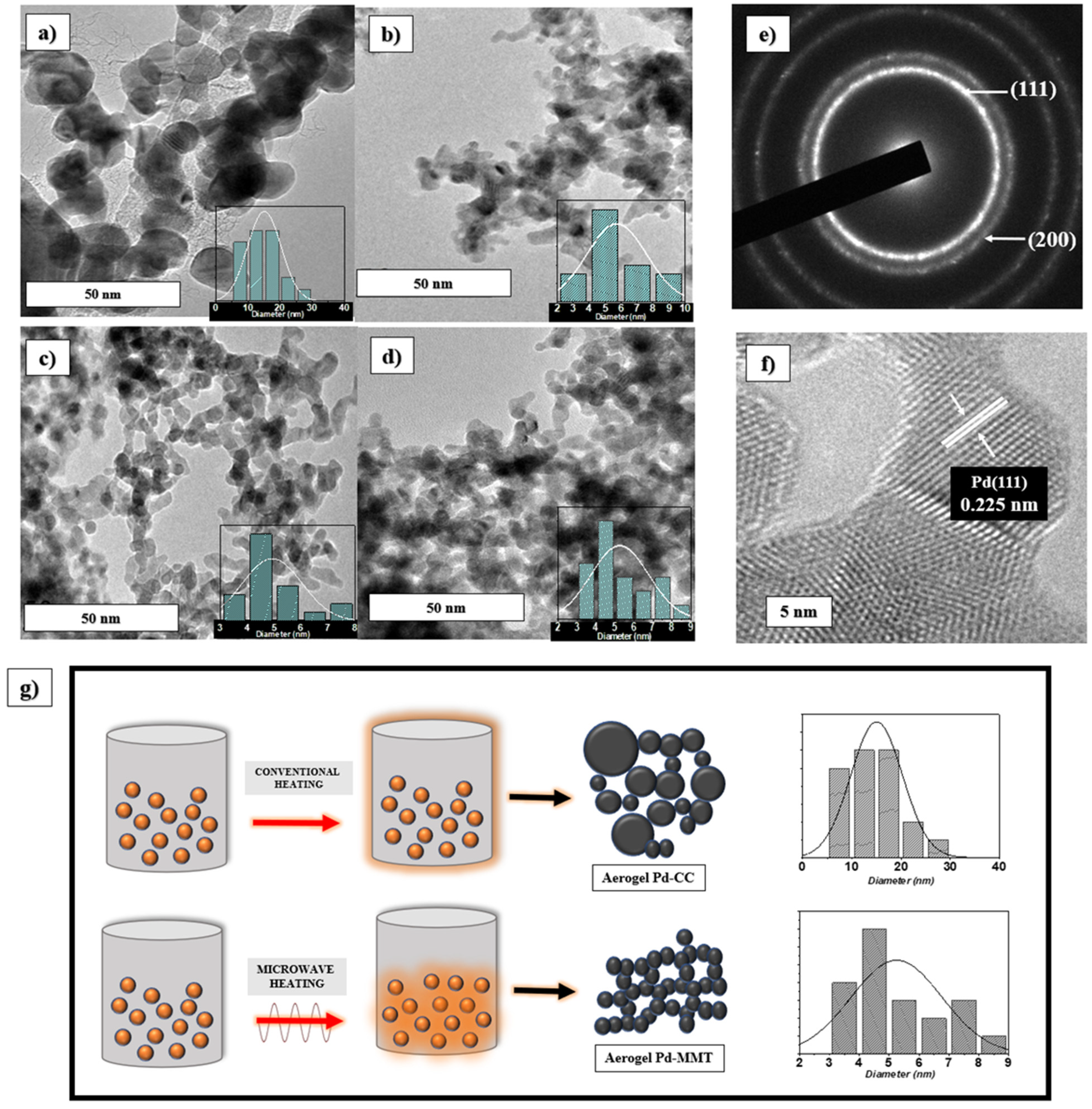
3.1. Physicochemical Characterization
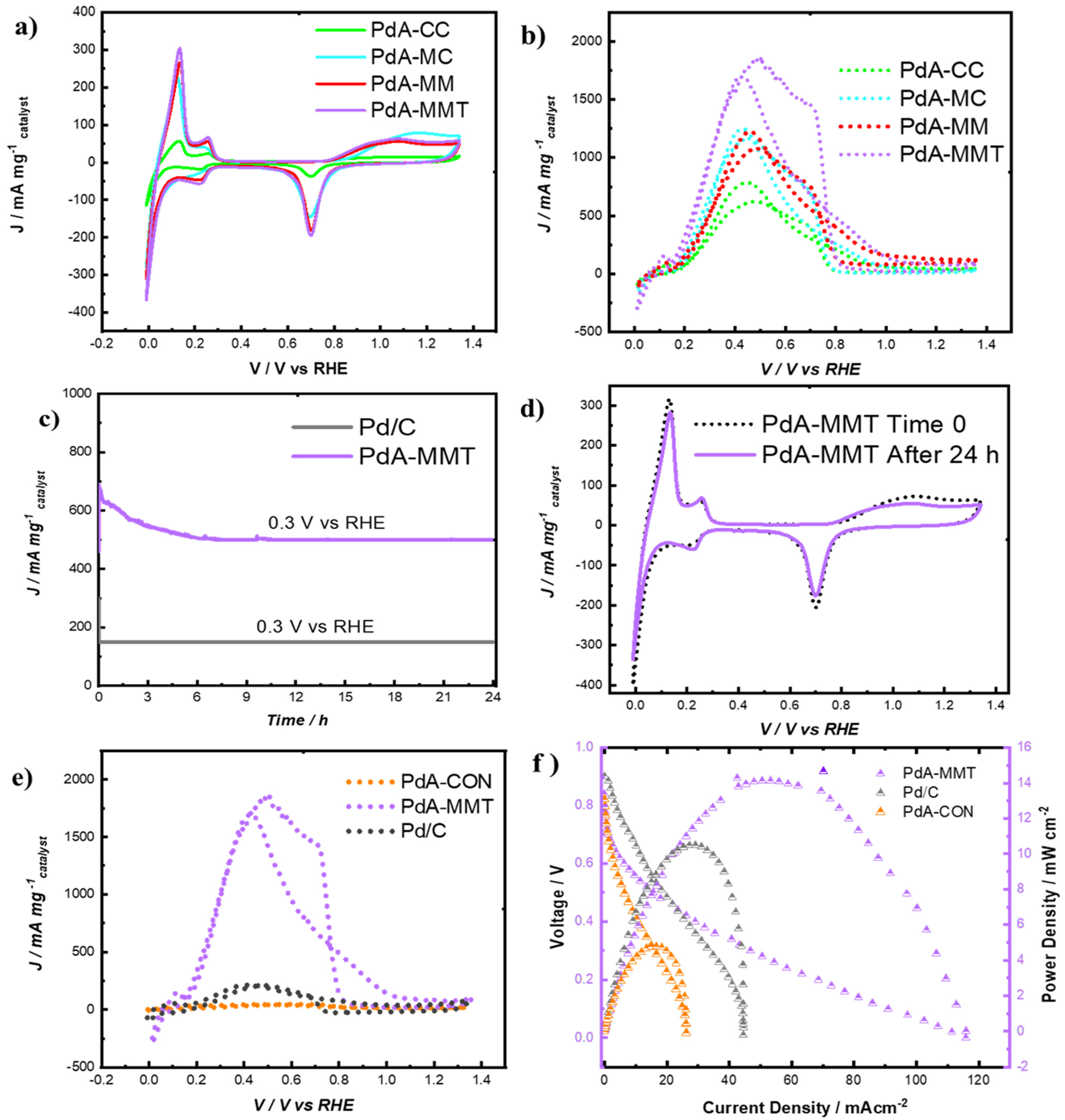
3.2. Electrocatalytic Performances
3.3. Microfluidic Fuel Cell Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, B.; Ren, L.; Xu, Z.; Cheng, N.; Lai, W.; Zhang, L.; Hao, W.; Chu, S.; Wang, Y.; Du, Y.; et al. Atomic Structural Evolution of Single-Layer Pt Clusters as Efficient Electrocatalysts. Small 2021, 1–8, 2100732. [Google Scholar] [CrossRef]
- Hidayah, N.; Irwan, M. Three-dimensional CFD modeling of a direct formic acid fuel cell. Int. J. Hydrogen Energy 2019, 44, 30627–30635. [Google Scholar]
- Fan, H.; Cheng, M.; Wang, L.; Song, Y.; Cui, Y.; Wang, R. Extraordinary electrocatalytic performance for formic acid oxidation by the synergistic effect of Pt and Au on carbon black. Nano Energy 2018, 48, 1–9. [Google Scholar] [CrossRef]
- Ying, L.; Ouyang, Y.; Wang, S.; Gong, Y.; Jiang, M. Ultrafast synthesis of uniform 4–5 atoms-thin layered tremella-like Pd nanostructure with extremely large electrochemically active surface area for formic acid oxidation. J. Power Sources 2020, 447, 227248. [Google Scholar]
- Choi, M.; Ahn, C.; Lee, H.; Kwan, J.; Oh, S.; Hwang, W.; Yang, S.; Kim, J.; Kim, O.; Choi, I. Bi-modified Pt supported on carbon black as electro-oxidation catalyst for 300 W formic acid fuel cell stack. Appl. Catal. B Environ. 2019, 253, 187–195. [Google Scholar] [CrossRef]
- Yang, Q.; Lin, H.; Wang, X.; Ying, L.; Jing, M.; Yuan, W. Dynamically self-assembled adenine-mediated synthesis of pristine graphene-supported clean Pd nanoparticles with superior electrocatalytic performance toward formic acid oxidation. J. Colloid Interface Sci. 2022, 613, 515–523. [Google Scholar] [CrossRef]
- Wang, H.; Chen, H.; Wang, H.Q.; Ou, C.R.; Li, R.; Liu, H.B. Synthesis of ultrafine low loading Pd–Cu alloy catalysts supported on graphene with excellent electrocatalytic performance for formic acid oxidation. Int. J. Hydrogen Energy 2020, 45, 10735–10744. [Google Scholar] [CrossRef]
- Douk, A.S.; Saravani, H.; Noroozifar, M. A fast method to prepare Pd-Co nanostructures decorated on graphene as excellent electrocatalyst toward formic acid oxidation. J. Colloid Interface Sci. 2018, 739, 882–891. [Google Scholar]
- Hu, S.; Che, F.; Khorasani, B.; Jeon, M.; Won, C. Improving the electrochemical oxidation of formic acid by tuning the electronic properties of Pd-based bimetallic nanoparticles. Appl. Catal. B Environ. 2019, 254, 685–692. [Google Scholar] [CrossRef]
- Kwon, T.; Choi, K.; Han, J. Separation of Ultra-High-Density Cell Suspension via Elasto-Inertial Microfluidics. Small 2021, 17, 2101880. [Google Scholar] [CrossRef]
- Hao, R.; Yu, Z.; Du, J.; Hu, S.; Yuan, C.; Guo, H.; Zhang, Y.; Yang, H. A High-Throughput Nanofluidic Device for Exosome Nanoporation to Develop Cargo Delivery Vehicles. Small 2021, 12, 2102150. [Google Scholar] [CrossRef] [PubMed]
- Moreno-zuria, A.; Ortiz-ortega, E.; Gurrola, M.P. Evolution of microfluidic fuel stack design as an innovative alternative to energy production. Int. J. Hydrogen Energy 2017, 42, 27929–27939. [Google Scholar] [CrossRef]
- Ouyang, T.; Chen, J.; Huang, G.; Lu, J.; Mo, C.; Chen, N. A novel two-phase model for predicting the bubble formation and performance in microfluidic fuel cells. J. Power Sources 2020, 457, 228018. [Google Scholar] [CrossRef]
- Gharib, A.; Arab, A. Electrodeposited Pd, Pdsingle bondCd, and Pdsingle bondBi nanostructures: Preparation, characterization, corrosion behavior, and their electrocatalytic activities for formic acid oxidation. J. Electroanal. Chem. 2020, 866, 114166. [Google Scholar] [CrossRef]
- Bao, Y.; Liu, H.; Liu, Z.; Wang, F.; Feng, L. Pd/FeP catalyst engineering via thermal annealing for improved formic acid electrochemical oxidation. Appl. Catal. B Environ. 2020, 274, 119106. [Google Scholar] [CrossRef]
- Rupa, P.; Harivignesh, R.; Sung, Y.; Kalai, R. Polyol assisted formaldehyde reduction of bi-metallic Pt-Pd supported agro-waste derived carbon spheres as an efficient electrocatalyst for formic acid and ethylene glycol oxidation. J. Colloid Interface Sci. 2020, 561, 358–371. [Google Scholar] [CrossRef]
- Bi, Y.; Liu, M.; Ren, H.; Shi, X.; He, S.; Zhang, Y.; Zhang, L. Investigation on gelation process and microstructure for copper-based aerogel prepared via sol–gel method. J. Non-Cryst. Solids 2015, 425, 195–198. [Google Scholar] [CrossRef]
- Suenaga, S.; Osada, M. Preparation of β-chitin nanofiber aerogels by lyophilization. Int. J. Biol. Macromol. 2019, 126, 1145–1149. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, A.K.; Formanek, P.; Borchardt, L.; Klose, M.; Giebeler, L.; Eckert, J.; Kaskel, S.; Gaponik, N.; Eychmüller, A. Multimetallic Aerogels by Template-Free Self-Assembly of Au, Ag, Pt, and Pd Nanoparticles. Chem. Mater. 2014, 26, 1074–1083. [Google Scholar] [CrossRef]
- Wang, H.; Fang, Q.; Gu, W.; Du, D.; Lin, Y.; Zhu, C. Noble Metal Aerogels. ACS Appl. Mater. Interfaces 2020, 12, 52234–52250. [Google Scholar] [CrossRef]
- Du, R.; Fan, X.; Jin, X.J. Emerging Noble Metal Aerogels: State of the Art and a Look Forward. Matter 2019, 1, 39–56. [Google Scholar] [CrossRef] [Green Version]
- Erdem, A.; Ngwabebhoh, F.A.; Yildiz, U. Novel macroporous cryogels with enhanced adsorption capability for the removal of Cu(II) ions from aqueous phase: Modelling, kinetics and recovery studies. J. Environ. Chem. Eng. 2017, 5, 1269–1280. [Google Scholar] [CrossRef]
- Osaki, T. Factors controlling catalytic CO oxidation activity on Pd/CeO2-ZrO2-Al2O3 cryogels. Mater. Res. Bull. 2019, 118, 110498. [Google Scholar] [CrossRef]
- Yildiz, S.; Aktas, N.; Sahiner, N. Metal nanoparticle-embedded super porous poly(3-sulfopropyl methacrylate) cryogel for H2 production from chemical hydride hydrolysis. Int. J. Hydrogen Energy 2014, 39, 14690–14700. [Google Scholar] [CrossRef]
- Baimenov, A.; Berillo, A.D.; Poulopoulos, G. A review of cryogels synthesis, characterization and applications on the removal of heavy metals from aqueous solutions. Adv. Coll. Int. Sci. 2020, 276, 102088. [Google Scholar] [CrossRef]
- Li, D.; Xu, H.; Zhang, L.; Leung, D.Y.; Vilela, F.; Wang, H. Boosting the performance of formic acid microfluidic fuel cell: Oxygen annealing enhanced Pd@graphene electrocatalyst. Int. J. Hydrogen Energy 2016, 41, 10249–10254. [Google Scholar] [CrossRef]
- Ortiz-Ortega, E.; Goulet, M.A.; Lee, J.W.; Guerra-Balcázar, M.; Arjona, N.; Kjeang, E.; Ledesma-García, J.; Arriaga, L.G. A nanofluidic direct formic acid fuel cell with a combined flow-through and air-breathing electrode for high performance. Lab Chip 2014, 14, 4596–4598. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Du, D.; Eychmüller, A.; Lin, Y. Engineering Ordered and Nonordered Porous Noble Metal Nanostructures: Synthesis, Assembly, and Their Applications in Electrochemistry. Chem. Rev. 2015, 115, 8896–8943. [Google Scholar] [CrossRef]
- Liu, W.; Herrmann, A.K.; Geiger, D.; Borchardt, L.; Simon, F.; Kaskel, S.; Gaponik, N.; Eychmüller, A. High-performance electrocatalysis on palladium aerogels. Angew. Chem. Int. Ed. Engl. 2012, 51, 5743–5747. [Google Scholar] [CrossRef]
- Zhu, C.; Shi, Q.; Fu, S.; Song, J.; Xia, H.; Du, D.; Lin, Y. Efficient Synthesis of MCu (M = Pd, Pt, and Au) Aerogels with Accelerated Gelation Kinetics and their High Electrocatalytic Activity. Adv. Mater. 2016, 28, 8779–8783. [Google Scholar] [CrossRef]
- Douk, A.S.; Saravani, H.; Abad, M.Z.Y.; Noroozifar, M. Three-Dimensional Engineering of Nanoparticles To Fabricate a Pd–Au Aerogel as an Advanced Supportless Electrocatalyst for Low-Temperature Direct Ethanol Fuel Cells. ACS Appl. Energy Mater. 2020, 3, 7527–7534. [Google Scholar] [CrossRef]
- Honma1, H.; Kobayashi, T.J. Electroless Copper Deposition Process Using Glyoxylic Acid as a Reducing Agent. Electrochem. Soc. 1994, 141, 730. [Google Scholar] [CrossRef]
- Maleki, H.; Hüsing, N. Current status, opportunities and challenges in catalytic and photocatalytic applications of aerogels: Environmental protection aspects. Appl. Catal. B Environ. 2018, 221, 530–555. [Google Scholar] [CrossRef]
- Liu, X.; Bu, Y.; Cheng, T.; Gao, W.; Jiang, Q. Flower-like carbon supported Pd–Ni bimetal nanoparticles catalyst for formic acid electrooxidation. Electrochim. Act. 2019, 324, 134816. [Google Scholar] [CrossRef]
- Caglar, A.; Selim, M. Effective carbon nanotube supported metal (M = Au, Ag, Co, Mn, Ni, V, Zn) core Pd shell bimetallic anode catalysts for formic acid fuel cells. Renew. Energy 2020, 150, 78–90. [Google Scholar] [CrossRef]
- Doustkhah, E.; Mohtasham, H.; Farajzadeh, M.; Rostamnia, S.; Wang, Y.; Arandiyan, H. Organosiloxane tunability in mesoporous organosilica and punctuated Pd nanoparticles growth; theory and experiment. Microporous Mesoporous Mater. 2020, 293, 109832. [Google Scholar] [CrossRef]
- Gao, N.; Ma, R.; Wang, X.; Jin, Z. Activating the Pd-Based catalysts via tailoring reaction interface towards formic acid dehydrogenation. Int. J. Hydrogen Energy 2020, 45, 17575–17582. [Google Scholar] [CrossRef]
- Saravani, H.; Farsadrooh, M.; Share, M. Two-dimensional engineering of Pd nanosheets as advanced electrocatalysts toward formic acid oxidation. Int. J. Hydrogen Energy 2020, 45, 21232–21240. [Google Scholar] [CrossRef]
- Herrero, E.; Feliu, J.M. Understanding formic acid oxidation mechanism on platinum single crystal electrodes. Curr. Opin. Electrochem. 2018, 9, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Douk, A.S.; Saravani, H.; Noroozifar, M. Three-dimensional assembly of building blocks for the fabrication of Pd aerogel as a high performance electrocatalyst toward ethanol oxidation. Electrochim. Act. 2018, 275, 182–191. [Google Scholar] [CrossRef]
- Yang, L.; Wang, X.; Liu, D.; Cui, G.; Dou, B.; Wang, J. Efficient anchoring of nanoscale Pd on three-dimensional carbon hybrid as highly active and stable catalyst for electro-oxidation of formic acid. Appl. Catal. B Environ. 2020, 263, 118304. [Google Scholar] [CrossRef]
- Morales-Acosta, D.; G, H.R.; Godinez, L.A.; Arriaga, L.G. Performance increase of microfluidic formic acid fuel cell using Pd/MWCNTs as catalyst. J. Power Sources 2010, 195, 1862–1865. [Google Scholar] [CrossRef]
- Morales-Acosta, D.; Morales-Acosta, M.D.; Godinez, L.A.; Álvarez-Contreras, L.; Duron-Torres, S.M.; Ledesma-García, J.; Arriaga, L.G. PdCo supported on multiwalled carbon nanotubes as an anode catalyst in a microfluidic formic acid fuel cell. J. Power Sources 2011, 196, 9270–9275. [Google Scholar] [CrossRef]
- Jindal, A.; Basu, S.; Chauhan, N.; Ukai, T.; Kumar, D.; Samudhyatha, K.T. Application of electrospun CNx nanofibers as cathode in microfluidic fuel cell. J. Power Sources 2017, 342, 165–174. [Google Scholar] [CrossRef]
- Zhang, H.; Xuan, J.; Leung, D.; Wang, H.; Xu, H.; Zhang, L. Advanced gas-emission anode design for microfluidic fuel cell eliminating bubble accumulation. J. Micromech. Microeng. 2017, 27, 105016. [Google Scholar] [CrossRef]
- Park, H.; Lee, K.; Sung, H.J. Performance of H-shaped membraneless micro fuel cells. J. Power Sources 2013, 226, 266–271. [Google Scholar] [CrossRef]
- Brushett, F.; Jayashree, R.; Zhou, W.; Kenis, P. Investigation of fuel and media flexible laminar flow-based fuel cells. Electrochim. Act. 2009, 54, 7099–7105. [Google Scholar] [CrossRef]
- Choban, E.; Markoski, L.; Wieckowski, A.; Kenis, P. Microfluidic fuel cell based on laminar flow. J. Power Sources 2004, 128, 54–60. [Google Scholar] [CrossRef]
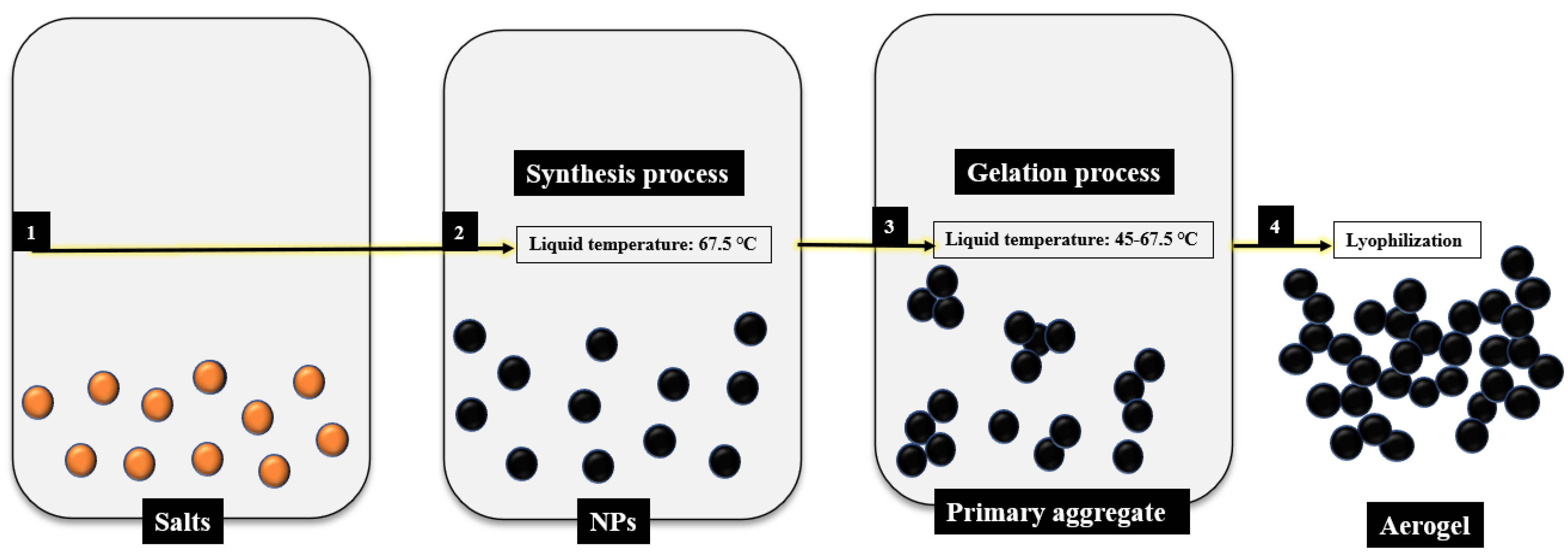
| Sample | Synthesis at 67.5 °C for 2 h | Heating Reduction Device | Reduction Conditions | Drying Device |
|---|---|---|---|---|
| PdA-CC | CON | CON | 24 h/45 °C | LYO |
| PdA-MC | MW | CON | 24 h/45 °C | LYO |
| PdA-MM | MW | MW | 7 h/45 °C | LYO |
| PdA-MMT | MW | MW | 7 h/67 °C | LYO |
| PdA-CON | CON | CON | 24 h/45 °C | CON |
| Sample | ECSA (m2/g) |
|---|---|
| PdA-CON | 1.3 |
| PdA-CC | 22.1 |
| PdA_MC | 22.1 |
| PdA-MM | 22.8 |
| PdA-MMT | 28.5 |
| Pd/C | 16 |
| Anodic Catalyst | Formic Acid Concentration | Mass Loading/mg cm−2 | OCV/V | J/mA cm−2 | W Max/mW cm2 | Reference |
|---|---|---|---|---|---|---|
| Pd/C | 0.5 M | 0.7 | 0.9 | 7.4 | 2.9 | [42] |
| Pd/C | 0.5 M | 0.1 | 0.9 | 43 | 10.5 | This work |
| Pd/MWCNT | 0.5 M | 1.3 | 0.9 | 9.1 | 2.3 | [43] |
| Pd50Co50/MWCNT | 0.5 M | 1.2 | 0.9 | 5.9 | 1.75 | [38] |
| PdA-MMT | 0.5 M | 0.1 | 0.88 | 118.3 | 14 | This work |
| Pd/graphene | 0.5 M | 2 | 0.7 | 30 | 15.2 | [26] |
| Pt/CNx | 0.5 M | 1 | 1.1 | 9.79 | 3.43 | [44] |
| Pt-Ru | 3 M | 3 | 0.47 | 1.2 | 12.5 | [45] |
| Au-Pt | 1 M | - | 1.2 | 28 | 12 | [46] |
| Pd | 0.5 M | 10 | 0.95 | 125 | 26 | [47] |
| Pt | 0.5 M | - | 1.1 | 8 | 2.2 | [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Lázaro, A.; Ramírez-Montoya, L.A.; Ledesma-García, J.; Montes-Morán, M.A.; Gurrola, M.P.; Menéndez, J.A.; Arenillas, A.; Arriaga, L.G. Facile Synthesis of Unsupported Pd Aerogel for High Performance Formic Acid Microfluidic Fuel Cell. Materials 2022, 15, 1422. https://doi.org/10.3390/ma15041422
Martínez-Lázaro A, Ramírez-Montoya LA, Ledesma-García J, Montes-Morán MA, Gurrola MP, Menéndez JA, Arenillas A, Arriaga LG. Facile Synthesis of Unsupported Pd Aerogel for High Performance Formic Acid Microfluidic Fuel Cell. Materials. 2022; 15(4):1422. https://doi.org/10.3390/ma15041422
Chicago/Turabian StyleMartínez-Lázaro, Alejandra, Luis A. Ramírez-Montoya, Janet Ledesma-García, Miguel A. Montes-Morán, Mayra P. Gurrola, J. Angel Menéndez, Ana Arenillas, and Luis G. Arriaga. 2022. "Facile Synthesis of Unsupported Pd Aerogel for High Performance Formic Acid Microfluidic Fuel Cell" Materials 15, no. 4: 1422. https://doi.org/10.3390/ma15041422
APA StyleMartínez-Lázaro, A., Ramírez-Montoya, L. A., Ledesma-García, J., Montes-Morán, M. A., Gurrola, M. P., Menéndez, J. A., Arenillas, A., & Arriaga, L. G. (2022). Facile Synthesis of Unsupported Pd Aerogel for High Performance Formic Acid Microfluidic Fuel Cell. Materials, 15(4), 1422. https://doi.org/10.3390/ma15041422









