Influence of Bi2O3 on Mechanical Properties and Radiation-Shielding Performance of Lithium Zinc Bismuth Silicate Glass System Using Phys-X Software
Abstract
:1. Introduction
2. Methodology
3. Results and Discussion
3.1. Mechanical Properties
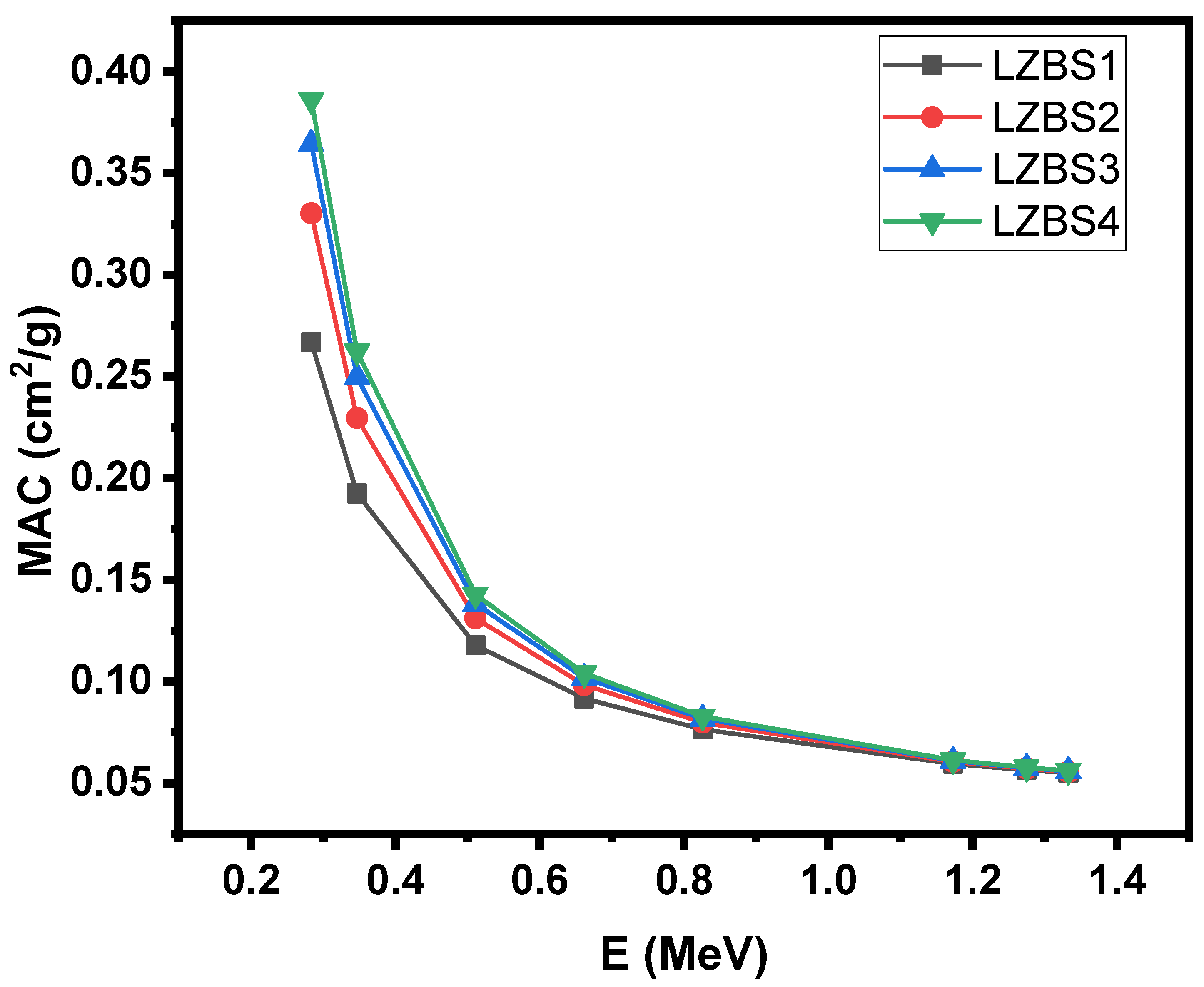
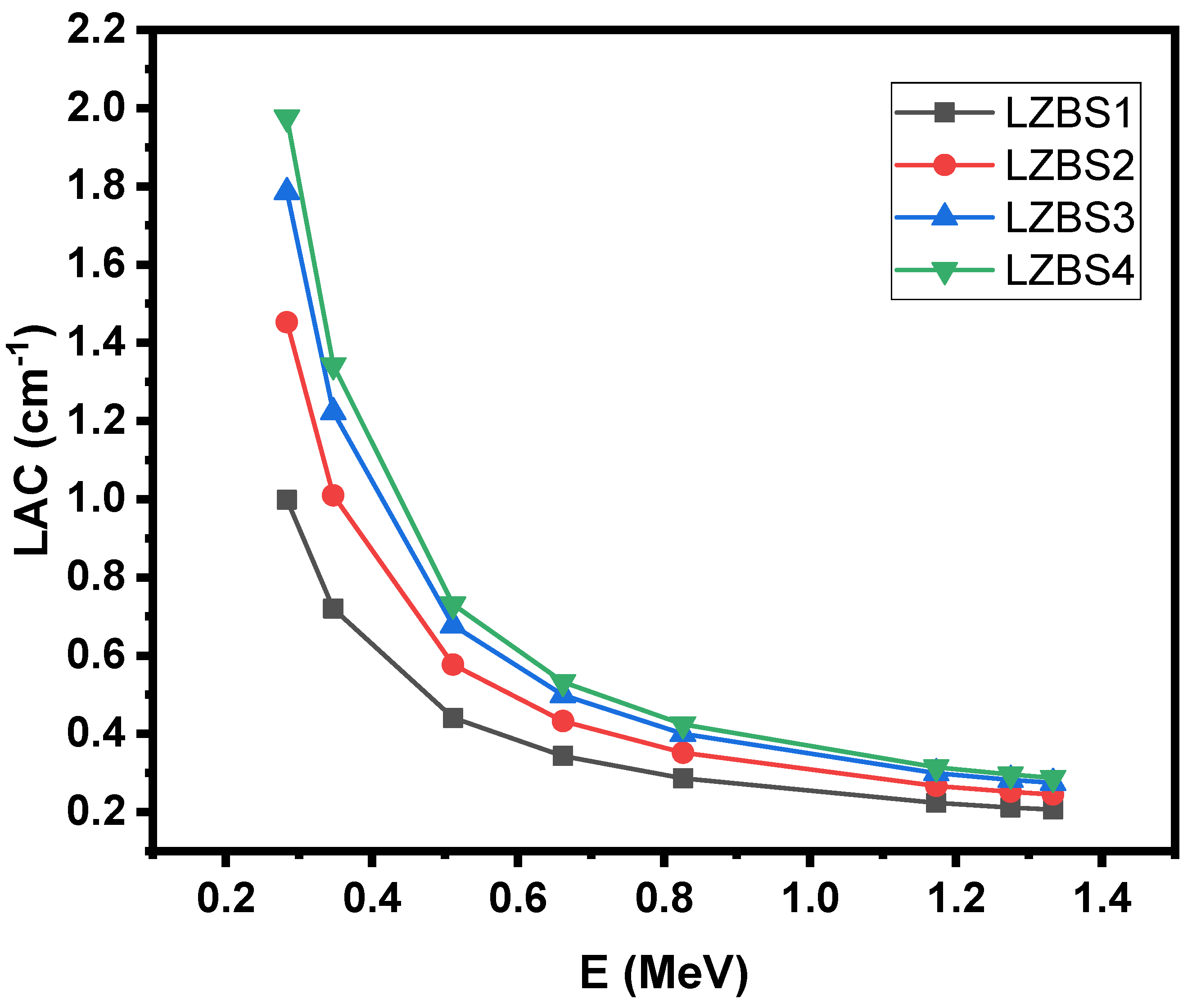
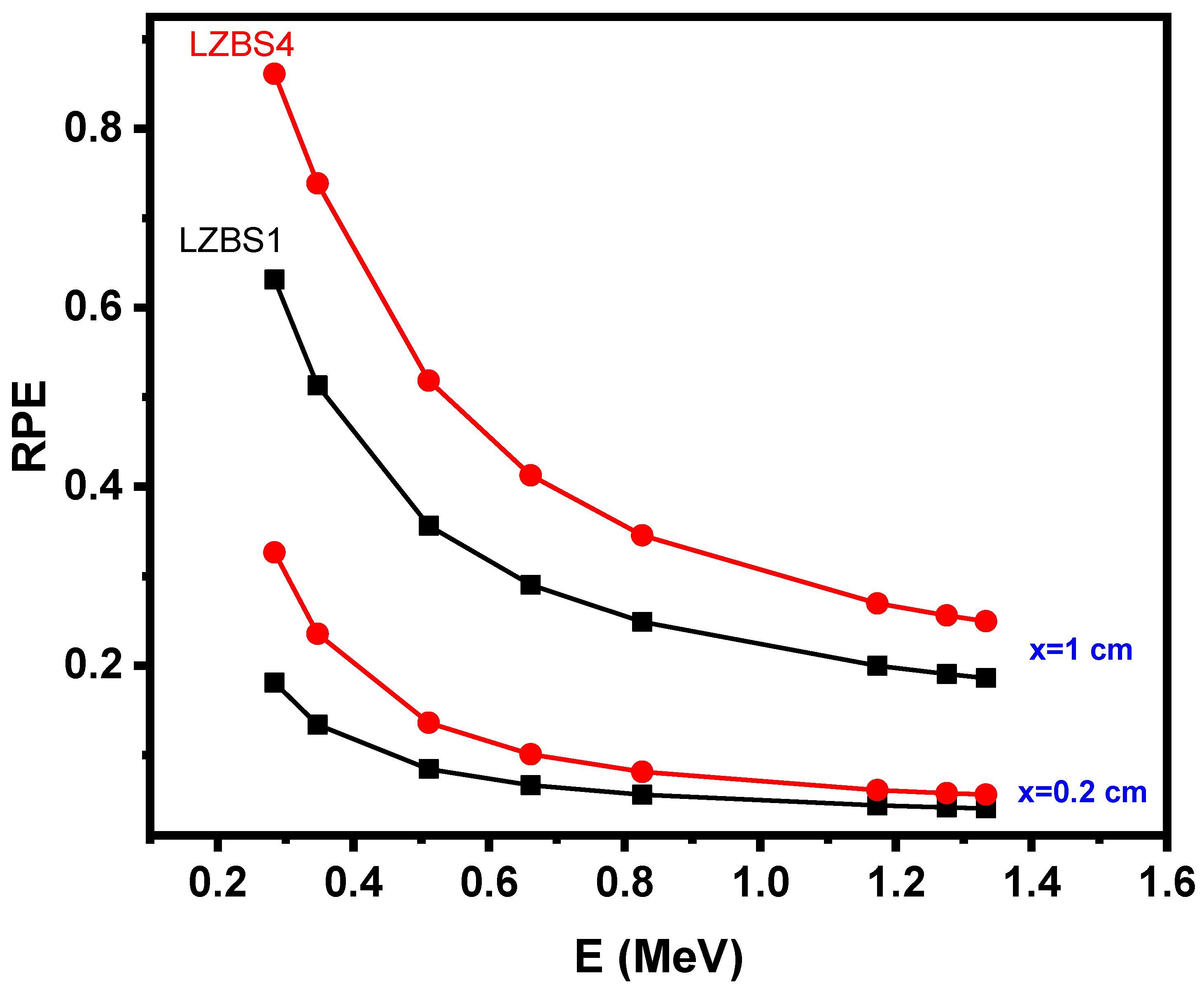
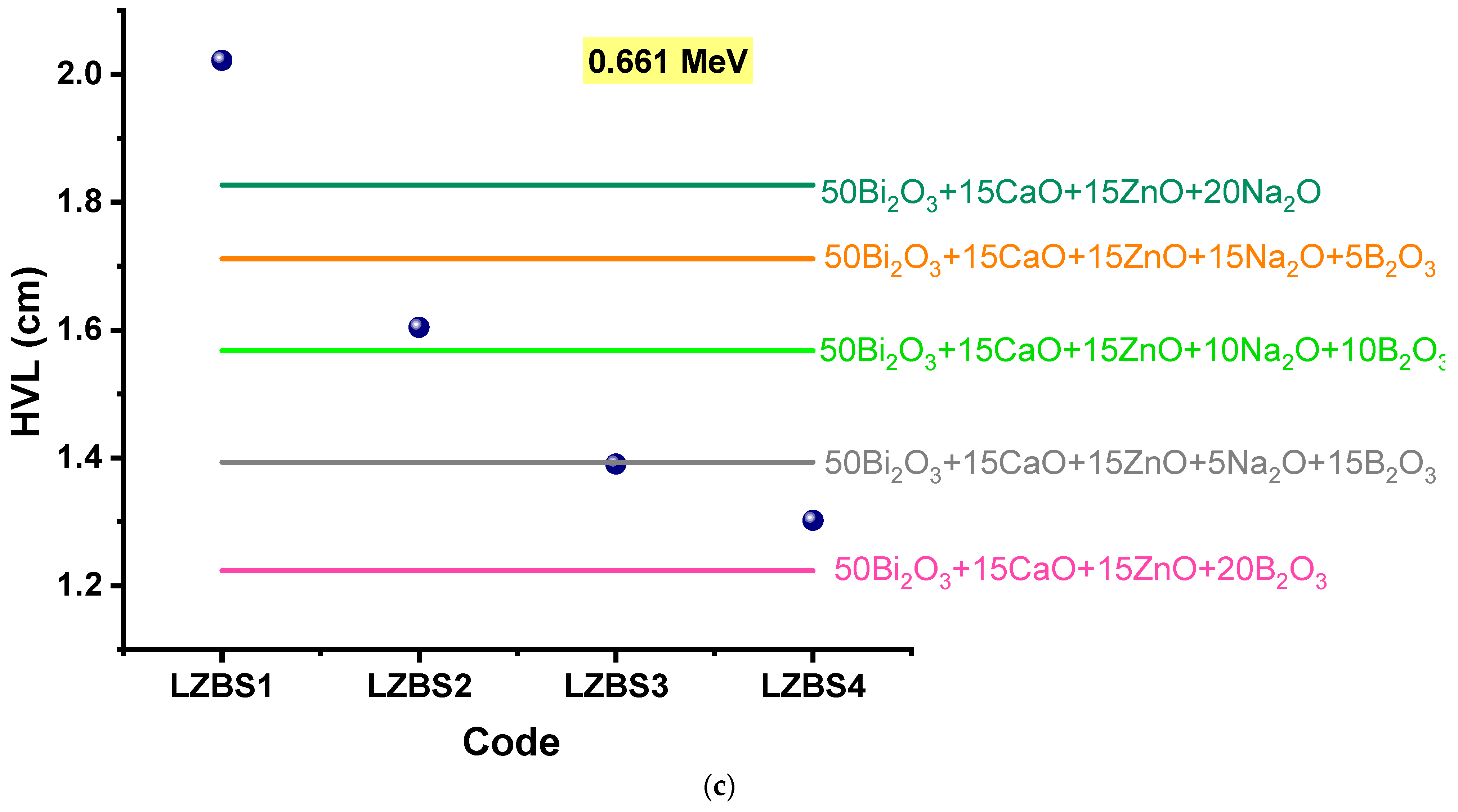
3.2. Radiation-Shielding Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamislioglu, M. An investigation into gamma radiation shielding parameters of the (Al:Si) and (Al + Na):Si-doped international simple glasses (ISG) used in nuclear waste management, deploying Phy-X/PSD and SRIM software. J. Mater. Sci. Mater. Electron. 2021, 32, 12690–12704. [Google Scholar] [CrossRef]
- Dong, M.; Zhou, S.; Xue, X.; Feng, X.; Sayyed, M.I.; Khandaker, M.U.; Bradley, D.A. The potential use of boron containing resources for protection against nuclear radiation. Radiat. Phys. Chem. 2021, 188, 109601. [Google Scholar] [CrossRef]
- Dong, M.; Xue, X.; Yang, H.; Liu, D.; Wang, C.; Li, Z. A novel comprehensive utilization of vanadium slag: As gamma ray shielding material. J. Hazard. Mater. 2016, 318, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Xue, X.; Yang, H.; Li, Z. Highly cost-effective shielding composite made from vanadium slag and boron-rich slag and its properties. Radiat. Phys. Chem. 2017, 141, 239–244. [Google Scholar] [CrossRef]
- Erdem, Ş.; Fırat Özpolat, Ö.; Alım, B.; Sayyed, M.I.; Kurudirek, M. Phy-X/PSD: Development of a user friendly online software for calculation of parameters relevant to radiation shielding and dosimetry. Radiat. Phys. Chem. 2020, 166, 108496. [Google Scholar]
- Yasmin, S.; Barua, B.S.; Khandaker, M.U.; Chowdhury, F.-U.-Z.; Rashid, A.; Bradley, D.A.; Olatunji, M.A.; Kamal, M. Studies of ionizing radiation shielding effectiveness of silica-based commercial glasses used in Bangladeshi dwellings. Results Phys. 2018, 9, 541–549. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Elmahroug, Y.; Elbashir, B.O.; Issa, S. Gamma-ray shielding properties of zinc oxide soda lime silica glasses. J. Mater. Sci. Mater. Electron. 2017, 28, 4064–4074. [Google Scholar] [CrossRef]
- Yasmin, S.; Rozaila, Z.S.; Khandaker, M.U.; Barua, B.S.; Chowdhury, F.-U.-Z.; Rashid, A.; Bradley, A.D. The radiation shielding offered by the commercial glass installed in Bangladeshi dwellings. Radiat. Eff. Defects Solids 2018, 173, 657–672. [Google Scholar] [CrossRef]
- Rammah, Y.S.; Abouhaswa, A.S.; Sayyed, M.I.; Tekin, H.O.; El-Mallawany, R. Structural, UV and shielding properties of ZBPC glasses. J. Non-Cryst. Solids 2019, 509, 99–105. [Google Scholar] [CrossRef]
- Gökçe, H.; Öztürk, B.C.; Çam, N.; Andiç-Çakır, Ö. Gamma-ray attenuation coefficients and transmission thickness of high consistency heavyweight concrete containing mineral admixture. Cem. Concr. Compos. 2018, 92, 56–69. [Google Scholar] [CrossRef]
- Gökçe, H.S.; Yalçınkaya, Ç.; Tuyan, M. Optimization of reactive powder concrete by means of barite aggregate for both neutrons and gamma rays. Constr. Build. Mater. 2018, 189, 470–477. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Akyildirim, H.; Al-Buriahi, M.S.; Lacomme, E.; Ayad, R.; Bonvicini, G. Oxyfluoro-tellurite-zinc glasses and the nuclear-shielding ability under the substitution of AlF3 by ZnO. Appl. Phys. A 2020, 126, 88. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Rammah, Y.S.; Abouhaswa, A.S.; Tekin, H.O.; Elbashir, B.O. ZnO-B2O3-PbO glasses: Synthesis and radiation shielding characterization. Phys. B Condens. Matter 2018, 548, 20–26. [Google Scholar] [CrossRef]
- Sayyed, M.; Almuqrin, A.H.; Kumar, A.; Jecong, J.; Akkurt, I. Optical, mechanical properties of TeO2-CdO-PbO-B2O3 glass systems and radiation shielding investigation using EPICS2017 library. Optik 2021, 242, 167342. [Google Scholar] [CrossRef]
- Kaewjaeng, S.; Kothan, S.; Chaiphaksa, W.; Chanthima, N.; Rajaramakrishna, R.; Kim, H.; Kaewkhao, J. High transparency La2O3-CaO-B2O3-SiO2 glass for diagnosis X-rays shielding material application. Radiat. Phys. Chem. 2019, 160, 41–47. [Google Scholar] [CrossRef]
- Chanthima, N.; Kaewkhao, J.; Limkitjaroenporn, P.; Tuscharoen, S.; Kothan, S.; Tungjai, M.; Kaewjaeng, S.; Sarachai, S.; Limsuwan, P. Development of BaO–ZnO–B2O3 glasses as a radiation shielding material. Radiat. Phys. Chem. 2017, 137, 72–77. [Google Scholar] [CrossRef]
- IssaS, A.; Saddeek, Y.; Sayyed, M.; Tekin, H.O.; Kilicoglu, O. Radiation shielding features using MCNPX code and mechanical properties of the PbO Na2O B2O3CaO Al2O3SiO2 glass systems. Compos. Part B Eng. 2019, 167, 231–240. [Google Scholar] [CrossRef]
- Vijayalakshmi, L.; Kumar, K.N.; Baek, J.D.; Hwang, P. Bright green fluorescence from Tb3+ activated lithium zinc borate glasses for solid-state laser and w-LEDs applications. Optik 2021, 248, 168219. [Google Scholar] [CrossRef]
- Bootjomchai, C.; Laopaiboon, J.; Yenchai, C.; Laopaiboon, R. Gamma-ray shielding and structural properties of barium–bismuth–borosilicate glasses. Radiat. Phys. Chem. 2012, 81, 785–790. [Google Scholar] [CrossRef]
- Al-Hadeethi, Y.; Sayyed, M. BaO–Li2O–B2O3 glass systems: Potential utilization in gamma radiation protection. Prog. Nucl. Energy 2020, 129, 103511. [Google Scholar] [CrossRef]
- Cheewasukhanont, W.; Limkitjaroenporn, P.; Kothan, S.; Kedkaew, C.; Kaewkhao, J. The effect of particle size on radiation shielding properties for bismuth borosilicate glass. Radiat. Phys. Chem. 2020, 172, 108791. [Google Scholar] [CrossRef]
- Sayyed, M.; Dong, M.; Tekin, H.; Lakshminarayana, G.; Mahdi, M. Comparative investigations of gamma and neutron radiation shielding parameters for different borate and tellurite glass systems using WinXCom program and MCNPX code. Mater. Chem. Phys. 2018, 215, 183–202. [Google Scholar] [CrossRef]
- Abouhaswa, A.; Kavaz, E. Bi2O3 effect on physical, optical, structural and radiation safety characteristics of B2O3Na2O-ZnO CaO glass system. J. Non-Cryst. Solids 2020, 535, 119993. [Google Scholar] [CrossRef]
- Al-Buriahi, M.; Alajerami, Y.; Abouhaswa, A.; Alalawi, A.; Nutaro, T.; Tonguc, B. Effect of chromium oxide on the physical, optical, and radiation shielding properties of lead sodium borate glasses. J. Non-Cryst. Solids 2020, 544, 120171. [Google Scholar] [CrossRef]
- Al-Buriahi, M.S.; Somaily, H.H.; Alalawi, A.; Alraddadi, S. Polarizability, Optical Basicity, and Photon Attenuation Properties of Ag2O–MoO3–V2O5–TeO2 Glasses: The Role of Silver Oxide. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1047–1056. [Google Scholar] [CrossRef]
- Al-Hadeethi, Y.; Sayyed, M.; Nune, M. Radiation shielding study of WO3–ZnO–PbO–B2O3 glasses using Geant4 and Phys-X: A comparative study. Ceram. Int. 2021, 47, 3988–3993. [Google Scholar] [CrossRef]
- Al-Buriahi, M.; Rammah, Y. Radiation sensing properties of tellurite glasses belonging to ZnO–TeO2–PbO system using Geant4 code. Radiat. Phys. Chem. 2020, 170, 108632. [Google Scholar] [CrossRef]
- Cheewasukhanont, W.; Limkitjaroenporn, P.; Sayyed, M.; Kothan, S.; Kim, H.; Kaewkhao, J. High density of tungsten gadolinium borate glasses for radiation shielding material: Effect of WO3 concentration. Radiat. Phys. Chem. 2021, 192, 109926. [Google Scholar] [CrossRef]
- Boukhris, I.; Kebaili, I.; Al-Buriahi, M.; Sayyed, M. Radiation shielding properties of tellurite-lead-tungsten glasses against gamma and beta radiations. J. Non-Cryst. Solids 2021, 551, 120430. [Google Scholar] [CrossRef]
- Rani, S.; Sanghi, S.; Ahlawat, N.; Agarwal, A. Influence of Bi2O3 on physical, electrical and thermal properties of Li2O·ZnO·Bi2O3·SiO2 glasses. J. Alloy. Compd. 2015, 619, 659–666. [Google Scholar] [CrossRef]
- Makishima, A.; MacKenzie, J.D. Calculation of bulk modulus, shear modulus and Poisson’s ratio of glass. J. Non-Cryst. Solids 1975, 17, 147–157. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, Internet Version 2005, 85th ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Elbashar, Y.; Rashad, M.; Rayan, D.A. Physical and Mechanical Properties of Neodymium Doped Zinc Borate Glass with Different Boron Content. Silicon 2016, 10, 115–122. [Google Scholar] [CrossRef]
- Kavaz, E.; Ekinci, N.; Tekin, H.O.; Sayyed, M.I.; Aygün, B.; Perişanoğlu, U. Estimation of gamma radiation shielding qualification of newly developed glasses by using WinXCOM and MCNPX code. Prog. Nucl. Energy 2019, 115, 12–20. [Google Scholar] [CrossRef]
- Sayyed, M.; Agar, O.; Kumar, A.; Tekin, H.O.; Gaikwad, D.; Obaid, S.S. Shielding behaviour of (20 + x) Bi2O3–20BaO–10Na2O–10MgO–(40-x) B2O3: An experimental and Monte Carlo study. Chem. Phys. 2020, 529, 110571. [Google Scholar] [CrossRef]
- Al-Hadeethi, Y.; Sayyed, M.I. A comprehensive study on the effect of TeO2 on the radiation shielding properties of TeO2–B2O3–Bi2O3–LiF–SrCl2 glass system using Phy-X/PSD software. Ceram. Int. 2020, 46, 6136–6140. [Google Scholar] [CrossRef]
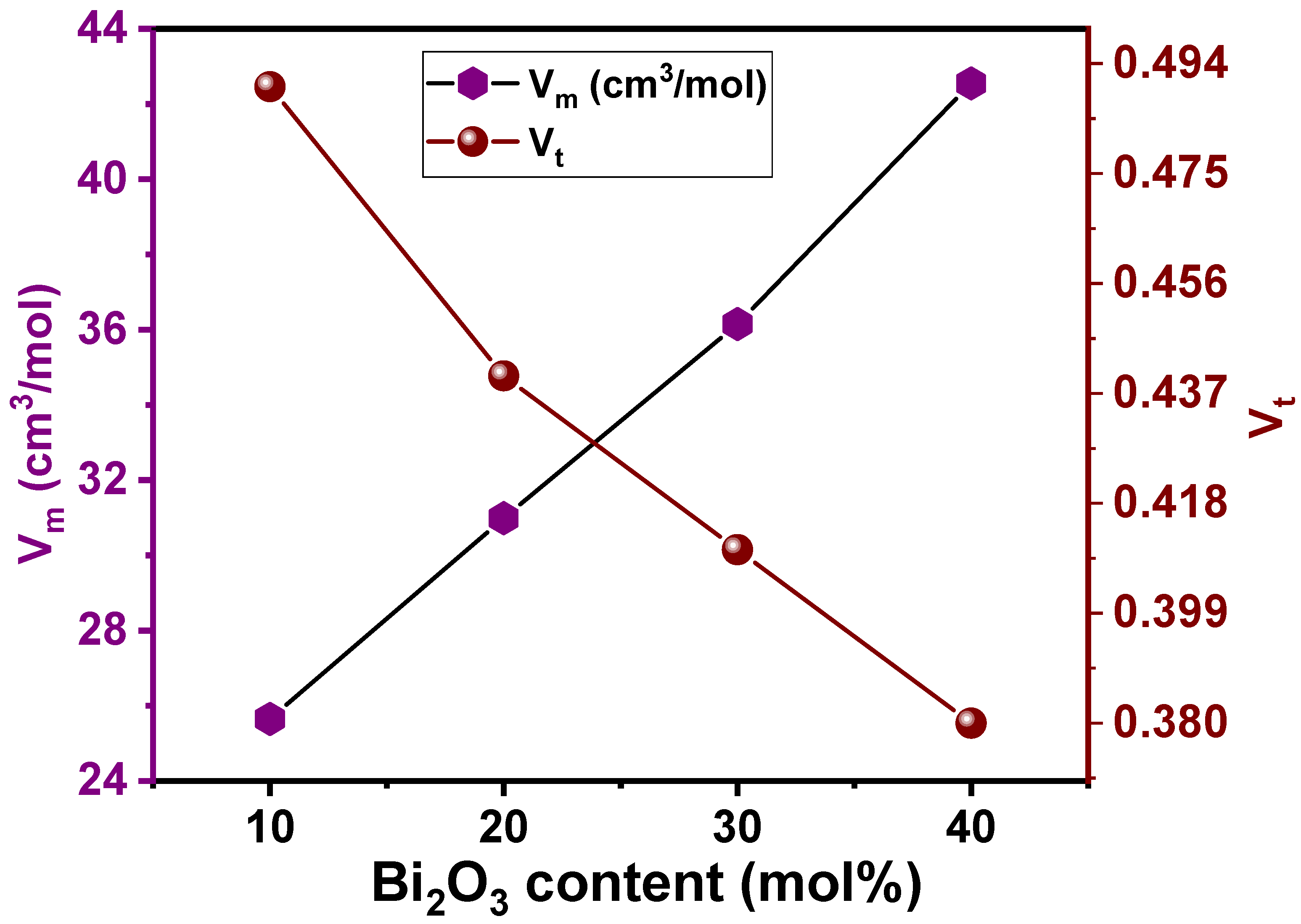

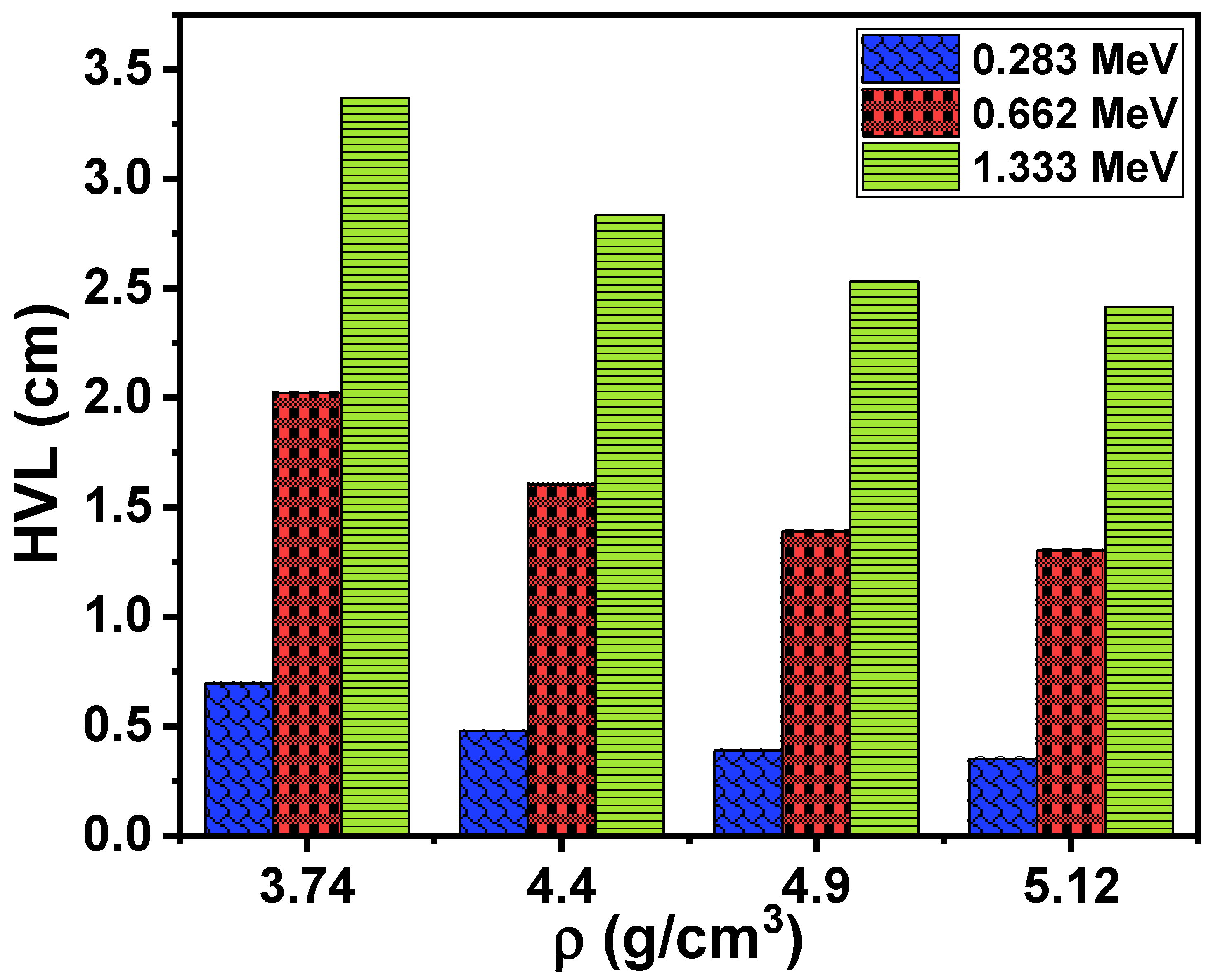
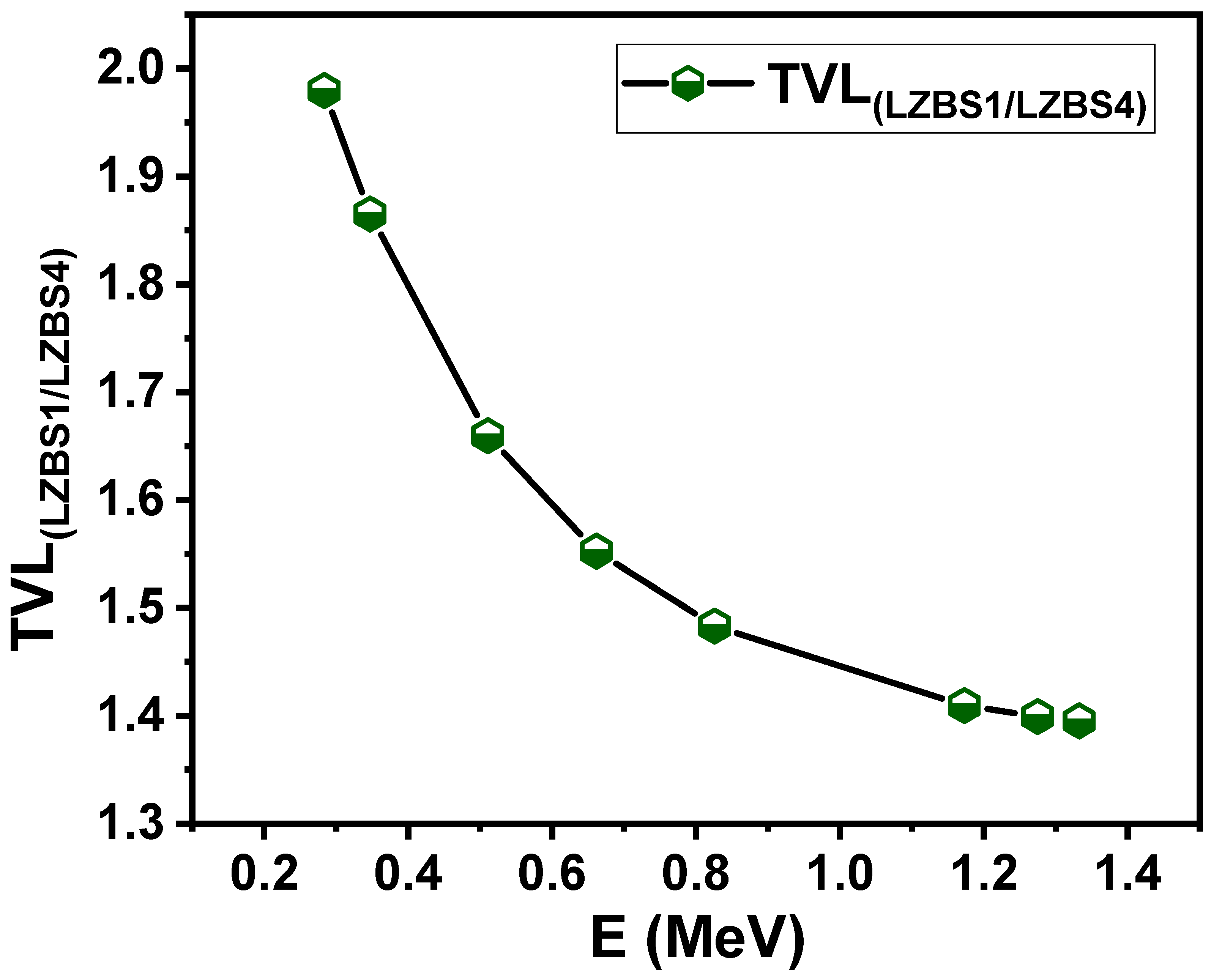
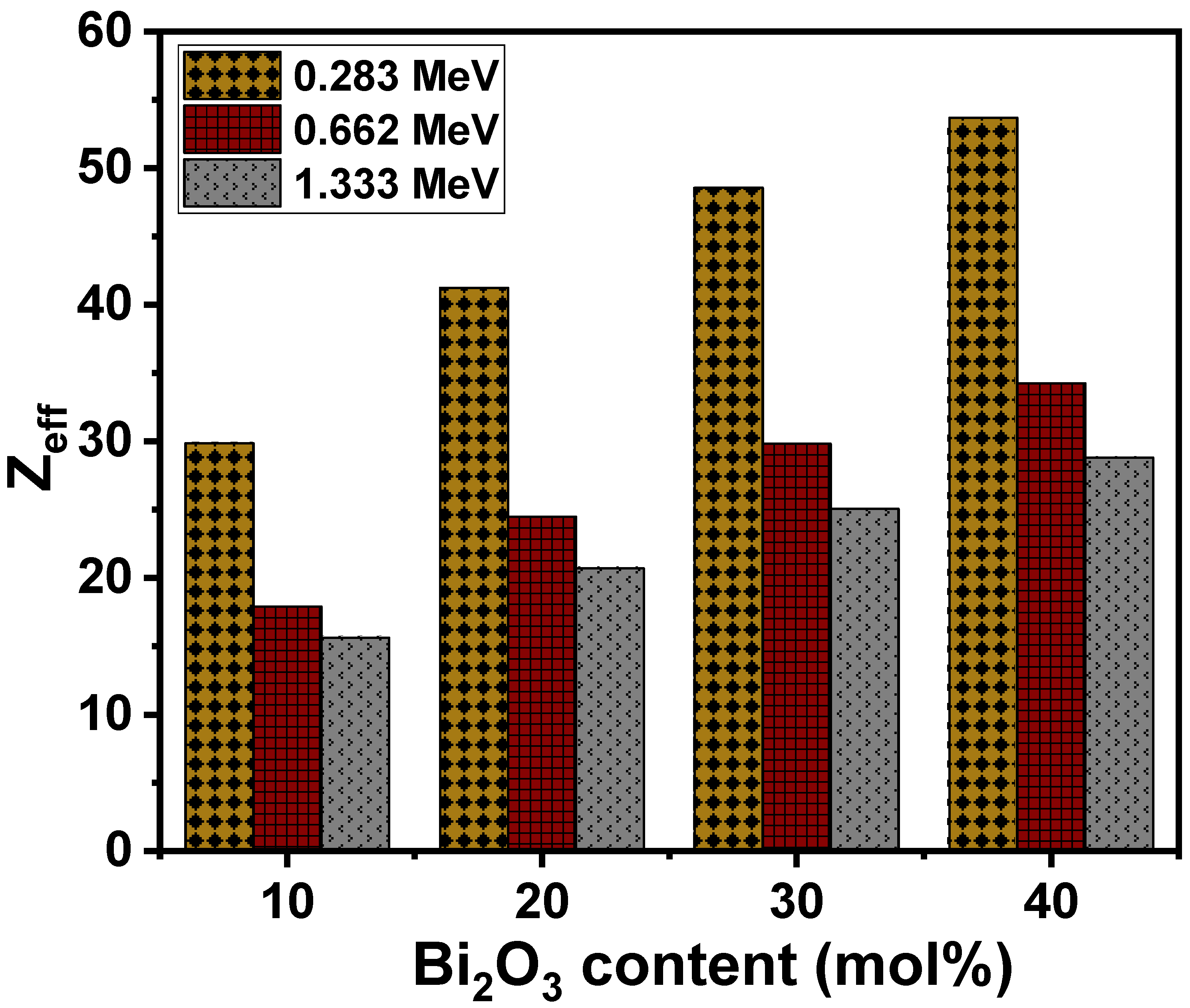
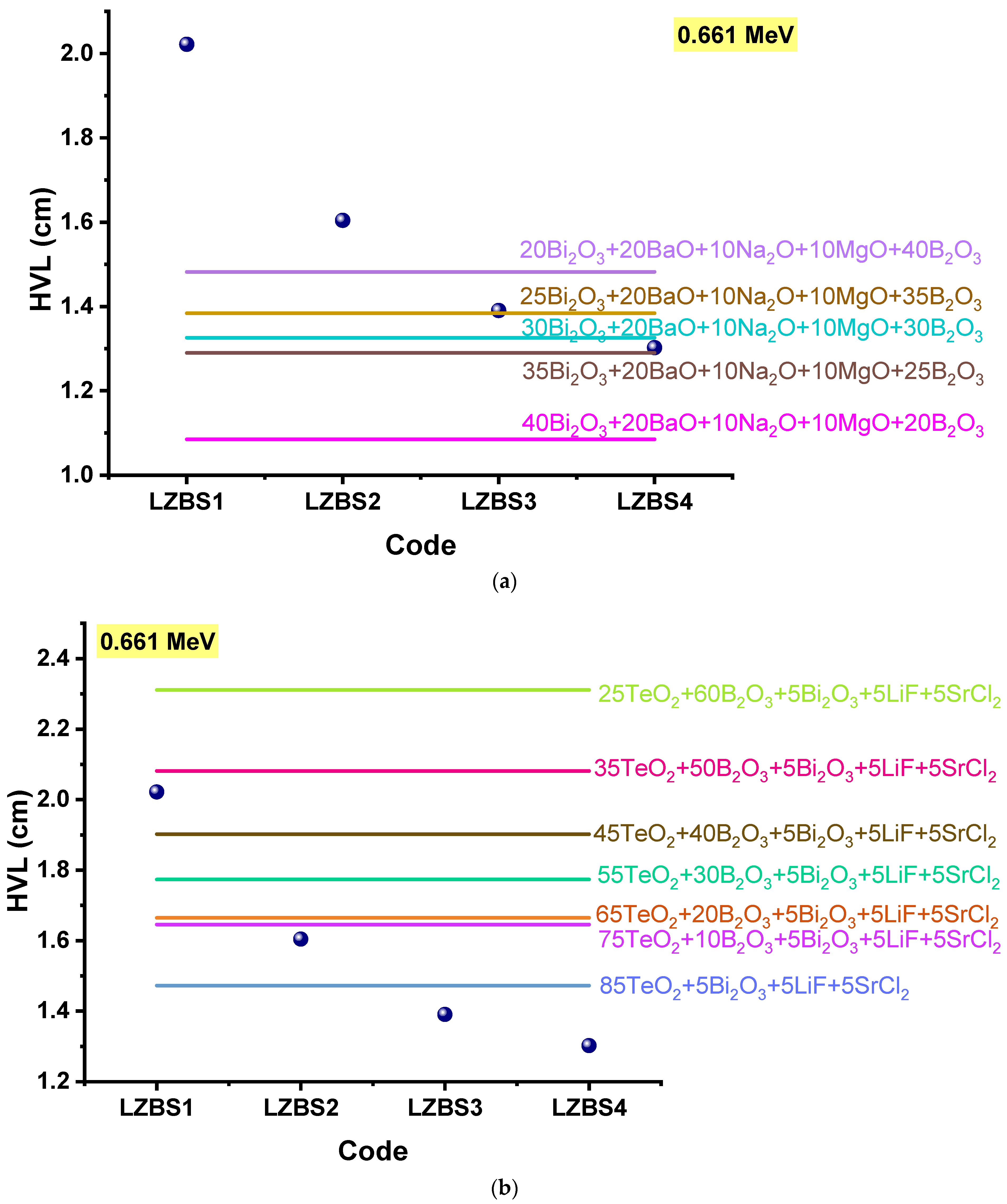
| Glass Composition [30] | Glass Code | ρ (g/cm3) [30] | Vm (cm3/mol) [30] | Vt | L (GPa) | H (GPa) | |||
|---|---|---|---|---|---|---|---|---|---|
| 30Li2O-20ZnO-10Bi2O3-40SiO2 | LZBS1 | 3.74 | 25.65 | 0.49 | 46 | 27 | 19 | 52 | 3.55 |
| 30Li2O-20ZnO-20Bi2O3-30SiO2 | LZBS2 | 4.40 | 30.99 | 0.44 | 40 | 21 | 17 | 43 | 3.48 |
| 30Li2O-20ZnO-30Bi2O3-20SiO2 | LZBS3 | 4.90 | 36.15 | 0.41 | 35 | 18 | 15 | 38 | 3.39 |
| 30Li2O-20ZnO-40Bi2O3-10SiO2 | LZBS4 | 5.12 | 42.55 | 0.38 | 31 | 14 | 14 | 32 | 3.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almuqrin, A.H.; Sayyed, M.I.; Prabhu, N.S.; Kamath, S.D. Influence of Bi2O3 on Mechanical Properties and Radiation-Shielding Performance of Lithium Zinc Bismuth Silicate Glass System Using Phys-X Software. Materials 2022, 15, 1327. https://doi.org/10.3390/ma15041327
Almuqrin AH, Sayyed MI, Prabhu NS, Kamath SD. Influence of Bi2O3 on Mechanical Properties and Radiation-Shielding Performance of Lithium Zinc Bismuth Silicate Glass System Using Phys-X Software. Materials. 2022; 15(4):1327. https://doi.org/10.3390/ma15041327
Chicago/Turabian StyleAlmuqrin, Aljawhara H., M. I. Sayyed, Nimitha S. Prabhu, and Sudha D. Kamath. 2022. "Influence of Bi2O3 on Mechanical Properties and Radiation-Shielding Performance of Lithium Zinc Bismuth Silicate Glass System Using Phys-X Software" Materials 15, no. 4: 1327. https://doi.org/10.3390/ma15041327
APA StyleAlmuqrin, A. H., Sayyed, M. I., Prabhu, N. S., & Kamath, S. D. (2022). Influence of Bi2O3 on Mechanical Properties and Radiation-Shielding Performance of Lithium Zinc Bismuth Silicate Glass System Using Phys-X Software. Materials, 15(4), 1327. https://doi.org/10.3390/ma15041327






