Porous Biomass Carbon Derived from Clivia miniata Leaves via NaOH Activation for Removal of Dye
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CMBC
2.3. Characterization
2.4. Adsorption Studies
2.5. Effect of Temperature and pH
2.6. Reusability Studies
3. Results and Discussion
3.1. Characterizations
3.2. Adsorption Experiments
3.2.1. Adsorption Kinetics
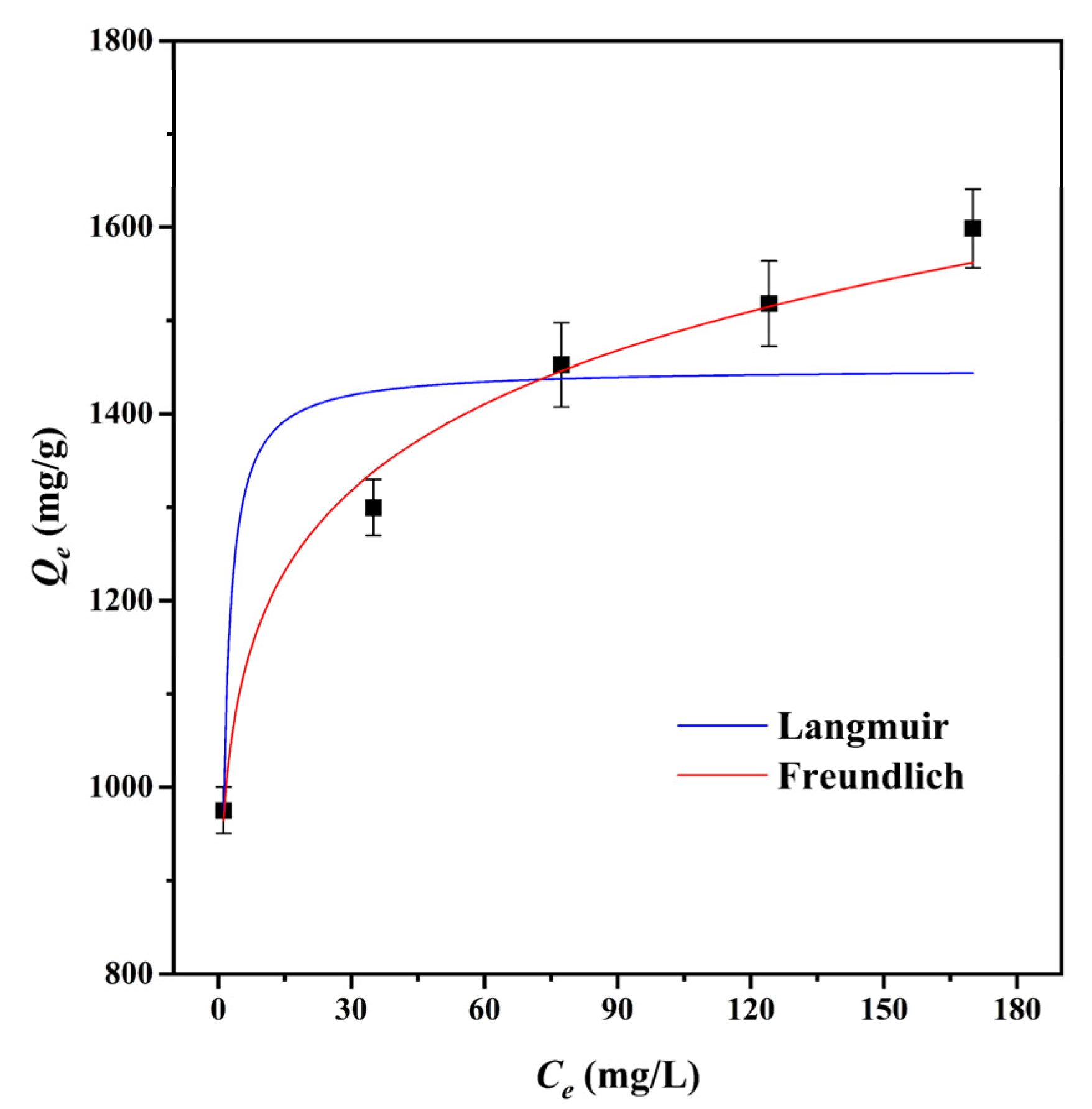
3.2.2. Adsorption Isotherms
3.3. Effect of Temperature and pH
3.4. Reusability of the CMBC-4-700-30 and Probable Mechanism Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rathinam, K.; Kou, X.; Hobby, R.; Panglisch, S. Sustainable development of magnetic chitosan core-shell network for the removal of organic dyes from aqueous solutions. Materials 2021, 14, 7701. [Google Scholar] [CrossRef] [PubMed]
- Santucci, V.; Fiore, S. Recovery of waste polyurethane from e-waste. Part II. investigation of the adsorption potential for wastewater treatment. Materials 2021, 14, 7587. [Google Scholar] [CrossRef] [PubMed]
- Luhar, I.; Luhar, S.; Abdullah, M.M.A.B.; Razak, R.A.; Vizureanu, P.; Sandu, A.V.; Matasaru, P. A State-of-the-art review on innovative geopolymer composites designed for water and wastewater treatment. Materials 2021, 14, 7456. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gao, X.; Xu, H.; Cai, Y.; Cui, J. Influence of extracellular polymeric substances (EPS) treated by combined ultrasound pretreatment and chemical re-flocculation on water treatment sludge settling performance. Chemosphere 2017, 170, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, L.; Tang, M.; Hong, L.; Sun, S.; Xing, W. Multi-component separation of small molecular/ionic pollutants with smart pH-gating membranes. Chem. Eng. Sci. 2021, 245, 116854. [Google Scholar] [CrossRef]
- Moreira, V.; Lebron, Y.; Paula, E.; Lucilaine, V.; Míriam, C. Recycled reverse osmosis membrane combined with pre-oxidation for improved arsenic removal from high turbidity waters and retrofit of conventional drinking water treatment process. J. Clean. Prod. 2021, 312, 127859. [Google Scholar] [CrossRef]
- Mukherjee, A.; Borugadda, V.; Dynes, J.; Niu, C.; Dalai, A. Carbon dioxide capture from flue gas in biochar produced from spent coffee grounds: Effect of surface chemistry and porous structure. J. Environ. Chem. Eng. 2021, 9, 106049. [Google Scholar] [CrossRef]
- Mokrzycki, J.; Gazinska, M.; Fedyna, M.; Karcz, R.; Lorenc-Grabowska, E.; Rutkowski, P. Pyrolysis and torrefaction of waste wood chips and cone-like flowers derived from black alder (Alnus glutinosa L. Gaertn.) for sustainable solid fuel production. Biomass Bioenerg. 2020, 143, 105842. [Google Scholar] [CrossRef]
- Cheng, H.; Sun, Y.; Wang, X.; Zou, S.; Ye, G.; Huang, H.; Ye, D. Hierarchical porous carbon fabricated from cellulose-degrading fungus modified rice husks: Ultrahigh surface area and impressive improvement in toluene adsorption. J. Hazard. Mater. 2020, 392, 122298. [Google Scholar] [CrossRef]
- Charoensook, K.; Huang, C.; Tai, H.; Lanjapall, V.; Chiang, L.; Hosseini, S.; Lin, Y.; Li, Y. Preparation of porous nitrogen-doped activated carbon derived from rice straw for high-performance supercapacitor application. J. Taiwan Inst. Chem. Eng. 2021, 120, 246–256. [Google Scholar] [CrossRef]
- Muhammad, A.; Lee, L.; Tang, S.; Yoshimasa, A.; Motoi, M. Effects of physical activation on pore textures and heavy metals removal of fiber-based activated carbons. Mater. Today Proc. 2021, 39, 917–921. [Google Scholar]
- Seyyed, A.; Behruz, B.; Mohammad, R.; Helder, T.; Zahra, N. Adsorption of diclofenac on mesoporous activated carbons: Physical and chemical activation, modeling with genetic programming and molecular dynamic simulation. Chem. Eng. Res. Des. 2021, 167, 116–128. [Google Scholar]
- Chin, H.H.; Varbanov, P.S.; Liew, P.Y.; Klemeš, J.J. Pinch-based targeting methodology for multi-contaminant material recycle/reuse. Chem. Eng. Sci. 2021, 230, 116129. [Google Scholar] [CrossRef]
- Govil, T.; Wang, J.; Samanta, D.; David, A.; Tripathi, A.; Rauniyar, S.; Salem, D.R.; Sani, R.K. Lignocellulosic feedstock: A review of a sustainable platform for cleaner production of nature’s plastics. J. Clean. Prod. 2020, 270, 122521. [Google Scholar] [CrossRef]
- Arevalo-Gallegos, A.; Ahmadb, Z.; Asgher, M.; Parra-Saldivar, R.; Iqbal, H.M.N. Lignocellulose: A sustainable material to produce value-added products with a zero waste approach-A review. Int. J. Biol. Macromol. 2017, 99, 308–318. [Google Scholar] [CrossRef]
- Rouzitalab, Z.; Maklavany, D.M.; Jafarinejad, S.; Rashidi, A. Lignocellulose-based adsorbents: A spotlight review of the effective parameters on carbon dioxide capture process. Chemosphere 2020, 246, 125756. [Google Scholar] [CrossRef]
- Gao, W.; Zhao, H.; Liu, Y.; Li, M.; Nurmamat, E.; Li, L.; Ren, Y.; Xiao, H. Isolation and identification of fourteen microsatellite markers in clivia miniata and clivia nobilis (Amaryllidaceae). Int. J. Mol. Sci. 2012, 13, 9609–9614. [Google Scholar] [CrossRef] [Green Version]
- Vealea, D.; Oliverb, D.; Arangiesa, N.; Furman, K. Preliminary isolated organ studies using an aqueous extract of Clivia miniata leaves. J. Ethnopharmacol. 1989, 27, 341–346. [Google Scholar] [CrossRef]
- Zhang, B.; Jin, Y.; Qi, J.; Chen, H.; Chen, G.; Tang, S. Porous carbon materials based on Physalis alkekengi L. husk and its application for removal of malachite green. Environ. Technol. Innov. 2021, 21, 101343. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Z.; Xia, Y.; Zhang, B.; Chen, H.; Chen, G.; Tang, S. Porous carbon material derived from fungal hyphae and its application for the removal of dye. RSC Adv. 2019, 9, 25480. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Xia, Y.; Zhang, B.; Chen, H.; Chen, G.; Tang, S. Disassembly of lignocellulose into cellulose, hemicellulose, and lignin for preparation of porous carbon materials with enhanced performances. J. Hazard. Mater. 2021, 408, 124956. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Jin, Y.; Qi, J.; Chen, H.; Chen, G.; Tang, S. Preparation of biomass carbon material based on Fomes fomentarius via alkali activation and its application for the removal of brilliant green in wastewater. Environ. Technol. Innov. 2021, 23, 101659. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmet, P.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Jagiello, J.; Jaroniec, M. 2D-NLDFT adsorption models for porous oxides with corrugated cylindrical pores. J. Colloid Interf. Sci. 2018, 532, 588–597. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption geloster stoffe. K. Sven. Vetenskapsakademiens. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Ball, W.; Roberts, P. Long-term sorption of halogenated organic chemicals by aquifer material. 2. Intraparticle diffusion. Environ. Sci. Technol. 1991, 25, 1223–1237. [Google Scholar] [CrossRef]
- Oh, S.; Yoo, D.; Shin, Y.; Kim, H.; Chung, Y.; Park, W.; Youk, J. Crystalline structure analysis of cellulose treated with sodiumhydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohyd. Res. 2005, 340, 2376–2391. [Google Scholar] [CrossRef]
- Baig, M.; Gul, I. Conversion of wheat husk to high surface area activated carbon for energy storage in high-performance supercapacitors. Biomas. Bioenerg. 2021, 144, 105909. [Google Scholar] [CrossRef]
- Tran, T.; Le, H.; Pham, T.; Nguyen, D.; La, D.; Chang, S.; Lee, S.; Chung, W.; Nguyen, D. Comparative study on methylene blue adsorption behavior of coffee husk-derived activated carbon materials prepared using hydrothermal and soaking methods. J. Environ. Chem. Eng. 2021, 9, 105362. [Google Scholar] [CrossRef]
- Aup-Ngoen, K.; Noipitak, M. Effect of carbon-rich biochar on mechanical properties of PLA-biochar composites. Sustain. Chem. Pharm. 2020, 15, 100204. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Cao, Y.; Han, L. Characteristics of tetracycline adsorption by cow manure biochar prepared at different pyrolysis temperatures. Bioresour. Technol. 2019, 285, 121348. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, R.; Cao, J.; Zhang, X.; Zhu, J.; Wei, X. N/O co-doped interlinked porous carbon nanoflakes derived from soybean stalk for high-performance supercapacitors. J. Electroanal. Chem. 2020, 871, 114288. [Google Scholar] [CrossRef]
- Shao, L.; Chen, H.; Li, Y.; Li, J.; Chen, G.; Wang, G. Pretreatment of corn stover via sodium hydroxide-urea solutions to improve the glucose yield. Bioresour. Technol. 2020, 307, 123191. [Google Scholar] [CrossRef]
- Koyuncu, D.D.E.; Okur, M. Investigation of dye removal ability and reusability of green and sustainable silica and carbon-silica hybrid aerogels prepared from paddy waste ash. Colloids Surface. A 2021, 628, 127370. [Google Scholar] [CrossRef]
- Awasthi, G.; Bhattarai, D.; Maharjan, B.; Kim, K.; Park, C.; Kim, C. Synthesis and characterizations of activated carbon from Wisteria sinensis seeds biomass for energy storage applications. J. Ind. Eng. Chem. 2019, 72, 265–272. [Google Scholar] [CrossRef]
- Kragovic, M.; Stojmenovic, M.; Petrovic, J.; Loredo, J.; Pasalic, S.; Nedeljkovic, A.; Ristovic, I. Influence of alginate encapsulation on point of zero charge (pHpzc) and thermodynamic properties of the natural and Fe (III)-modified Zeolite. Proced. Manuf. 2019, 32, 286–293. [Google Scholar] [CrossRef]
- Zhou, K.; Ma, W.; Zeng, Z.; Chen, R.; Xu, X.; Liu, B.; Li, H.; Li, H.; Li, L. Waste biomass-derived oxygen and nitrogen co-doped porous carbon/MgO composites as superior acetone adsorbent: Experimental and DFT study on the adsorption behavior. Chem. Eng. J. 2020, 387, 124173. [Google Scholar] [CrossRef]
- Feizi, F.; Sarmah, A.K.; Rangsivek, R. Adsorption of pharmaceuticals in a fixed-bed column using tyre-based activated carbon: Experimental investigations and numerical modelling. J. Hazard. Mater. 2021, 417, 126010. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, B.; Xia, Y.; Chen, H.; Chen, G.; Tang, S. Influence of mixed alkali on the preparation of edible fungus substrate porous carbon material and its application for the removal of dye. Colloids Surface. A 2021, 609, 125675. [Google Scholar] [CrossRef]
- Zhao, M.; Hou, Z.; Lian, Z.; Qin, D.; Ge, C. Direct extraction and detection of malachite green from marine sediments by magnetic nano-sized imprinted polymer coupled with spectrophotometric analysis. Mar. Pollut. Bull. 2020, 158, 111363. [Google Scholar] [CrossRef]
- Bagheri, R.; Ghaedi, M.; Asfaram, A.; Dil, E.; Javadian, H. RSM-CCD design of malachite green adsorption onto activated carbon with multimodal pore size distribution prepared from Amygdalus scoparia: Kinetic and isotherm studies. Polyhedron 2019, 171, 464–472. [Google Scholar] [CrossRef]
- Gunes, B.; Jaquet, Y.; Sánchez, L.; Pumarino, R.; McGlade, D.; Quilty, B.; Morrissey, A.; Gholamvand, Z.; Nolan, K.; Lawler, J. Activated graphene oxide-calcium alginate beads for adsorption of methylene blue and pharmaceuticals. Materials 2021, 14, 6343. [Google Scholar] [CrossRef] [PubMed]
- Krsti, S.; Kragovi, M.; Pagnacco, M.; Dodevski, V.; Kaluđerovi, B.; Momcilovi, M.; Ristovic, I.; Stojmenovi, M. Hydrothermal synthesized and alkaline activated carbons prepared from glucose and fructose-detailed characterization and testing in heavy metals and methylene blue removal. Minerals 2018, 8, 246. [Google Scholar] [CrossRef] [Green Version]
- Bien, T.; Kołodynska, D.; Franus, W. Functionalization of Zeolite NaP1 for simultaneous acid red 18 and Cu (II) removal. Materials 2021, 14, 7817. [Google Scholar] [CrossRef] [PubMed]
- Oyekanmi, A.; Ahmad, A.; Setapar, S.; Alshammari, M.; Jawaid, M.; Hanafiah, M.; Khalil, H.; Vaseashta, A. sustainable durio zibethinus-derived biosorbents for congo red removal from aqueous solution: Statistical optimization, isotherms and mechanism studies. Sustainability 2021, 13, 13264. [Google Scholar] [CrossRef]
- Delpiano, G.; Tocco, D.; Medda, L.; Magner, E.; Salis, A. Adsorption of malachite green and alizarin red S dyes using Fe-BTC metal organic framework as adsorbent. Int. J. Mol. Sci. 2021, 22, 788. [Google Scholar] [CrossRef]
- Ahmad, A.; Setapar, S.; Yaqoob, A.; Ibrahim, M. Synthesis and characterization of GO-Ag nanocomposite for removal of malachite dye from aqueous solution. Mater. Today Proc. 2021, 47, 1359–1365. [Google Scholar] [CrossRef]
- Kazmierczak-Razna, J.; Zioła-Frankowska, A.; Nowicki, P.; Frankowski, M.; Wolski, R.; Pietrzak, R. Removal of heavy metal ions from one- and two-component solutions via adsorption on N-doped activated carbon. Materials 2021, 14, 7045. [Google Scholar] [CrossRef]
- Santamaría, L.; Korili, S.A.; Gil, A. Solketal Removal from aqueous solutions using activated carbon and a metal–organic framework as adsorbents. Materials 2021, 14, 6852. [Google Scholar] [CrossRef]
- Jalilvand, H.; Feyzi, F.; Dehghani, M. Adsorption of dimethyl sulfide from model fuel on raw and modified activated carbon from walnut and pistachio shell origins: Kinetic and thermodynamic study. Colloids Surface. A 2020, 593, 124620. [Google Scholar] [CrossRef]
- Gohr, M.; Abd-Elhamid, A.; El-Shanshory, A.; Soliman, H. Adsorption of cationic dyes onto chemically modified activated carbon: Kinetics and thermodynamic study. J. Mol. Liq. 2022, 346, 118227. [Google Scholar] [CrossRef]
- Liu, Z.; Mu, Q.; Sun, Y.; Gao, P.; Yu, Y.; Gao, J.; Shi, W.; Wen, X.; Fei, Z. Effective adsorption of chloroanilines from aqueous solution by m-phenylenediamine modified hyper-cross-linked resin: Kinetic, equilibrium, and thermodynamic studies. Colloids Surface. A 2020, 601, 124996. [Google Scholar] [CrossRef]
- Marrakchi, F.; Hameed, B.; Bouaziz, M. Mesoporous and high-surface-area activated carbon from defatted olive cake by-products of olive mills for the adsorption kinetics and isotherm of methylene blue and acid blue 29. J. Environ. Chem. Eng. 2020, 8, 104199. [Google Scholar] [CrossRef]


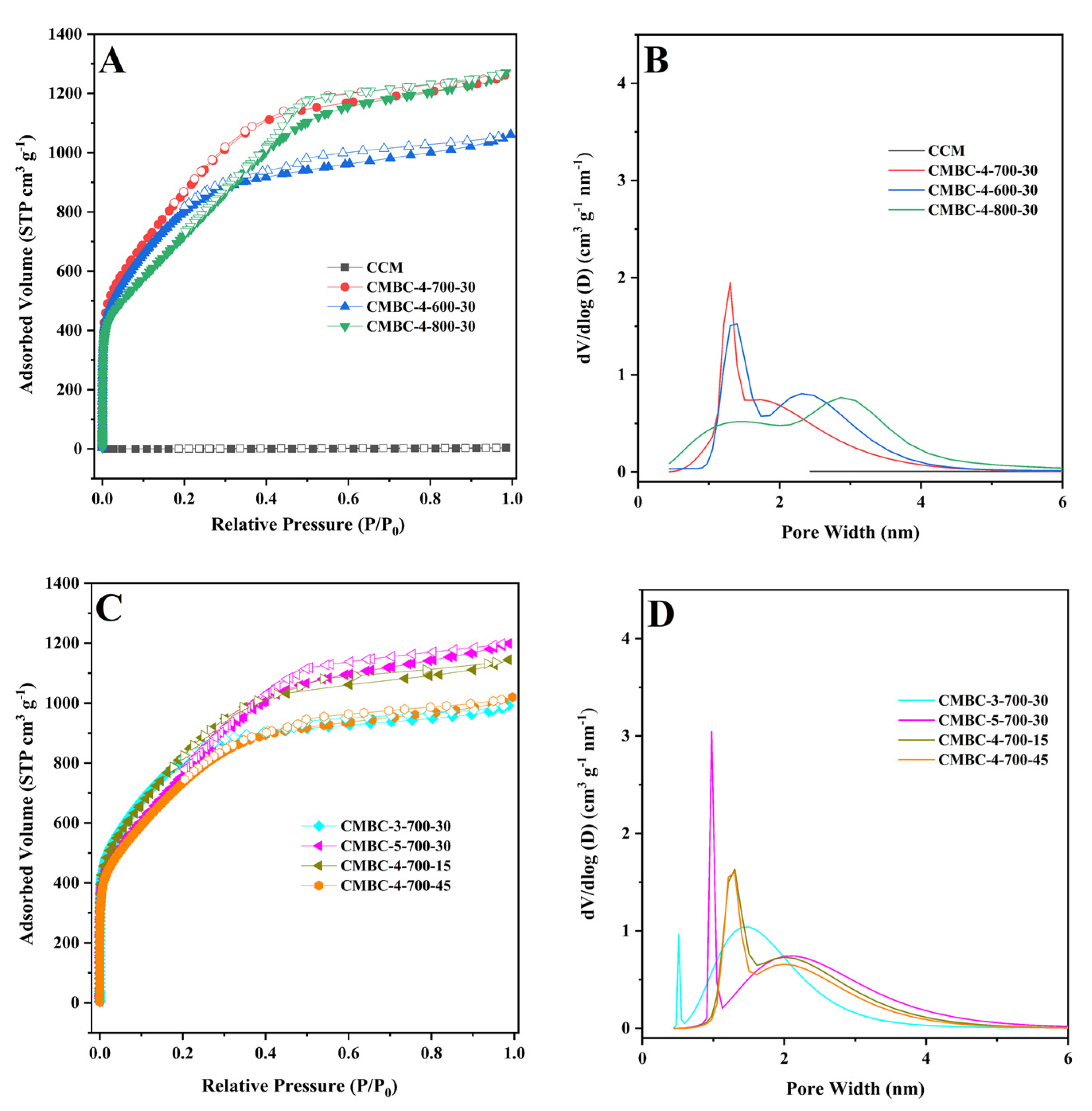



| Samples | Conditions | SBET (m2/g) | SMicro (m2/g) | VMicro (cm3/g) | Vtotal (cm3/g) | ||
|---|---|---|---|---|---|---|---|
| NaOH:Carbon | T (°C) | t (min) | |||||
| CCM | - | 500 | 60 | 3 | - | - | 0.0062 |
| CMBC-4-600-30 | 4:1 | 600 | 30 | 2759 | 1755 | 0.82 | 1.63 |
| CMBC-4-700-30 | 4:1 | 700 | 30 | 2716 | 1001 | 0.69 | 1.95 |
| CMBC-4-800-30 | 4:1 | 800 | 30 | 2354 | 348 | 0.39 | 1.96 |
| CMBC-3-700-30 | 3:1 | 700 | 30 | 2772 | 1958 | 0.91 | 1.53 |
| CMBC-5-700-30 | 5:1 | 700 | 30 | 2468 | 722 | 0.53 | 1.85 |
| CMBC-4-700-15 | 4:1 | 700 | 15 | 2689 | 1282 | 0.73 | 1.77 |
| CMBC-4-700-45 | 4:1 | 700 | 45 | 2429 | 1224 | 0.66 | 1.57 |
| C0 (mg/L) | Qe (mg/g) | Pseudo-First-Order Kinetic | Pseudo-Second-Order Kinetic | Intra-Particle Diffusion | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| k1 (min−1) | Qe.cat (mg/g) | R2 | k2 (g mg−1 min−1) | Qe.cat (mg/g) | R2 | k3-1 (mg g−1 min−1) | R2 | k3-2 (mg g−1 min−1) | R2 | ||
| 50 | 1051.5 | 0.1465 | 1061.8 | 0.9367 | 0.0003 | 1070.8 | 0.9932 | 83.39 | 0.9997 | 19.45 | 0.9509 |
| 150 | 1306.5 | 0.1874 | 1318.2 | 0.9428 | 0.0003 | 1323.9 | 0.9927 | 80.80 | 0.9992 | 20.62 | 0.8024 |
| 250 | 1692.1 | 0.1991 | 1707.9 | 0.9810 | 0.0002 | 1745.4 | 0.9999 | 136.65 | 0.9301 | 15.22 | 0.9160 |
| Isotherm Models | Constants | |
|---|---|---|
| Langmuir | Qm (mg/g) | 1483.1 |
| KL (L/mg) | 1.50 | |
| R2 | 0.8296 | |
| Freundlich | KF (mg g−1(L mg−1)1/n) | 936.2 |
| n | 9.8425 | |
| R2 | 0.9888 |
| Temperature (K) | Qe (mg/g) | ΔG (kJ mol−1) | ΔH (kJ mol−1) | ΔS (J mol−1 K−1) |
|---|---|---|---|---|
| 293 | 1501.8 | −3.73 | −5.62 | 13.36 |
| 303 | 1598.7 | −4.05 | ||
| 313 | 1689.8 | −4.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, W. Porous Biomass Carbon Derived from Clivia miniata Leaves via NaOH Activation for Removal of Dye. Materials 2022, 15, 1285. https://doi.org/10.3390/ma15041285
Gao W. Porous Biomass Carbon Derived from Clivia miniata Leaves via NaOH Activation for Removal of Dye. Materials. 2022; 15(4):1285. https://doi.org/10.3390/ma15041285
Chicago/Turabian StyleGao, Wei. 2022. "Porous Biomass Carbon Derived from Clivia miniata Leaves via NaOH Activation for Removal of Dye" Materials 15, no. 4: 1285. https://doi.org/10.3390/ma15041285
APA StyleGao, W. (2022). Porous Biomass Carbon Derived from Clivia miniata Leaves via NaOH Activation for Removal of Dye. Materials, 15(4), 1285. https://doi.org/10.3390/ma15041285





