Overcoming Antibiotic Resistance: Playing the ‘Silver Nanobullet’ Card
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of ZnAl-Br LDH
2.3. Preparation of Acetate LDH
2.4. Intercalation of Antibiotics into LDH
2.5. Synthesis of Ag/ZnAl-CFZ and Ag/ZnAl-NAL
2.6. Techniques
2.7. Release Assay
2.8. Microbial Strains and Growth Conditions
2.9. Minimum Inhibitory Concentration Assay
2.10. Assessment of the Synergy of Antimicrobial Activity of Antibiotics and Silver
3. Results and Discussion
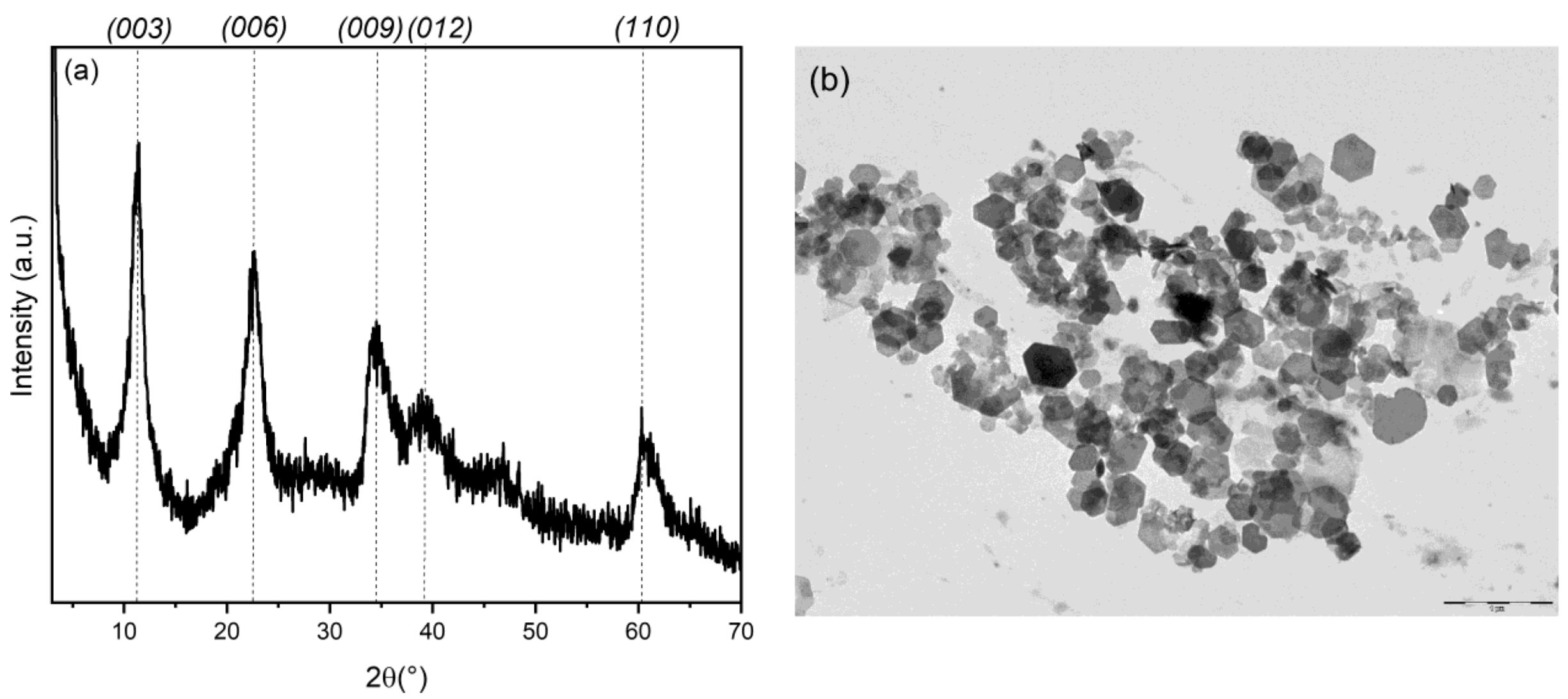
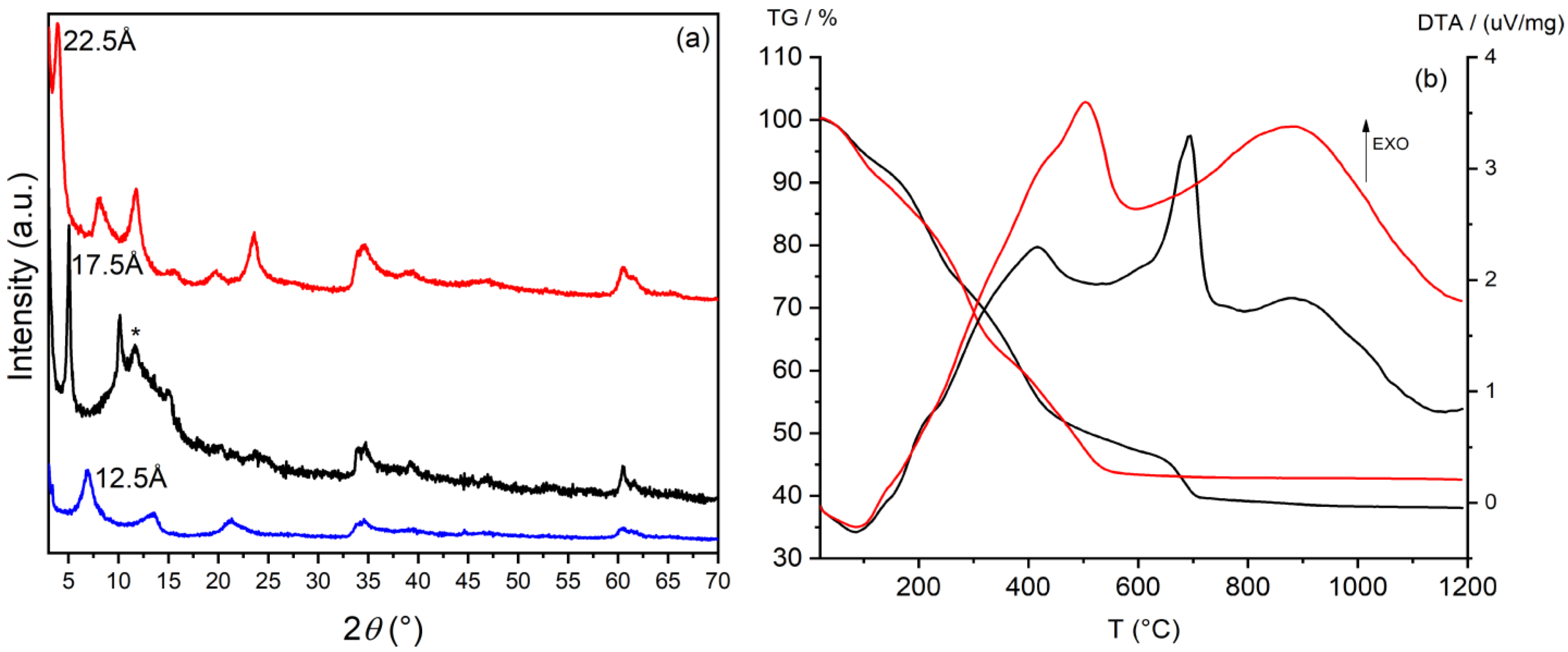
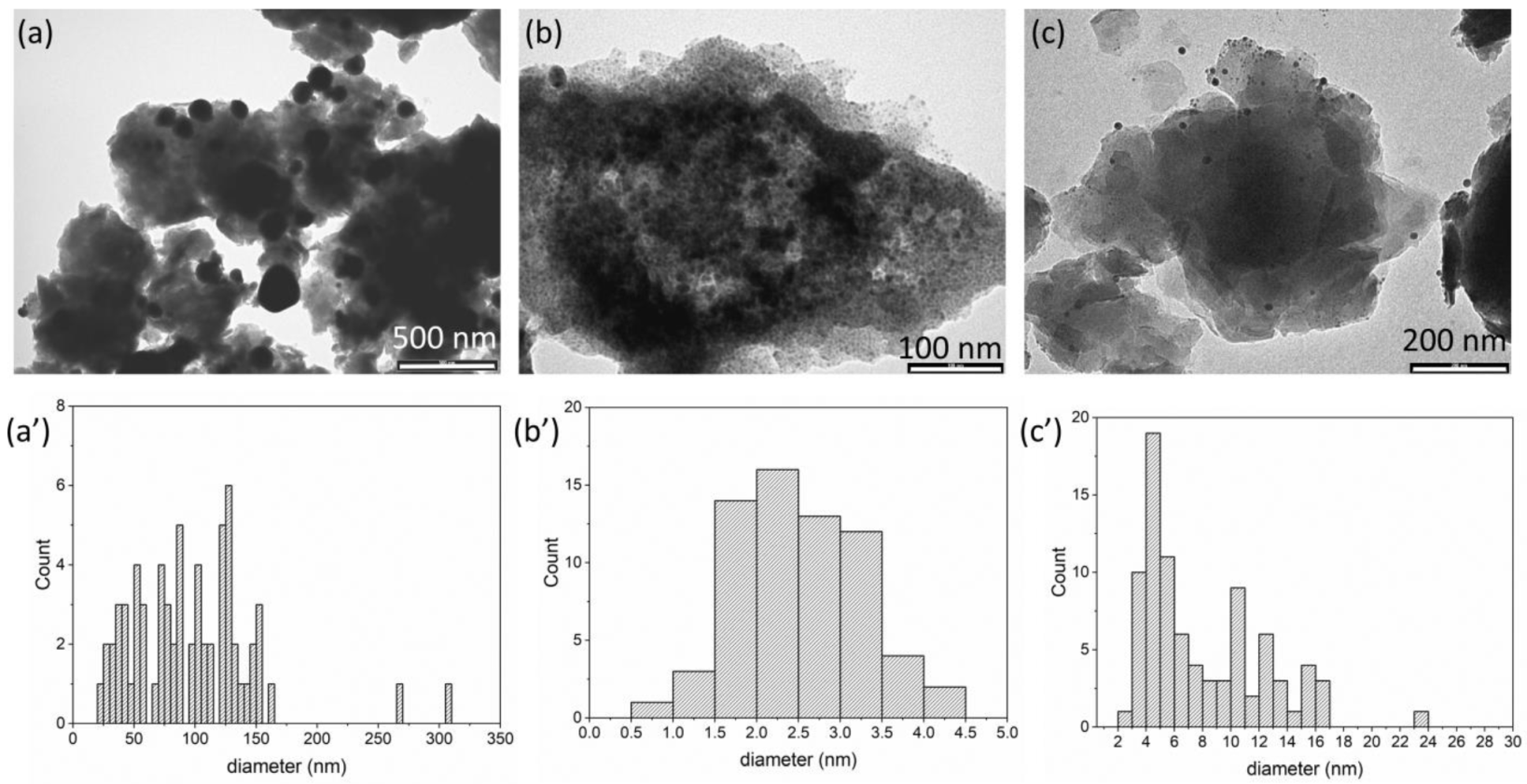
3.1. Preparation and Characterization of Silver/Antibiotic Loaded LDH
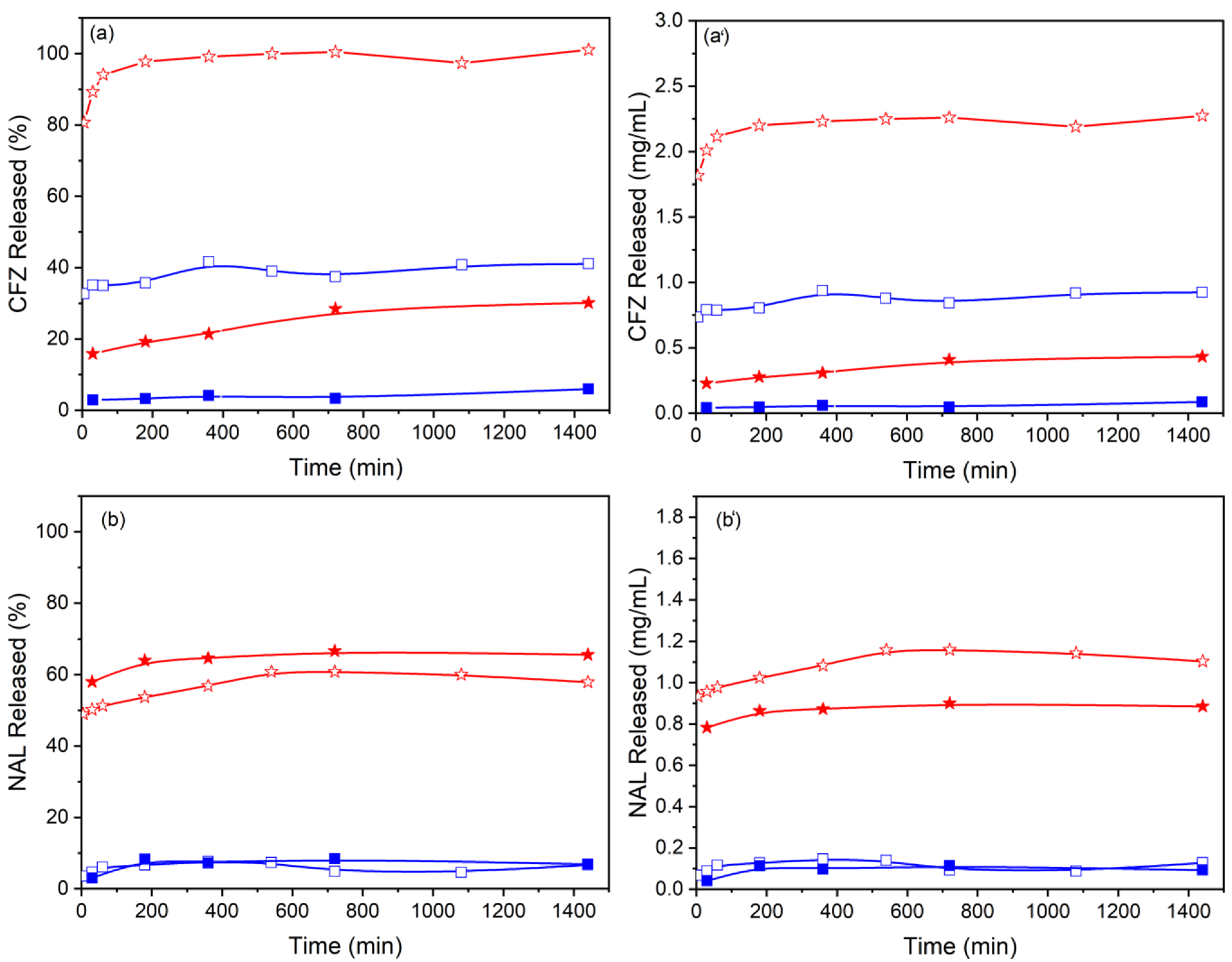
3.2. Release Profiles
3.3. Antimicrobial Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spellberg, B.; Gilbert, D.N. The future of antibiotics and resistance: A tribute to a career of leadership by John Bartlett. Clin. Infect. Dis. 2014, 59 (Suppl. S2), S71–S75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaman, S.B.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A review on antibiotic resistance: Alarm bells are ringing. Cureus 2017, 9, e1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alanis, A.J. Resistance to antibiotics: Are we in the post-antibiotic era? Arch. Med. Res. 2005, 36, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.M.; Seiple, I.B.; Myers, A.G.J.A.C. Zur Rolle der chemischen Synthese in der Entwicklung antibakterieller Wirkstoffe. Angew. Chem. 2014, 126, 8984–9014. [Google Scholar] [CrossRef]
- File, T.M. Solutions to the problem of bacterial resistance. Treat. Respir. Med. 2005, 4, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Blaskovich, M.A.; Butler, M.S.; Cooper, M.A. Polishing the tarnished silver bullet: The quest for new antibiotics. Essays Biochem. 2017, 61, 103–114. [Google Scholar]
- Raymond, B. Five rules for resistance management in the antibiotic apocalypse, a road map for integrated microbial management. Evol. Appl. 2019, 12, 1079–1091. [Google Scholar] [CrossRef]
- Tyers, M.; Wright, G.D. Drug combinations: A strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 2019, 17, 141–155. [Google Scholar] [CrossRef]
- Payne, D.J.; Gwynn, M.N.; Holmes, D.J.; Pompliano, D.L. Drugs for bad bugs: Confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 2007, 6, 29–40. [Google Scholar] [CrossRef]
- Pica, M.; Messere, N.; Felicetti, T.; Sabatini, S.; Pietrella, D.; Nocchetti, M. Biofunctionalization of Poly (lactide-co-glycolic acid) Using Potent NorA Efflux Pump Inhibitors Immobilized on Nanometric Alpha-Zirconium Phosphate to Reduce Biofilm Formation. Materials 2021, 14, 670. [Google Scholar] [CrossRef]
- Dibrov, P.; Dzioba, J.; Gosink, K.K.; Häse, C.C. Chemiosmotic mechanism of antimicrobial activity of Ag+ in Vibrio cholerae. Antimicrob. Agents Chemother. 2002, 46, 2668–2670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qayyum, S.; Oves, M.; Khan, A.U. Obliteration of bacterial growth and biofilm through ROS generation by facilely synthesized green silver nanoparticles. PLoS ONE 2017, 12, e0181363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Qu, F.; Xu, H.; Lai, W.; Wang, Y.A.; Aguilar, Z.P.; Wei, H. Role of reactive oxygen species in the antibacterial mechanism of silver nanoparticles on Escherichia coli O157: H7. Biometals 2012, 25, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Barros, C.H.; Fulaz, S.; Stanisic, D.; Tasic, L. Biogenic nanosilver against multidrug-resistant bacteria (MDRB). Antibiotics 2018, 7, 69. [Google Scholar] [CrossRef] [Green Version]
- Saha, B.; Bhattacharya, J.; Mukherjee, A.; Ghosh, A.; Santra, C.; Dasgupta, A.K.; Karmakar, P. In vitro structural and functional evaluation of gold nanoparticles conjugated antibiotics. Nanoscale Res. Lett. 2007, 2, 614–622. [Google Scholar] [CrossRef] [Green Version]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-strategies to fight multidrug resistant bacteria—“A Battle of the Titans”. Front. Microbiol. 2018, 9, 1441. [Google Scholar] [CrossRef] [Green Version]
- Dar, M.A.; Ingle, A.; Rai, M. Enhanced antimicrobial activity of silver nanoparticles synthesized by Cryphonectria sp. evaluated singly and in combination with antibiotics. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 105–110. [Google Scholar] [CrossRef]
- Smekalova, M.; Aragon, V.; Panacek, A.; Prucek, R.; Zboril, R.; Kvitek, L. Enhanced antibacterial effect of antibiotics in combination with silver nanoparticles against animal pathogens. Vet. J. 2016, 209, 174–179. [Google Scholar] [CrossRef]
- De Souza, A.; Mehta, D.; Leavitt, R. Bactericidal activity of combinations of Silver–Water Dispersion TM with 19 antibiotics against seven microbial strains. Curr. Sci. 2006, 91, 926–929. [Google Scholar]
- Wu, D.; Fan, W.; Kishen, A.; Gutmann, J.L.; Fan, B. Evaluation of the antibacterial efficacy of silver nanoparticles against Enterococcus faecalis biofilm. J. Endod. 2014, 40, 285–290. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kora, A.J.; Rastogi, L. Enhancement of antibacterial activity of capped silver nanoparticles in combination with antibiotics, on model gram-negative and gram-positive bacteria. Bioinorg. Chem. Appl. 2013, 2013, 871097. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Kumar, R. Enhanced bactericidal efficacy of polymer stabilized silver nanoparticles in conjugation with different classes of antibiotics. RSC Adv. 2019, 9, 1095–1105. [Google Scholar] [CrossRef] [Green Version]
- Mei, X.; Xu, S.; Hu, T.; Peng, L.; Gao, R.; Liang, R.; Wei, M.; Evans, D.G. Layered double hydroxide monolayers for controlled loading and targeted delivery of anticancer drugs. Nano Res. 2017, 11, 195–205. [Google Scholar] [CrossRef]
- Cunha, V.R.R.; Souza, R.B.; da Fonseca Martins, A.M.; Koh, I.H.J.; Constantino, V.R.L.C. Accessing the biocompatibility of layered double hydroxide by intramuscular implantation: Histological and microcirculation evaluation. Sci. Rep. 2016, 6, 30547. [Google Scholar] [CrossRef]
- Nocchetti, M.; Pica, M.; Ridolfi, B.; Donnadio, A.; Boccalon, E.; Zampini, G.; Pietrella, D.; Casciola, M. AgCl-ZnAl layered double hydroxides as catalysts with enhanced photodegradation and antibacterial activities. Inorganics 2019, 7, 120. [Google Scholar] [CrossRef] [Green Version]
- Nocchetti, M.; Donnadio, A.; Ambrogi, V.; Andreani, P.; Bastianini, M.; Pietrella, D.; Latterini, L. Ag/AgCl nanoparticle decorated layered double hydroxides: Synthesis, characterization and antimicrobial properties. J. Mater. Chem. B 2013, 1, 2383–2393. [Google Scholar] [CrossRef]
- Rives, V. (Ed.) Layered Double Hydroxides: Present and Future; Nova Science Publishers: New York, NY, USA, 2001. [Google Scholar]
- Segura-Pérez, V.; Lobo-Sánchez, M.; Velázquez-Herrera, F.D.; Frías-Vázquez, D.A.; Reyes-Cervantes, E.; Fetter, G. Hydrotalcite/hydroxyapatite composites with high bacterial activity against clinical bacteria. A new alternative to prevent osteomyelitis diseases. Micropor. Mesopor. Mat. 2020, 298, 110069. [Google Scholar] [CrossRef]
- Donnadio, A.; Bini, M.; Centracchio, C.; Mattarelli, M.; Caponi, S.; Ambrogi, V.; Pietrella, D.; Di Michele, A.; Vivani, R.; Nocchetti, M. Bioinspired reactive interfaces based on layered double hydroxides-Zn rich hydroxyapatite with antibacterial activity. ACS Biomater. Sci. Eng. 2021, 7, 1361–1373. [Google Scholar] [CrossRef]
- Badar, M.; Rahim, M.I.; Kieke, M.; Ebel, T.; Rohde, M.; Hauser, H.; Behrens, P.; Mueller, P.P. Controlled drug release from antibiotic-loaded layered double hydroxide coatings on porous titanium implants in a mouse model: Antibiotic-Loaded Layered Double Hydroxide Coatings. J. Biomed. Mater. Res. 2015, 103, 2141–2149. [Google Scholar] [CrossRef]
- Cherif, N.F.V.; Constantino, R.L.; Hamdaoui, O.; Leroux, F.; Taviot-Gueho, C. New insights into two ciprofloxacin-intercalated arrangements for layered double hydroxide carrier materials. New J. Chem. 2020, 44, 10076–10086. [Google Scholar] [CrossRef]
- Bellezza, F.; Cipiciani, A.; Costantino, U.; Nocchetti, M.; Posati, T. Hydrotalcite-Like Nanocrystals from Water-in-Oil Microemulsions. Eur. J. Inorg. Chem. 2009, 2603–2611. [Google Scholar] [CrossRef]
- Choi, G.; Choy, J.H. Recent progress in layered double hydroxides as a cancer theranostic nanoplatform. WIREs Nanomed. Nanobiotechnol. 2021, 13, e1679. [Google Scholar] [CrossRef] [PubMed]
- Shirin, V.K.A.; Sankar, R.; Johnson, A.P.; Gangadharappa, H.V.; Pramod, K. Advanced drug delivery applications of layered double hydroxide. J. Control. Release 2021, 330, 398–426. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.-J.; Jung, H.; Oh, J.-M.; Lee, J.-K.; Choy, J.-H. Layered double hydroxide as novel antibacterial drug delivery system. J. Phys. Chem. Solids 2010, 71, 685–688. [Google Scholar] [CrossRef]
- Trikeriotis, M.; Ghanotakis, D.F. Intercalation of hydrophilic and hydrophobic antibiotics in layered double hydroxides. J. Int. J. Pharm. 2007, 332, 176–184. [Google Scholar] [CrossRef]
- Anacona, J.R.; Alvarez, P. Synthesis and antibacterial activity of metal complexes of cefazolin. Transit. Met. Chem. 2002, 27, 856–860. [Google Scholar] [CrossRef]
- Nabipour, H.; Sadra, M.H.; Thomas, N. Synthesis, controlled release and antibacterial studies of nalidixic acid–zinc hydroxide nitrate nanocomposites. New J. Chem. 2016, 40, 238–244. [Google Scholar] [CrossRef]
- Debnath, A.; Mogha, N.K.; Masram, D.T. Metal Complex of the First-Generation Quinolone Antimicrobial Drug Nalidixic Acid: Structure and Its Biological Evaluation. Appl. Biochem. Biotechnol. 2015, 175, 2659–2667. [Google Scholar] [CrossRef]
- Ross, D.L.; RileyAqueous, C.M. Solubilities of some various substituted quinolone antimicrobials. Int. J. Pharm. 1990, 63, 237–250. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 4421, Nalidixic Acid. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Nalidixic-acid (accessed on 16 January 2022).
- Perioli, L.; Posati, T.; Nocchetti, M.; Bellezza, F.; Costantino, U.; Cipiciani, A. Intercalation and release of antiinflammatory drug diclofenac into nanosized ZnAl hydrotalcite-like compound. Appl. Clay Sci. 2011, 53, 374–378. [Google Scholar] [CrossRef]
- Fernandes, R.; Amador, P.; Prudêncio, C. β-Lactams: Chemical structure, mode of action and mechanisms of resistance. Rev. Med. Microbiol. 2013, 24, 7–17. [Google Scholar] [CrossRef] [Green Version]
- Fàbrega, A.; Madurga, S.; Giralt, E.; Vila, J. Mechanism of action of and resistance to quinolones. Microb. Biotechnol. 2009, 2, 40–61. [Google Scholar] [CrossRef] [Green Version]
- Dakal, T.; Kumar, A.; Majumdar, R.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun’an Qing, L.C.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311. [Google Scholar]
- Vazquez-Muñoz, R.; Meza-Villezcas, A.; Fournier, P.; Soria-Castro, E.; Juarez-Moreno, K.; Gallego-Hernández, A.; Bogdanchikova, N.; Vazquez-Duhalt, R.; Huerta-Saquero, A. Enhancement of antibiotics antimicrobial activity due to the silver nanoparticles impact on the cell membrane. PLoS ONE 2019, 14, e0224904. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Yu, H.; Ma, K.; Chen, Y.; Zhang, X.; Wang, T.; Li, S.; Zhu, X.; Wang, X. Flower-like surface of three-metal-component layered double hydroxide composites for improved antibacterial activity of lysozyme. Bioconjug. Chem. 2018, 29, 2090–2099. [Google Scholar] [CrossRef]


| Sample | A | Composition [Zn0.72Al0.28(OH)2](A)x(OH)y·nH2O | A (wt%) | ||
|---|---|---|---|---|---|
| x | y | n | |||
| ZnAl-CFZ | CFZ | 0.19 | 0.09 | 0.8 | 45.0 |
| ZnAl-NAL | NAL | 0.28 | 0 | 0.9 | 38.1 |
| Sample | Antibiotic (wt%) | Ag (wt%) | Ag/AgCl NPs Diameter (nm) |
|---|---|---|---|
| Ag/ZnAl-Ac | - | 8.2 | 96.3 ± 51.0 |
| Ag/ZnAl-CFZ | 28.7 | 8.6 | 2.5 ± 0.7 |
| Ag/ZnAl-NAL | 27.0 | 10.3 | 8.0 ± 4.3 |
| Sample | MIC(µg/mL) | |||||
|---|---|---|---|---|---|---|
| S. aureus | S. pneumoniae | P. aeruginosa | ||||
| AgNO3 | 10.8 | 10.8 | 31.6 | |||
| CFZ | 6.2 | 26.0 | 1000.0 | |||
| NAL | 83.3 | 3.3 | 250.0 | |||
| Ag/ZnAl-Ac | 375.0 | 1000.0 | 666.7 | |||
| ZnAl-CFZ | 1.3 | 333.3 | 1000.0 | |||
| Ag/ZnAl-CFZ | 26.9 (a) | 8.0 (b) | 35.9 (a) | 10.7 (b) | 40.4 (a) | 12.1 (b) |
| ZnAl-NAL | 190.3 | 7.93 | 253.7 | |||
| Ag/ZnAl-NAL | 67.5 (a) | 25.6 (b) | 2.3 (a) | 0.9 (b) | 56.3 (a) | 21.4 (b) |
| Antibacterial Combination | FICI (mg/mL) | |
|---|---|---|
| AgNO3/NAL | AgNO3/CFZ | |
| S. pneumoniae | 1.4 (ID) | 1.2 (ID) |
| S. aureus | 0.9 (PS) | 1 (AD) |
| P. aeruginosa | 1.4 (ID) | 0.75 (PS) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nocchetti, M.; Boccalon, E.; Pica, M.; Giordano, N.M.R.; Finori, F.; Pietrella, D.; Cipiciani, A. Overcoming Antibiotic Resistance: Playing the ‘Silver Nanobullet’ Card. Materials 2022, 15, 932. https://doi.org/10.3390/ma15030932
Nocchetti M, Boccalon E, Pica M, Giordano NMR, Finori F, Pietrella D, Cipiciani A. Overcoming Antibiotic Resistance: Playing the ‘Silver Nanobullet’ Card. Materials. 2022; 15(3):932. https://doi.org/10.3390/ma15030932
Chicago/Turabian StyleNocchetti, Morena, Elisa Boccalon, Monica Pica, Nicoletta Maria Rosaria Giordano, Francesco Finori, Donatella Pietrella, and Antonio Cipiciani. 2022. "Overcoming Antibiotic Resistance: Playing the ‘Silver Nanobullet’ Card" Materials 15, no. 3: 932. https://doi.org/10.3390/ma15030932
APA StyleNocchetti, M., Boccalon, E., Pica, M., Giordano, N. M. R., Finori, F., Pietrella, D., & Cipiciani, A. (2022). Overcoming Antibiotic Resistance: Playing the ‘Silver Nanobullet’ Card. Materials, 15(3), 932. https://doi.org/10.3390/ma15030932








