Effect of Internal Donors on Raman and IR Spectroscopic Fingerprints of MgCl2/TiCl4 Nanoclusters Determined by Machine Learning and DFT
Abstract
:1. Introduction
2. Computational Models and DFT Calculations Details
3. Results and Discussion
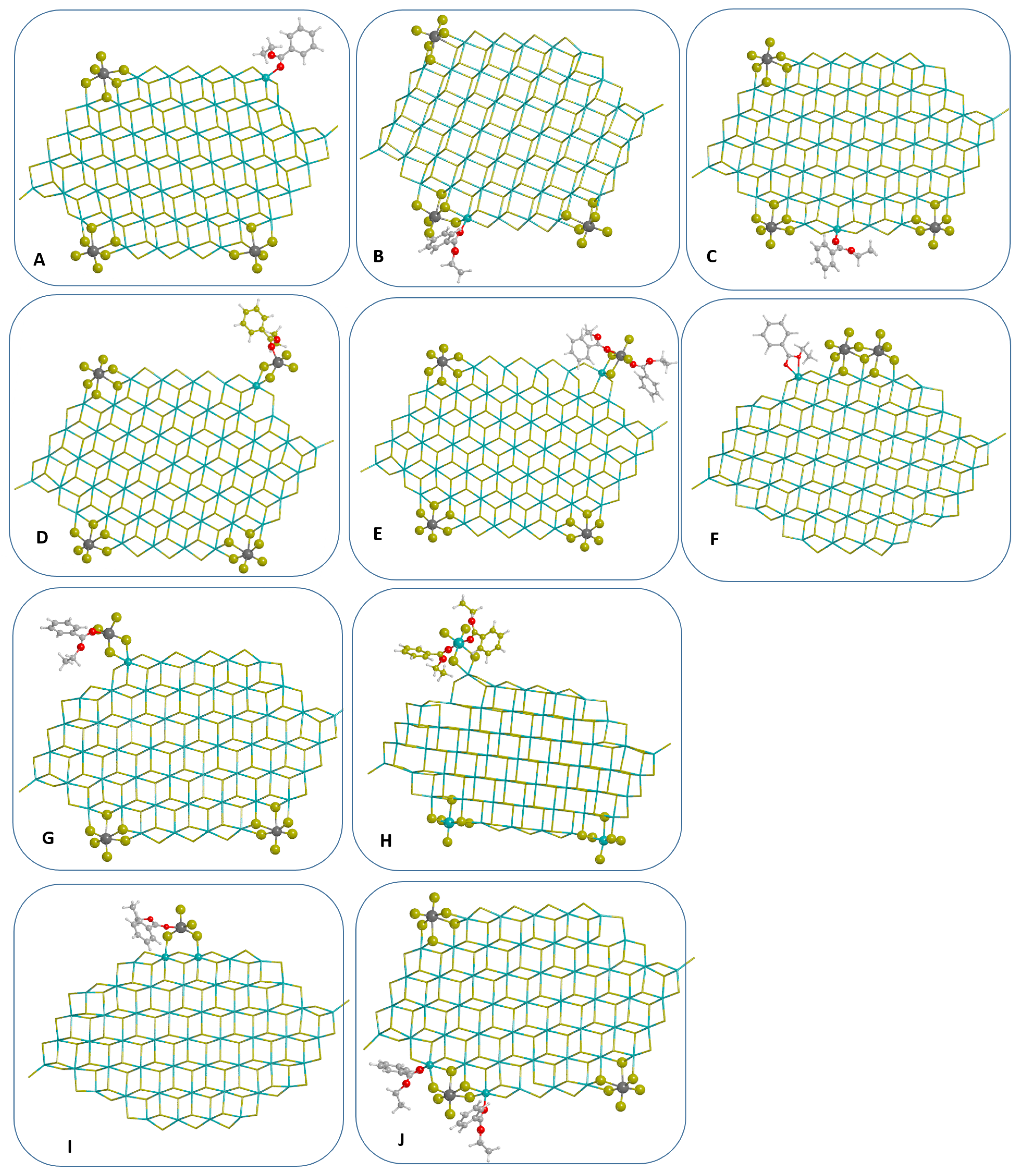
3.1. Models of Ternary Systems MgCl/TiCl/EB
3.2. Adsorption Properties of Adducts on MgCl Nanoplatelets
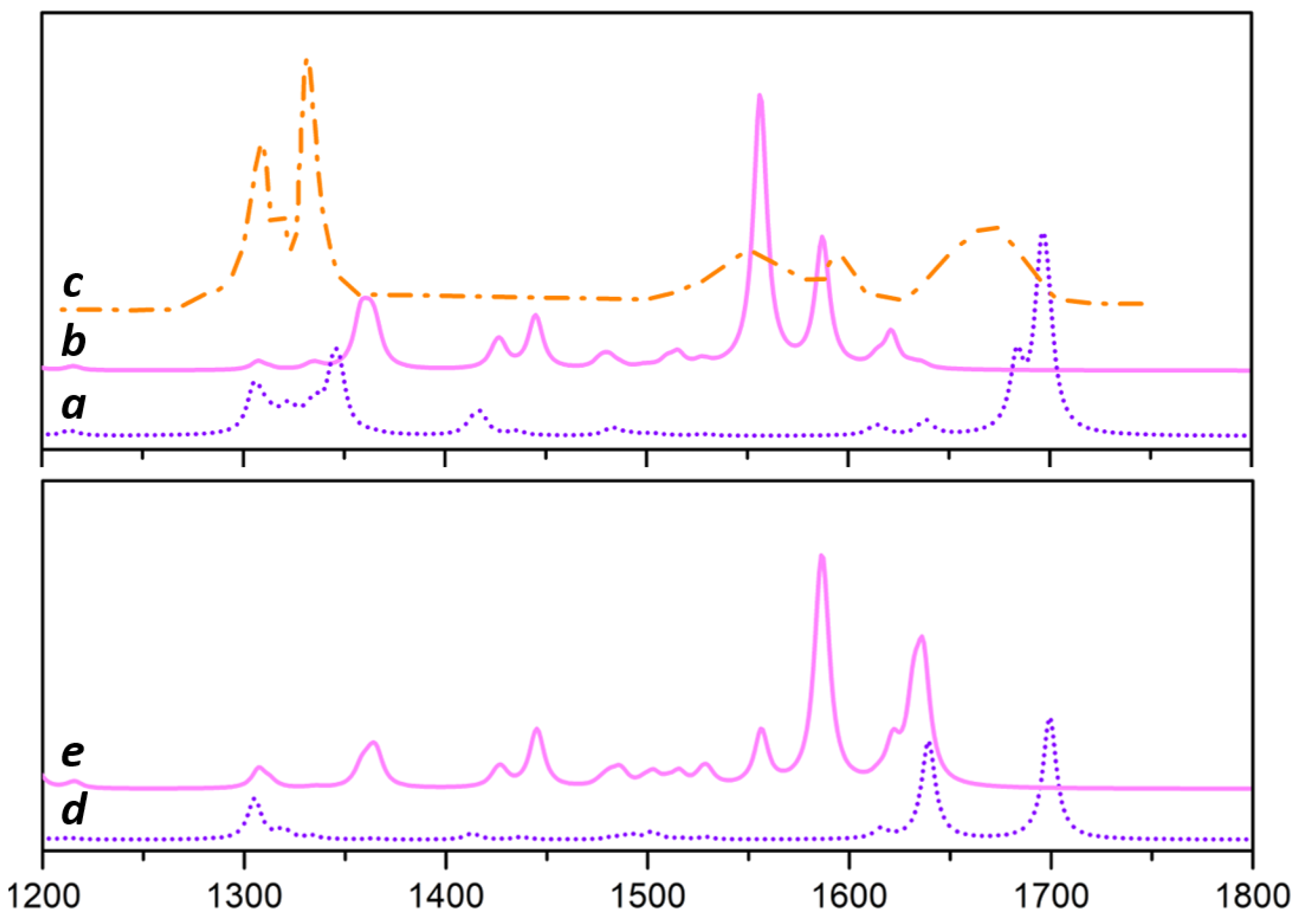
3.3. IR and Raman Response of EB/TiCl on Nanoplatelet
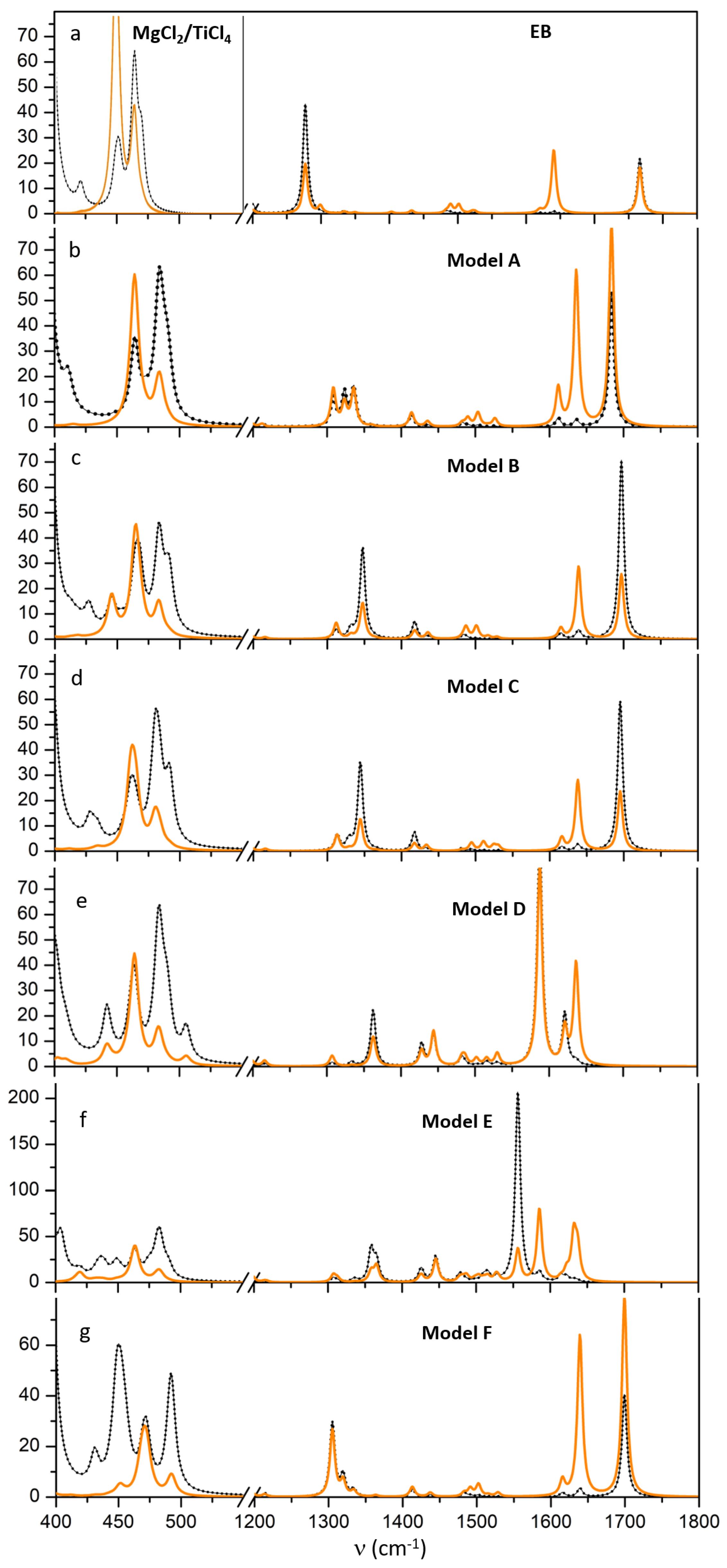
3.3.1. IR Simulations
- the (C=O) vibration that dominates the IR spectrum and is located at 1683, 1697 and 1695 cm for models A–C, downshifted by 83, 69 and 71 cm in reference to gas phase, respectively. Two very weak satellite bands are also osberved at 1638 and 1615 cm that correspond to C-C stretching modes of the phenyl ring. In the presence of a TiClEB adduct (model D) the C=O stretching mode undergoes a huge redshift to 1587 cm (medium); it is further redshifted to 1556 cm and coupled with a very weak band at 1585 cm in the case of adduct E: they correspond to the antisymmetric and to the symmetric coupling of the two C=O stretchings;
- the (C-O) signal drops its intensity with respect to free EB and is splitted into three components at 1308, 1323, 1336; 1312, 1332, 1348 and 1313, 1329, 1344 cm for models A–C, respectively due to coupling with CH twisting and phenyl H modes; however for model A the triplet of bands have comparable intensity, whereas for model B and C the band at higher wavenumber dominates in the triplet. A similar group of bands is observed for models D and E with the most intense peak located at 1362 cm;
- a new weak band appears at 1414, 1418, 1417 cm for models A–C, and at 1427 cm for models D–E, is associated to the wagging of -CH and therefore slightly perturbed by different EB binding mode; the band is weak but it can be clearly identified in the IR spectra;
- in the spectral region 400–500 cm region, for A and B models the Ti-Cl stretching mode at 465 and 485 cm are only slightly perturbed by the presence of EB; for model C, the coupling with EB mode causes the splitting of Ti-Cl symmetric stretching mode in components at 464, 465, 468 cm and antisymmetric Ti-Cl stretching at 483, 484, also partially coupled with Mg-Cl modes of tetrahedral Mg at 490 cm. For models D–E two further bands, not visible in Raman, pop-up at 437 cm and at 449 cm due to bending modes of O-Ti-O and Ti-O-C, respectively. Weak bands at 588, 645 cm assigned to Ti-O-C appear.
3.3.2. Raman Characterization MgCl/TiCl/EB Adducts
3.4. Discussion
3.5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Chadwick, J.C. Advances in propene polymerization using MgCl2-supported catalysts. Fundamental aspects and the role of electron donors. Macromol. Symp. 2001, 173, 21–36. [Google Scholar] [CrossRef]
- Cecchin, G.; Morini, G.; Pelliconi, A. Polypropene product innovation by reactor granule technology. Macromol. Symp. 2001, 173, 195–210. [Google Scholar] [CrossRef]
- Busico, V. Giulio Natta and the development of stereoselective propene polymerization. Adv. Polym. Sci. 2013, 257, 37–58. [Google Scholar]
- Albizzati, E.; Giannini, U.; Collina, G.; Noristi, L.; Resconi, L. Catalysts and polymerizations. In Polypropylene Handbook; Moore, E.P.J., Ed.; Hanser-Gardner Publications: Cincinnati, OH, USA, 1996. [Google Scholar]
- Taniike, T.; Terano, M. The use of donors to increase the isotacticity of polypropylene. Adv. Polym. Sci. 2013, 257, 81–97. [Google Scholar]
- Busico, V.; Corradini, P.; De Martino, L.; Proto, A.; Savino, V.; Albizzati, E. Polymerization of propene in the presence of MgCl2-supported Ziegler-Natta catalysts 1. The role of ethyl benzoate as “internal” and “external” base. Makromol. Chem. 1985, 186, 1279–1288. [Google Scholar] [CrossRef]
- Busico, V.; Chadwick, J.C.; Cipullo, R.; Ronca, S.; Talarico, G. Propene/ethene-[1-C-13] copolymerization as a tool for investigating catalyst regioselectivity. MgCl2/internal donor/TiCl4-external donor AlR3 systems. Macromolecules 2004, 37, 7437–7443. [Google Scholar] [CrossRef]
- Soga, K.; Shiono, T.; Doi, Y. Influence of internal and external donors on activity and stereospecificity of Ziegler-Natta catalysts. Makromol. Chem. 1988, 189, 1531–1541. [Google Scholar] [CrossRef]
- Sacchi, M.C.; Tritto, I.; Shan, C.; Mendichi, R.; Noristi, L. Role of the pair of internal and external donors in MgCl2-supported Ziegler-Natta catalysts. Macromolecules 1991, 24, 6823–6826. [Google Scholar] [CrossRef]
- Sacchi, M.C.; Forlini, F.; Tritto, I.; Mendichi, R.; Zannoni, G.; Noristi, L. Activation effect of alkoxysilanes as external donors in MgCl2-supported Ziegler-Natta catalysts. Macromolecules 1992, 25, 5914–5918. [Google Scholar]
- Sacchi, M.C.; Forlini, F.; Tritto, I.; Locatelli, P.; Morini, G.; Noristi, L.; Albizzati, E. Polymerization stereochemistry with Ziegler-Natta catalysts containing dialkylpropane diethers: A tool for understanding internal/external donor relationships. Macromolecules 1996, 29, 3341–3345. [Google Scholar] [CrossRef]
- Noristi, L.; Barbe, P.C.; Baruzzi, G. External donor pair in high-yield catalysts for propylene polymerization, 1. Catalysts-Cocatalyst Interactions. Makromol. Chem. 1991, 192, 1115–1127. [Google Scholar] [CrossRef]
- Noristi, L.; Barbe, P.C.; Baruzzi, G. Effect of the internal/external donor pair in high-yield catalysts for propylene polymerization, 2. Polymerization results. Makromol. Chem. 1992, 193, 229–241. [Google Scholar]
- D’Amore, M.; Auriemma, F.; De Rosa, C.; Barone, V. Disordered Chain Conformations of Poly(tetrafluoroethylene) in the High-Temperature Crystalline Form I. Macromolecules 2004, 37, 9473–9480. [Google Scholar] [CrossRef]
- Chadwick, J.C.; Van Kessel, G.M.M.; Sudmeijer, O. Regiospecificity and stereospecificity in propene polymerization with MgCl2-supported Ziegler-Natta catalysts - Effects of hydrogen and the external donor. Macromol. Chem. Phys. 1995, 196, 1431–1437. [Google Scholar] [CrossRef]
- Chadwick, J.C.; Van Kessel, G.M.M.; Sudmeijer, O. Influence of Ziegler-Natta catalyst regioselectivity on polypropylene molecular weight distribution and rheological and crystallization behavior. Macromolecules 2004, 37, 9722–9727. [Google Scholar] [CrossRef]
- Mori, H.; Endo, M.; Terano, M. Deviation of hydrogen response during propene polymerization with various Ziegler-Natta catalysts. J. Mol. Catal. A 1999, 145, 211. [Google Scholar] [CrossRef]
- Taniike, T.; Terano, M. Coadsorption and support-mediated interaction of ti species with ethyl benzoate in MgCl2-supported heterogeneous ziegler-natta catalysts studied by density functional calculations. Macromol. Rapid Commun. 2007, 28, 1918–1922. [Google Scholar] [CrossRef]
- Pirinen, S.; Pakkanen, T.T. Polyethers as potential electron donors for Ziegler-Natta ethylene polymerization catalysts. J. Mol. Catal. A 2015, 398, 177–183. [Google Scholar] [CrossRef]
- Ratanasak, M.; Rungrotmongkol, T.; Saengsawang, O.; Hannongbua, S.; Parasuk, V. Towards the design of new electron donors for Ziegler-Natta catalyzed propylene polymerization using QSPR modeling. Polymer 2015, 56, 340–345. [Google Scholar] [CrossRef]
- Vittoria, A.; Meppelder, A.; Friederichs, N.; Busico, V.; Cipullo, R. Demystifying Ziegler-Natta catalysts: The origin of stereoselectivity. ACS Catal. 2017, 7, 4509–4518. [Google Scholar] [CrossRef]
- Cecchin, G.; Morini, G.; Piemontesi, F. Encyclopedia of Chemical Technology; John Wiley and Sons: New York, NY, USA, 2006; Volume 26, pp. 502–554. [Google Scholar]
- Moore, E.P. Polypropylene Handbook: Polymerization, Characterization, Properties, Applications; Hanser Publishers: Munich, Germany, 1996. [Google Scholar]
- Chien, J.C.W.; Wu, J.C.; Kuo, C. Magnesium chloride supported high-mileage catalysts for olefin polymerization. IV. FTIR and quantitative analysis of modifiers in the catalysts. J. Polym. Sci. Polym. Chem. Ed. 1983, 21, 725–736. [Google Scholar] [CrossRef]
- Stukalov, D.V.; Zakharov, V.A.; Zilberberg, I.L. Adsorption species of ethyl benzoate in MgCl2-supported Ziegler-Natta catalysts. A density functional theory study. J. Phys. Chem. C 2010, 114, 429–435. [Google Scholar] [CrossRef]
- Correa, A.; Piemontesi, F.; Morini, G.; Cavallo, L. Key elements in the structure and function relationship of the MgCl2/TiCl4/Lewis base Ziegler-Natta catalytic system. Macromolecules 2007, 40, 9181–9189. [Google Scholar] [CrossRef]
- Correa, A.; Credendino, R.; Pater, J.; Morini, G.; Cavallo, L. Theoretical investigation of active sites at the corners of MgCl2 crystallites in supported Ziegler-Natta catalysts. Macromolecules 2015, 45, 3695–3701. [Google Scholar] [CrossRef]
- Credendino, R.; Pater, J.T.M.; Liguori, D.; Morini, G.; Cavallo, L. Investigating alkoxysilane coverage and dynamics on the (104) and (110) surfaces of MgCl2-supported Ziegler-Natta catalysts. J. Phys. Chem. C 2012, 116, 22980–22986. [Google Scholar] [CrossRef]
- Credendino, R.; Liguori, D.; Fan, Z.; Morini, G.; Cavallo, L. A Unified Model Explaining Heterogeneous Ziegler-Natta Catalysis. ACS Catal. 2015, 5, 5431–5435. [Google Scholar] [CrossRef] [Green Version]
- Capone, F.; Rongo, L.; D’Amore, M.; Budzelaar, P.H.M.; Busico, V. A Periodic Hybrid DFT Approach (Including Dispersion) to MgCl2-supported Ziegler-Natta catalysts 2: Model Electron Donor Adsorption on MgCl2 Crystal Surfaces. J. Phys. Chem. C 2013, 117, 24345–24353. [Google Scholar] [CrossRef]
- Seth, M.; Margl, P.M.; Ziegler, T. Polymerization properties of heterogeneous Ziegler-Natta catalyst modified by a base: A theoretical study. Macromolecules 2003, 36, 6613–6623. [Google Scholar] [CrossRef]
- Sacchi, M.C.; Forlini, F.; Tritto, I.; Locatelli, P. Stereochemistry of the initiation step in Ziegler-Natta catalysts containing dialkyl propane diethers: A tool for distinguishing the role of internal and external donors. Macromol. Symp. 1995, 89, 91–100. [Google Scholar] [CrossRef]
- Rytter, E.; Nirisen, Ø.; Ystenes, M.; Øye, H.A. FTIR Spectroscopy of Ethyl Benzoate-Titanium Tetrachloride Complexes with Application to Supported Ziegler-Natta Catalysts. Mikrochim. Acta 1988, 95, 85–87. [Google Scholar] [CrossRef]
- Credendino, R.; Minenkov, Y.; Liguori, D.; Piemontesi, F.; Melchior, A.; Morini, G.; Tolazzi, M.; Cavallo, L. Accurate experimental and theoretical enthalpies of association of TiCl4 with typical Lewis bases used in heterogeneous Ziegler-Natta catalysis. Phys. Chem. Chem. Phys. 2017, 19, 26996–27006. [Google Scholar] [CrossRef] [Green Version]
- Terano, M.; Kataoka, T.; Keii, T. A study on the states of ethyl benzoate and TiCl4 in MgCl2-supported high-yield catalysts. Makromol. Chem. 1987, 188, 1477–1487. [Google Scholar] [CrossRef]
- Corradini, P.; Barone, V.; Fusco, R.; Guerra, G. A possible model of catalytic sites for the stereospecific polymerization of alpha olefins on 1st-generation and supported Ziegler-Natta catalysts. Gazz. Chim. Ital. 1983, 113, 601. [Google Scholar]
- Busico, V.; Cipullo, R. A possible model of catalytic sites for the stereospecific polymerization of alpha olefins on 1st-generation and supported Ziegler-Natta catalysts. Prog. Polym. Sci. 2001, 26, 443–533. [Google Scholar] [CrossRef]
- Corradini, P.; Guerra, G.; Cavallo, L. Do New Century Catalysts Unravel the Mechanism of Stereocontrol of Old Ziegler-Natta catalysts? Acc. Chem. Res. 2004, 37, 231–241. [Google Scholar] [CrossRef]
- Correa, A.; Bahri-Laleh, N.; Cavallo, L. How Well Can DFT Reproduce Key Interactions in Ziegler–Natta Systems? Macromol. Chem. Phys. 2013, 214, 1980–1989. [Google Scholar] [CrossRef]
- Wada, T.; Takasao, G.; Piovano, A.; D’Amore, M.; Thakur, A.; Chammingkwan, P.; Bruzzese, P.C.; Terano, M.; Civalleri, B.; Bordiga, S.; et al. Revisiting the Identity of δ-MgCl2: Part I. Structural Disorder Studied by Synchrotron X-ray Total Scattering. J. Catal. 2020, 385, 76–86. [Google Scholar] [CrossRef]
- Piovano, A.; D’Amore, M.; Wada, T.; Bruzzese, P.C.; Takasao, G.; Thakur, A.; Chammingkwan, P.; Terano, M.; Civalleri, B.; Bordiga, S.; et al. Revisiting the Identity of δ-MgCl2: Part II. Morphology and Exposed Surfaces Studied by Vibrational Spectroscopies and DFT Calculation. J. Catal. 2020, 387, 1–11. [Google Scholar] [CrossRef]
- Corradini, P.; Barone, V.; Fusco, R.; Guerra, G. Analysis of models for the Ziegler-Natta stereospecific polymerization on the basis of non-bonded interactions at the catalytic site—I. The Cossee model. Eur. Polym. J. 1979, 15, 1133–1141. [Google Scholar] [CrossRef]
- Boero, M.; Parrinello, M.; Terakura, K. First Principles Molecular Dynamics Study of Ziegler-Natta Heterogeneous Catalysis. J. Am. Chem. Soc. 1998, 120, 2746–2752. [Google Scholar] [CrossRef]
- Boero, M.; Parrinello, M.; Hüffer, S.; Weiss, H. First Principles Study of Propene Polymerization in Ziegler-Natta Heterogeneous Catalysis. J. Am. Chem. Soc. 2000, 122, 501–509. [Google Scholar] [CrossRef]
- Monaco, G.; Toto, M.; Guerra, G.; Cavallo, L. Geometry and stability of titanium chloride species adsorbed on the (100) and (110) cuts of the MgCl2 support of the heterogeneous Ziegler-Natta catalysts. Macromolecules 2000, 33, 8953–8962. [Google Scholar] [CrossRef]
- Boero, M.; Parrinello, M.; Weiss, H.; Hüffer, S. A First Principles Exploration of a Variety of Active Surfaces and Catalytic Sites in Ziegler-Natta Heterogeneous Catalysis. J. Phys. Chem. A 2001, 105, 5096–5105. [Google Scholar] [CrossRef]
- Seth, M.; Margl, P.M.; Ziegler, T. A Density Functional Embedded Cluster Study of Proposed Active Sites in Heterogeneous Ziegler-Natta Catalysts. Macromolecules 2002, 35, 7815–7829. [Google Scholar] [CrossRef]
- Taniike, T.; Terano, M. Reductive Formation of Isospecific Ti Dinuclear Species on a MgCl2 (110) Surface in Heterogeneous Ziegler-Natta Catalysts. Macromol. Rapid Commun. 2008, 29, 1472–1476. [Google Scholar] [CrossRef]
- D’Amore, M.; Credendino, R.; Budzelaar, P.H.M.; Causá, M.; Busico, V. A Periodic Hybrid DFT Approach (Including Dispersion) to MgCl2-supported Ziegler-Natta catalysts 1:TiCl4 Adsorption on MgCl2 Crystal Surfaces. J. Catal. 2012, 286, 103–110. [Google Scholar] [CrossRef]
- Breuza, E.; Antinucci, G.; Budzelaar, P.H.M.; Busico, V.; Correa, A.; Ehm, C. MgCl2-supported Ziegler-Natta catalysts: A DFT-D ‘flexible-cluster’ approach. TiCl4 and probe donor adducts. Int. J. Quantum Chem. 2018, 118, e25721. [Google Scholar] [CrossRef]
- Breuza, E.; Antinucci, G.; Budzelaar, P.H.M.; Busico, V.; Correa, A.; Ehm, C. MgCl2-Supported Ziegler–Natta Catalysts: A DFT-D “Flexible-Cluster” Approach to Internal Donor Adducts. J. Phys. Chem. C 2018, 122, 9046–9053. [Google Scholar] [CrossRef]
- Credendino, R.; Pater, J.T.M.; Correa, A.; Morini, G.; Cavallo, L. Thermodynamics of Formation of Uncovered and Dimethyl Ether Covered MgCl2 Crystallites. Consequences in the Structure of Ziegler-Natta Heterogeneous Catalysts. J. Phys. Chem. C 2011, 115, 13322–13328. [Google Scholar] [CrossRef]
- D’Amore, M.; Thushara, K.; Piovano, A.; Causá, M.; Bordiga, S.; Groppo, E. Surface Investigation and Morphological Analysis of Structurally Disordered MgCl2 and MgCl2/TiCl4 Ziegler-Natta Catalysts. ACS Catal. 2016, 6, 5786–5796. [Google Scholar] [CrossRef]
- D’Amore, M.; Piovano, A.; Vottero, E.; Rudic, S.; Groppo, E.; Bordiga, S.; Civalleri, B. Insights on inelastic neutron scattering data of MgCl2 ZN-catalyst support from ab initio modelling of nano-sized and disordered models. ACS Appl. Nano Mater. 2020, 3, 11118–11128. [Google Scholar] [CrossRef]
- D’ Amore, M.; Takasao, G.; Chikuma, H.; Wada, T.; Taniike, T.; Pascale, F.; Ferrari, A.M. Spectroscopic Fingerprints of MgCl2/TiCl4 Nanoclusters Determined by Machine Learning and DFT. J. Phys. Chem. C 2021, 125, 20048–20058. [Google Scholar] [CrossRef]
- Thushara, K.; D’Amore, M.; Piovano, A.; Bordiga, S.; Groppo, E. The Influence of Alcohols in Driving the Morphology of Magnesium Chloride Nanocrystals. ChemCatChem 2017, 9, 1782–1787. [Google Scholar] [CrossRef]
- Takasao, G.; Wada, T.; Thakur, A.; Chammingkwan, P.; Terano, M.; Taniike, T. Machine Learning-Aided Structure Determination for TiCl4-Capped MgCl2 Nanoplate of Heterogeneous Ziegler-Natta Catalyst. ACS Catal. 2019, 9, 2599–2609. [Google Scholar] [CrossRef]
- Takasao, G.; Wada, T.; Thakur, A.; Chammingkwan, P.; Terano, M.; Taniike, T. Insight into Structural Distribution of Heterogeneous Ziegler–Natta Catalyst from Non-empirical Structure Determination. J. Catal. 2021, 394, 299–306. [Google Scholar] [CrossRef]
- Piovano, A.; D’Amore, M.; Thushara, K.; Groppo, E. Spectroscopic Evidences for TiCl4/Donor Complexes on the Surfaceof MgCl2-Supported Ziegler-Natta Catalysts. J. Phys. Chem. C 2018, 122, 5615–5626. [Google Scholar] [CrossRef]
- Potapov, A.G.; Bukatov, G.D.; Zakharov, V.A. DRIFT study of internal donors in supported Ziegler-Natta catalysts. J. Mol. Catal. A 2006, 246, 248–254. [Google Scholar] [CrossRef]
- Potapov, A.G.; Bukatov, G.D.; Zakharov, V.A. DRIFTS study of the interaction of the AlEt3 cocatalyst with the internal donor ethyl benzoate in supported Ziegler-Natta catalysts. J. Mol. Catal. A 2009, 301, 18–23. [Google Scholar] [CrossRef]
- Di Noto, V.; Fregonese, D.; Marigo, A.; Bresadola, S. High Yield MgCl2-supported catalysts for propene polymerization: Effects of ethyl propionate as internal donor on the activity and stereospecificity. Macromol. Chem. Phys. 1998, 199, 633–640. [Google Scholar] [CrossRef]
- Piovano, A.; Groppo, E. Flexible ligands in heterogeneous catalysts for olefin polymerization: Insights from spectroscopy. Coord. Chem. Rev. 2022, 451, 214258. [Google Scholar] [CrossRef]
- Wada, T.; Funako, T.; Thakur, A.; Matta, A.; Terano, M.; Taniike, T. Structure-performance relationship of Mg(OEt)2-based Ziegler-Natta catalysts. J. Catal. 2021, 389, 525–532. [Google Scholar] [CrossRef]
- Taniike, T.; Terano, M. Coadsorption Model for First-Principle Description of Roles of Donors in Heterogeneous Ziegler-Natta Propylene Polymerization. J. Catal. 2012, 293, 39–50. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864. [Google Scholar] [CrossRef] [Green Version]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133. [Google Scholar] [CrossRef] [Green Version]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Yang, W.; Parr, R. Development of the Colle-Salvetti Correlation-Energy Formula Into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Dovesi, R.; Erba, A.; Orlando, R.; Zicovich-Wilson, C.M.; Civalleri, B.; Maschio, L.; Rérat, M.; Casassa, S.; Baima, J.; Salustro, S.; et al. Quantum-Mechanical Condensed Matter Simulations with CRYSTAL. WIREs Comput. Mol. Sci. 2018, 8, e1360. [Google Scholar] [CrossRef]
- Dovesi, R.; Pascale, F.; Civalleri, B.; Doll, K.; Harrison, N.M.; Bush, I.; D’Arco, P.; Noël, Y.; Rérat, M.; Carbonnière, P.; et al. The CRYSTAL code, 1976–2020 and beyond, a long story. J. Chem. Phys. 2020, 152, 204111. [Google Scholar] [CrossRef]
- Grimme, S.S. Semiempirical GGA-Type Density Functional Constructed with a Long-Range Dispersion Correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Fornaro, T.; Brucato, J.R.; Feuillie, C.; Sverjensky, D.A.; Hazen, R.M.; Brunetto, R.; D’Amore, M.; Barone, V. Binding of Nucleic Acid Components to the Serpentinite-Hosted Hydrothermal Mineral Brucite. Astrobiology 2018, 18, 989–1007. [Google Scholar] [CrossRef]
- Signorile, M.; Vitillo, J.G.; D’Amore, M.; Crocellà, V.; Ricchiardi, G.; Bordiga, S. Characterization and Modeling of Reversible CO2 Capture from Wet Streams by a MgO/Zeolite Y Nanocomposite. J. Phys. Chem. C 2019, 123, 17214–17224. [Google Scholar] [CrossRef]
- Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Schafer, A.; Horn, H.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Salustro, S.; Colasuonno, F.; Ferrari, A.M.; D’Amore, M.; Mackrodt, W.C.; Dovesi, R. Substitutional boron and nitrogen pairs in diamond. A quantum mechanical vibrational analysis. Carbon 2019, 146, 709–716. [Google Scholar] [CrossRef]
- Platonenko, A.; Gentile, F.S.; Pascal, F.; Ferrari, A.M.; D’Amore, M.; Mackrodt, W.C.; Dovesi, R. Nitrogen substitutional defects in silicon. A quantum mechanical investigation of the structural, electronic and vibrational properties. Phys. Chem. Chem. Phys. 2019, 21, 20939–20950. [Google Scholar] [CrossRef]
- Ashuiev, A.; Humbert, M.; Norsic, S.; Blahut, J.; Gajan, D.; Searles, K.; Klose, D.; Lesage, A.; Pintacuda, G.; Raynaud, J.; et al. Spectroscopic Signature and Structure of the Active Sites in Ziegler–Natta Polymerization Catalysts Revealed by Electron Paramagnetic Resonance. J. Am. Chem. Soc. 2021, 143, 9791–9797. [Google Scholar] [CrossRef]
- Bahri-Laleh, N.; Correa, A.; Mehdipour-Ataei, S.; Arabi, H.; Haghighi, M.N.; Zohuri, G.; Cavallo, L. Moving up and down the Titanium Oxidation State in Ziegler–Natta Catalysis. Macromolecules 2011, 44, 778–783. [Google Scholar] [CrossRef]
| Reaction | Model | E (B3LYP-D2) | E (M06) |
|---|---|---|---|
| EB + (MgClTiCl) → (MgClTiClEB) | A | −79.3 | −81.6 |
| “ | B | −78.9 | −80.9 |
| ” | C | −77.5 | −79.5 |
| TiClEB + (MgClTiCl) → (MgClTiClEB) | D | −66.3 | −75.4 |
| TiClEB + (MgClTiCl) → (MgClTiClEB) | E | −181.2 | −185.8 |
| EB + (MgClTiCl) → ((MgClTiClEB) | F | −99.3 | −96.0 |
| TiClEB + (MgClTiCl) → (MgClTiClEB) | G | −79.3 | −94.9 |
| TiClEB + (MgClTiCl) → (MgClTiClEB) | H | −172.1 | −191.6 |
| TiClEB + (MgClTiCl) → (MgClTiClEB) | I | −79.5 | −95.3 |
| EB + (MgClTiClEB) → (MgClTiClEB) | J | −121.9 | −123.6 |
| (MgClTiClEB) → (MgClTiClEB) | G | 35.5 | 60.9 |
| (MgClTiClEB) → (MgClTiClEB) | H | 55.5 | 67.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Amore, M.; Taniike, T.; Terano, M.; Ferrari, A.M. Effect of Internal Donors on Raman and IR Spectroscopic Fingerprints of MgCl2/TiCl4 Nanoclusters Determined by Machine Learning and DFT. Materials 2022, 15, 909. https://doi.org/10.3390/ma15030909
D’Amore M, Taniike T, Terano M, Ferrari AM. Effect of Internal Donors on Raman and IR Spectroscopic Fingerprints of MgCl2/TiCl4 Nanoclusters Determined by Machine Learning and DFT. Materials. 2022; 15(3):909. https://doi.org/10.3390/ma15030909
Chicago/Turabian StyleD’Amore, Maddalena, Toshiaki Taniike, Minoru Terano, and Anna Maria Ferrari. 2022. "Effect of Internal Donors on Raman and IR Spectroscopic Fingerprints of MgCl2/TiCl4 Nanoclusters Determined by Machine Learning and DFT" Materials 15, no. 3: 909. https://doi.org/10.3390/ma15030909
APA StyleD’Amore, M., Taniike, T., Terano, M., & Ferrari, A. M. (2022). Effect of Internal Donors on Raman and IR Spectroscopic Fingerprints of MgCl2/TiCl4 Nanoclusters Determined by Machine Learning and DFT. Materials, 15(3), 909. https://doi.org/10.3390/ma15030909