Research on the Morphology Reconstruction of Deep Cryogenic Treatment on PtRu/nitrogen-Doped Graphene Composite Carbon Nanofibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of PtRu/NG Composite Carbon Nanofibers
- (1)
- Preparation of precursor solution
- (2)
- Preparation of fibrous membrane
2.2.2. DCT of PtRu/NG Composite Carbon Nanofibers
2.3. Performance Characterization
3. Results and Discussion
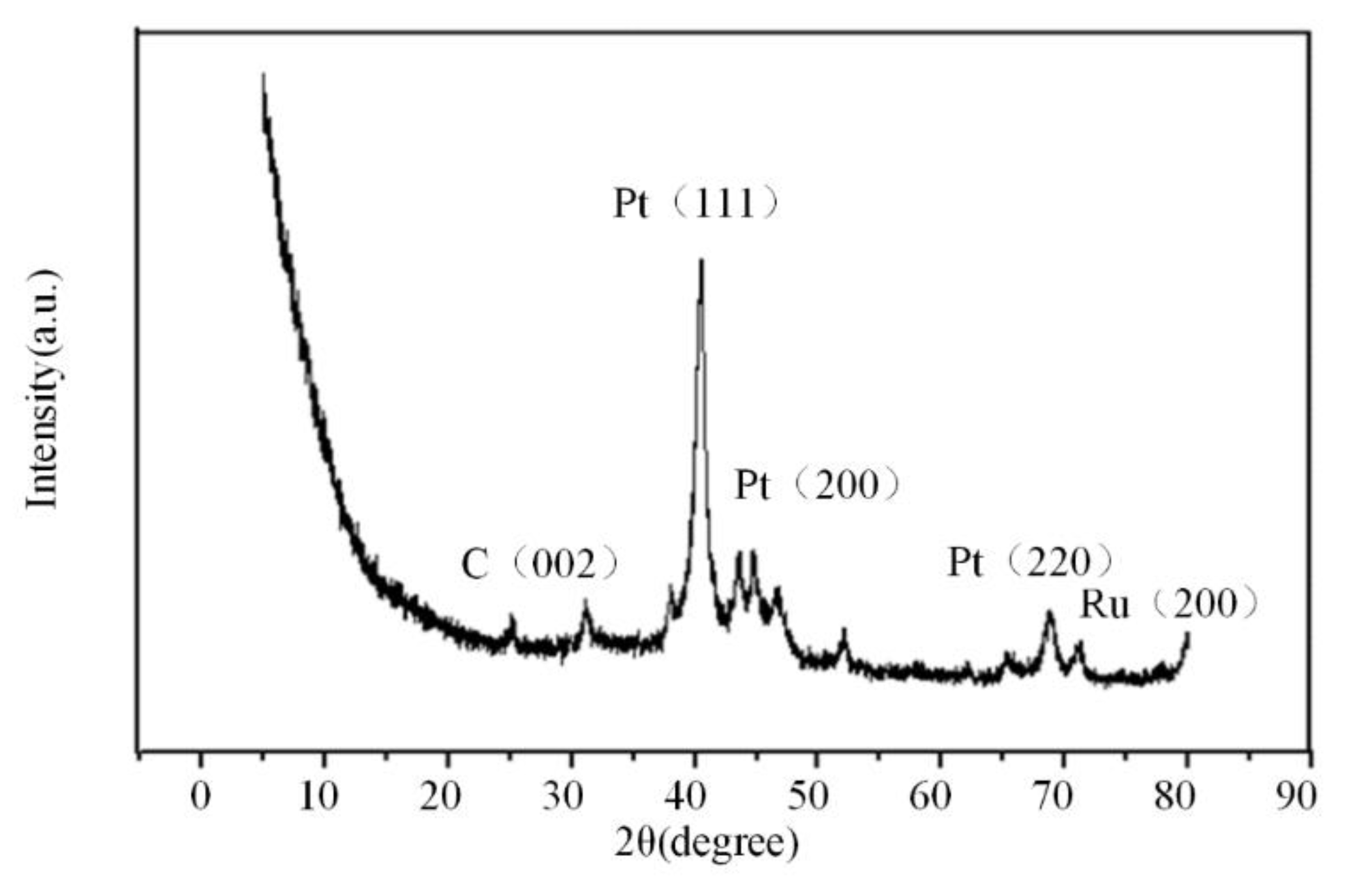
3.1. Analysis of Existing Form of NG
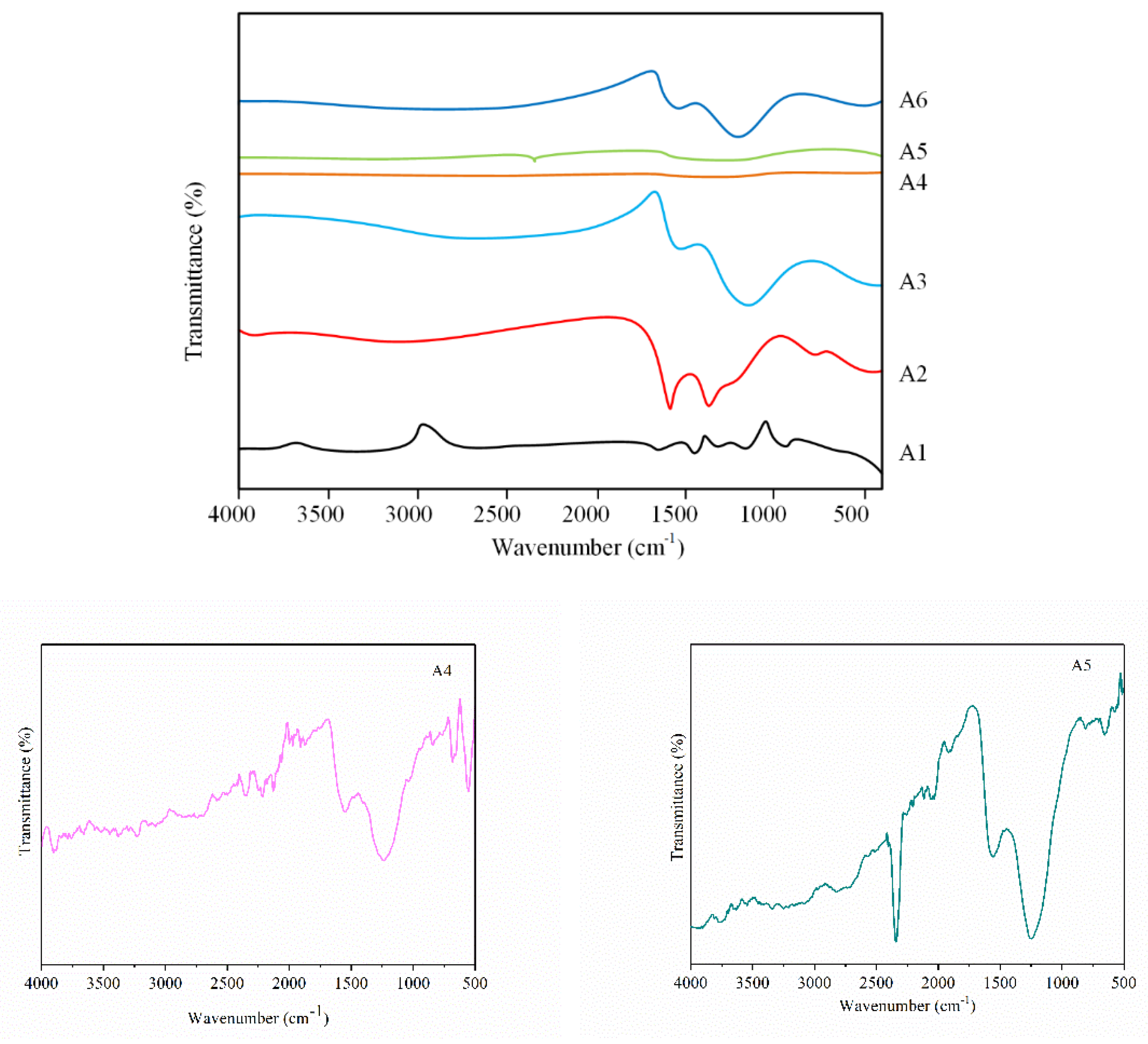
3.2. Analysis of Group Transformation for PtRu/NG Composite Carbon Nanofiber
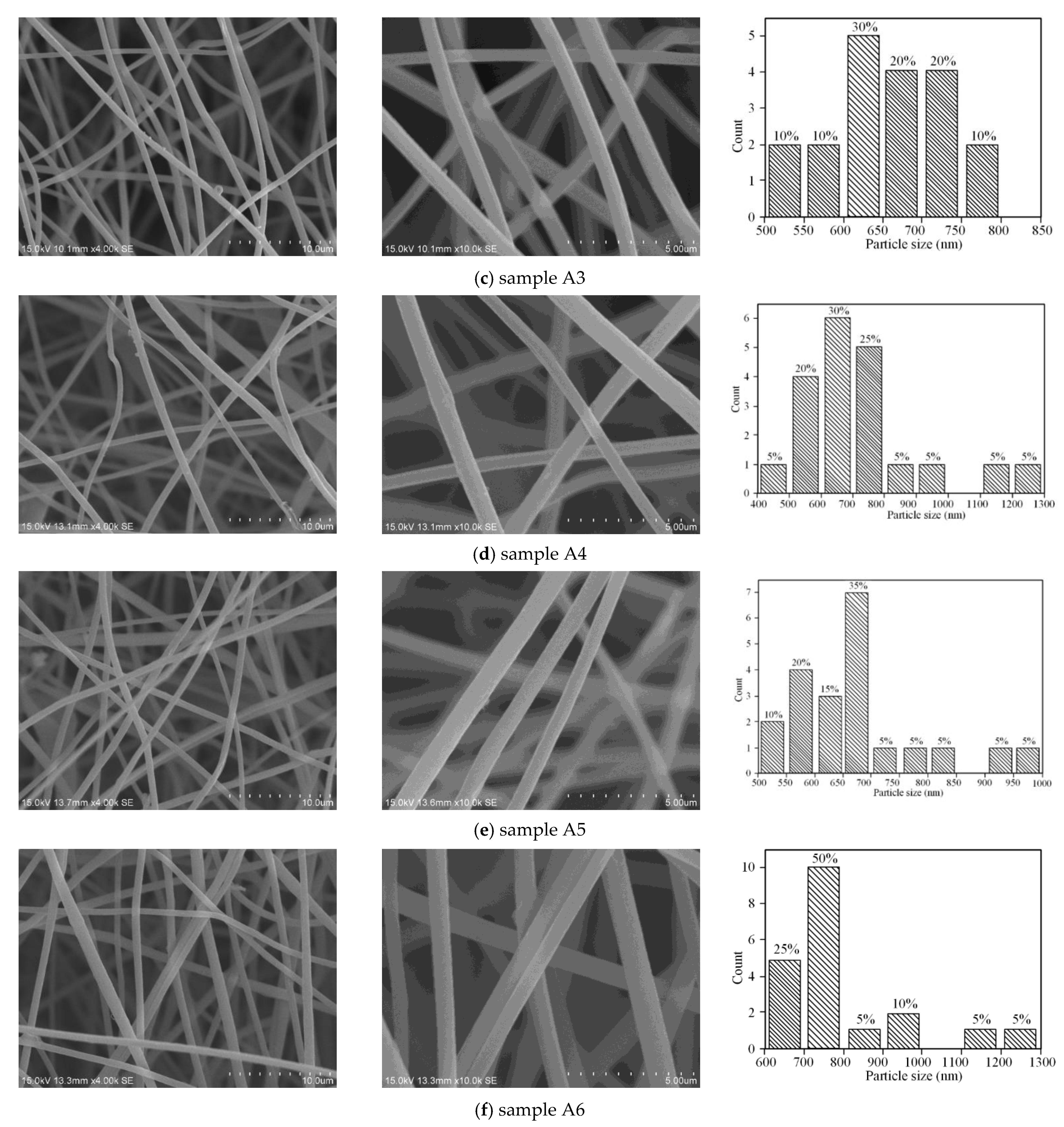
3.3. Morphology Reconstruction of PtRu/NG Composite Carbon Nanofiber
4. Conclusions
- (1)
- Through the combination of electrostatic spinning, carbonization, and DCT, the morphology reconstruction of platinum ruthenium/nitrogen-doped graphene composite carbon nanofibers is carried out, thus realizing the application of cryogenic modification fibers;
- (2)
- DCT can effectively improve molecular interaction and material crystallinity, providing the possibility for the preparation of nanoscale materials;
- (3)
- Cryogenic time should not be too long under the condition of treating the fiber at the same cryogenic temperature. The longer the cryogenic time, the greater the environmental impact, and the more likely the fiber is to produce defects.
- (4)
- DCT has a significant effect on the nanofiber, and there have not been many studies on the mechanism and application of DCT in nanofiber materials. Therefore, an in depth exploration of the mechanism is of great importance in order to obtain universal laws and applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Filiz, B.; Elamis, Y.; Bektas, I.; Figen, A. Fabrication of stable electrospun blended chitosan-poly(vinyl alcohol) nanofibers for designing naked-eye colorimetric glucose biosensor based on GOx/HRP. Int. J. Biol. Macromol. 2021, 192, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Atashgahi, M.; Ghaemi, B.; Valizadeh, A.; Mishiri, A.; Nekoofar, M.; Amani, A. Epinephrine-entrapped chitosan nanoparticles covered by gelatin nanofibers: A bi-layer nano-biomaterial for rapid hemostasis. Int. J. Pharm. 2021, 608, 121074. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.; Kim, S.; Yoon, S.; Kim, K.; Kim, G.; An, T.; Lim, G. Highly flexible and transparent film heater with electrospun copper conductive network via junction-free structure. J. Alloys Compd. 2021, 886, 161191. [Google Scholar] [CrossRef]
- Bhullar, V.; Sardana, S.; Mahajan, A. Size modeling of TiO2 nanofibers for efficient TiO2 sensitized mesoscopic solar cells. Sol. Energy 2021, 230, 177–185. [Google Scholar] [CrossRef]
- Lee, S.; Park, J.; Lee, K.; Ju, Y. Anodic properties of Ni-Fe bimetallic nanofiber for solid oxide fuel cell using LaGaO3 electrolyte. J. Alloys Compd. 2021, 875, 159911. [Google Scholar] [CrossRef]
- Banitaba, S.; Ehrmann, A. Application of electrospun nanofibers for fabrication of versatile and highly efficient electrochemical devices: A review. Polymers 2021, 13, 1741. [Google Scholar] [CrossRef]
- Patricia, J.; Tjasa, K.; Matic, J.; Tadeja, K.; Bojan, P. Influence of the deep cryogenic treatment on AISI 52100 and AISI D3 steel’s corrosion resistance. Materials 2021, 14, 6357. [Google Scholar]
- Mavi, A.; Kaplan, Y.; AKsoz, S. Effects of aging and deep cryogenic treatment on wear behavior of Al7075 Alloy. J. Tribol. 2021, 143, 121702. [Google Scholar] [CrossRef]
- Singh, T.; Singla, A.; Singh, J.; Singh, K.; Gupta, M.; Ji, H.; Song, Q.; Liu, Z.; Pruncu, C. Abrasive wear behavior of cryogenically treated boron steel (30MnCrB4) used for rotavator blades. Materials 2020, 13, 436. [Google Scholar] [CrossRef] [Green Version]
- Funk, P.; Kanaan, A.; Shank, C.; Cooke, P.; Sevostianov, I.; Thomas, J.; Pate, M. Quantifying deep cryogenic treatment extent and its effect on steel properties. Int. J. Eng. Sci. 2021, 167, 103521. [Google Scholar] [CrossRef]
- Fan, S.; Hao, H.; Zhang, X. Effect of deep cryogenic treatment on the microstructure and wear resistance of a novel nanobainite steel. Steel Res. Int. 2021, 92, 2000554. [Google Scholar] [CrossRef]
- Ozden, R.; Anik, M. Enhancement of the mechanical properties of EN52CrMoV4 spring steel by deep cryogenic treatment. Mater. Werkst. 2020, 51, 422–431. [Google Scholar] [CrossRef]
- Padmakumar, M.; Dinakaran, D. A review on cryogenic treatment of tungsten carbide (WC-Co) tool material. Mater. Manuf. Process. 2021, 36, 637–659. [Google Scholar] [CrossRef]
- Cong, R.; Park, H.; Jo, M.; Lee, H.; Lee, C. Synthesis and electrochemical performance of electrostatic self-assembled nano-Silicon@N-doped reduced graphene oxide/carbon nanofibers composite as anode material for lithium-ion batteries. Molecules 2021, 26, 4831. [Google Scholar] [CrossRef] [PubMed]
- Kamedulski, P.; Lukaszewicz, J.; Witczak, L.; Szroeder, P.; Ziolkowski, P. The importance of structural factors for the electrochemical performance of graphene/carbon nanotube/melamine powders towards the catalytic activity of oxygen reduction reaction. Materials 2021, 14, 2448. [Google Scholar] [CrossRef]
- Xiong, Y.; You, M.; Liu, F.; Wu, M.; Cai, C.; Ding, L.; Zhou, C.; Hu, M.; Deng, W.; Wang, S. Pt-decorated, nanocarbon-intercalated, and N-doped graphene with enhanced activity and stability for oxygen reduction reaction. ACS Appl. Energy Mater. 2020, 3, 2490–2495. [Google Scholar] [CrossRef]
- Oscar, A.; Royer, V.; Agustin, B.; Ramses, A.; Marina, E. Sb2O3 nanoparticles anchored on N-doped graphene nanoribbons as improved anode for sodium-ion batteries. RSC Adv. 2021, 11, 31566–31571. [Google Scholar]
- Irshad, H.; Hakeem, A.; Raza, K.; Baroud, T.; Ehsan, M.; Ali, S.; Tahir, M. Design, development and evaluation of thermal properties of polysulphone-CNT/GNP nanocomposites. Nanomaterials 2021, 11, 2080. [Google Scholar] [CrossRef]
- Antonini, M.; Cov, P.; Delmonte, N.; Castellazzi, A. GaN transistors efficient cooling by graphene foam. Microelectron. Reliab. 2018, 88–90, 812–816. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, J.; Zhao, M.; Hu, T.; Jia, J.; Wu, H. Hydrogenated graphene as support of Pd nanoparticles with improved electrocatalytic activity for ethanol oxidation reaction in alkaline media. Electrochim. Acta 2019, 297, 856–863. [Google Scholar] [CrossRef]
- Ansari, A.; Ansari, S.; Parveen, N.; Ansari, M.; Osman, Z. Silver nanoparticles embedded on reduced graphene Oxide@Copper oxide nanocomposite for High Performance Supercapacitor Applications. Materials 2021, 14, 5032. [Google Scholar] [CrossRef]
- Sanad, M.; Shenouda, A. Impact of sulphur-containing compounds on the electrochemical capabilities of spinel carbon-coated Sb2SnS4 nano-sheets as alternative anodes in lithium ion batteries. J. Mater. Sci.-Mater. Electron. 2021, 32, 20489–20498. [Google Scholar] [CrossRef]
- Ozkan, S.; Karpacheva, G.; Efimov, M.; Vasilev, A.; Muratov, D.; Petrov, V.; Chernavskii, P.; Pankina, G. One-step synthesis, characterization and properties of novel hybrid electromagnetic nanomaterials based on polydiphenylamine and Co–Fe particles in the absence and presence of single-walled carbon nanotubes. RSC Adv. 2021, 11, 24772–24786. [Google Scholar] [CrossRef]
- Jacob, A.; Wahab, R.; Misson, M. Operational stability, regenerability, and thermodynamics studies on biogenic silica/magnetite/graphene oxide nanocomposite-activated candida rugosa lipase. Polymers 2021, 13, 3854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, F.; Zhang, C.; Wang, J.; Jia, Z.; Hui, D.; Qiu, Y. Tensile and interfacial properties of polyacrylonitrile-based carbon fiber after different cryogenic treated condition. Compos. Part B-Eng. 2021, 99, 358–365. [Google Scholar] [CrossRef]
- Blinn, B.; Winter, S.; Weber, M.; Demmler, M.; Krausel, V.; Beck, T. Analyzing the influence of a deep cryogenic treatment on the mechanical properties of blanking tools by using the short-time method PhyBaLCHT. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2021, 824, 141846. [Google Scholar] [CrossRef]
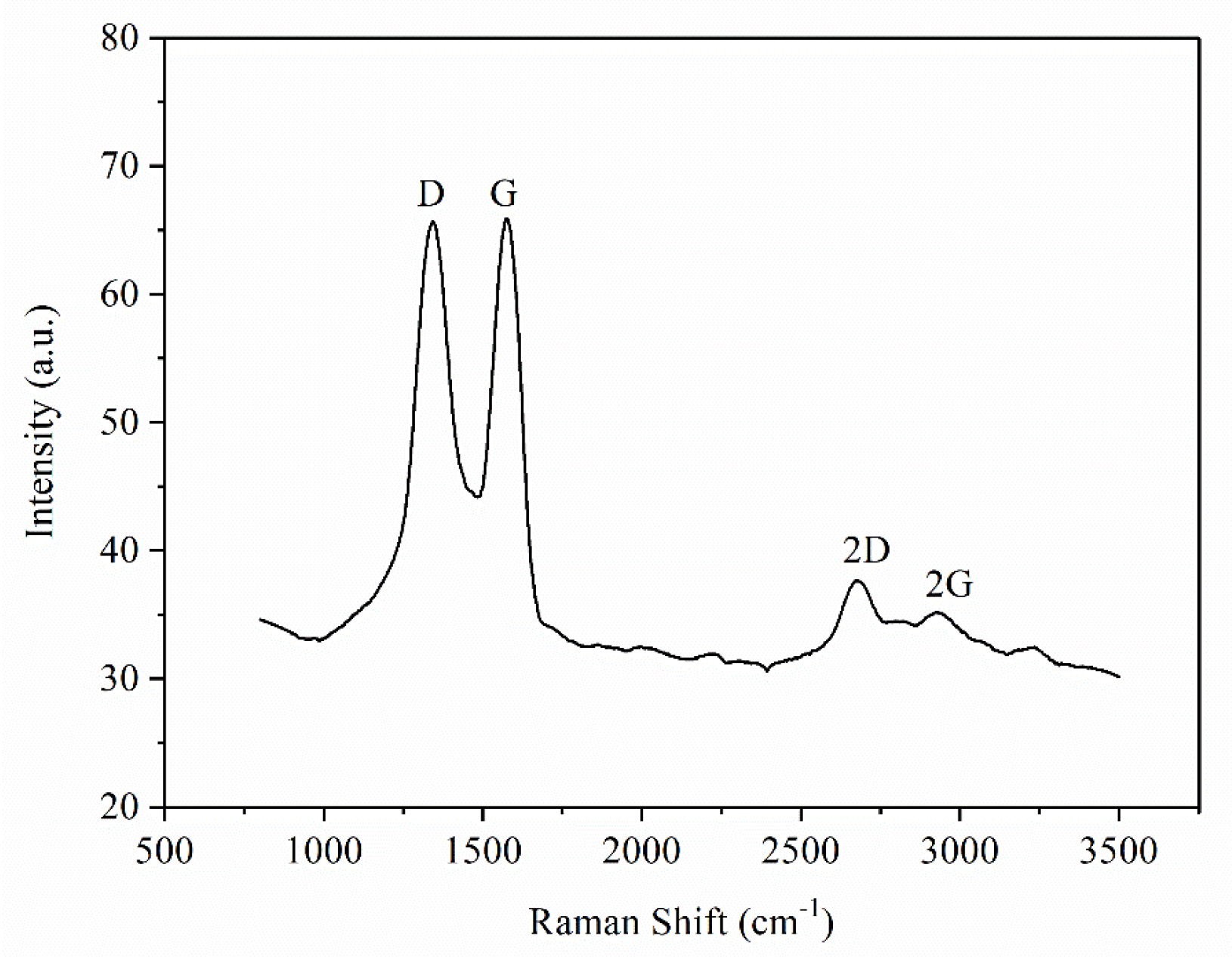
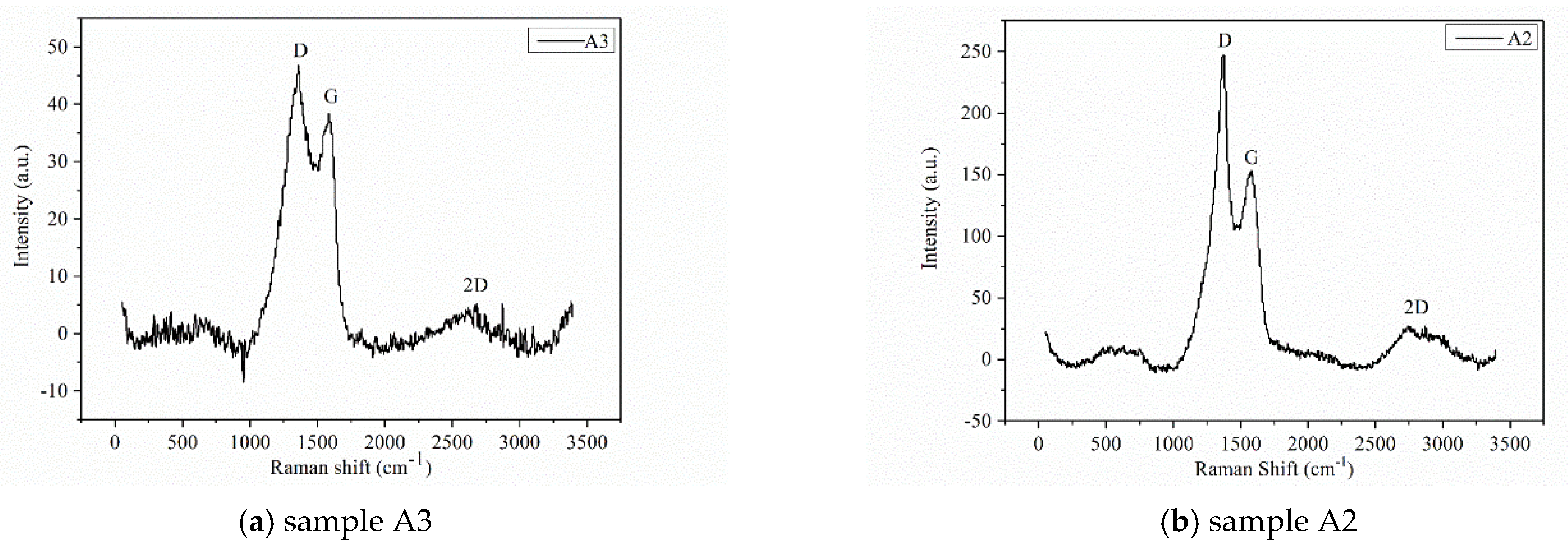
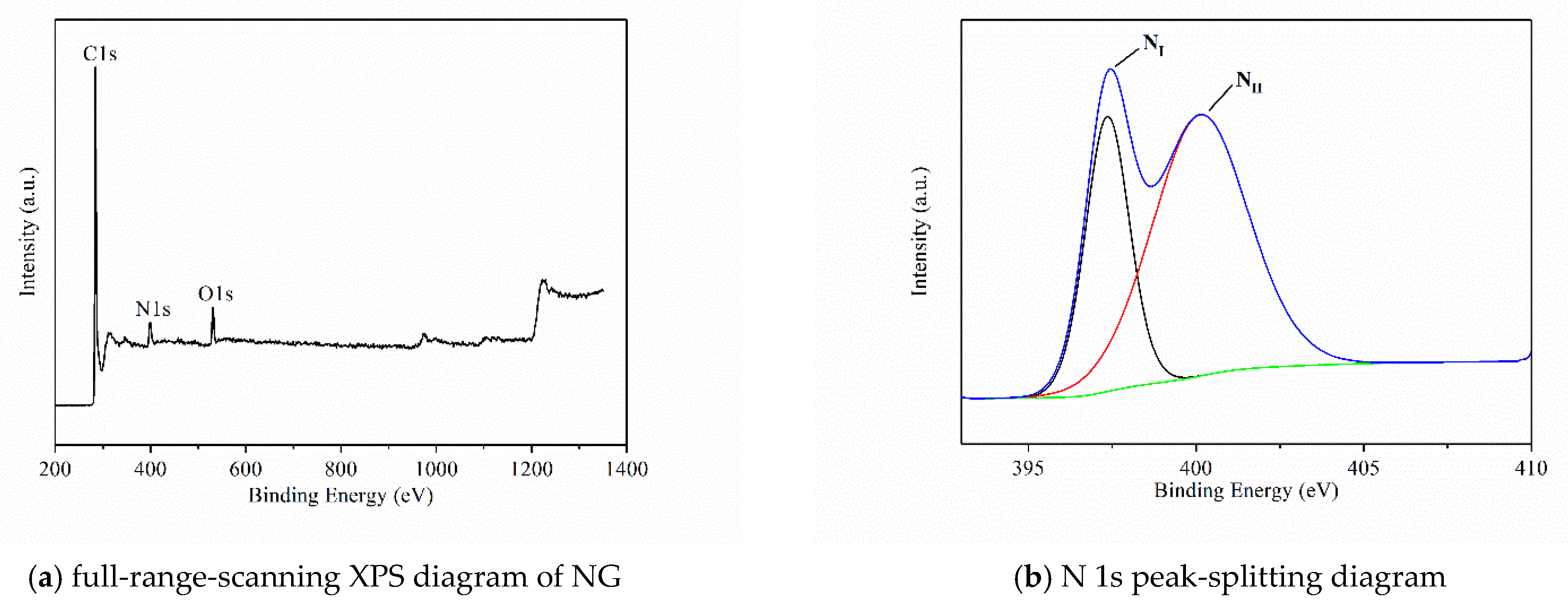
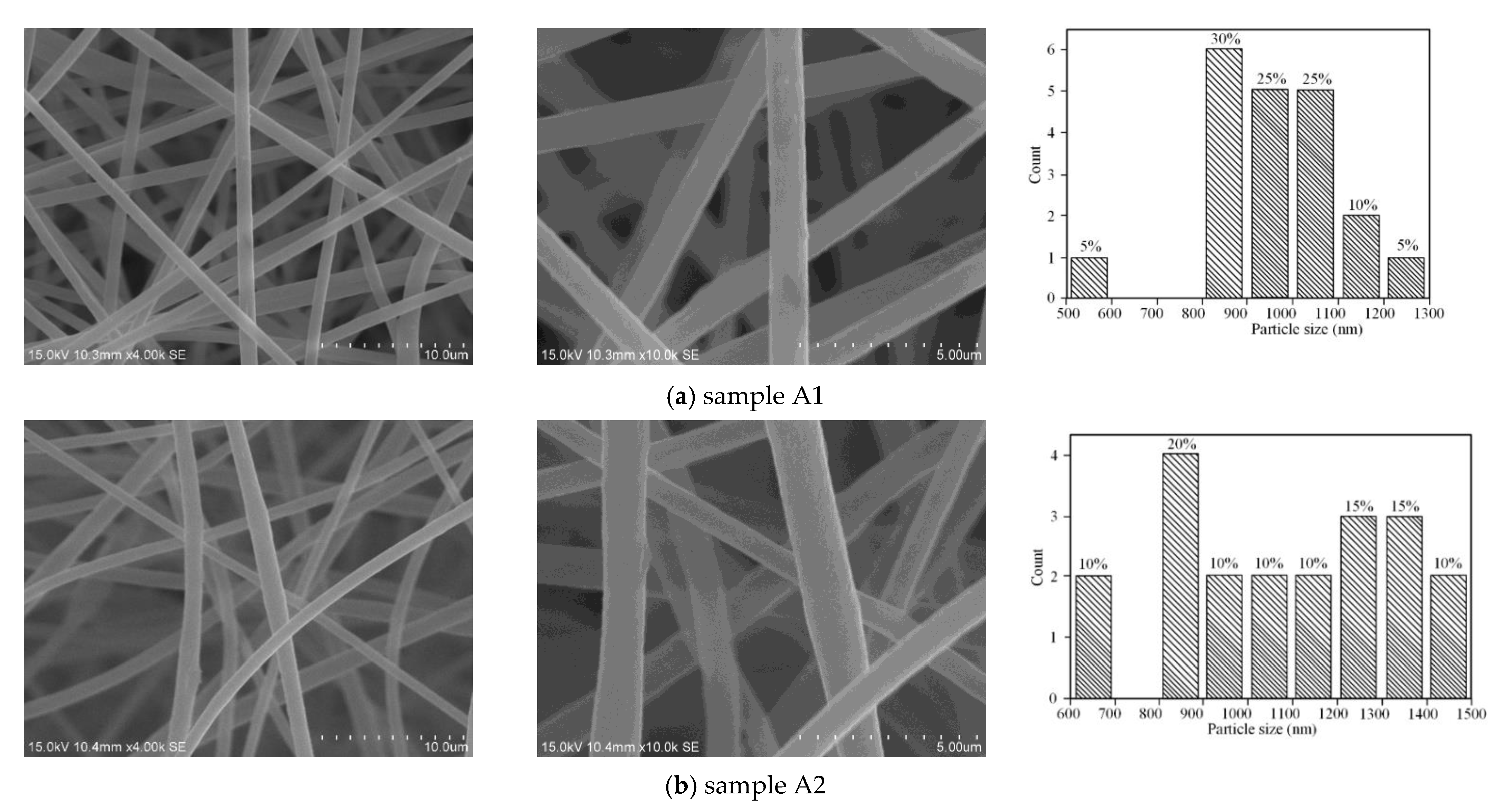
| Sample | C 1s | O 1s | N 1s | |||
|---|---|---|---|---|---|---|
| EB/eV | Atomic Fraction/% | EB/eV | Atomic Fraction/% | EB/eV | Atomic Fraction/% | |
| NG | 284.28 | 88.30 | 531.21 | 5.18 | 399.18 | 6.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, S.; Zhu, Y.; Wang, X.; Zhu, Y.; Wang, K.; Ni, H.; Gu, R. Research on the Morphology Reconstruction of Deep Cryogenic Treatment on PtRu/nitrogen-Doped Graphene Composite Carbon Nanofibers. Materials 2022, 15, 908. https://doi.org/10.3390/ma15030908
Lv S, Zhu Y, Wang X, Zhu Y, Wang K, Ni H, Gu R. Research on the Morphology Reconstruction of Deep Cryogenic Treatment on PtRu/nitrogen-Doped Graphene Composite Carbon Nanofibers. Materials. 2022; 15(3):908. https://doi.org/10.3390/ma15030908
Chicago/Turabian StyleLv, Shuaishuai, Yangyang Zhu, Xingxing Wang, Yu Zhu, Kaixuan Wang, Hongjun Ni, and Ruobo Gu. 2022. "Research on the Morphology Reconstruction of Deep Cryogenic Treatment on PtRu/nitrogen-Doped Graphene Composite Carbon Nanofibers" Materials 15, no. 3: 908. https://doi.org/10.3390/ma15030908
APA StyleLv, S., Zhu, Y., Wang, X., Zhu, Y., Wang, K., Ni, H., & Gu, R. (2022). Research on the Morphology Reconstruction of Deep Cryogenic Treatment on PtRu/nitrogen-Doped Graphene Composite Carbon Nanofibers. Materials, 15(3), 908. https://doi.org/10.3390/ma15030908






