Evaluation of Disposal Stability for Cement Solidification of Lime Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods and Evaluation
2.2.1. Maximum Content of Lime Waste
2.2.2. Mixing Ratio of Cement Waste Form
2.2.3. Workability Test
2.2.4. Preparation and Curing of Cement Waste Form
2.2.5. Cement Waste Form Property Tests
3. Results
3.1. Characteristics of Lime Waste
3.2. Operating Range and Optimum Conditions
3.3. Evaluation of Disposal Feasibility
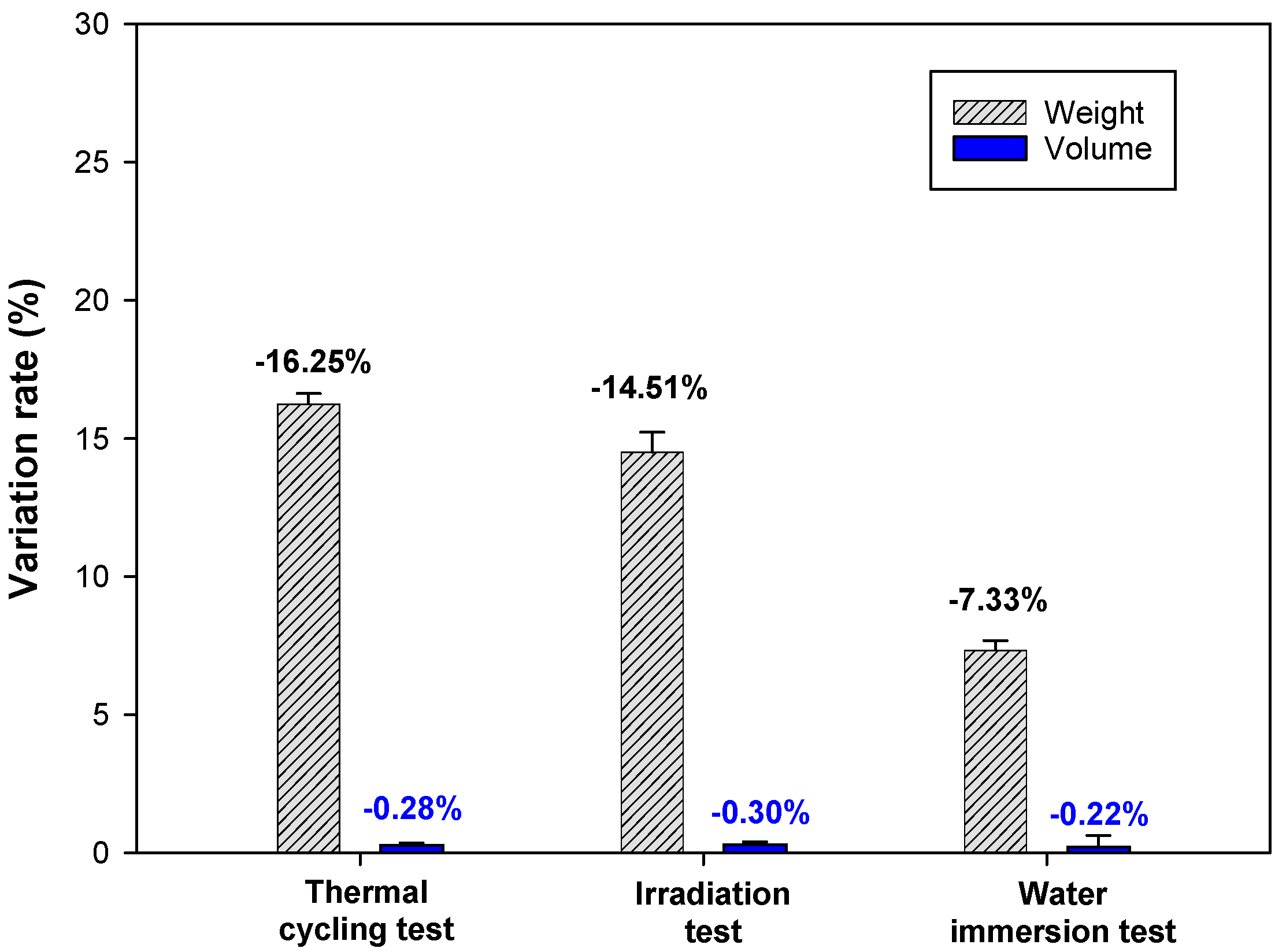
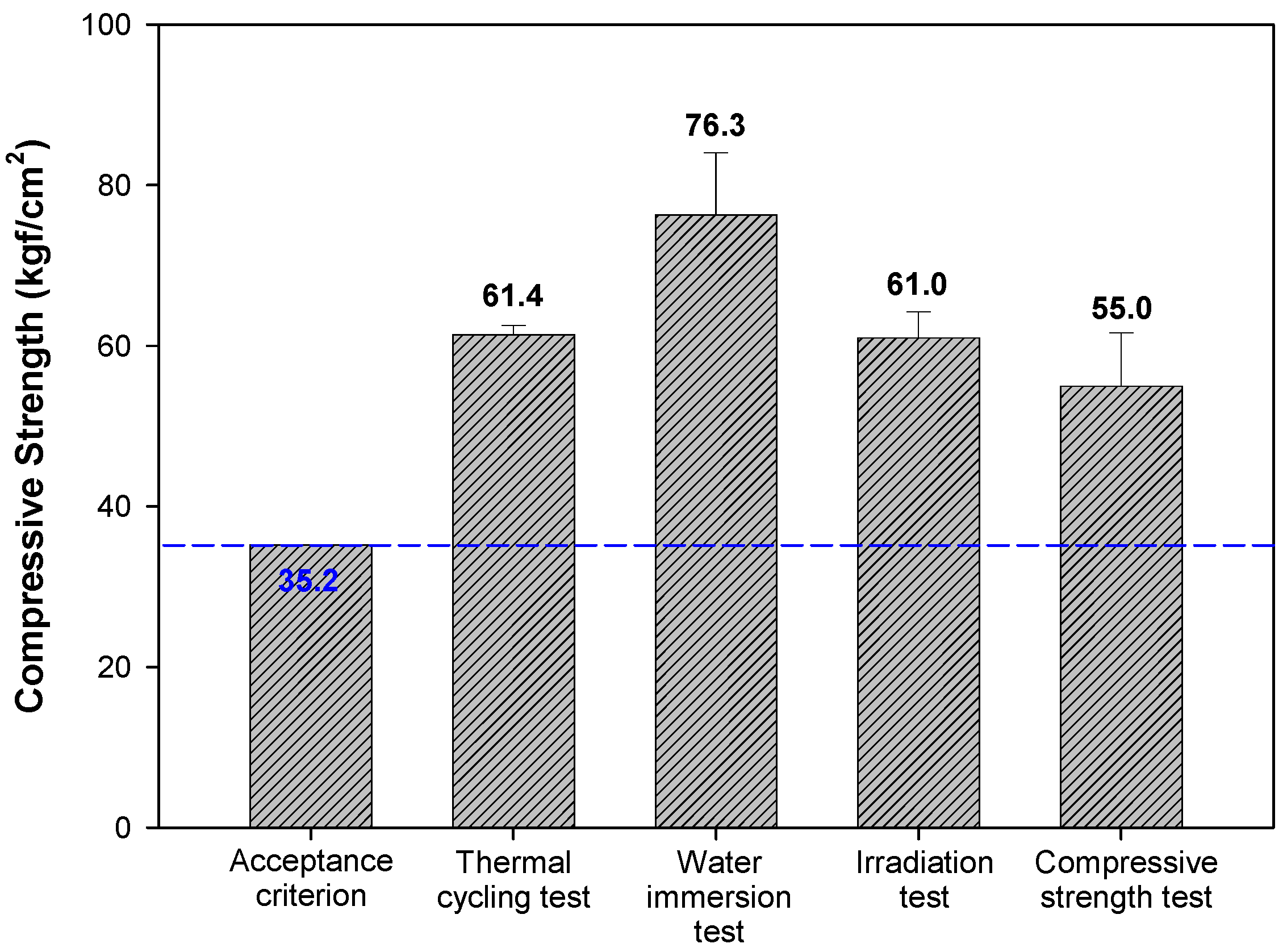
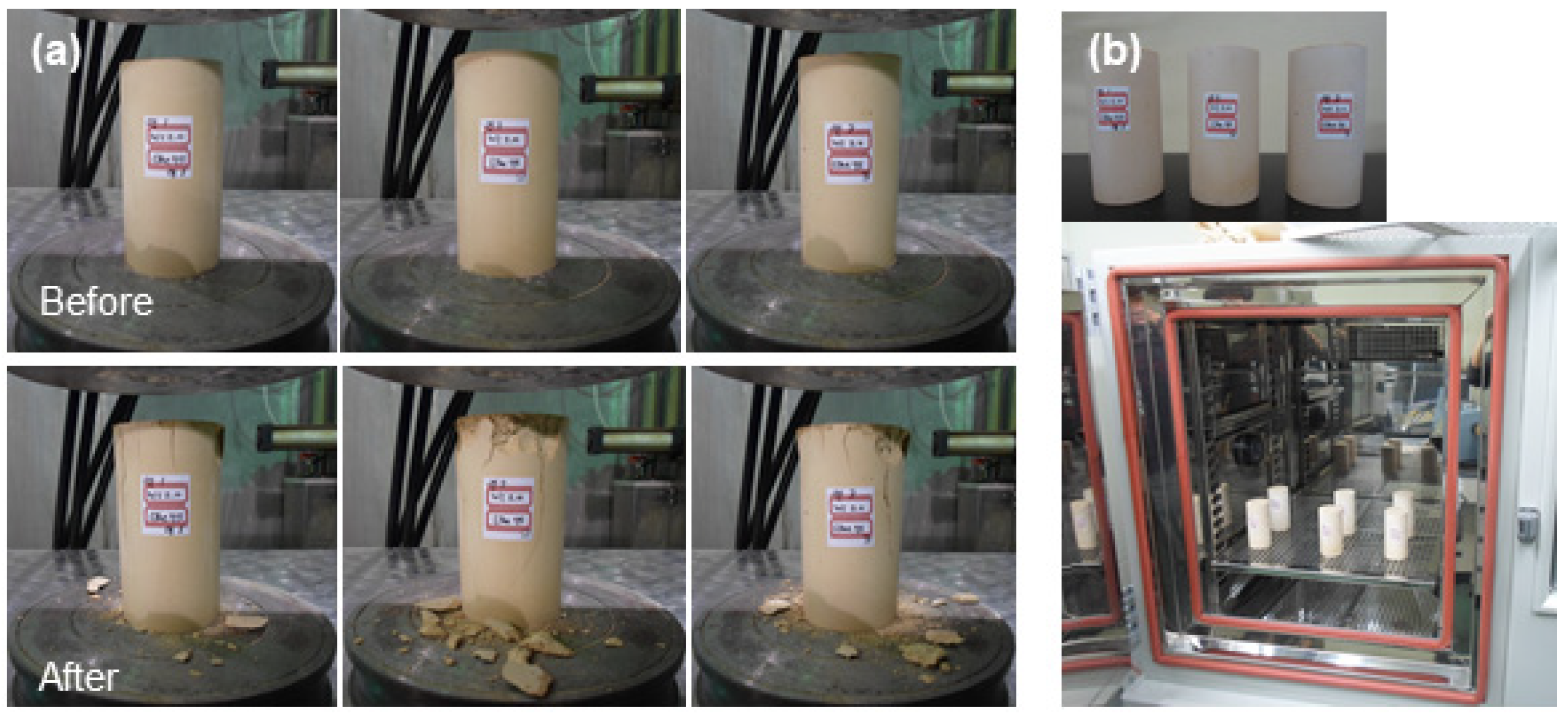
3.3.1. Structural Stability of Cement Waste Form
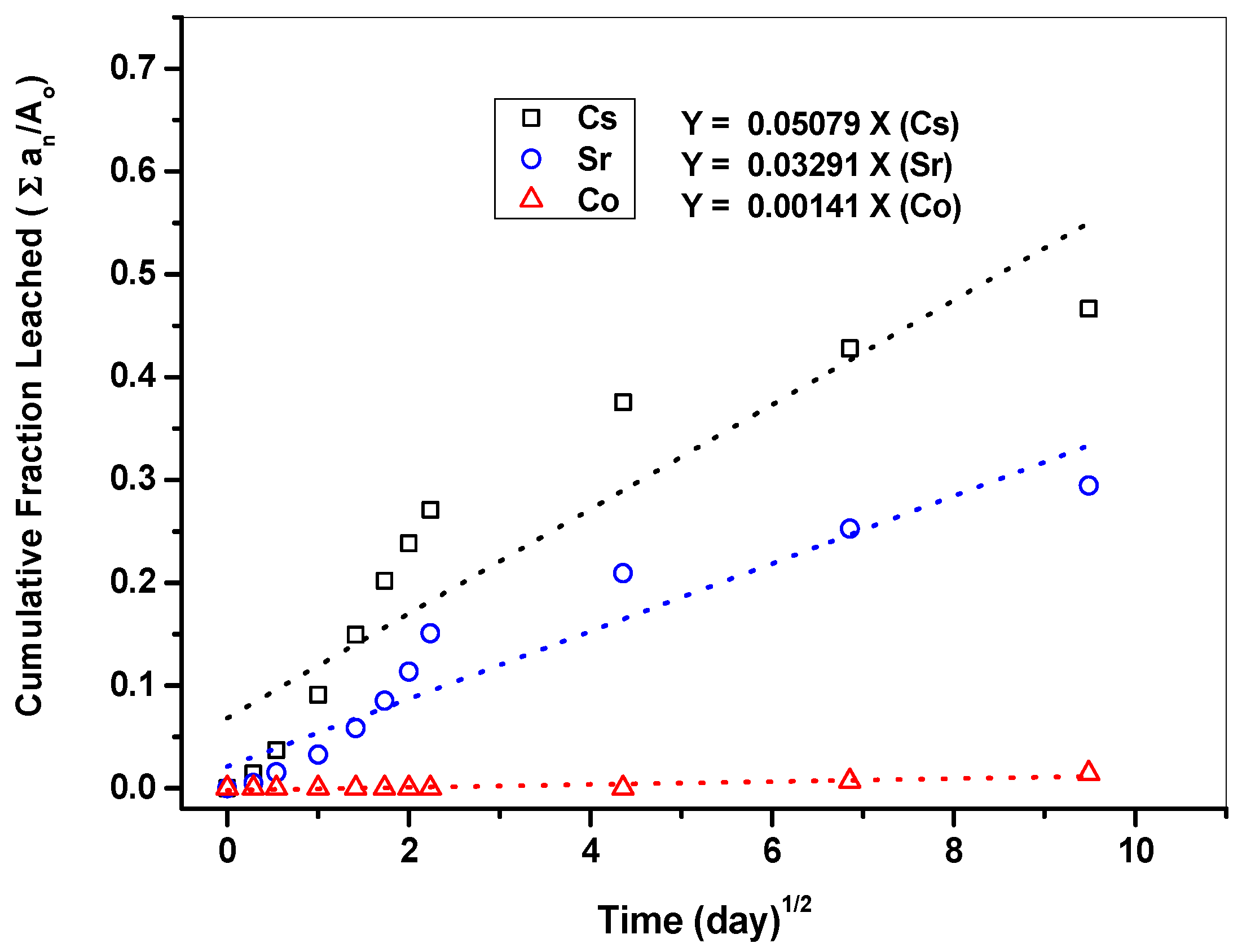
3.3.2. Leaching Stability of Cement Waste Form
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeong, K.C.; Kim, T.J.; Choi, J.H.; Park, J.H.; Hwang, S.T. A study on the waste treatment from a nuclear fuel powder conversion plant. J. Korean Ind. Eng. Chem. 1996, 7, 1164–1173. [Google Scholar]
- Hwang, D.S.; Park, J.H.; Lee, K.I.; Hwang, S.T.; Jeon, K.J. Radiological properties of uranium sludges generated from the uranium conversion plant. In Proceedings of the KNS-KARP Joint Spring Meeting, Gwangju, Korea, 23–24 May 2002. [Google Scholar]
- Korea Radioactive Wastes Agency. Safety Analysis Report of Low- and Intermediate-Level Radioactive Waste Disposal Facility; Korea Radioactive Wastes Agency: Gyeongju, Korea, 2008. [Google Scholar]
- Qian, Z.; Liu, X.; Qiao, Y.; Wang, S.; Qin, Q.; Shi, L.; Peng, H. Effect of fluorine on stabilization/solidification of radioactive fluoride liquid waste in magnesium potassium phosphate cement. J. Radioanal. Nucl. Chem. 2019, 319, 393–399. [Google Scholar] [CrossRef]
- Koo, D.-S.; Sung, H.-H.; Kim, S.-S.; Kim, G.-N.; Choi, J.-W. Characteristics of cement solidification of metal hydroxide waste. Nucl. Eng. Technol. 2017, 49, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Bai, B.; Zhou, R.; Cai, G.; Hu, W.; Yang, G. Coupled thermo-hydro-mechanical mechanism in view of the soil particle rearrangement of granular thermodynamics. Comput. Geotech. 2021, 137, 104272. [Google Scholar] [CrossRef]
- Bai, B.; Yang, G.-C.; Li, T.; Yang, G.-S. A thermodynamic constitutive model with temperature effect based on particle rearrangement for geomaterials. Mech. Mater. 2019, 139, 103180. [Google Scholar] [CrossRef]
- Rogue, R.; Lerch, W. Hydration of Portland cement compounds. Ind. Eng. Chem. 1934, 26, 837–847. [Google Scholar] [CrossRef]
- Glasser, F.P. Progress in the immobilization of radioactive wastes in cement. Cem. Concr. Res. 1992, 22, 201–216. [Google Scholar] [CrossRef]
- Bediako, M.; Amankwah, E.O. Analysis of chemical composition of Portland cement in Ghana: A key to understand the behavior of cement. Adv. Mater. Sci. Eng. 2015, 2015, 349401. [Google Scholar] [CrossRef] [Green Version]
- Soroka, I. Portland Cement Paste and Concrete; Macmillan International Higher Education: London, UK, 1979. [Google Scholar]
- Osborne, G. BRECEM: A rapid hardening cement based on high alumina cement. Proc. Inst. Civ. Eng.-Struct. Build. 1994, 104, 93–100. [Google Scholar] [CrossRef]
- Goberis, S.; Pundiene, I. Some Aspects of Influence of Microsilica and Admixtures on Hydration Kinetics of the Alumina Cement “Gorkal-40”. Mater. Sci. 2004, 10, 50–54. [Google Scholar]
- Helmy, I.; El-Didamony, H.; Ali, A.; El-Sokkary, T. Influence of silica fume on the properties of sulphate resisting cement and alumina cement mixture. Ceram. Silik. 2002, 46, 56–62. [Google Scholar]
- Li, J.; Li, G.; Yu, Y. The influence of compound additive on magnesium oxychloride cement/urban refuse floor tile. Constr. Build. Mater. 2008, 22, 521–525. [Google Scholar] [CrossRef]
- KS L 5109; Testing Method for Mechanical Mixing of Hydraulic Cement Pastes and Mortars of Plastic Consistency. Korean Agency for Technology and Standard: Eumseong-gun, Korea, 2012.
- Shon, J.-S.; Kim, G.-Y.; Im, J. Evaluation of efficient cementation of fission 99Mo production process waste and feasibility disposal of cement waste form. Ann. Nucl. Energy 2019, 124, 342–348. [Google Scholar] [CrossRef]
- Characteristics of Lagoon Sludge; TR-2138/2002; Korea Atomic Energy Research Institute: Daejeon, Korea, 2002; p. 34.
- Chen, M.; Shen, Y.; Zhu, S.; Li, P. Digital phase diagram and thermophysical properties of KNO3-NaNO3-Ca(NO3)2 ternary system for solar energy storage. Vacuum 2017, 145, 225–233. [Google Scholar] [CrossRef]
- Roy, A.; Bhattacharya, J. Microwave assisted synthesis of CaO nanoparticles and use in waste water treatment. Nano Technol. 2011, 3, 565–568. [Google Scholar]
- Wang, X.; Shi, L.; Zhang, J.; Cheng, J.; Wang, X. In situ formation of surface-functionalized ionic calcium carbonate nanoparticles with liquid-like behaviours and their electrical properties. R. Soc. Open Sci. 2018, 5, 170732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, K.Q.; Satomi, T.; Takahashi, H. Improvement of mechanical behavior of cemented soil reinforced with waste cornsilk fibers. Constr. Build. Mater. 2018, 178, 204–210. [Google Scholar] [CrossRef]
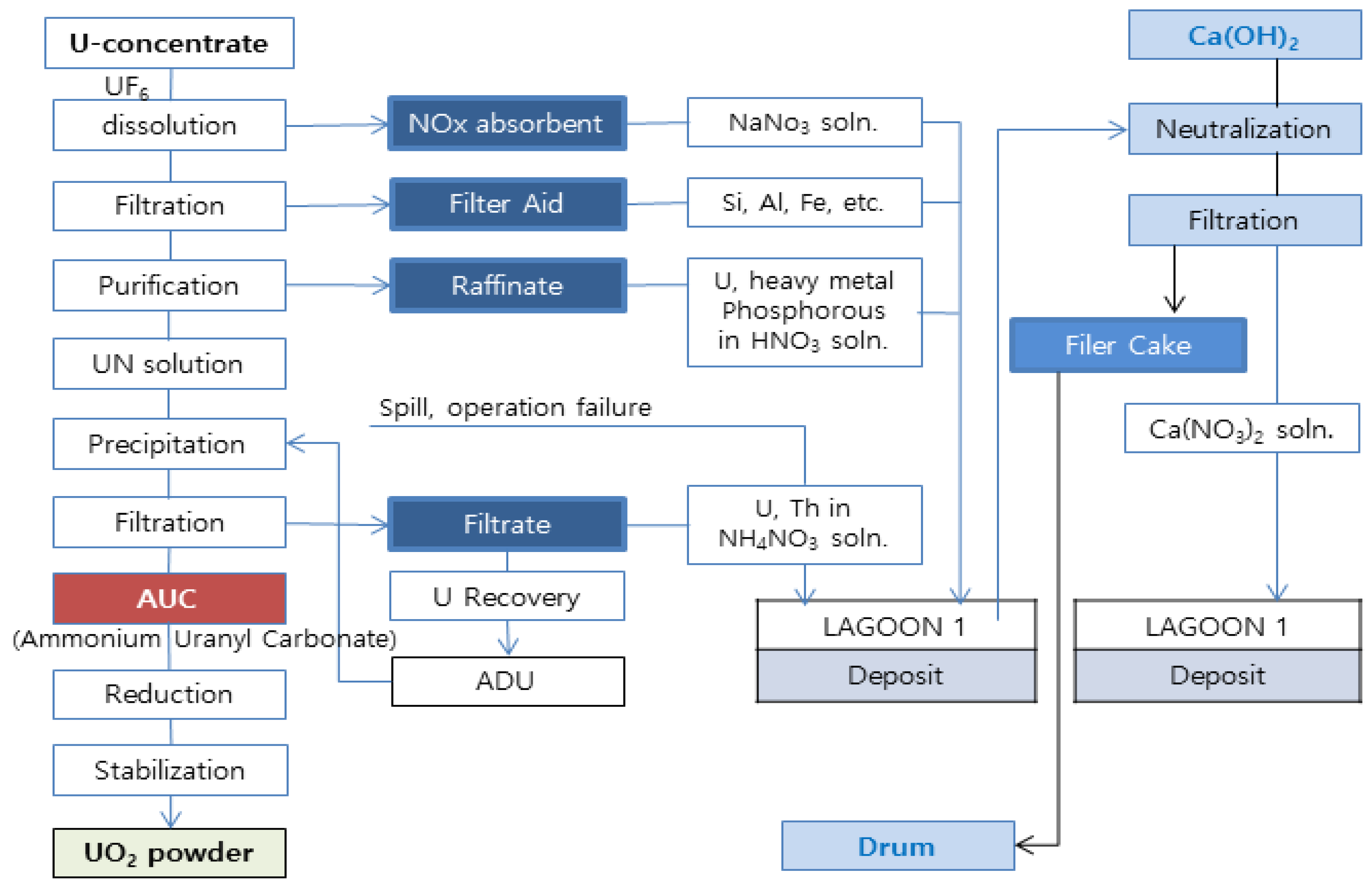

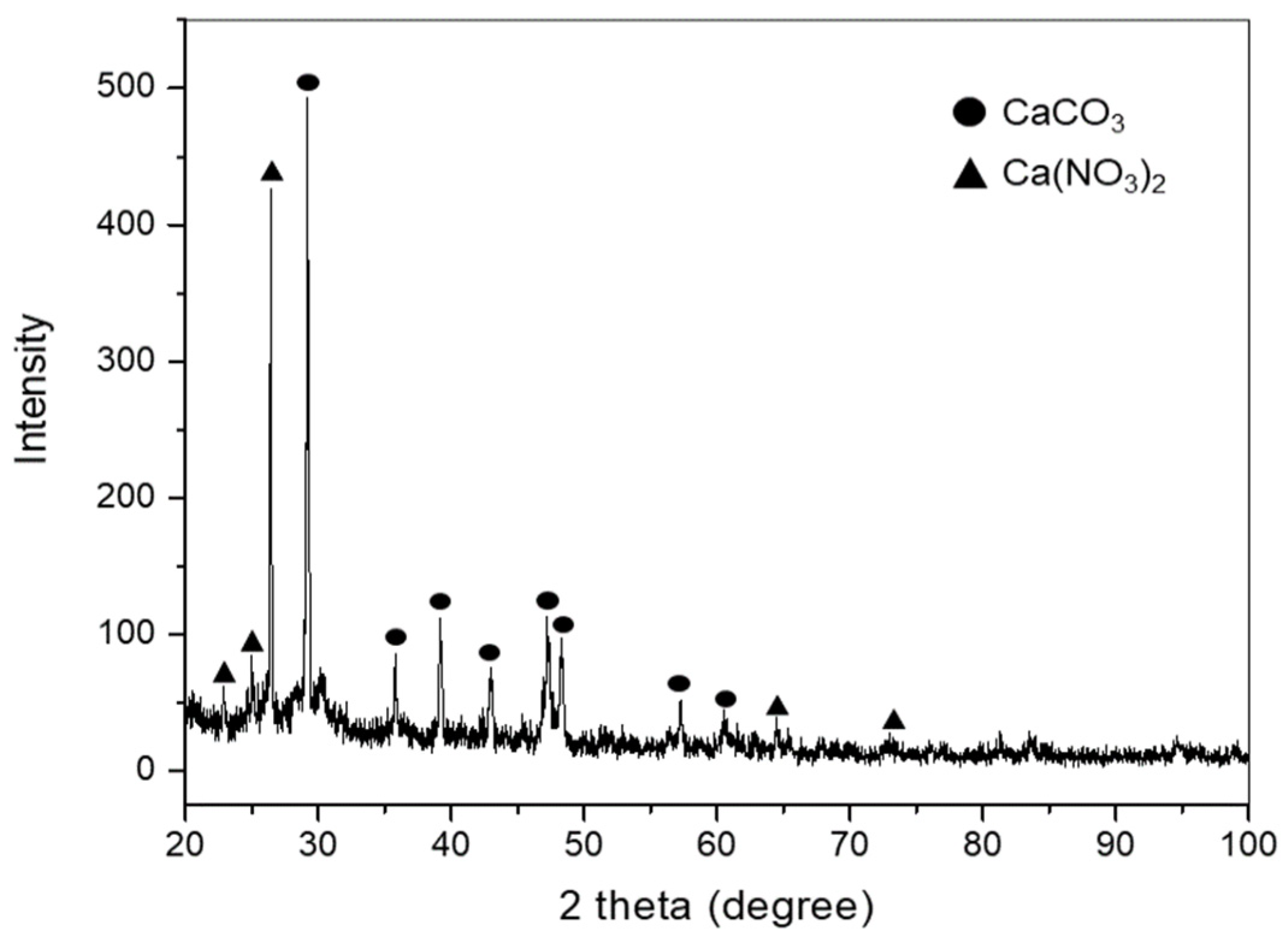

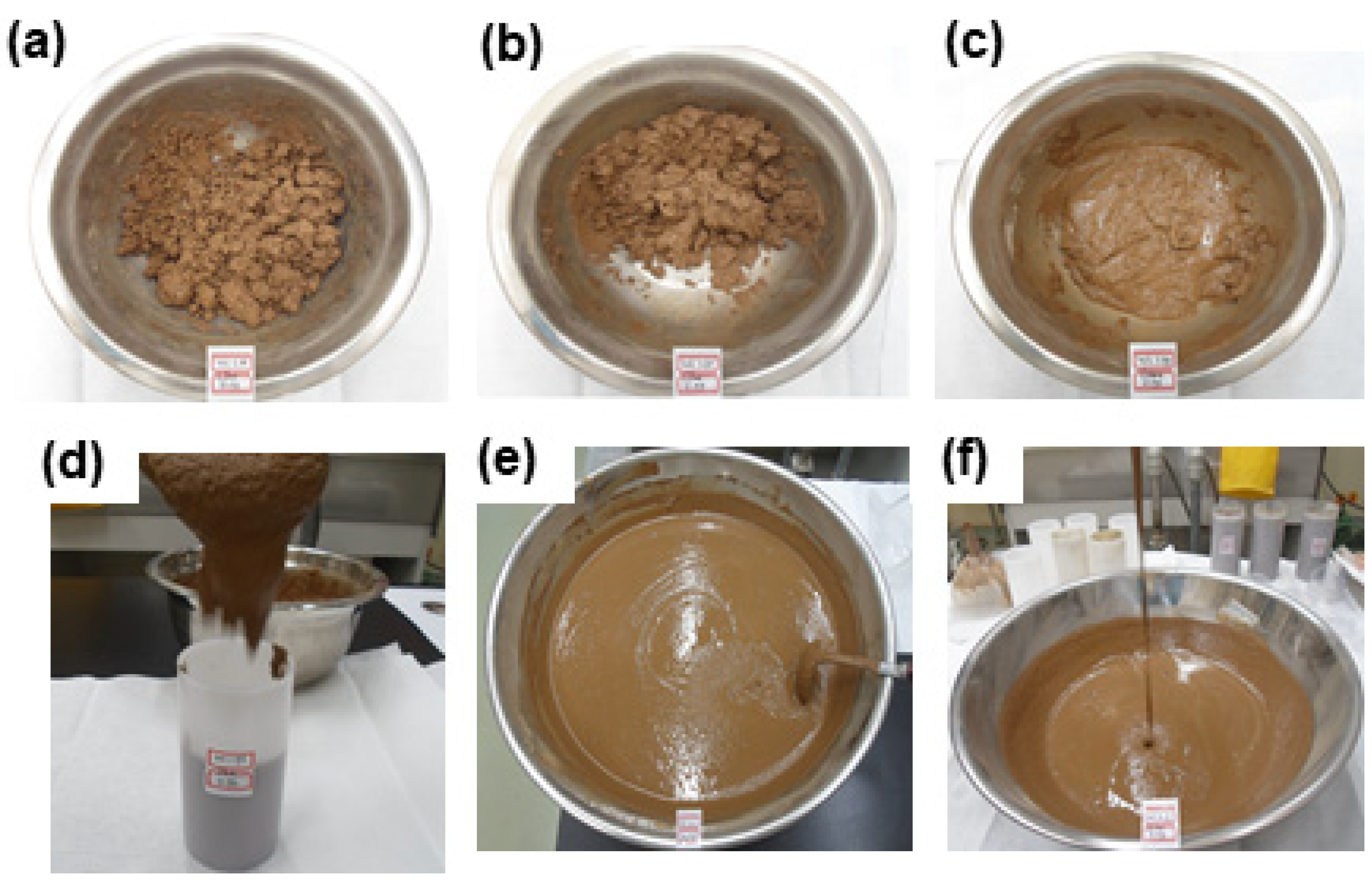

| Storage Volume [200 L drum] | Radioactivity [Bq/g] | Components | pH |
|---|---|---|---|
| 435 | U-235: 55.4 Cs-137 < 0.200 | CaCO3 Ca(NO3)2 | 8.2 |
| Item | Test | Standard Method | Test Method | Criteria |
|---|---|---|---|---|
| Structural stability | Compressive strength test | KS F2405 | - | ≥35.2 kgf/cm2 (3.44 Mpa) |
| Water immersion test (90 days) | NRC 1 | Compressive strength after immersion test | ≥35.2 kgf/cm2 | |
| Thermal cycling test (28 days) | ASTM B553 | Compressive strength after thermal cycling test | ≥35.2 kgf/cm2 | |
| Irradiation test | NRC 1 | Compressive strength after irradiation (1.0 × 107 Gy) | ≥35.2 kgf/cm2 | |
| Leachability | Leaching test (90 days) | ANS 16.1 | Cs, Sr, Co | Leachability index ≥ 6 |
| Free standing water test | Sample | EPA 2 | - | >0.5 vol.% |
| 200 L drum | ANS 55.1 | - | >0.5 vol.% |
| Ratio | Workability | No. Sample | Free Standing Water | Water Immersion Test | ||
|---|---|---|---|---|---|---|
| w/c | Waste (wt.%) | Measurement (mL) | Criteria (≤0.5 vol/%) | No Cracks/ Collapses | ||
| 1.55 | 75 | No workability | ||||
| 1.65 | 75 | No workability | ||||
| 1.75 | 75 | No workability | ||||
| 1.85 | 75 | Bad | 1 | 0.0 | Less than 0.5 vol.% | o |
| 2 | 0.0 | o | ||||
| 3 | 0.0 | o | ||||
| 1.95 | 75 | Good | 1 | 0.0 | o | |
| 2 | 0.0 | o | ||||
| 3 | 0.0 | o | ||||
| 2.00 | 75 | Very good | 1 | 0.0 | o | |
| 2 | 0.0 | o | ||||
| 3 | 0.0 | o | ||||
| 2.20 | 75 | Very good | 1 | 0.8 | o | |
| 2 | 0.0 | o | ||||
| 3 | 0.0 | o | ||||
| 2.40 | 75 | Very good | 1 | 2.6 | More than 0.5 vol.% | o |
| 2 | 3.1 | o | ||||
| 3 | 2.5 | o | ||||
| Cement | Ratio (Weight) | Cement Waste Form | ||
|---|---|---|---|---|
| Portland cement type I | w/c | Lime/ (lime + cement) | Cement/ (lime + cement) | Density [g/cm3] |
| 2.00 | 0.75 | 0.25 | 1.445 | |
| No. | Σ(Δt)n (Day) | Σ(Δt)n (Day)1/2 | Cumulative Leached Fraction [∑an/Ao] | Co Concentration, an (mg/L) | ||
|---|---|---|---|---|---|---|
| Cs | Sr | Co | ||||
| 1 | 0.1 | 0.29 | 0.01 | 0.01 | 0.000 | <0.05 |
| 2 | 0.3 | 0.54 | 0.04 | 0.02 | 0.000 | <0.05 |
| 3 | 1 | 1.00 | 0.09 | 0.03 | 0.000 | <0.05 |
| 4 | 2 | 1.41 | 0.15 | 0.06 | 0.000 | <0.05 |
| 5 | 3 | 1.73 | 0.20 | 0.09 | 0.000 | <0.05 |
| 6 | 4 | 2.00 | 0.24 | 0.11 | 0.000 | <0.05 |
| 7 | 5 | 2.24 | 0.27 | 0.15 | 0.000 | <0.05 |
| 8 | 19 | 4.36 | 0.38 | 0.21 | 0.000 | <0.05 |
| 9 | 47 | 6.86 | 0.43 | 0.25 | 0.007 | 1.53 |
| 10 | 90 | 9.49 | 0.47 | 0.30 | 0.015 | 1.60 |
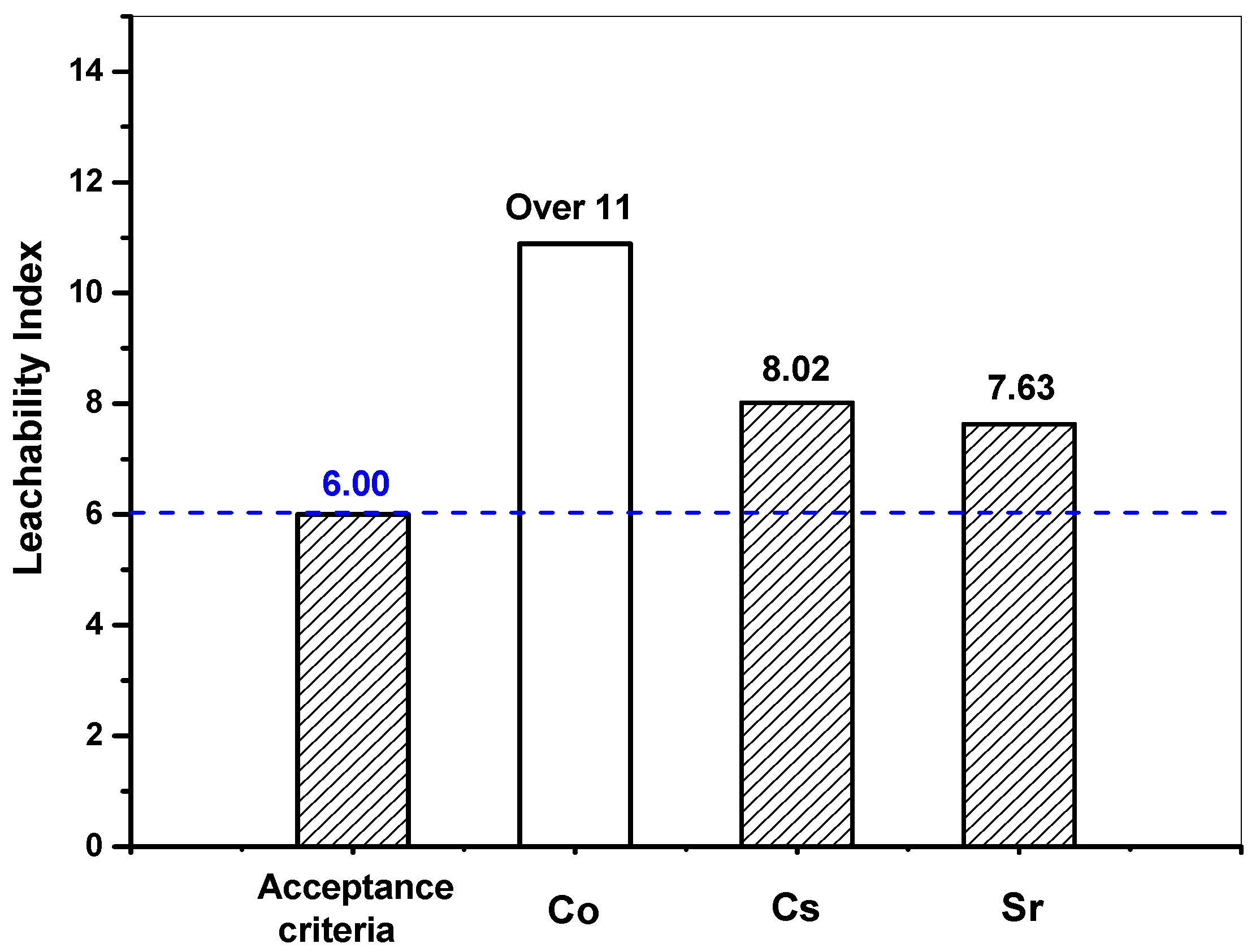
| Nuclides | Sample No. | V/S (cm) | Slope | De (cm2/day) | De (cm2/sec) | LX |
|---|---|---|---|---|---|---|
| Co | S1 | 0.999 | 4.87 × 10−4 | 1.86 × 10−7 | 2.15 × 10−12 | 11.67 |
| S2 | 1.000 | 1.89 × 10−3 | 2.80 × 10−6 | 3.25 × 10−11 | 10.49 | |
| S3 | 1.000 | 1.86 × 10−3 | 2.72 × 10−6 | 3.14 × 10−11 | 10.50 | |
| Average | 1.000 | 1.41 × 10−3 | 1.90 × 10−6 | 2.20 × 10−11 | 10.89 | |
| Sr | S1 | 0.999 | 3.14 × 10−2 | 7.74 × 10−4 | 8.96 × 10−9 | 8.05 |
| S2 | 1.000 | 3.43 × 10−2 | 9.24 × 10−4 | 1.07 × 10−8 | 7.97 | |
| S3 | 1.000 | 3.22 × 10−2 | 8.12 × 10−4 | 9.40 × 10−9 | 8.03 | |
| Average | 1.000 | 3.26 × 10−2 | 8.37 × 10−4 | 9.68 × 10−9 | 8.02 | |
| Cs | S1 | 0.999 | 5.05 × 10−2 | 2.00 × 10−3 | 2.31 × 10−8 | 7.64 |
| S2 | 1.000 | 5.13 × 10−2 | 2.06 × 10−3 | 2.39 × 10−8 | 7.62 | |
| S3 | 1.000 | 5.06 × 10−2 | 2.01 × 10−3 | 2.33 × 10−8 | 7.63 | |
| Average | 1.000 | 5.08 × 10−2 | 2.02 × 10−3 | 2.34 × 10−8 | 7.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shon, J.-S.; Lee, H.-K.; Kim, G.-Y.; Kim, T.-J.; Ahn, B.-G. Evaluation of Disposal Stability for Cement Solidification of Lime Waste. Materials 2022, 15, 872. https://doi.org/10.3390/ma15030872
Shon J-S, Lee H-K, Kim G-Y, Kim T-J, Ahn B-G. Evaluation of Disposal Stability for Cement Solidification of Lime Waste. Materials. 2022; 15(3):872. https://doi.org/10.3390/ma15030872
Chicago/Turabian StyleShon, Jong-Sik, Hyun-Kyu Lee, Gi-Yong Kim, Tack-Jin Kim, and Byung-Gil Ahn. 2022. "Evaluation of Disposal Stability for Cement Solidification of Lime Waste" Materials 15, no. 3: 872. https://doi.org/10.3390/ma15030872
APA StyleShon, J.-S., Lee, H.-K., Kim, G.-Y., Kim, T.-J., & Ahn, B.-G. (2022). Evaluation of Disposal Stability for Cement Solidification of Lime Waste. Materials, 15(3), 872. https://doi.org/10.3390/ma15030872






