Application of Activated Carbon Banana Peel Coated with Al2O3-Chitosan for the Adsorptive Removal of Lead and Cadmium from Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagent
2.2. Instrumentation
2.3. Synthesis of Banana Peel Activated Carbon (BPAC)
2.4. Activation of the Banana Peel Powder
2.5. Preparation of BPAC@Al2O3@chitosan Nanocomposite
2.6. Adsorption Experiments
2.7. Isotherm Studies
2.8. Adsorption Kinetics
2.9. Application in Real Wastewater
2.10. Reusability of BPAC@Al2O3@chitosan Composite
3. Results
3.1. Preliminary Studies
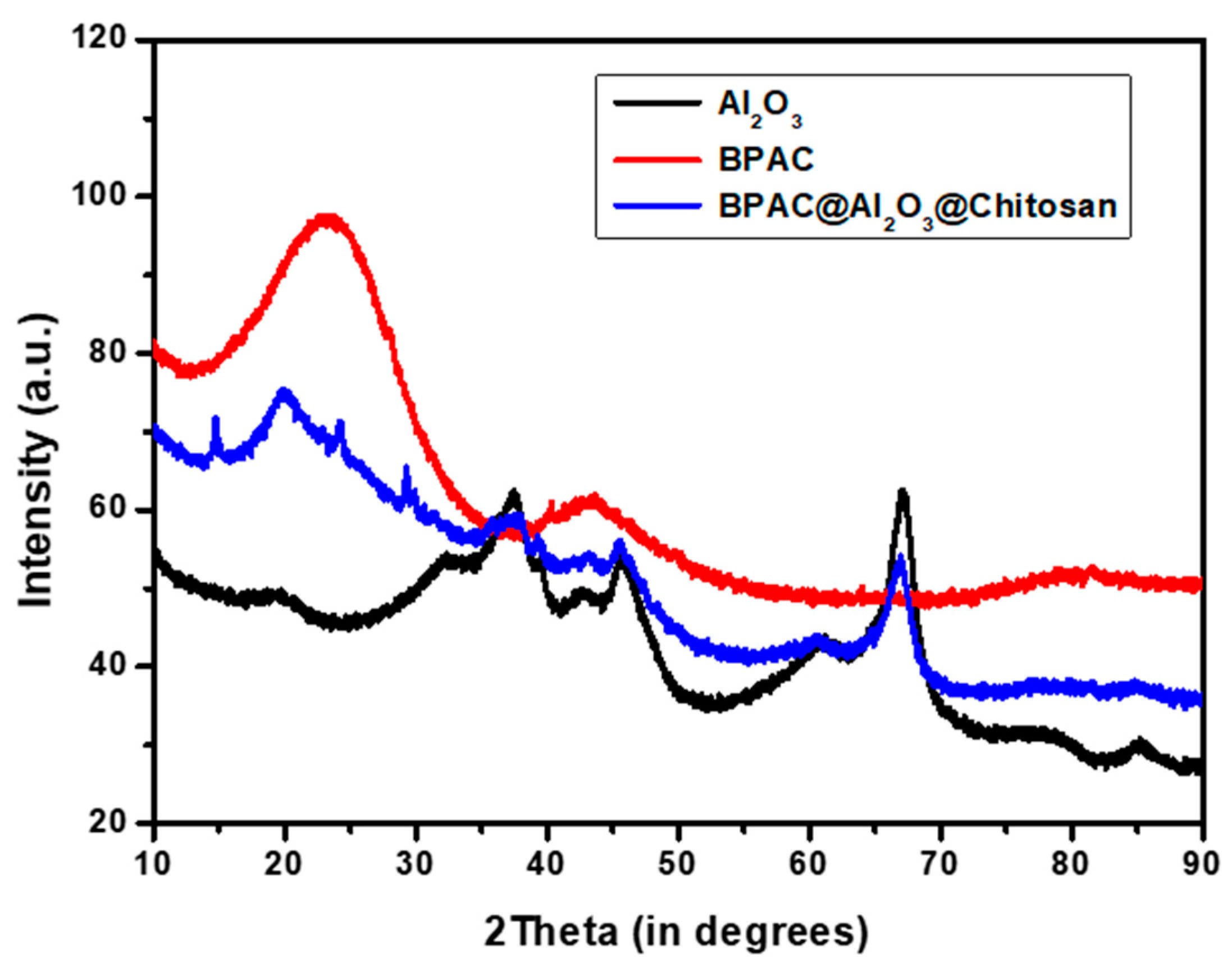
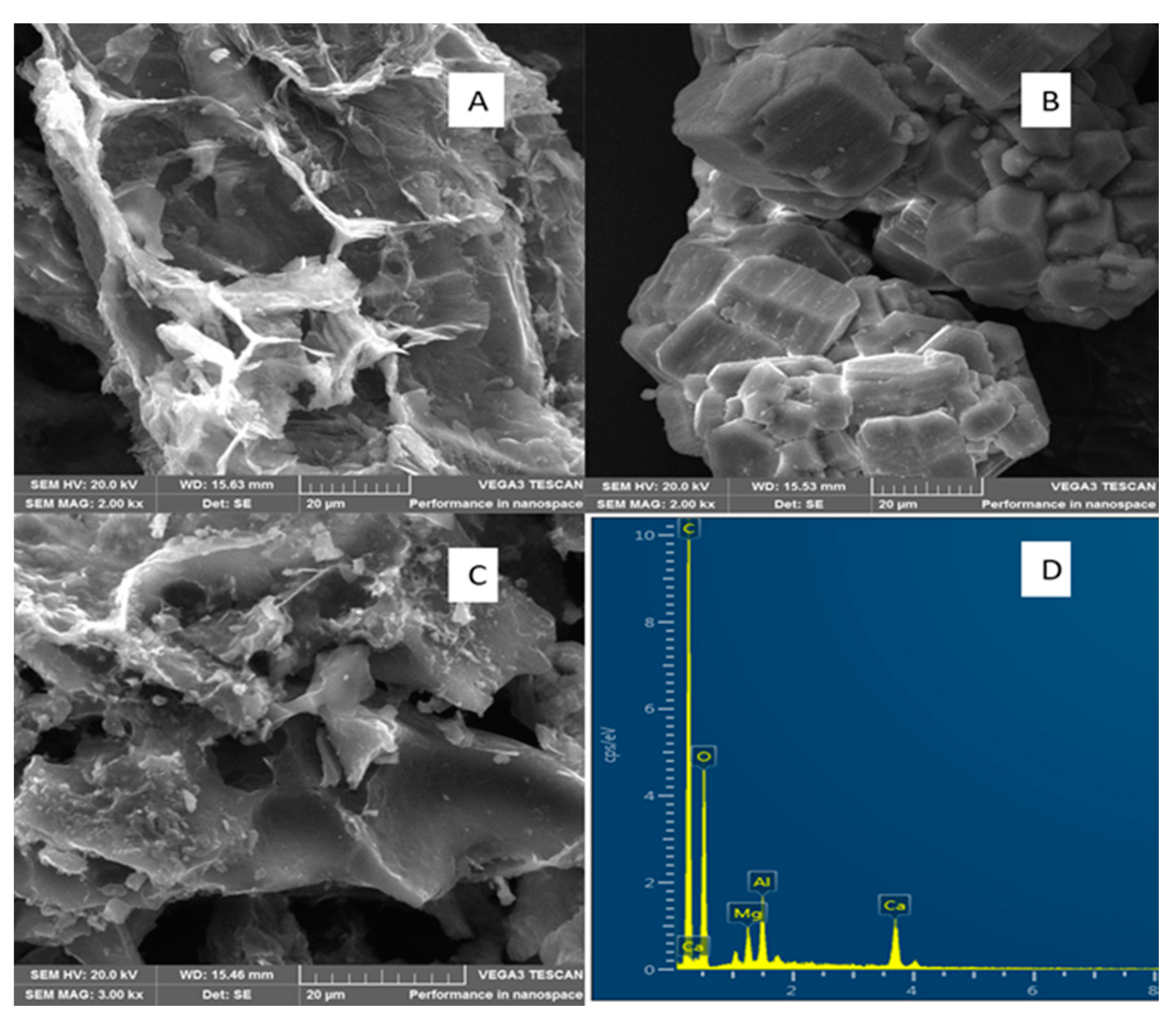
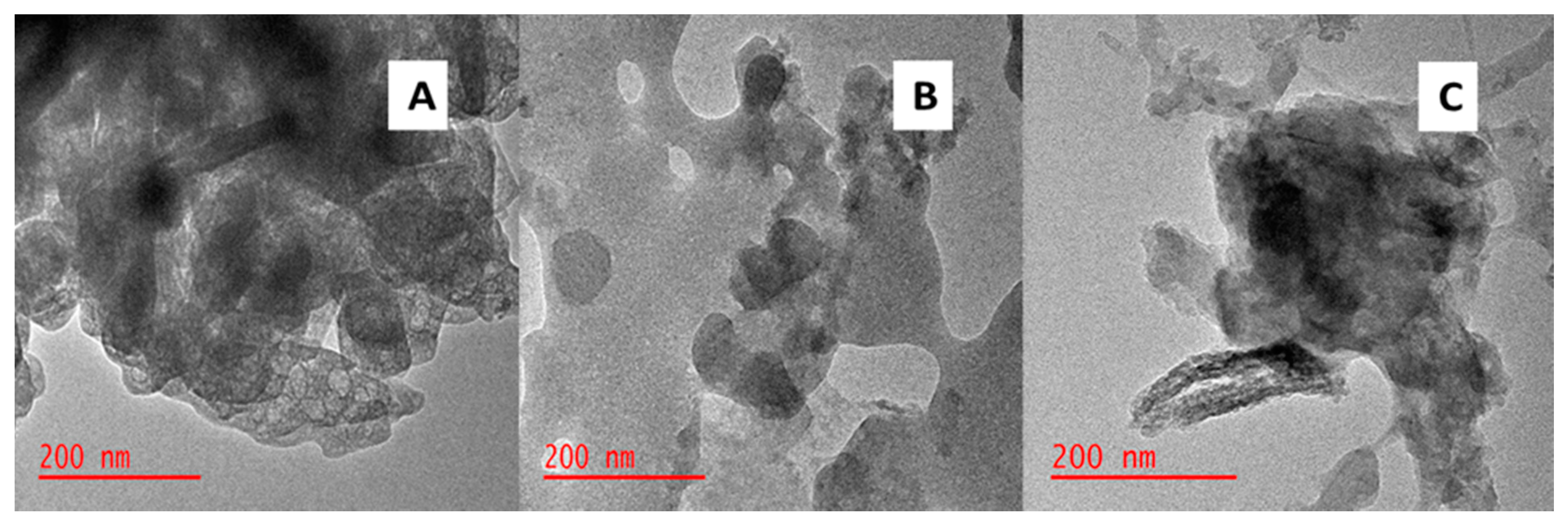
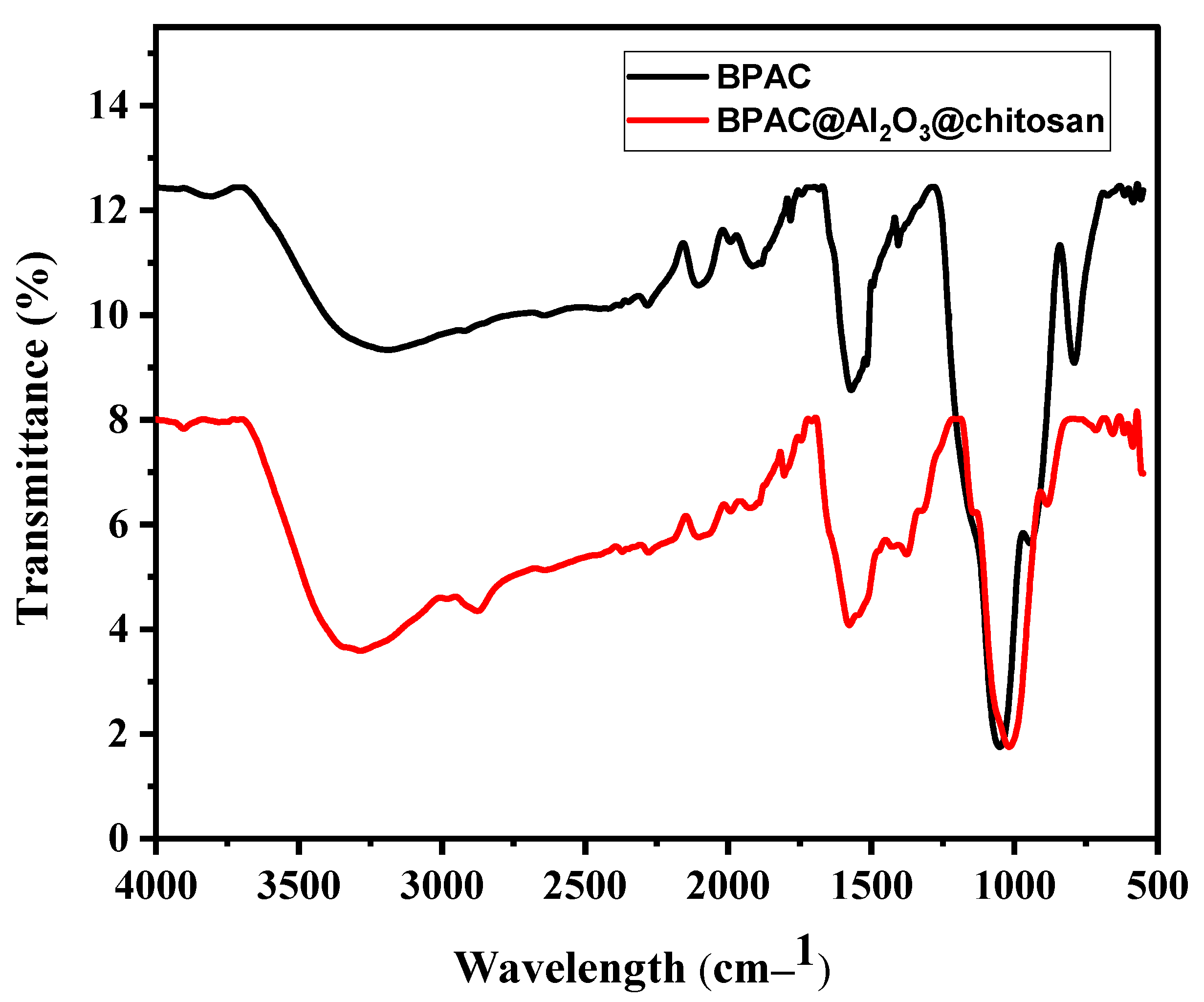
3.2. Physicochemical Properties of the Adsorbents
3.3. Adsorption Studies
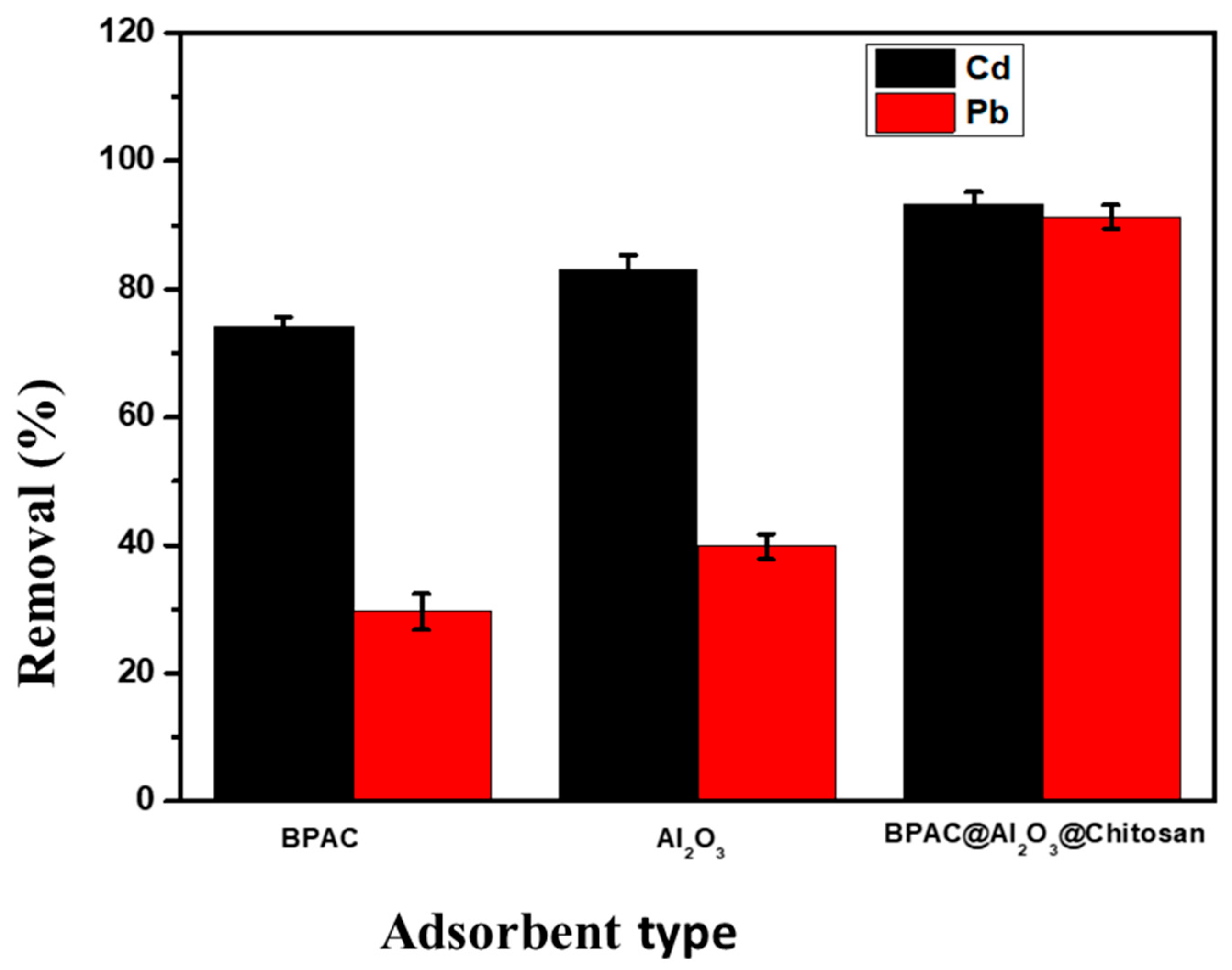
Selection of Adsorbent
3.4. Optimization of the Adsorption Batch Method
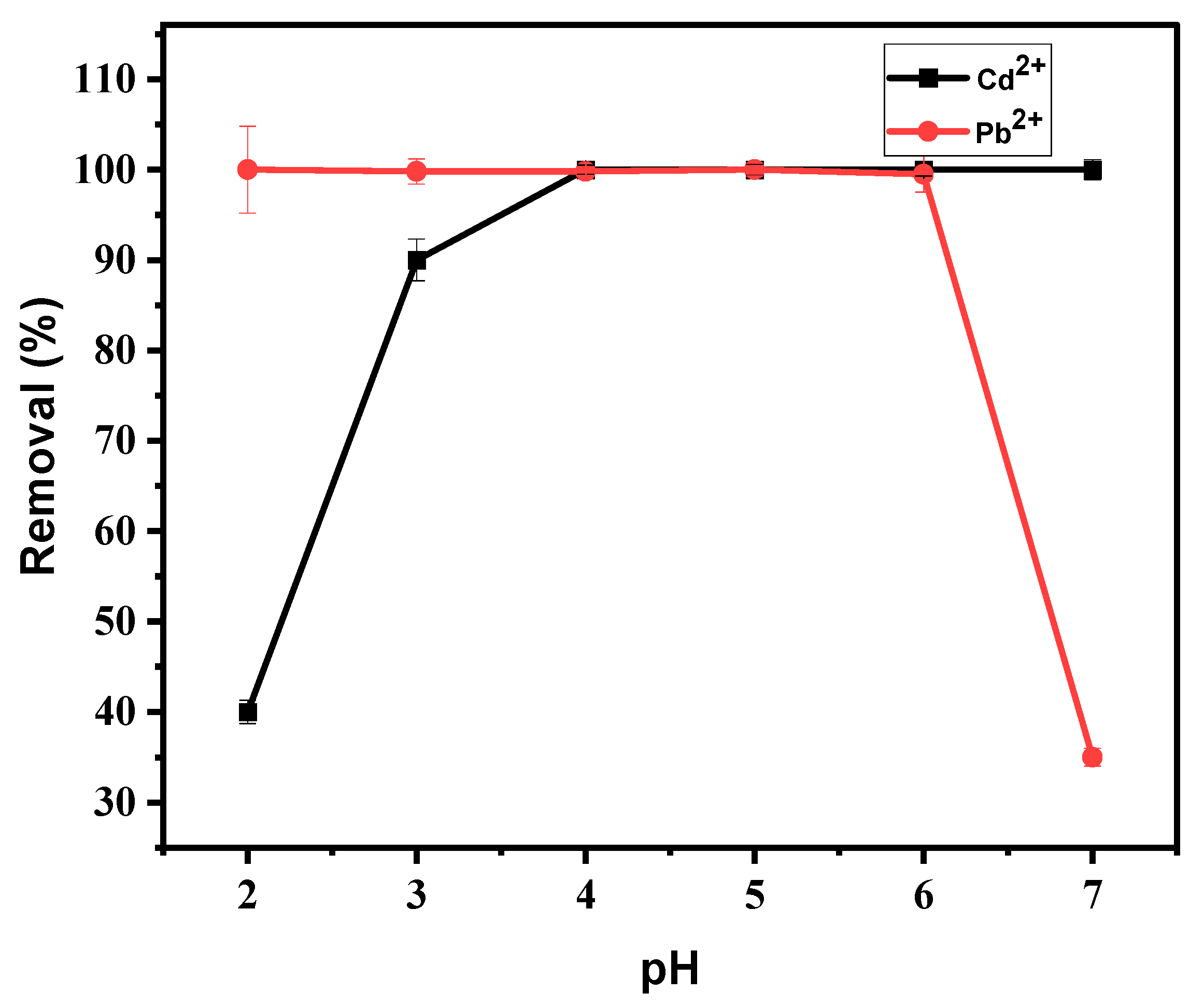
3.4.1. Effect of pH
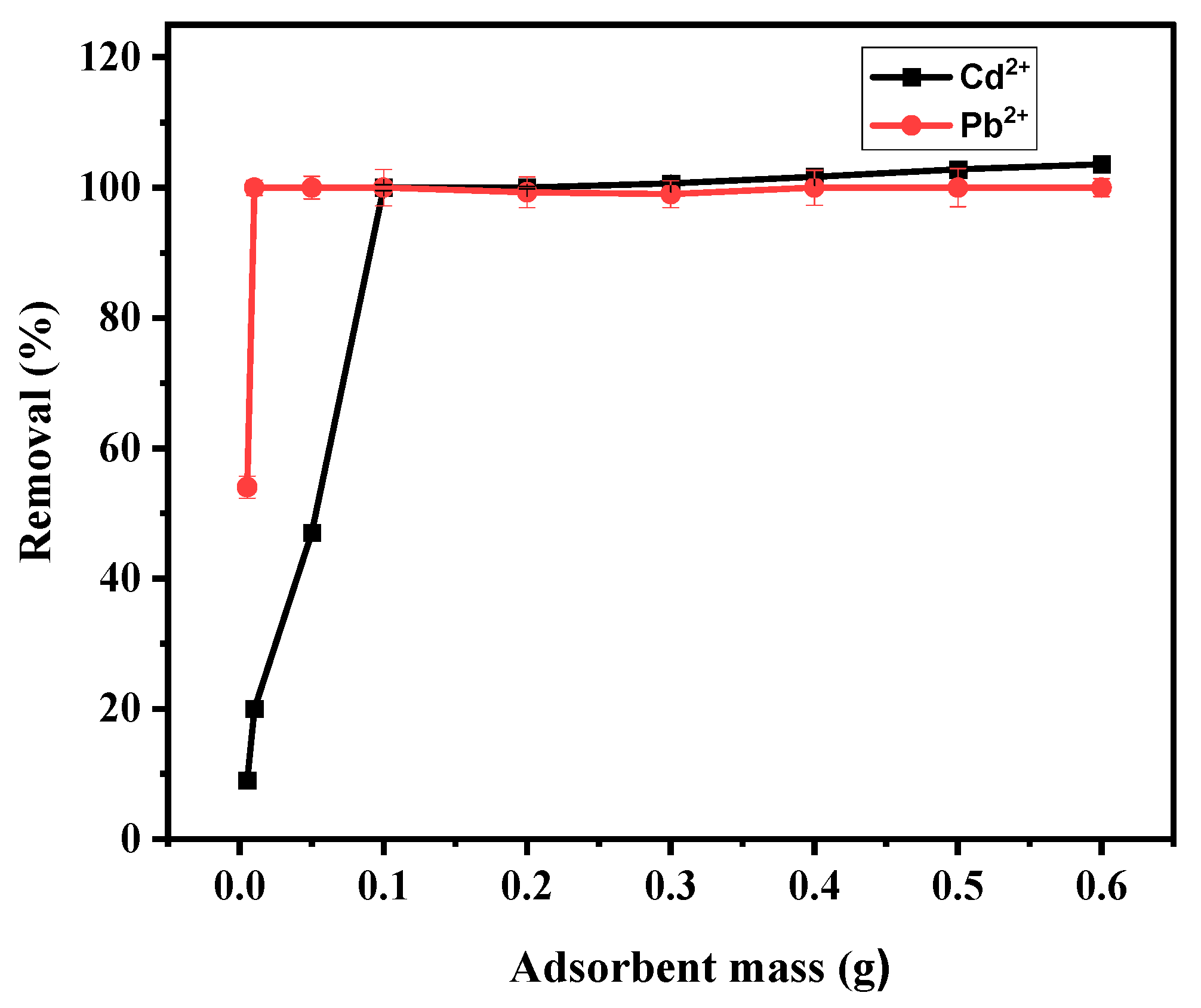
3.4.2. Effect of Adsorbent Mass
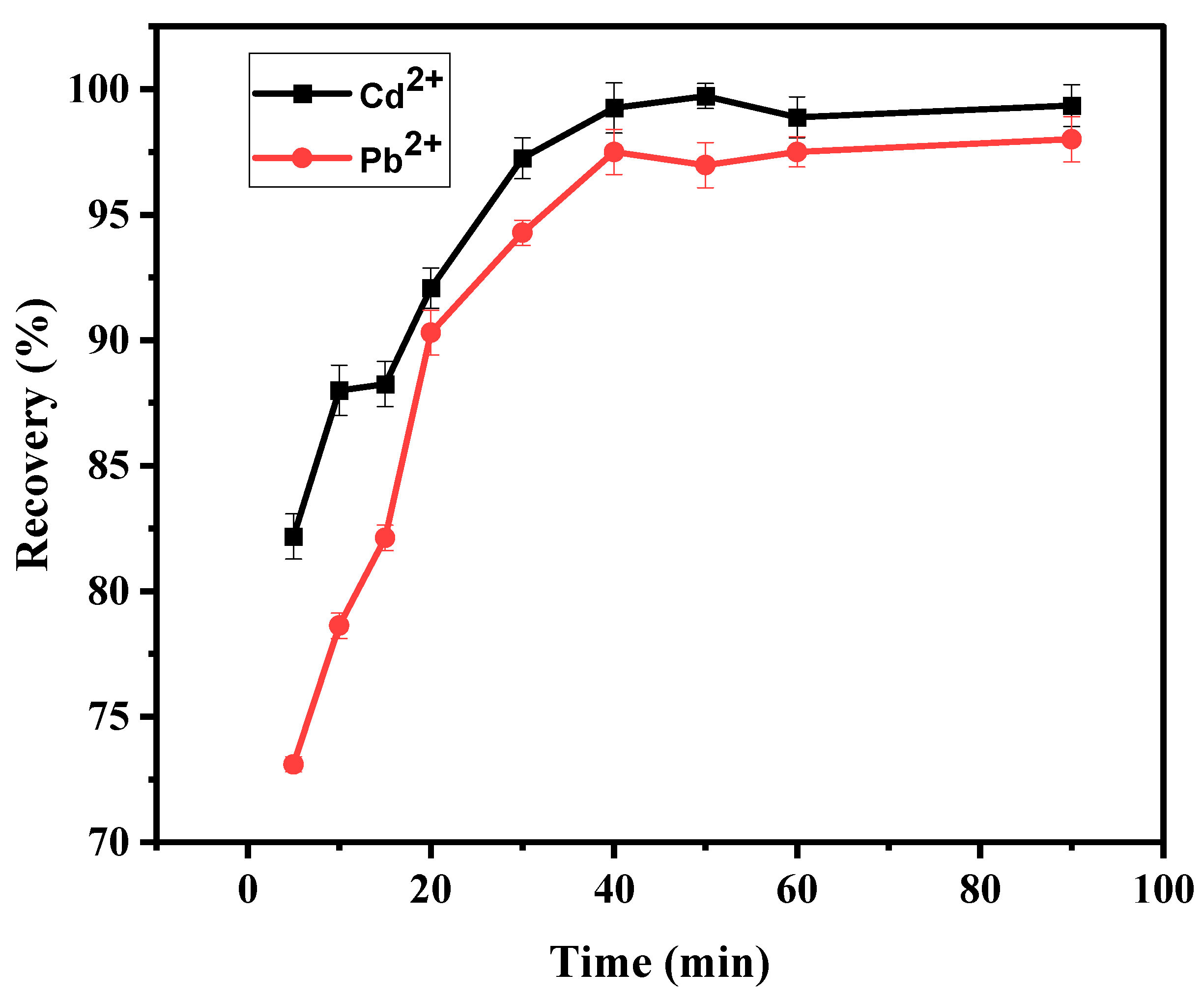
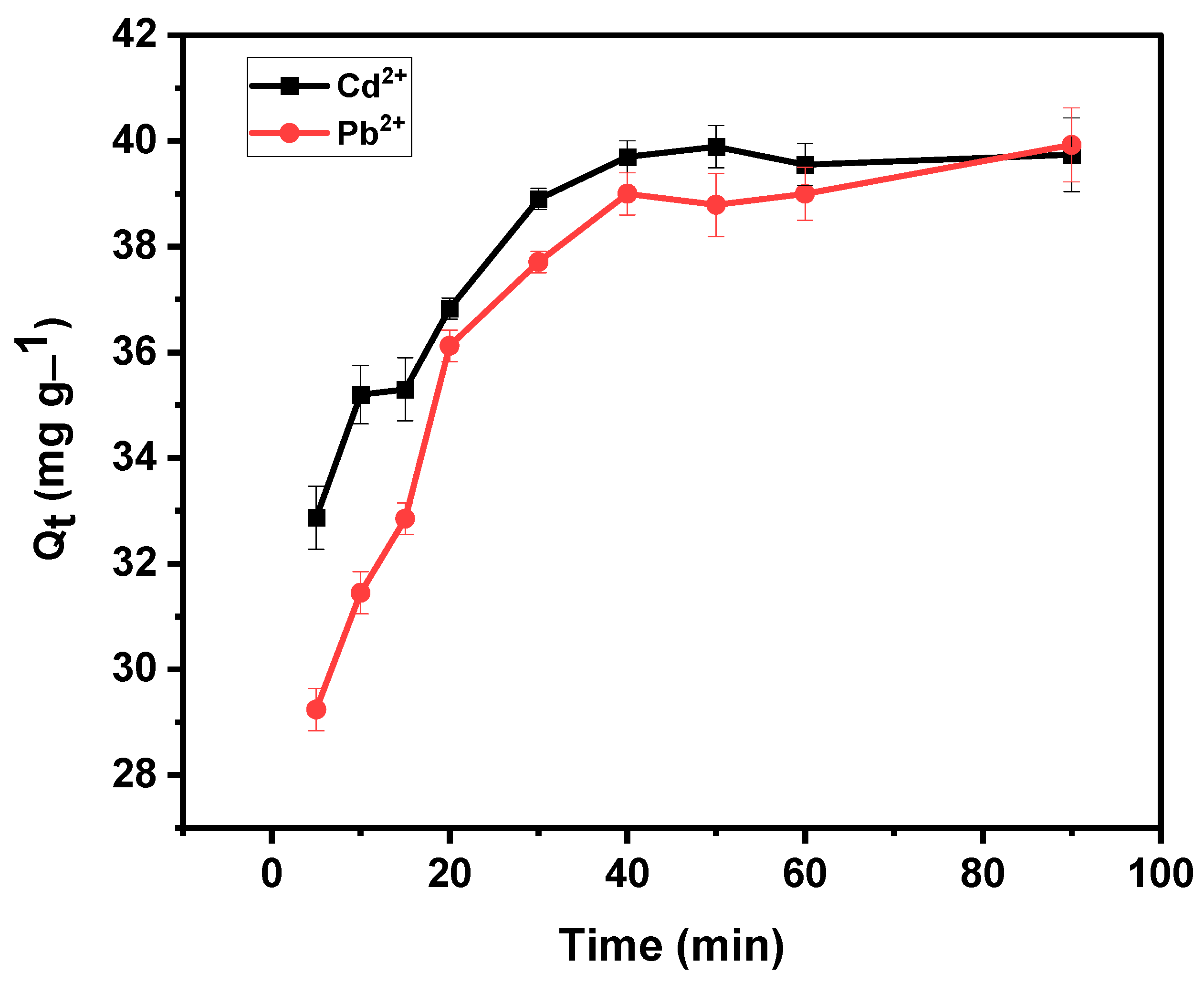
3.4.3. Effect of Contact Time
4. Discussion
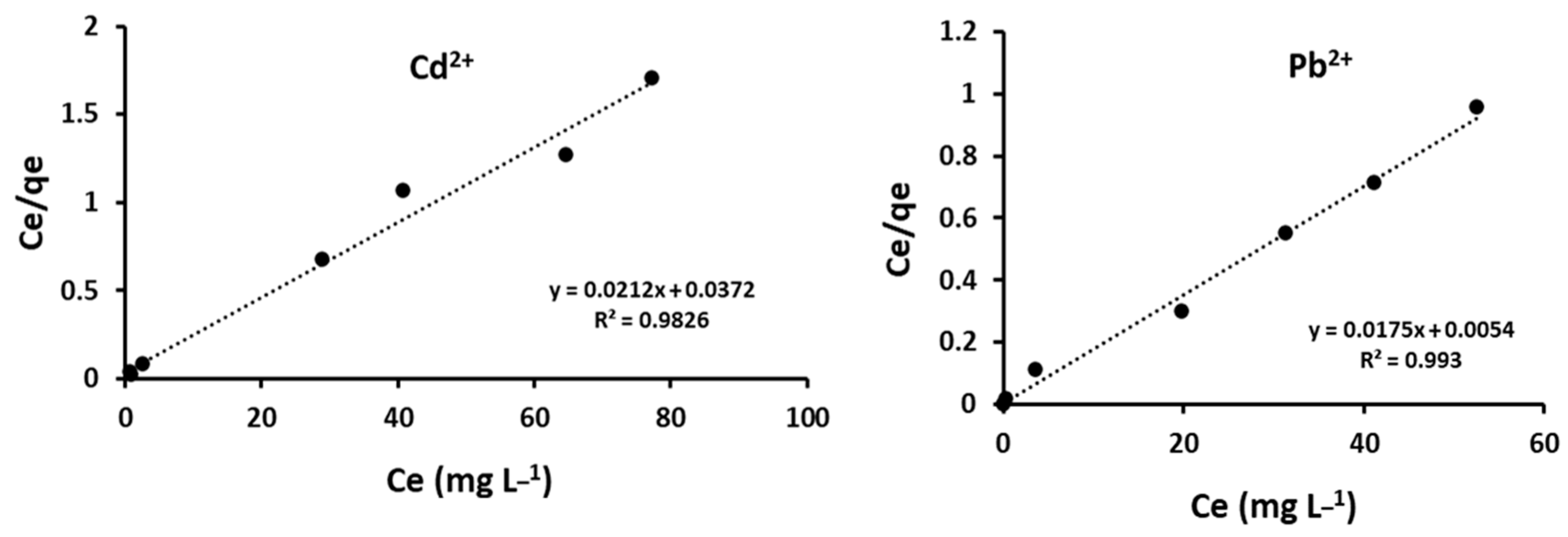
4.1. Sorption Isotherms
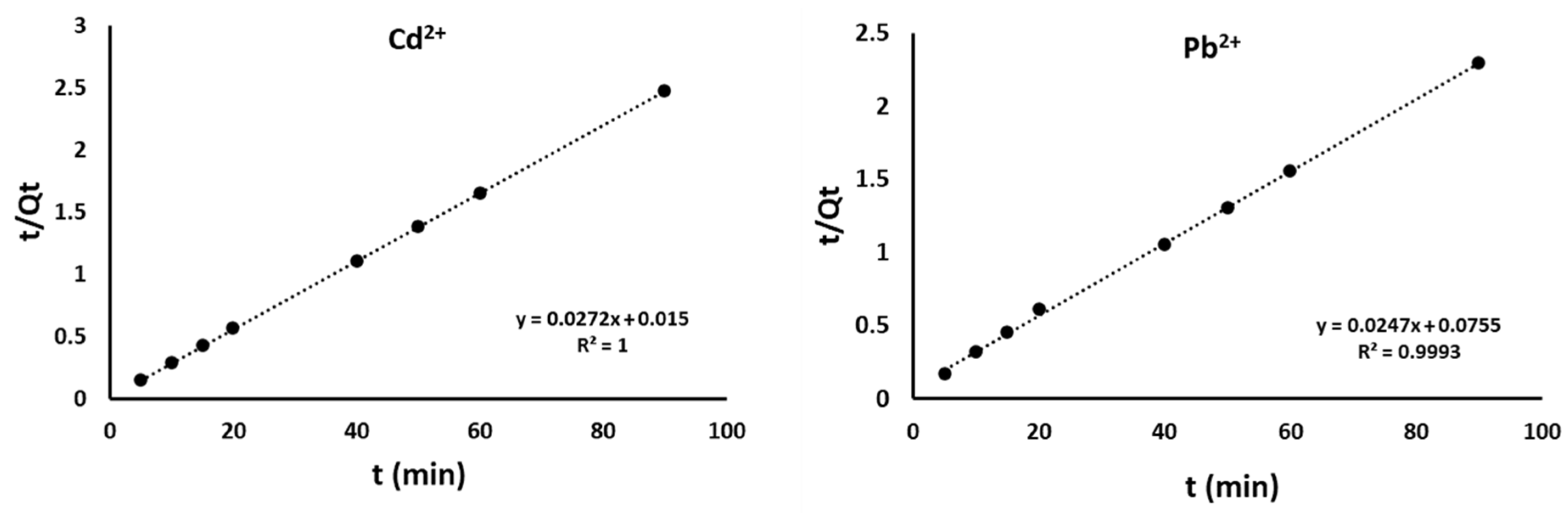
4.2. Adsorption Kinetics
4.3. Application of Real Water Samples
4.4. Adsorption–Desorption Studies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, J.; Hou, B.; Wang, J.; Tian, B.; Bi, J.; Wang, N.; Li, X.; Huang, X. Nanomaterials for the removal of heavy metals from wastewater. Nanomaterials 2019, 9, 424. [Google Scholar] [CrossRef] [Green Version]
- Sharififard, H.; Shahraki, Z.H.; Rezvanpanah, E.S.; Rad, H. A novel natural chitosan/activated carbon/iron bio-nano composite: Sonochemical synthesis, characterization, and application for cadmium removal in batch and continuous adsorption process. Bioresour. Technol. 2018, 270, 562–569. [Google Scholar] [CrossRef]
- Millers, D.; Grigorjeva, L.; Chernov, S.; Popov, A.; Lecoq, P.; Auffray, E. The temperature dependence of scintillation parameters in PbWO4 crystals. Phys. Status Solidi B 1997, 203, 585–589. [Google Scholar] [CrossRef]
- Nagirnyi, V.; Feldbach, E.; Jönsson, L.; Kirm, M.; Kotlov, A.; Lushchik, A.; Nagornaya, L.L.; Savikhin, F.; Svensson, G. Study of oriented CdWO4 scintillating crystals using synchrotron radiation. Radiat. Meas. 2001, 33, 601–604. [Google Scholar] [CrossRef]
- Michail, C.; Koukou, V.; Martini, N.; Saatsakis, G.; Kalyvas, N.; Bakas, A.; Kandarakis, I.; Fountos, G.; Panayiotakis, G.; Valais, I. Luminescence Efficiency of Cadmium Tungstate. Crystals 2020, 10, 429. [Google Scholar] [CrossRef]
- Ayati, A.; Tanhaei, B.; Sillanpää, M. Lead (II)-ion removal by ethylenediaminetetraacetic acid ligand functionalized magnetic chitosan–aluminum oxide–iron oxide nanoadsorbents and microadsorbents: Equilibrium, kinetics, and thermodynamics. J. Appl. Polym. Sci. 2017, 134, 1–10. [Google Scholar] [CrossRef]
- Calabrò, P.S.; Bilardi, S.; Moraci, N. Advancements in the use of filtration materials for the removal of heavy metals from multicontaminated solutions. Curr. Opin. Environ. Sci. Health 2021, 20, 100241. [Google Scholar] [CrossRef]
- Bashir, A.; Malik, L.A.; Ahad, S.; Manzoor, T.; Bhat, M.A.; Dar, G.N.; Pandith, A.H. Removal of heavy metal ions from aqueous system by ion-exchange and biosorption methods. Environ. Chem. Lett. 2019, 17, 729–754. [Google Scholar] [CrossRef]
- Corroto, C.; Iriel, A.; Cirelli, A.F.; Carrera, A.L.P. Constructed wetlands as an alternative for arsenic removal from reverse osmosis effluent. Sci. Total Environ. 2019, 691, 1242–1250. [Google Scholar] [CrossRef]
- Minas, F.; Chandravanshi, B.S.; Leta, S. Chemical precipitation method for chromium removal and its recovery from tannery wastewater in Ethiopia. Chem. Int. 2017, 3, 291–305. [Google Scholar] [CrossRef]
- Ramutshatsha, D.; Ngila, J.C.; Ndungu, P.G.; Nomngongo, P.N. Simultaneous removal of Na, Ca, K and mg from synthetic brine and seawater using Fe2O3–SiO2 mixed oxide nanostructures: Kinetics and isotherms studies. Desalin. Water Treat. 2018, 104, 206–216. [Google Scholar] [CrossRef]
- Abussaud, B.; Asmaly, H.A.; Ihsanullah; Saleh, T.A.; Gupta, V.K.; Laoui, T.; Atieh, M.A. Sorption of phenol from waters on activated carbon impregnated with iron oxide, aluminum oxide, and titanium oxide. J. Mol. Liq. 2016, 213, 351–359. [Google Scholar] [CrossRef]
- Ramutshatsha, D.; Catherine Ngila, J.; Ndungu, P.G.; Nomngongo, P.N. Application of response surface methodology for simultaneous removal of major cations from seawater using metal oxide nanostructures. Water SA 2020, 46, 313–321. [Google Scholar] [CrossRef]
- Adam, A.M.; Saad, H.A.; Atta, A.A.; Alsawat, M.; Hegab, M.S.; Altalhi, T.A.; Refat, M.S. An Environmentally friendly method for removing Hg (II), Pb (II), Cd (II) and Sn (II) Heavy Metals from Wastewater Using Novel Metal—Carbon-Based Composites. Crystals 2021, 11, 882. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Zhang, C.; Wen, T.; Zhuang, L.; Wang, X.; Song, G.; Chen, D.; Ai, Y.; Hayat, T.; et al. Porous Fe2O3 microcubes derived from metal organic frameworks for efficient elimination of organic pollutants and heavy metal ions. Chem. Eng. J. 2018, 336, 241–252. [Google Scholar] [CrossRef]
- El-Azim, H.; Mourad, F. Removal of Heavy Metals Cd (II), Fe (III) and Ni (II), from Aqueous Solutions by Natural (Clinoptilolite) Zeolites and Application to Industrial Wastewater. Asian J. Environ. Ecol. 2018, 7, 1–13. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Yuan, X.; Zhang, J.; Chew, J.W. Metal-organic framework membranes for wastewater treatment and water regeneration. Coord. Chem. Rev. 2020, 404, 213116. [Google Scholar] [CrossRef]
- Diagboya, P.N.; Olu-Owolabi, B.I.; Mtunzi, F.M.; Adebowale, K.O. Clay-carbonaceous material composites: Towards a new class of functional adsorbents for water treatment. Surf. Interfaces 2020, 19, 100506. [Google Scholar] [CrossRef]
- Eskandari, P.; Farhadian, M.; Solaimany Nazar, A.R.; Goshadrou, A. Cyanide adsorption on activated carbon impregnated with ZnO, Fe2O3, TiO2 nanometal oxides: A comparative study. Int. J. Environ. Sci. Technol. 2021, 18, 297–316. [Google Scholar] [CrossRef]
- Shahrokhi-Shahraki, R.; Benally, C.; El-Din, M.G.; Park, J. High efficiency removal of heavy metals using tire-derived activated carbon vs commercial activated carbon: Insights into the adsorption mechanisms. Chemosphere 2021, 264, 128455. [Google Scholar] [CrossRef]
- Ndjientcheu Yossa, L.M.; Ouiminga, S.K.; Sidibe, S.S.; Ouedraogo, I.W.K. Synthesis of a cleaner potassium hydroxide-activated carbon from baobab seeds hulls and investigation of adsorption mechanisms for diuron: Chemical activation as alternative route for preparation of activated carbon from baobab seeds hulls and adsorption. Sci. Afr. 2020, 9, e00476. [Google Scholar] [CrossRef]
- Alghamdi, A.A.; Al-Odayni, A.B.; Saeed, W.S.; Al-Kahtani, A.; Alharthi, F.A.; Aouak, T. Efficient adsorption of lead (II) from aqueous phase solutions using polypyrrole-based activated carbon. Materials 2019, 12, 2020. [Google Scholar] [CrossRef] [Green Version]
- Karnjanakom, S.; Maneechakr, P. Adsorption behaviors and capacities of Cr(VI) onto environmentally activated carbon modified by cationic (HDTMA and DDAB) surfactants. J. Mol. Struct. 2019, 1186, 80–90. [Google Scholar] [CrossRef]
- Hami, H.K.; Abbas, R.F.; Eltayef, E.M.; Mahdi, N.I. Applications of aluminum oxide and nano aluminum oxide as adsorbents: Review. Samarra J. Pure Appl. Sci. 2021, 2, 19–32. [Google Scholar] [CrossRef]
- Mishra, A.K. (Ed.) Nanomaterials for Water Remediation: Inorganic Oxide Materials; Smithers Rapra: Shrewsbury, UK, 2016; Volume 2, p. 5. [Google Scholar]
- Young, C.; Wang, J.; Kim, J.; Sugahara, Y.; Henzie, J.; Yamauchi, Y. Controlled Chemical Vapor Deposition for Synthesis of Nanowire Arrays of Metal-Organic Frameworks and Their Thermal Conversion to Carbon/Metal Oxide Hybrid Materials. Chem. Mater. 2018, 30, 3379–3386. [Google Scholar] [CrossRef]
- Rakhi, C.; Preetha, K.C. An investigation on the effect of complexing agents on zirconium based ternary metal oxide nanoparticles by co-precipitation method. J. Mater. Sci. Mater. Electron. 2019, 30, 19587–19597. [Google Scholar] [CrossRef]
- Shashanka, R.; Kamaci, Y.; Tas, R.; Ceylan, Y.; Bülbül, A.S.; Uzun, O.; Karaoglanli, A.C. Antimicrobial investigation of CuO and ZnO nanoparticles prepared by a rapid combustion method. Phys. Chem. Res. 2019, 7, 799–812. [Google Scholar] [CrossRef]
- Bayal, N.; Jeevanandam, P. Synthesis of TiO2-MgO mixed metal oxide nanoparticles via a sol-gel method and studies on their optical properties. Ceram. Int. 2014, 40, 15463–15477. [Google Scholar] [CrossRef]
- Tabesh, S.; Davar, F.; Loghman-Estarki, M.R. Preparation of γ-Al2O3 nanoparticles using modified sol-gel method and its use for the adsorption of lead and cadmium ions. J. Alloys Compd. 2018, 730, 441–449. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Ibrahim, F.A.; Shaban, S.A.; Youssef, N.A. Adsorption of heavy metal ion from aqueous solution by nickel oxide nano catalyst prepared by different methods. Egypt. J. Pet. 2015, 24, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Liu, J.; Li, N.; Wang, W.; Nan, J.; Zhao, Z.; Cui, F. Highly efficient removal of bivalent heavy metals from aqueous systems by magnetic porous Fe3O4-MnO2: Adsorption behavior and process study. Chem. Eng. J. 2016, 304, 737–746. [Google Scholar] [CrossRef]
- Ramutshatsha-Makhwedzha, D.; Ngila, J.C.; Ndungu, P.G.; Nomngongo, P.N. Ultrasound assisted adsorptive removal of Cr, Cu, Al, Ba, Zn, Ni, Mn, Co and Ti from seawater using Fe2O3-SiO2-PAN nanocomposite: Equilibrium kinetics. J. Mar. Sci. Eng. 2019, 7, 133. [Google Scholar] [CrossRef] [Green Version]
- Mashile, G.P.; Mpupa, A.; Nqombolo, A.; Dimpe, K.M.; Nomngongo, P.N. Recyclable magnetic waste tyre activated carbon-chitosan composite as an effective adsorbent rapid and simultaneous removal of methylparaben and propylparaben from aqueous solution and wastewater. J. Water Process Eng. 2020, 33, 101011. [Google Scholar] [CrossRef]
- Gupta, H.; Kumar, R. Removal of PAH Anthracene from Aqueous Media using Banana Peel Activated Carbon. Int. J. Sci. Res. Environ. Sci. 2016, 4, 109–114. [Google Scholar] [CrossRef]
- Dimpe, K.M.; Ngila, J.C.; Nomngongo, P.N. Preparation and application of a tyre-based activated carbon solid phase extraction of heavy metals in wastewater samples. Phys. Chem. Earth. 2018, 105, 161–169. [Google Scholar] [CrossRef]
- Alasadi, A.M.; Khaili, F.I.; Awwad, A.M. Adsorption of Cu (II), Ni (II) and Zn (II) ions by nano kaolinite: Thermodynamics and kinetics studies. Chem. Int. 2020, 5, 258–268. [Google Scholar] [CrossRef]
- Naseem, K.; Begum, R.; Wu, W.; Usman, M.; Irfan, A.; Al-Sehemi, A.G.; Farooqi, Z.H. Adsorptive removal of heavy metal ions using polystyrene-poly(N-isopropylmethacrylamide-acrylic acid) core/shell gel particles: Adsorption isotherms and kinetic study. J. Mol. Liq. 2019, 277, 522–531. [Google Scholar] [CrossRef]
- Rasmey, A.H.; Aboseidah, A.A.; Youssef, A.K. Application of Langmuir and Freundlich isotherm models on Biosorption of Pb2+ by Freezdried Biomass of Pseudomonas aeruginosa. Egypt. J. Microbiol. 2018, 53, 37–48. [Google Scholar] [CrossRef]
- Gonte, R.; Balasubramanian, K. Heavy and toxic metal uptake by mesoporous hypercrosslinked SMA beads: Isotherms and kinetics. J. Saudi Chem. Soc. 2016, 20, S579–S590. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.S.K.; Jiang, S.J. Chitosan-functionalized graphene oxide: A novel adsorbent an efficient adsorption of arsenic from aqueous solution. J. Environ. Chem. Eng. 2016, 4, 1698–1713. [Google Scholar] [CrossRef]
- Lee, S.P.; Ali, G.A.M.; Algarni, H.; Chong, K.F. Flake size-dependent adsorption of graphene oxide aerogel. J. Mol. Liq. 2019, 277, 175–180. [Google Scholar] [CrossRef]
- Setshedi, K.Z.; Bhaumik, M.; Songwane, S.; Onyango, M.S.; Maity, A. Exfoliated polypyrrole-organically modified montmorillonite clay nanocomposite as a potential adsorbent for Cr(VI) removal. Chem. Eng. J. 2013, 222, 186–197. [Google Scholar] [CrossRef]
- Vijayalakshmi, K.; Devi, B.M.; Latha, S.; Gomathi, T.; Sudha, P.N.; Venkatesan, J.; Anil, S. Batch adsorption and desorption studies on the removal of lead (II) from aqueous solution using nanochitosan/sodium alginate/microcrystalline cellulose beads. Int. J. Biol. Macromol. 2017, 104, 1483–1494. [Google Scholar] [CrossRef]
- Pap, S.; Radonić, J.; Trifunović, S.; Adamović, D.; Mihajlović, I.; Vojinović Miloradov, M.; Turk Sekulić, M. Evaluation of the adsorption potential of eco-friendly activated carbon prepared from cherry kernels for the removal of Pb2+, Cd2+ and Ni2+ from aqueous wastes. J. Environ. Manag. 2016, 184, 297–306. [Google Scholar] [CrossRef]
- Foroutan, R.; Mohammadi, R.; Farjadfard, S.; Esmaeili, H.; Saberi, M.; Sahebi, S.; Dobaradaran, S.; Ramavandi, B. Characteristics and performance of Cd, Ni, and Pb bio-adsorption using Callinectes sapidus biomass: Real wastewater treatment. Environ. Sci. Pollut. Res. 2019, 26, 6336–6347. [Google Scholar] [CrossRef] [PubMed]
- El-Said, W.A.; El-Khouly, M.E.; Ali, M.H.; Rashad, R.T.; Elshehy, E.A.; Al-Bogami, A.S. Synthesis of mesoporous silica-polymer composite for the chloridazon pesticide removal from aqueous media. J. Environ. Chem. Eng. 2018, 6, 2214–2221. [Google Scholar] [CrossRef]
- Sadegh, H.; Ali, G.A.M.; Makhlouf, A.S.H.; Chong, K.F.; Alharbi, N.S.; Agarwal, S.; Gupta, V.K. MWCNTs-Fe3O4 nanocomposite for Hg (II) high adsorption efficiency. J. Mol. Liq. 2018, 258, 345–353. [Google Scholar] [CrossRef]
- Igberase, E.; Ofomaja, A.; Osifo, P.O. Enhanced heavy metal ions adsorption by 4-aminobenzoic acid grafted on chitosan/epichlorohydrin composite: Kinetics, isotherms, thermodynamics and desorption studies. Int. J. Biol. Macromol. 2019, 123, 664–676. [Google Scholar] [CrossRef]
- Nomngongo, P.N.; Ngila, J.C.; Msagati, T.A.M.; Moodley, B. Kinetics and Equilibrium Studies for the Removal of Cobalt, Manganese, and Silver in Ethanol using Dowex 50W-x8 Cation Exchange Resin. Sep. Sci. Technol. 2014, 49, 1848–1859. [Google Scholar] [CrossRef]
- Mashile, P.P.; Dimpe, M.K.; Nomngongo, P.N. Application of waste tyre-based powdered activated carbon for the adsorptive removal of cylindrospermopsin toxins from environmental matrices: Optimization using response surface methodology and desirability function. J. Environ. Sci. Health Part A 2019, 54, 679–685. [Google Scholar] [CrossRef]
- Sajjadi, S.A.; Meknati, A.; Lima, E.C.; Dotto, G.L.; Mendoza-Castillo, D.I.; Anastopoulos, I.; Alakhras, F.; Unuabonah, E.I.; Singh, P.; Hosseini-Bandegharaei, A. A novel route for the preparation of chemically activated carbon from pistachio wood for highly efficient Pb (II) sorption. J. Environ. Manag. 2019, 236, 34–44. [Google Scholar] [CrossRef]
- Nejadshafiee, V.; Islami, M.R. Intelligent-activated carbon prepared from pistachio shells precursor for effective adsorption of heavy metals from industrial waste of copper mine. Environ. Sci. Pollut. Res. 2020, 27, 1625–1639. [Google Scholar] [CrossRef]
- Norouzi, S.; Heidari, M.; Alipour, V.; Rahmanian, O.; Fazlzadeh, M.; Mohammadi-moghadam, F.; Nourmoradi, H.; Goudarzi, B.; Dindarloo, K. Preparation, characterization and Cr(VI) adsorption evaluation of NaOH-activated carbon produced from Date Press Cake; an agro-industrial waste. Bioresour. Technol. 2018, 258, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Van Tran, T.; Bui, Q.T.P.; Nguyen, T.D.; Le, N.T.H.; Bach, L.G.A. comparative study on the removal efficiency of metal ions (Cu2+, Ni2+, and Pb2+) using sugarcane bagasse-derived ZnCl2-activated carbon by the response surface methodology. Adsorpt. Sci. Technol. 2017, 35, 72–85. [Google Scholar] [CrossRef]
- Van Thuan, T.; Quynh, B.T.P.; Nguyen, T.D.; Ho, V.T.T.; Bach, L.G. Response surface methodology approach for optimization of Cu2+, Ni2+ and Pb2+ adsorption using KOH-activated carbon from banana peel. Surf. Interfaces 2017, 6, 209–217. [Google Scholar] [CrossRef]
- Alslaibi, T.M.; Abustan, I.; Ahmad, M.A.; Foul, A.A. Cadmium removal from aqueous solution using microwaved olive stone activated carbon. J. Environ. Chem. Eng. 2013, 1, 589–599. [Google Scholar] [CrossRef]

| Activation | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) |
|---|---|---|---|
| BPAC was calcined at 600 °C | 2.275 | 0.155 | 256.0 |
| BPAC-H2SO4 | 361.86 | 0.2294 | 25.36 |
| BPAC-KOH | 283.92 | 0.2245 | 31.63 |
| Langmuir Parameters | |||||
| Cations | Qmax ex | qmax (mg g−1) | KL (L mg−1) | RL | R2 |
| Cd2+ | 43.0 | 46.9 | 0.57 | 0.03 | 0.983 |
| Pb2+ | 57 | 57.1 | 1.75 | 0.01 | 0.993 |
| Freundlich Parameters | |||||
| KF | n | R2 | |||
| Cd2+ | 26.61 | 7.59 | 0.226 | ||
| Pb2+ | 14.67 | 1.60 | 0.684 | ||
| Cd2+ | Pb2+ | ||
|---|---|---|---|
| qt exp | 38 | 39.0 | |
| Pseudo-First order | k1 (min−1) | 0.22 | 0.085 |
| qe (mg g−1) | 11.2 × 1028 | 1.0 × 1028 | |
| R2 | 0.7155 | 0.886 | |
| Pseudo-Second order | k2 (g mg−1 min−1) | 0.22 | 0.01 |
| qt (mg g−1) | 40.65 | 41 | |
| R2 | 0.999 | 0.999 | |
| h (mg g−1 min−1) | 33.05 | 15.3 | |
| t1/2 (min) | 1.2 | 2.7 | |
| Intraparticle Diffusion | kid1(g mg−1 min−1) | 11.5 | 1.524 |
| C (mg g−1) | 28.46 | 27.56 | |
| R2 | 0.966 | 0.847 |
| Analytes | Influent Conc. (mg L−1) | %Re | Effluent Conc. (mg L−1) | %Re |
|---|---|---|---|---|
| Cd2+ | 2.5 | 99.8 | 2.3 | 99.3 |
| Pb2+ | 8.58 | 97.4 | 4.1 | 92.8 |
| Analytes | Adsorbents | Removal Efficiency (%) | Ref. |
|---|---|---|---|
| Pb (II) | Pistachio wood | 99 | [52] |
| Cu (II) | Magnetic activated carbon prepared from pistachio shells | 94.5 | [53] |
| Cr (VI) | Date press cake | >90 | [54] |
| Cu2+, Ni2+, Pb2+ | Sugarcane bagasse derived ZnCl2 | 66.4, 90, 99.9 | [55] |
| Cu2+, Ni2+, Pb2+ | KOH-activated carbon from banana peel | 98.8, 99.2, 100 | [56] |
| Cd (II) | Olive stones | 95.3 | [57] |
| Cd2+, Pb2+ | Cherry pits | 92.4, 94.5 | [45] |
| Pb2+, Cd2+ | BPAC @Al2O3@chitosan | <99 <92 | This Work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramutshatsha-Makhwedzha, D.; Mbaya, R.; Mavhungu, M.L. Application of Activated Carbon Banana Peel Coated with Al2O3-Chitosan for the Adsorptive Removal of Lead and Cadmium from Wastewater. Materials 2022, 15, 860. https://doi.org/10.3390/ma15030860
Ramutshatsha-Makhwedzha D, Mbaya R, Mavhungu ML. Application of Activated Carbon Banana Peel Coated with Al2O3-Chitosan for the Adsorptive Removal of Lead and Cadmium from Wastewater. Materials. 2022; 15(3):860. https://doi.org/10.3390/ma15030860
Chicago/Turabian StyleRamutshatsha-Makhwedzha, Denga, Richard Mbaya, and Mapula Lucey Mavhungu. 2022. "Application of Activated Carbon Banana Peel Coated with Al2O3-Chitosan for the Adsorptive Removal of Lead and Cadmium from Wastewater" Materials 15, no. 3: 860. https://doi.org/10.3390/ma15030860
APA StyleRamutshatsha-Makhwedzha, D., Mbaya, R., & Mavhungu, M. L. (2022). Application of Activated Carbon Banana Peel Coated with Al2O3-Chitosan for the Adsorptive Removal of Lead and Cadmium from Wastewater. Materials, 15(3), 860. https://doi.org/10.3390/ma15030860





