Effect of Hydroxyapatite Nanoparticles and Nitrogen Plasma Treatment on Osteoblast Biological Behaviors of 3D-Printed HDPE Scaffold for Bone Tissue Regeneration Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nitrogen Plasma Treatment of the 3D HDPE/n-HAp Scaffold
2.3. Surface Characterizations
2.4. Cell Culture
2.5. Evaluations of Cell Bioactivity
3. Results
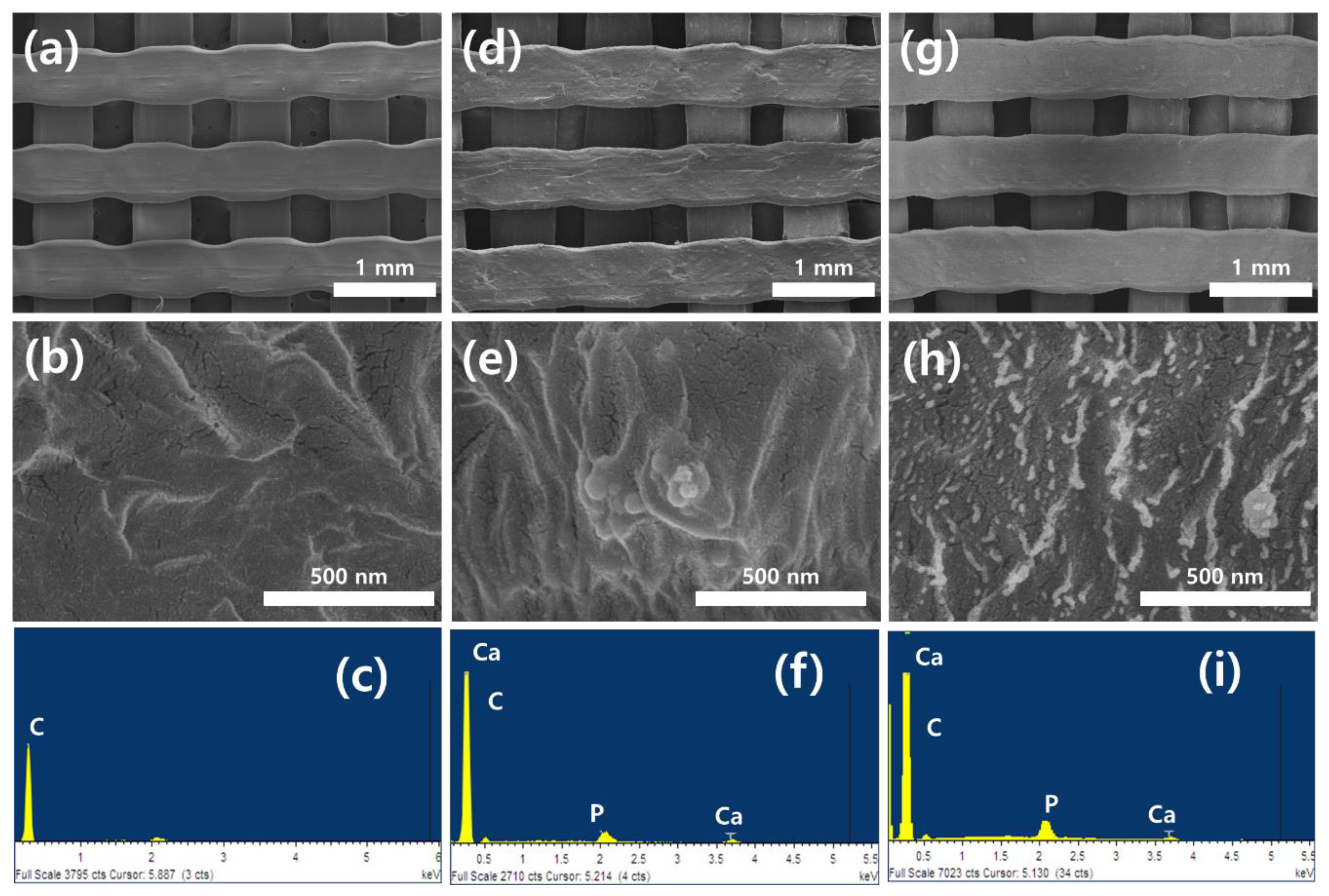
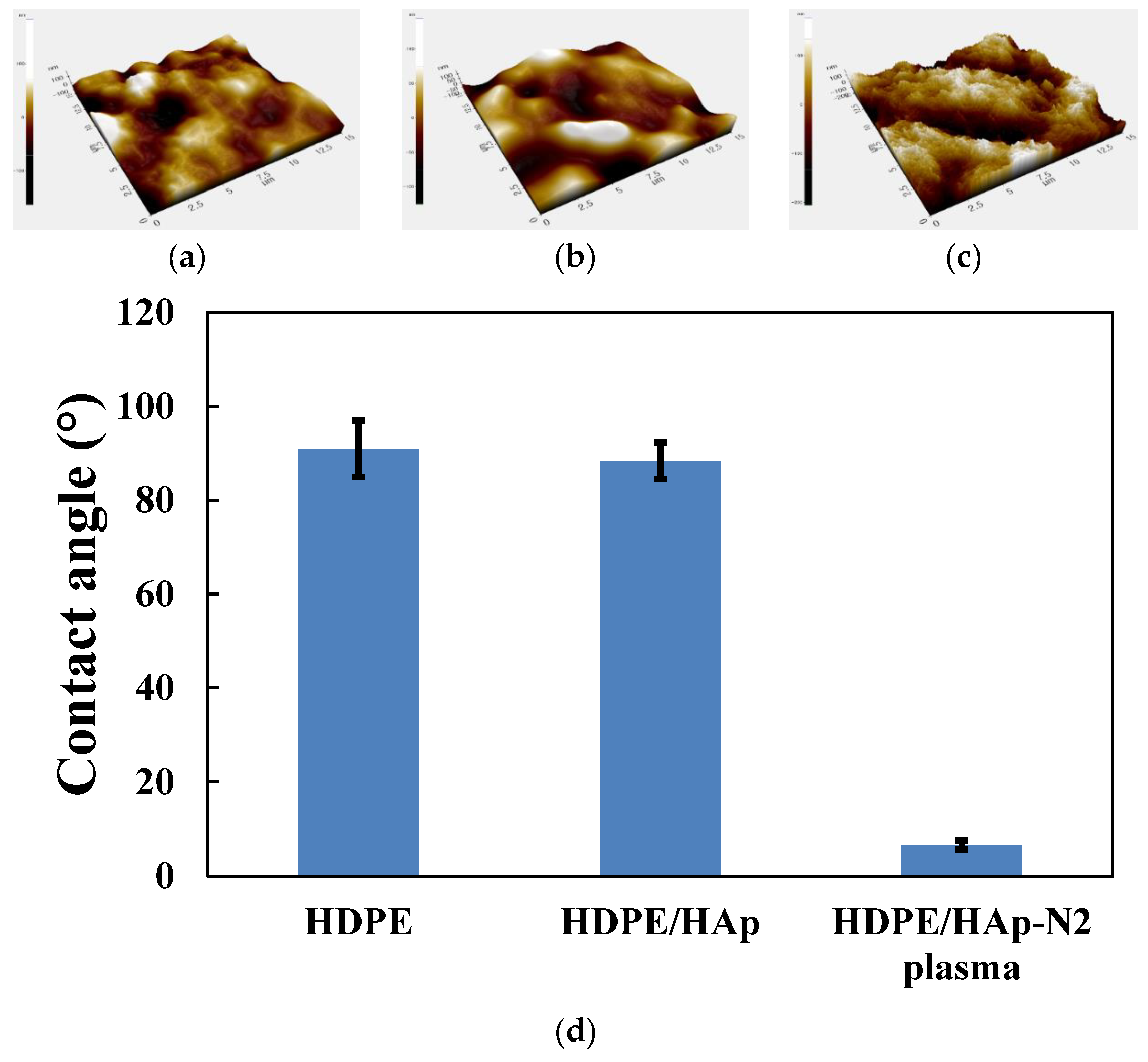
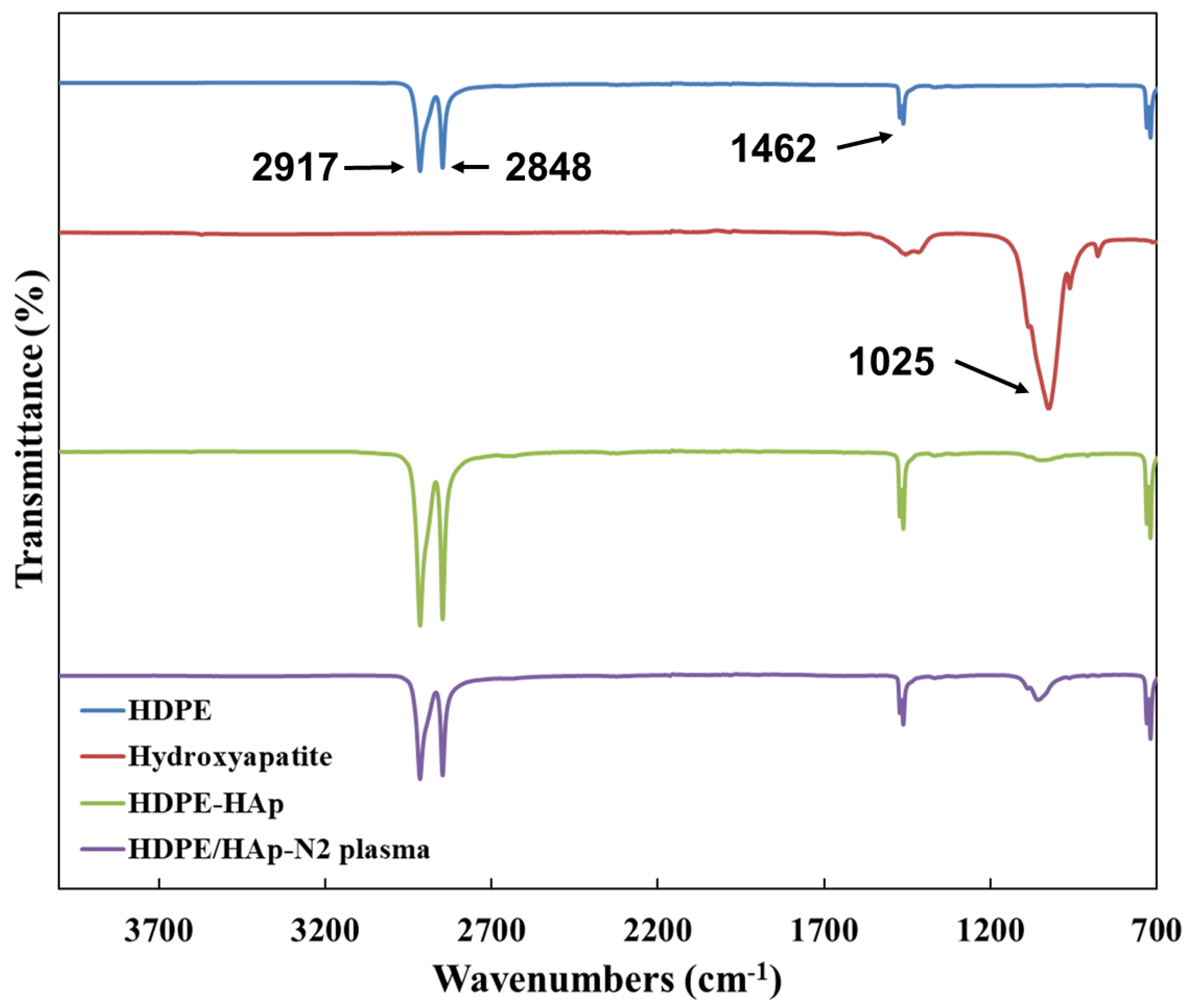
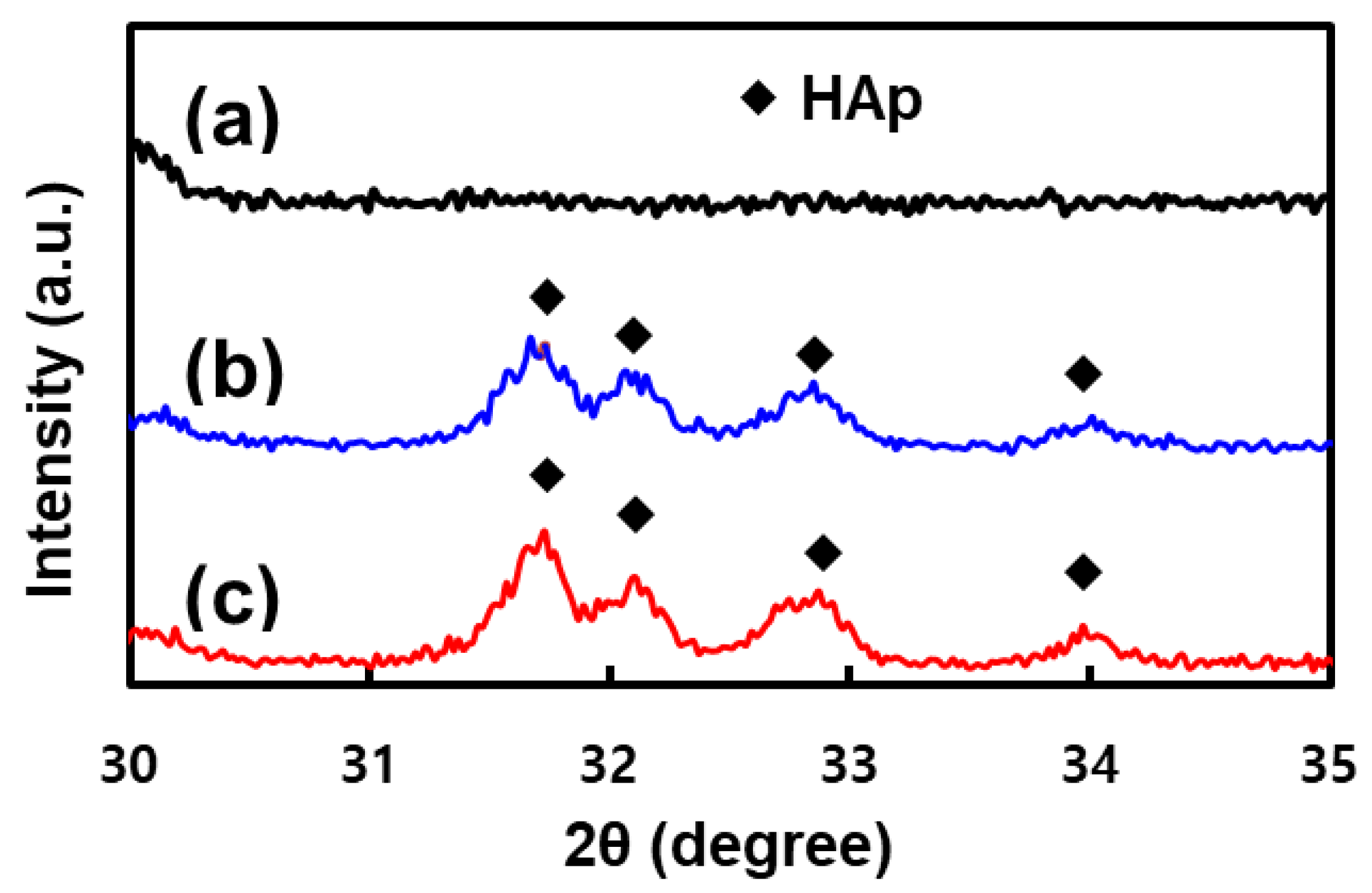
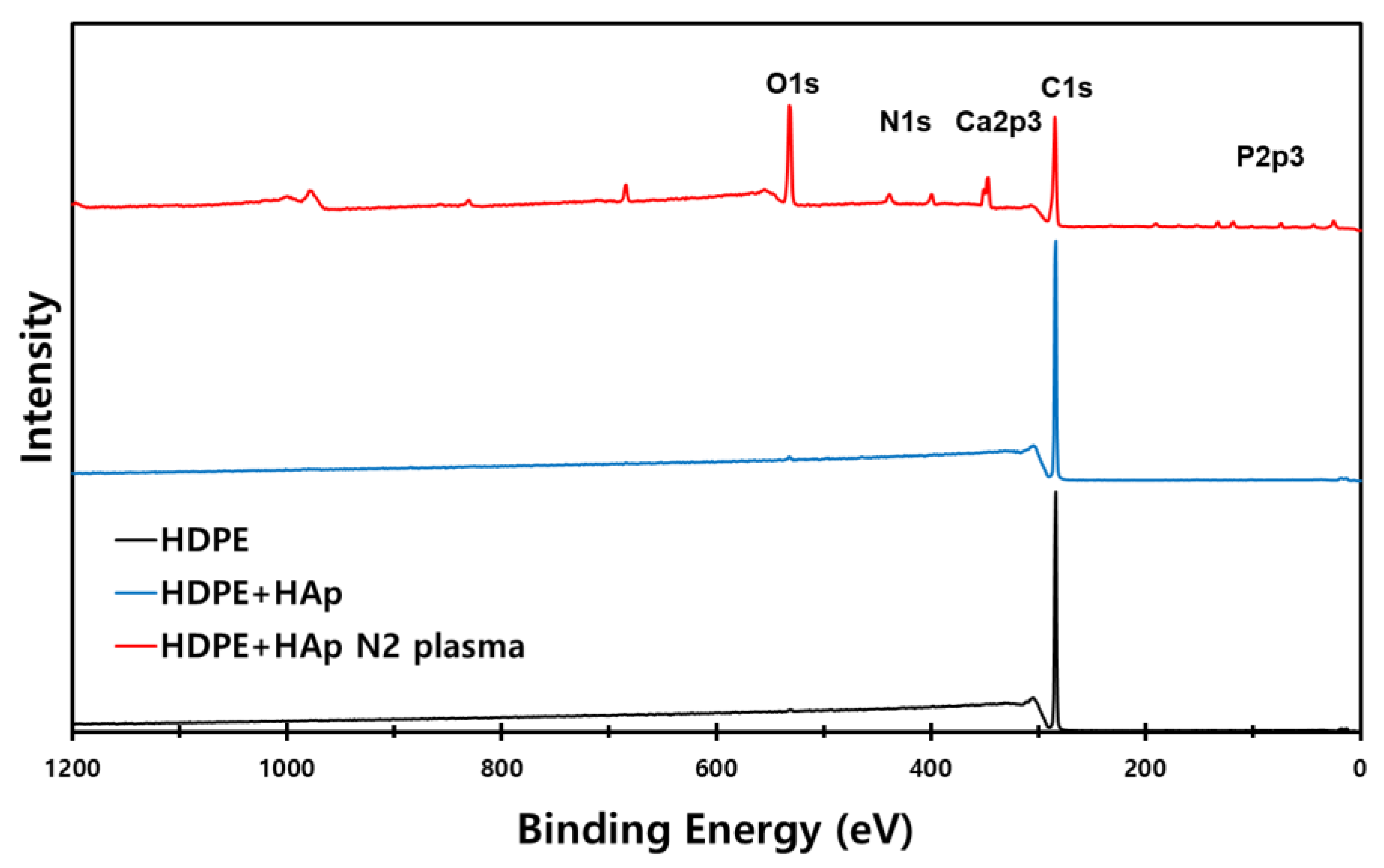
3.1. Surface Characterization
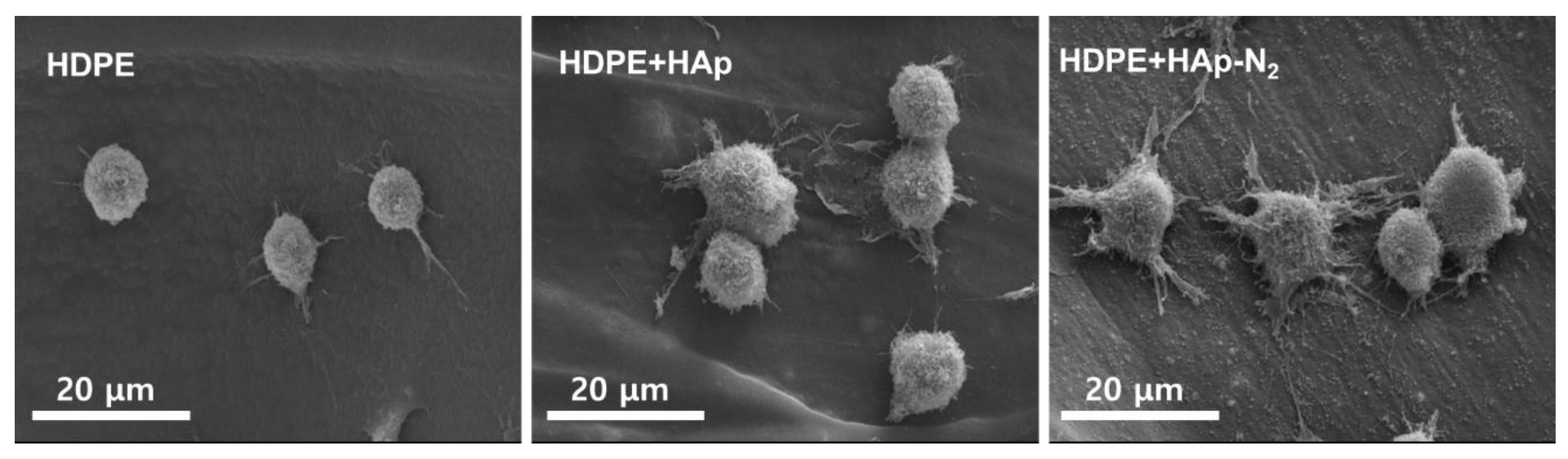
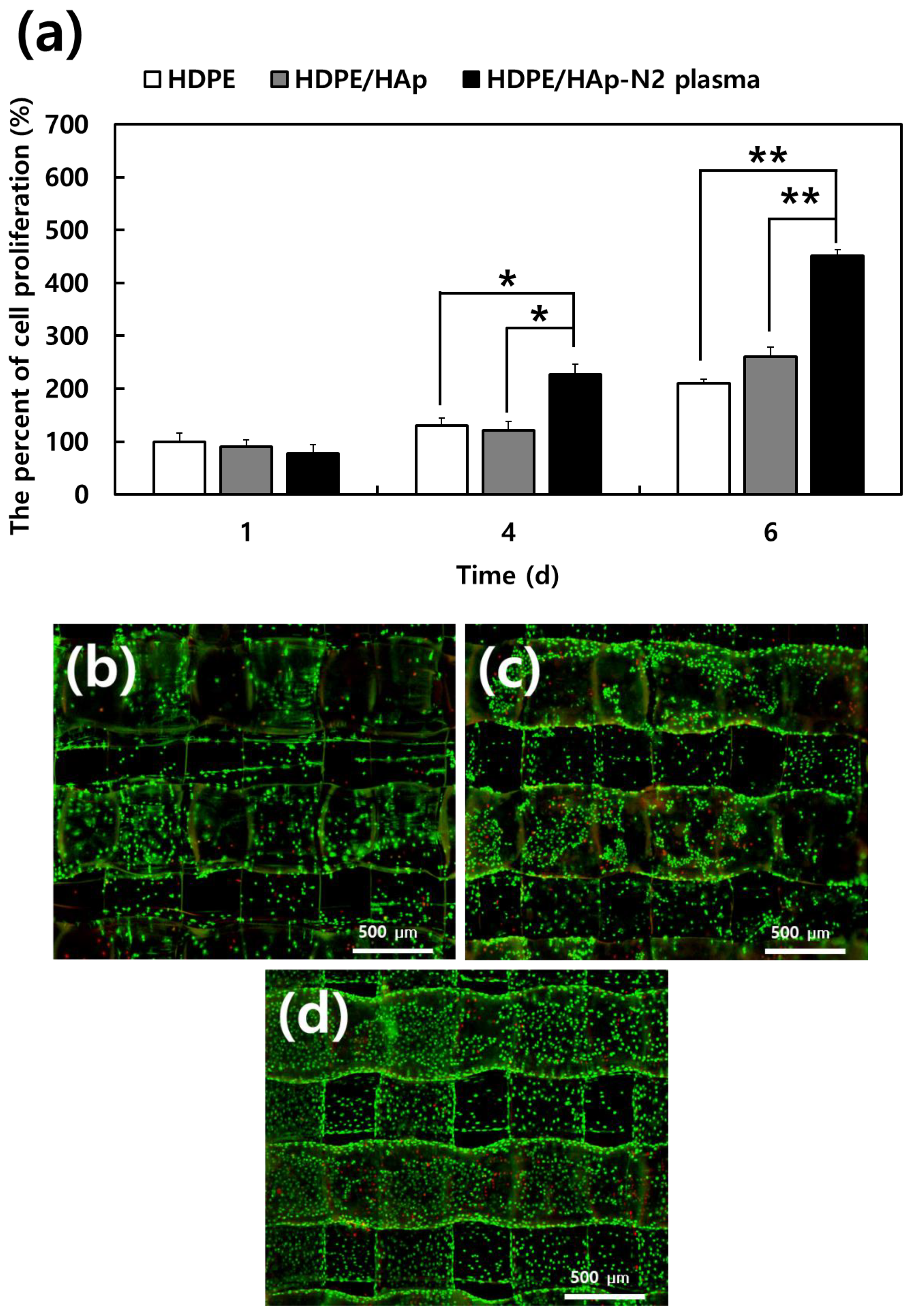
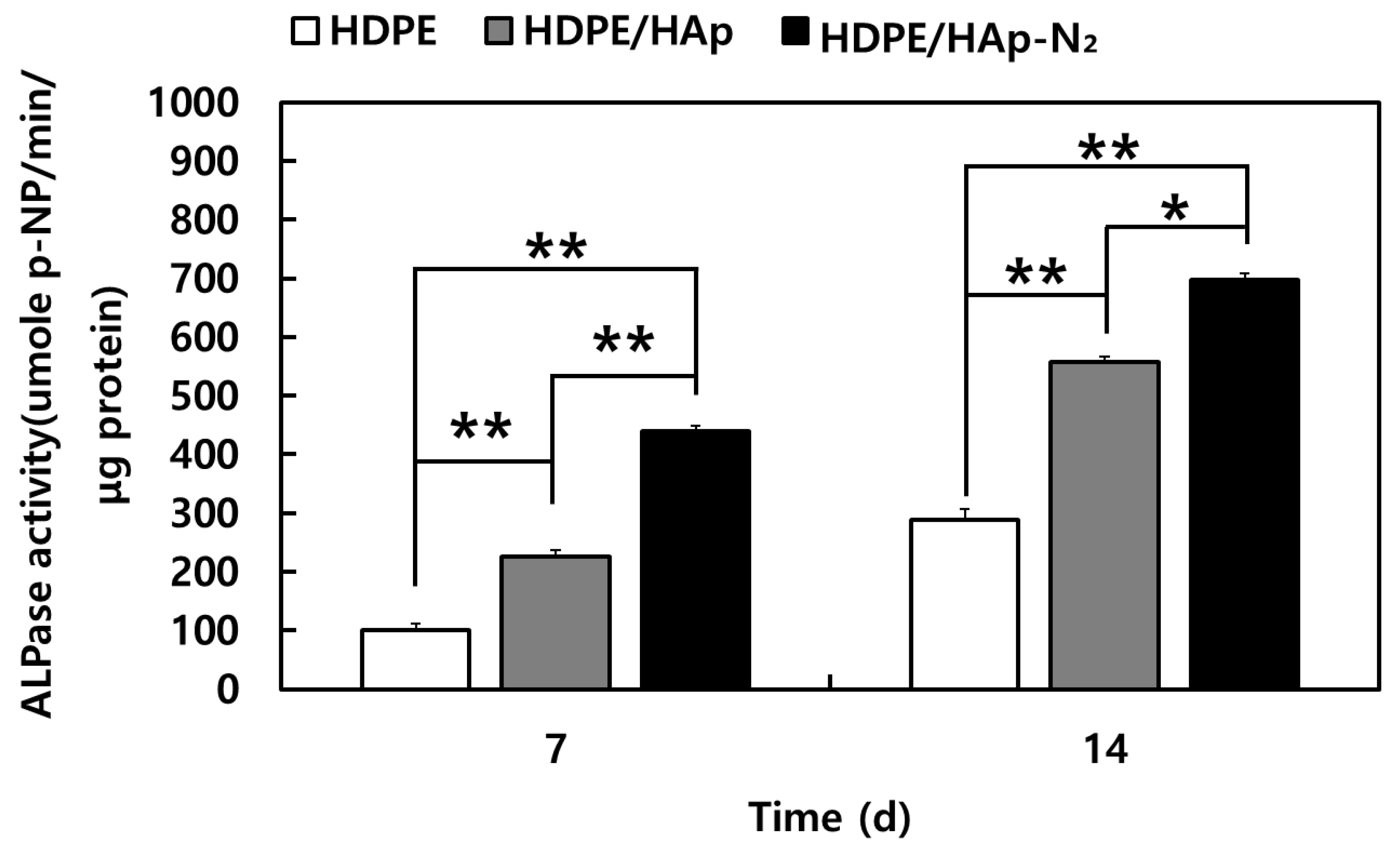
3.2. Evaluations of MC3T3-E1 Cell Bioactivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, P.C.; Mikos, A.G.; Fisher, J.P.; Jansen, J.A. Strategic directions in tissue engineering. Tissue Eng. 2007, 13, 2827–2837. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Lee, C.H.; Tan, J.; Lu, C.; Mendelson, A.; Chen, M.; Embree, M.C.; Kong, K.; Shah, B.; Wang, S.; et al. Mus culoskeletal tissue engineering by endogenous stem cells. Cell Tissue Res. 2012, 347, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; Zaky, S.; Ray, H.; Sfeir, C. Porous magnesium/PLGA composite scaffolds for enhanced bone regeneration following tooth extraction. Acta Biomater. 2015, 11, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.T.; Scott, D.D. A review of bone substitutes. Oral Maxillofac. Surg. Clin. N. Am. 2007, 19, 513–521. [Google Scholar] [CrossRef]
- Evaniew, N.; Tan, V.; Parasu, N.; Jurriaans, E.; Finlay, K.; Deheshi, B.; Ghert, M. Use of a calcium sulfate-calcium phosphate synthetic bone graft composite in the surgical management of primary bone tumors. Orthopedics 2013, 36, e216–e222. [Google Scholar] [CrossRef]
- Kirkpatrick, J.S.; Cornell, C.N.; Hoang, B.H.; Hsu, W.; Watson, J.T.; Watters, W.C., 3rd; Turkelson, C.M.; Wies, J.L.; Anderson, S. Bone void fillers. J. Am. Acad. Orthop. Surg. 2010, 18, 576–579. [Google Scholar] [CrossRef]
- Titsinides, S.; Agrogiannis, G.; Karatzas, T. Bone grafting materials in dentoalveolar reconstruction: A comprehensive review. Jpn. Dent. Sci. Rev. 2019, 55, 26–32. [Google Scholar] [CrossRef]
- Niechajev, I. Facial Reconstruction Using Porous High-Density Polyethylene (Medpor): Long-Term Results. Aesthetic Plast. Surg. 2012, 36, 917–927. [Google Scholar] [CrossRef]
- Lim, J.S.; Kook, M.S.; Jung, S.; Park, H.J.; Ohk, S.H.; Oh, H.K. Plasma Treated High-Density Polyethylene (HDPE) Medpor Implant Immobilized with rhBMP-2 for Improving the Bone Regeneration. J. Nanomater. 2014, 2014, 810404. [Google Scholar] [CrossRef]
- Andrade, N.N.; Raikwar, K. Medpor in maxillofacial deformities: Report of three cases. J. Maxillofac. Oral Surg. 2009, 8, 192–195. [Google Scholar] [CrossRef][Green Version]
- Bhattacharjee, A.; Fang, Y.; Hooper, T.J.N.; Kelly, N.L.; Gupta, D.; Balani, K.; Manna, I.; Baikie, T.; Bishop, P.T.; White, T.J.; et al. Crystal chemistry and antibacterial properties of cupriferous hydroxyapatite. Materials 2019, 12, 1814. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; Pinheiro, A.; De Oliveira, R.; Goes, J.C.; Aranha, N.; De Oliveira, L.; Sombra, A. Properties and in vivo investigation of nanocrystalline hydroxyapatite obtained by mechanical alloying. Mater. Sci. Eng. C 2004, 24, 549–554. [Google Scholar] [CrossRef]
- Gaihre, B.; Jayasuriya, A.C. Comparative investigation of porous nano-hydroxyapatite/chitosan, nanozirconia/chitosan and novel nano-calcium zirconate/chitosan composite scaffolds for their potential applications in bone regeneration. Mater. Sci. Eng. C 2018, 91, 330–339. [Google Scholar] [CrossRef]
- Roether, J.A.; Boccaccini, A.R.; Hench, L.L.; Maquet, V.; Gautier, S.; Jérôme, R. Development and in vitro characterisation of novel bioresorbable and bioactive composite materials based on polylactide foams and Bioglass® for tissue engineering applications. Biomaterials 2002, 23, 3871–3878. [Google Scholar] [CrossRef]
- Larrañaga, A.; Alonso-Varona, A.; Palomares, T.; Rubio-Azpeitia, E.; Aldazabal, P.; Martin, F.J.; Sarasua, J.R. Effect of bioactive glass particles on osteogenic differentiation of adipose-derived mesenchymal stem cells seeded on lactide and caprolactone based scaffolds. J. Biomed. Mater. Res. A 2015, 103, 3815–3824. [Google Scholar] [CrossRef] [PubMed]
- Haaparanta, A.M.; Haimi, S.; Ellä, V.; Hopper, N.; Miettinen, S.; Suuronen, R.; Kellomäki, M. Porous polylactide/β-tricalcium phosphate composite scaffolds for tissue engineering applications. J. Tissue Eng. Regen. Med. 2010, 4, 366–373. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, B.; Wu, B.; Lee, M. Customized biomimetic scaffolds created by indirect three-dimensional printing for tissue engineering. Biofabrication 2013, 5, 045003. [Google Scholar] [CrossRef]
- Reed, S.; Lau, G.; Delattre, B.; Lopez, D.D.; Tomsia, A.P.; Wu, B.M. Macro- and micro-designed chitosan-alginate scaffold architecture by three-dimensional printing and directional freezing. Biofabrication 2016, 8, 015003. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef]
- Salmoria, G.V.; Fancello, E.A.; Roesler, C.R.M.; Dabbas, F. Functional graded scaffold of HDPE/HA prepared by selective laser sintering: Microstructure and mechanical properties. Int. J. Adv. Manuf. Technol. 2013, 65, 1529–1534. [Google Scholar] [CrossRef]
- Suwanprateeb, J.; Thammarakcharoen, F.; Wongsuvan, V.; Chokevivat, W. Development of porous powder printed high density polyethylene for personalized bone implants. J. Porous Mater. 2012, 19, 623–632. [Google Scholar] [CrossRef]
- Kim, C.S.; Jung, K.H.; Kim, H.; Kim, C.B.; Kang, I.K. Collagen-grafted porous HDPE/PEAA scaffolds for bone reconstruction. Biomater. Res. 2016, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, T.; Morent, R.; Geyter, N.D.; Dubruel, P.; Leys, C. Plasma Surface Modification of Biomedical Polymers: Influence on Cell-Material Interaction. Plasma Chem. Plasma Processing 2012, 32, 1039–1073. [Google Scholar] [CrossRef]
- Nedela, O.; Slepicka, P.; Svorcik, V. Surface Modification of Polymer Substrates for Biomedical Applications. Materials 2017, 10, 1115. [Google Scholar] [CrossRef] [PubMed]
- Davoodi, A.; Zadeh, H.H.; Joupari, M.D.; Sahebalzamani, M.A.; Khani, M.R.; Shahabi, S. Physicochemical- and biocompatibility of oxygen and nitrogen plasma treatment using a PLA scaffold. AIP Adv. 2020, 10, 125205. [Google Scholar] [CrossRef]
- Roh, H.S.; Jung, S.C.; Kook, M.S.; Kim, B.H. In vitro study of 3D PLGA/n-HAp/β-TCP composite scaffolds with etched oxygen plasma surface modification in bone tissue engineering. Appl. Surf. Sci. 2016, 388, 321–330. [Google Scholar] [CrossRef]
- Charles, J.; Ramkumaar, G.R. Qualitative Analysis of High Density Polyethylene Using FTIR Spectroscopy. Asian J. Chem. 2009, 21, 4477–4484. [Google Scholar]
- Lin, J.H.; Pan, Y.J.; Liu, C.F.; Huang, C.L.; Hsieh, C.T.; Chen, C.K.; Lin, Z.I.; Lou, C.W. Preparation and Compatibility Evaluation of Polypropylene/High Density Polyethylene Polyblends. Materials 2015, 8, 8850–8859. [Google Scholar] [CrossRef]
- Khan, S.M.; Muhammad, N.G.; Munawar, A.; Islam, A.; Zia, S.; Shafiq, M.; Sabir, A.; Awais, S.M.; Butt, M.A.; Butt, M.T.Z.; et al. 2D carbon fiber reinforced high density polyethylene multi-layered laminated composite panels structural, mechanical, thermal and morphological profile. J. Mater. Sci. Technol. 2016, 32, 1077–1082. [Google Scholar] [CrossRef]
- Bianco, A.; Cacciotti, I.; Lombardi, M.; Montanaro, L.; Bemporad, E.; Sebastiani, M. F-substituted hydroxyapatite nanopowders: Thermal stability, sintering behaviour and mechanical properties. Ceram. Int. 2010, 36, 313–322. [Google Scholar] [CrossRef]
- Slosarczyk, A.; Paszkiewicz, Z.; Paluszkiewicz, C. FTIR and XRD evaluation of carbonated hydroxyapatite powders synthesized by wet methods. J. Mol. Struct. 2005, 744–747, 657–661. [Google Scholar] [CrossRef]
- Kannan, S.; Rebelo, A.; Ferreira, J.M.F. Novel synthesis and structural characterization of fluorine and chlorine co-substituted hydroxyapatites. J. Inorg. Biochem. 2006, 100, 1692–1697. [Google Scholar] [CrossRef] [PubMed]
- Pulyala, P.; Singh, A.; Dias-Netipanyj, M.F.; Cogo, S.C.; Santos, L.S.; Soares, P.; Gopal, V.; Suganthan, V.; Manivasagam, G.; Popat, K.C. In-Vitro cell adhesion and proliferation of adipose derived stem cell on hydroxyapatite composite surfaces. Mater. Sci. Eng. C 2017, 75, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Li, P.; Lu, X.; Fang, L.; Lü, X.; Ren, F. A strong, tough, and osteoconductive hydroxyapatite mineralized polyacrylamide/dextran hydrogel for bone tissue regeneration. Acta Biomater. 2019, 88, 503–513. [Google Scholar] [CrossRef]
- Dai, C.; Li, Y.; Pan, W.; Wang, G.; Huang, R.; Bu, Y.; Liao, X.; Guo, K.; Gao, F. Three-dimensional high-porosity chitosan/honeycomb porous carbon/hydroxyapatite scaffold with enhanced osteoinductivity for bone regeneration. ACS Biomater. Sci. Eng. 2019, 6, 575–586. [Google Scholar] [CrossRef]
- Pertile, R.A.N.; Andrade, F.K.; Alves, C., Jr.; Gama, M. Surface modification of bacterial cellulose by nitrogen-containing plasma for improved interaction with cells. Carbohydr. Polym. 2010, 82, 692–698. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Desmet, T.; Dubruel, P.; Leys, C. Plasma Surface Modification of Biodegradable Polymers: A Review. Plasma Processes Polym. 2011, 8, 171–190. [Google Scholar] [CrossRef]
- Vlachopoulou, M.E.; Kokkoris, G.; Cardinaud, C.; Gogolides, E.; Tserepi, A. Plasma etching of poly(dimethylsiloxane): Roughness formation, mechanism, control, and application in the fabrication of microfluidic structures. Plasma Processes Polym. 2013, 10, 29–40. [Google Scholar] [CrossRef]
- Jacobs, T.; De Geyter, N.; Morent, R.; Desmet, T.; Dubruel, P.; Leys, C. Plasma treatment of polycaprolactone at medium pressure. Surf. Coat. Technol. 2011, 205, S543–S547. [Google Scholar] [CrossRef]
- Vlad, M.D.; Aguado, E.F.; González, S.G.; Ivanov, I.C.; Şindilar, E.V.; Poeată, I.; Iencean, A.Ş.; Butnaru, M.; Avădănei, E.R.; López, J.L. Novel titanium-apatite hybrid scaffolds with spongy bone-like micro architecture intended for spinal application: In vitro and in vivo study. Mater. Sci. Eng. C 2020, 110, 110658. [Google Scholar] [CrossRef]
- Nezafati, N.; Farokhi, M.; Heydari, M.; Hesaraki, S.; Nasab, N.A. In vitro bioactivity and cytocompatablity of an injectable calcium phosphate cement/silanated gelatin microsphere composite bone cement. Compos. B Eng. 2019, 175, 107146. [Google Scholar] [CrossRef]
- Chou, Y.; Huang, W.; Dunn, J.C.Y.; Miller, T.A.; Wu, B.M. The Effect of Biomimetic Apatite Structure on Osteoblast Viability, Proliferation, and Gene Expression. Biomaterials 2005, 26, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.; Konrad, L.; Gotzen, L.; Printz, H.; Ramaswamy, A.; Hofmann, C. Bioengineered Human Bone Tissue Using Autogenous Osteoblasts Cultured on Different Biomatrices. J. Biomed. Mater. Res. 2003, 67A, 191–199. [Google Scholar] [CrossRef]
- Inoue, M.; LeGeros, R.Z.; Inoue, M.; Tsujigiwa, H.; Nagatsuka, H.; Yamamoto, T.; Nagai, N. In Vitro Response of Osteoblast-Like and Odontoblast-Like Cells to Unsubstituted and Substituted Apatites. J. Biomed. Mater. Res. 2004, 70A, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Ogata, K.; Imazato, S.; Ehara, A.; Ebisu, S.; Kinomoto, Y.; Nakano, T.; Umakoshi, Y. Comparison of Osteoblast Responses to Hydroxyapatite and Hydroxyapatite/Soluble Calcium Phosphate Composites. J. Biomed. Mater. Res. 2005, 72A, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.J.; Fairbrother, D.H.; Reniers, F.A. Comparison of PE surfaces modified by plasma generated neutral nitrogen species and nitrogen ions. Plasmas Polym. 2003, 8, 119–134. [Google Scholar] [CrossRef]
- Zhang, W.; Luo, Y.J.; Chu, P.K. Biocompatibility of metal and gas plasma modified polyethylene with antibacterial properties. Acta Biomater. 2008, 4, 2028–2036. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Ji, J.H.; Zhang, W.; Jiang, J.; Zhang, Y.H.; Pu, S.H.; Chu, P.K. Osteoblast behavior on plasma-treated biodegradable poly(butylene succinate). Acta Biomater. 2009, 5, 279–287. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Oyane, A.; Tsurushima, H.H.; Chu, P.K. Osteoblast differentiation and disinfection induced by nitrogen plasma-treated surfaces. Biomed. Mater. Eng. 2011, 21, 75–82. [Google Scholar] [CrossRef]
- Mohsenimehr, S.; Khani, M.R.; Fani, N.; Eslaminejad, M.R.B.; Shokri, B.; Ghassami, A. Surface modification of PLA scaffold using radio frequency (RF) nitrogen plasma in tissue engineering application. Surf. Topogr. Metrol. Prop. 2020, 8, 015012. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-C.; Ryu, J.; Jung, S.; Park, H.-J.; Oh, H.-K.; Kook, M.-S. Effect of Hydroxyapatite Nanoparticles and Nitrogen Plasma Treatment on Osteoblast Biological Behaviors of 3D-Printed HDPE Scaffold for Bone Tissue Regeneration Applications. Materials 2022, 15, 827. https://doi.org/10.3390/ma15030827
Park H-C, Ryu J, Jung S, Park H-J, Oh H-K, Kook M-S. Effect of Hydroxyapatite Nanoparticles and Nitrogen Plasma Treatment on Osteoblast Biological Behaviors of 3D-Printed HDPE Scaffold for Bone Tissue Regeneration Applications. Materials. 2022; 15(3):827. https://doi.org/10.3390/ma15030827
Chicago/Turabian StylePark, Hyun-Chul, Jaeyoung Ryu, Seunggon Jung, Hong-Ju Park, Hee-Kyun Oh, and Min-Suk Kook. 2022. "Effect of Hydroxyapatite Nanoparticles and Nitrogen Plasma Treatment on Osteoblast Biological Behaviors of 3D-Printed HDPE Scaffold for Bone Tissue Regeneration Applications" Materials 15, no. 3: 827. https://doi.org/10.3390/ma15030827
APA StylePark, H.-C., Ryu, J., Jung, S., Park, H.-J., Oh, H.-K., & Kook, M.-S. (2022). Effect of Hydroxyapatite Nanoparticles and Nitrogen Plasma Treatment on Osteoblast Biological Behaviors of 3D-Printed HDPE Scaffold for Bone Tissue Regeneration Applications. Materials, 15(3), 827. https://doi.org/10.3390/ma15030827





