The Useful Life of Reinforced Concrete Structures with Reinforcement Corrosion Due to Carbonation in Non-Aggressive and Normal Exposures in the Spanish Mediterranean
Abstract
:1. Introduction
1.1. Background
1.2. State of Affairs
2. Objectives
- The detection, measurement and analysis of corrosion processes in reinforced concrete elements in non-aggressive and normal environments where corrosion by chlorides does not occur. For this purpose, a sample of ten buildings was analyzed, studying elements of their structure damaged by the corrosion of their rebars.
- The application of the EHE instruction regulating the execution and control of structural concrete elements for the studied environments that considers a certain start time for the corrosion process, simulating the conditions that occur in the coating of the reinforcement in an attack by carbonation up to the initial state of the propagation process on the rebar, and later, when it is in its propagation period, that is, in full development. In this phase, and for the media studied, it is necessary to consider the decrease in pH at the level of the steel-concrete interface produced by the hydrolysis of Fe++ions, resulting in the oxidation of the steel, which contributes to the increase in the speed of the cathodic process.
- Study of the initiation functions of carbonation corrosion.
- Study of the propagation functions of corrosion by carbonation.
- Theoretical depth of carbonation according to the age of the structure.
- Analysis of the factors that influence the process.
- Evolution of the corrosion rate [34].
3. Methodology
3.1. Structural Elements in the Different Exposure Environments
3.2. Data Collection from Experiments
- Compression resistance tests. In all the seventeen elements studied, and in each case for the corresponding laboratory, we proceeded with the extraction of test specimen controls by means of a 75 mm diameter rotating probe, in accordance with the UNE-EN 12504-1 and UNE-EN 12390-3 standards to determine the compressive strength of the concrete of the said elements.
- In many cases, non-destructive information tests were carried out by measuring the speed of ultrasound propagation in the elements with specimen extraction and in others to know their resistance from a comparative study. This information has not been used in this article and is the subject of future research.
- Carbonation depth test, taking a sample from the reinforced concrete element and applying an alcoholic phenolphthalein solution that produces a pink hue if the sample is not carbonated (pH greater than 9). Simple visual inspection reveals the carbonate area as the one that remains colorless (Figure 3).
- Coating of concrete. Given the conditions of execution of the element (verticality, irregularities, absence of spacers, state of the reinforcements) and the measurement errors by the flexometer of +/−5 mm, minimum coverings of 20 mm were considered in all cases.
- Diameter of the reinforcements. In all the columns, the behavior of both the transverse reinforcements (6 and 8 mm in diameter) and the longitudinal reinforcements (16 and 20 mm in diameter) was studied. A total of 34 trusses in 17 columns were analyzed.
- Building age. All the buildings are the product of extensive professional experience in the Spanish Mediterranean area, with the examples studied having ages from 9 to 44 years, and they can be considered a representative sample of buildings of their type. Their age was obtained from the data provided by the property and checked with the construction date that appears in the land registry file (Virtual Headquarters of the Catastro) in each case. They mostly date back to the 1970s, when the regulations for concrete stipulated characteristic strengths of 150 Kg/cm2 and minimum coverings greater than 10 mm or the diameter of the longitudinal bars.
3.3. Carbonation Corrosion
3.4. Initiation of Corrosion Period
3.5. Corrosion Rate
3.6. Propagation of Corrosion Period
- t = propagation time in years
- x = depth of concrete considered (reinforcement cover) in mm
- ∅ = reinforcement diameter in mm
- V = rate of corrossion in μm/year
3.7. Estimation of the Structure’s Useful Life in View of the Corrosion of the Reinforcements
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Wang, H.; Wang, J.; Liu, J. Experimental Analysis of Reinforcement Rust in Cement under Corrosive Environment. Coatings 2021, 11, 241. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, A.; Zhang, L.; Liu, J.; Han, Y.; Wang, J. Research on the Influence of Carbonation on the Content and State of Chloride Ions and the Following Corrosion Resistance of Steel Bars in Cement Paste. Coatings 2020, 10, 1071. [Google Scholar] [CrossRef]
- Song, D.; Yang, F.; Guo, M.; Zhao, S.; Hao, J.; Chen, Z.; Sun, J.; Xu, Y.; Jiang, J. Surface Modification of Rusted Rebar and Enhanced Passivation/Anticorrosion Performance in Simulated Concrete Pore Solutions with Different Alk. Metals 2019, 9, 1050. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Choe, D.-E.; Castro-Borges, P.; Castaneda, H. Probabilistic Corrosion Initiation Model for Coastal Concrete Structures. Corros. Mater. Degrad. 2020, 1, 328–344. [Google Scholar] [CrossRef]
- Garces, P.; Andrade, M.C.; Saez, A.; Alonso, M.C. Corrosion of reinforcing steel in neutral and acid solutions simulating the electrolytic environments in the micropores of concrete in the propagation period. Corros. Sci. 2005, 47, 289–306. [Google Scholar] [CrossRef]
- Garcés, P.; Saura, P.; Méndez, A.; Zornoza, E.; Andrade, C. Effect of nitrite in corrosion of reinforcing steel in neutral and acid solutions simulating the electrolytic environments of micropores of concrete in the propagation period. Corros. Sci. 2008, 50, 498–509. [Google Scholar] [CrossRef]
- Garcés, P.; Saura, P.; Zornoza, E.; Andrade, C. Influence of pH on the nitrite corrosion inhibition of reinforcing steel in simulated concrete pore solution. Corros. Sci. 2011, 53, 3991–4000. [Google Scholar] [CrossRef]
- Saura, P.; Zornoza, E.; Andrade, C.; Garcés, P. Steel Corrosion-Inhibiting Effect of Sodium Nitrate in Simulated Concrete Pore Solutions. Corrosion 2011, 67, 075005-1. [Google Scholar] [CrossRef]
- Shi, J.; Wu, M.; Ming, J. Degradation effect of carbonation on electrochemical behavior of 2304 duplex stainless steel in simulated concrete pore solutions. Corros. Sci. 2020, 177, 109006. [Google Scholar] [CrossRef]
- Moreno, M.; Morris, W.; Alvarez, M.; Duffó, G. Corrosion of reinforcing steel in simulated concrete pore solutions: Effect of carbonation and chloride content. Corros. Sci. 2004, 46, 2681–2699. [Google Scholar] [CrossRef]
- Andrade, C. Whitney Award Lecture: Probabilistic Treatment of the Reinforcement Corrosion. Corrosion 2014, 70, 643–651. [Google Scholar] [CrossRef]
- Xiao, M.; Zhang, S.; Tang, Y.; Lin, Z.; Chen, J. A study of a real-time online monitoring system for the durability of concrete structures. Anti-Corros. Methods Mater. 2016, 63, 184–189. [Google Scholar] [CrossRef]
- Garcés, P.; Climent, M.A.; Zornoza, E. Corrosión de Armaduras en Estructuras de Hormigón Armado; Club Universitario: Alicante, Spain, 2008. [Google Scholar]
- Tutti, K. Corrosion of Steel in Concrete; Swedish Foundation for Concrete Research: Estocolmo, Sweden, 1982. [Google Scholar]
- Melchers, R. Experience-Based Physico-Chemical Models for Long-Term Reinforcement Corrosion. Corros. Mater. Degrad. 2021, 2, 100–119. [Google Scholar] [CrossRef]
- Yan, Y.; Ling, W.; Folker, W. Publications on Durability of Reinforced Concrete Structures under Combined Mechanical Loads and Environmental Actions: An Annotated Bibliography; Aedificatio Publishers: Shinjuku, Tokyo, 2013; ISBN 978-3-942052-03-0. [Google Scholar]
- Guía Para la Inspección y Evaluación Complementaria de Estructuras de Hormigón en Edificios Existentes; Consellería de Medio Ambiente, Agua, Urbanismo y Vivienda; Instituto Valenciano de la Edificación: Valencia, Spain; Generallitat Valenciana: Valencia, Spain, 2008.
- Instrucción para el Proyecto y Ejecución de Obras de Hormigón; Orden de 3 de febrero de; Ministerio de Obras Públicas: Santiago, Chile, 1939.
- Instrucción Definitiva para el Proyecto y Ejecución de Obras de Hormigón; Orden de 23 de marzo de; Ministerio de Obras Públicas: Santiago, Chile, 1944.
- Instrucción para el Proyecto y Ejecución de Obras de Hormigón en Masa y Armado; Decreto 2987/1968 de 20 de febrero; Presidencia del Gobierno: Madrid, Spain, 1968.
- Instrucción Para el Proyecto y Ejecución de Obras de Hormigón en Masa o Armado; Decreto 3062/1973 de 20 de febrero, Instrucción EH-73; Presidencia del Gobierno: Madrid, Spain, 1973.
- Instrucción para el Proyecto y Ejecución de Obras de Hormigón en Masa o Armado (EH-80); Real Decreto 2868/1980 de 17 de octubre; Ministerio de Obras Públicas y Urbanismo: Madrid, Spain, 1973.
- Instrucción para el Proyecto y Ejecución de Obras de Hormigón en masa o armado (EH-82); Real Decreto 2262/1982 de 24 de julio; Ministerio de Obras Públicas y Urbanismo: Madrid, Spain, 1982.
- Instrucción Para el Proyecto y Ejecución de Obras de Hormigón en Masa o Armado (EH-88) y la Instrucción para el Proyecto y la Ejecución de Forjados Unidireccionales de Hormigón Armado o Pretensado (EF-88); Real Decreto; Ministerio de Obras Públicas y Urbanismo: Madrid, Spain, 1988.
- Instrucción para el Proyecto y Ejecución de Obras de Hormigón en masa o armado (EH-91); Real Decreto 1039/1991 de 28 de junio; Ministerio de Obras Públicas y Transportes: Madrid, Spain, 1991.
- Instrucción de Hormigón Estructural (ECH-99); Ministerio de Fomento; Real Decreto 2661/1998 de 11 de diciembre; Publisher: Madrid, Spain, 1998.
- Instrucción de Hormigón Estructural (EHE-08); Real Decreto 1247/2008 de 18 de julio; Ministerio de la Presidencia: Madrid, Spain, 2008.
- Ebell, G.; Burkert, A.; Mietz, J. Detection of Reinforcement Corrosion in Reinforced Concrete Structures by Potential Mapping: Theory and Practice. Int. J. Corros. 2018, 2018, 1–6. [Google Scholar] [CrossRef]
- Rodríguez, J.; Andrade, C.; Izquierdo, D.; Tanner, P. El Eurocódigo 2 y la evaluación de estructuras de hormigón armado con armaduras corroídas. Hormigón y Acero 2014, 65, 123–132. [Google Scholar] [CrossRef]
- L’Hostis, V. Long-term corrosion of rebars submitted to concrete carbonation. Mater. Corros. 2020, 71, 777–785. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, Z.; Wang, H.; Chen, Y.; Xu, L. Probabilistic Generalization of a Comprehensive Model for the Deterioration Prediction of RC Structure under Extreme Corrosion Environments. Sustainability 2018, 10, 3051. [Google Scholar] [CrossRef] [Green Version]
- Geng, J.; Liu, J.; Yan, J.; Ba, M.; He, Z.; Li, Y. Chemical Composition of Corrosion Products of Rebar Caused by Carbonation and Chloride. Int. J. Corros. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Burtuujin, G.; Son, D.; Jang, I.; Yi, C.; Lee, H. Corrosion Behavior of Pre-Rusted Rebars in Cement Mortar Exposed to Harsh Environments. Appl. Sci. 2020, 10, 8705. [Google Scholar] [CrossRef]
- Figueira, R.B. Electrochemical Sensors for Monitoring the Corrosion Conditions of Reinforced Concrete Structures: A Review. Appl. Sci. 2017, 7, 1157. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.-K.; Park, J.-S. Experimental Evaluation of the Durability of Concrete Repair Materials. Appl. Sci. 2021, 11, 2303. [Google Scholar] [CrossRef]
- Schroeder, R.; Müller, I. Stress corrosion cracking and hydrogen embrittlement susceptibility of an eutectoid steel employed in prestressed concrete. Corros. Sci. 2003, 45, 1969–1983. [Google Scholar] [CrossRef]
- Proverbio, E.; Longo, P. Failure mechanisms of high strength steels in bicarbonate solutions under anodic polarization. Corros. Sci. 2003, 45, 2017–2030. [Google Scholar] [CrossRef]
- Alonso, C.; Andrade, C. Life time of rebars in carbonated concrete. In Progress in Understanding and Prevention of Corrosion; Costa, J.M., Mercer, A.D., Eds.; Institute of Materials: Londres, UK, 1994; p. 624. [Google Scholar]
- Sergi, G. Corrosion of Steel in Concrete: Cement Matrix Variables. Ph.D. Thesis, Universidad de Aston, Birmingham, UK, 1986. [Google Scholar]
- Pu, Q.; Jiang, L.; Xu, J.; Chu, H.; Xu, Y.; Zhang, Y. Evolution of pH and chemical composition of pore solution in carbonated concrete. Constr. Build. Mater. 2012, 28, 519–524. [Google Scholar] [CrossRef]
- Yoon, I.-S.; Chang, C.-H. Time Evolution of CO2 Diffusivity of Carbonated Concrete. Appl. Sci. 2020, 10, 8910. [Google Scholar] [CrossRef]
- Alonso, C.; Andrade, C.; Balancán, M.; Barra, M.; Bonilla, M.; Borrachero, M.V.; Castro Borges, P.; Climent, M.A.; Fernández Cánovas, M.; De Vera, G.; et al. Procesos de Degradación Físico-Químicos en Estructuras de Hormigón Armado; Garcés, P., Ed.; Publicacions Universitat d’Alacant: Alicante, Spain, 2021. [Google Scholar]
- Stefanoni, M.; Angst, U.; Elsener, B. Corrosion rate of carbon steel in carbonated concrete—A critical review. Cem. Concr. Res. 2018, 103, 35–48. [Google Scholar] [CrossRef]
- Wierig, H.J. Long-time studies on the carbonation of concrete under normal outdoor exposure. In Proceedings of the Rilem Seminar on Durability of Concrete Structures under Normal Outdoor Exposure, Hannover, Germany, 26–27 March 1984. [Google Scholar]
- Ho, D.; Lewis, R. Carbonation of concrete and its prediction. Cem. Concr. Res. 1987, 17, 489–504. [Google Scholar] [CrossRef]
- Parrott, L.J. Carbonation, moisture and empty pores. Adv. Cem. Res. 1992, 4, 111–118. [Google Scholar] [CrossRef]
- Al-Kadhimi, T.K.H.; Banfill, P.F.G.; Millard, S.G.; Bungey, J.H. An accelerated carbonation procedure for studies on concrete. Adv. Cem. Res. 1996, 8, 47–59. [Google Scholar] [CrossRef]
- Page, C.L.; Treadaway, K.W.J. Aspects of the electrochemistry of steel in concrete. Nat. Cell Biol. 1982, 297, 109–115. [Google Scholar] [CrossRef]
- Chen, C.; Liu, R.; Zhu, P.; Liu, H.; Wang, X. Carbonization Durability of Two Generations of Recycled Coarse Aggregate Concrete with Effect of Chloride Ion Corrosion. Sustainability 2020, 12, 10544. [Google Scholar] [CrossRef]
- Glass, G.; Page, C.; Short, N. Factors affecting the corrosion rate of steel in carbonated mortars. Corros. Sci. 1991, 32, 1283–1294. [Google Scholar] [CrossRef]
- Liu, X.; Niu, D.; Li, X.; Lv, Y.; Fu, Q. Pore Solution pH for the Corrosion Initiation of Rebars Embedded in Concrete under a Long-Term Natural Carbonation Reaction. Appl. Sci. 2018, 8, 128. [Google Scholar] [CrossRef] [Green Version]
- Sohail, M.G.; Laurens, S.; Deby, F.; Balayssac, J.P. Significance of macrocell corrosion of reinforcing steel in partially carbonated concrete: Numerical and experimental investigation. Mater. Struct. 2015, 48, 217–233. [Google Scholar] [CrossRef]
- Sohail, M.G.; Laurens, S.; Deby, F.; Balayssac, J.P.; Al Nuaimi, N. Electrochemical corrosion parameters for active and passive reinforcing steel in carbonated and sound concrete. Mater. Corros. 2021, 72, 1854–1871. [Google Scholar] [CrossRef]
- Bossio, A.; Faella, G.; Frunzio, G.; Guadagnuolo, M.; Serpieri, R. Diagnostic Reliability in the Assessment of Degradation in Precast Concrete Elements. Infrastructures 2021, 6, 164. [Google Scholar] [CrossRef]
- Wang, C. Explicitly Assessing the Durability of RC Structures Considering Spatial Variability and Correlation. Infrastructures 2021, 6, 156. [Google Scholar] [CrossRef]













| General Exposure Class | Description | Examples | |||
|---|---|---|---|---|---|
| Class | Subclass | Designation | Process Type | ||
| Non-aggressive | I | Nothing | Interiors of buildings, not subjected to condensation. Mass concrete elements. | Structural elements of buildings, including floors that are protected from the elements. | |
| Normal | High humidity | IIa | Corrosion not caused by chlorides | Interiors subject to high average relative humidity (>65%) or condensation. Exteriors in the absence of chlorides and exposed to rain in areas with average annual rainfall greater than 600 mm. Buried or submerged elements. | Structural elements in non-ventilated basements. Foundations, bridge abutments, piers and decks in unsealed areas with average annual rainfall greater than 600 mm. Waterproofed bridge decks, in areas with de-icing salts or average annual rainfall greater than 600 mm. Concrete elements found outdoors or on the roofs of buildings in areas with average annual rainfall greater than 600 mm. Forged elements in a sanitary chamber or in the interior of kitchens and bathrooms or on unprotected roofs. |
| Medium humidity | IIb | Corrosion not caused by chlorides | Exteriors in the absence of chlorides subjected to the action of rainwater in areas with average annual rainfall less than 600 mm. | Structural elements in outdoor buildings protected from rain. Bridge decks and piers in areas with average annual rainfall less than 600 mm. | |
| Binder | Instruction Cement RC 03 | a | b |
|---|---|---|---|
| Portland Cement | CEM I CEM II/A CEM/II/B-S CEM II/B-L CEM II/B-LL CEM II/B-M CEM/V | 1800 | −1.7 |
| Portland Cement + 28% fly ash | CEM II/B-P CEM II/B-V CEM IV/A CEM IV/B | 360 | −1.2 |
| Portland Cement + 9% silica fume | CEM II/A-D | 400 | −1.2 |
| Portland Cement + 65% slag | CEM III/A CEM III/B | 360 | −1.2 |
| Environment | Building | C | C | a | b | f | f = f + 8 | K = c. c. a. f | ti = x2/Kc2 |
|---|---|---|---|---|---|---|---|---|---|
| I | 1D | ||||||||
| I | 1D_Pillar 8_interior_Level 0 | 1.00 | 0.70 | 1800 | −1.70 | 12.30 | 20.30 | 7.54 | ti = x/56.92 |
| I | 1D_Pillar 16_int. wall_Level 0 | 1.00 | 0.70 | 1800 | −1.70 | 18.90 | 26.90 | 4.68 | ti = x/21.86 |
| I | 2G | ||||||||
| I | 2G_Pillar 1_interior_Level 0 | 1.00 | 0.70 | 1800 | −1.70 | 9.70 | 17.70 | 9.52 | ti = x/90.71 |
| I | 2G_Pillar 2_exterior wall_Level 0 | 1.00 | 0.70 | 1800 | −1.70 | 12.60 | 20.60 | 7.36 | ti = x/54.15 |
| I | 3T | ||||||||
| I | 3T_Pillar 2_interior wall_Level 0 | 1.00 | 0.70 | 1800 | −1.70 | 29.60 | 37.60 | 2.65 | ti = x/7 |
| IIa | 4T | ||||||||
| IIa | 4T_Pillar 2_interior_basement | 1.00 | 0.70 | 1800 | −1.70 | 22.70 | 30.70 | 3.73 | ti = x/13.95 |
| IIa | 4T_Pillar 8_int._basement | 1.00 | 0.70 | 1800 | −1.70 | 23.80 | 31.80 | 3.52 | ti = x/12.37 |
| I | 5T | ||||||||
| I | 5T_Pillar 3_exterior wall_level 0 | 1.00 | 0.70 | 1800 | −1.70 | 33.00 | 41.00 | 2.28 | ti = x/5.22 |
| I | 5T_Pillar 14_exterior wall_level 0 | 1.00 | 0.70 | 1800 | −1.70 | 46.2 | 54.20 | 1.42 | ti = x/2.02 |
| I, II | 6D | ||||||||
| IIb | 6D_Pillar 1_exterior wall_level 0 | 1.00 | 0.70 | 1800 | −1.70 | 9.40 | 17.40 | 9.80 | ti = x/96.13 |
| I | 6D_Pillar 5_interior_level 0 | 1.00 | 0.70 | 1800 | −1.70 | 12.20 | 20.20 | 7.61 | ti = x/57.88 |
| I | 7R | ||||||||
| I | 7R_Pillar 1_Interior_level 0 | 1.00 | 0.70 | 1800 | −1.70 | 9.30 | 17.30 | 9.90 | ti = x/98.04 |
| I | 7R_Pillar 2_Interior_level 0 | 1.00 | 0.70 | 1800 | −1.70 | 10.90 | 18.90 | 8.52 | ti = x/72.57 |
| II | 8A | ||||||||
| IIa | 8A_Pillar 1_interior_basement | 1.00 | 1.00 | 1800 | −1.70 | 45.30 | 53.30 | 2.09 | ti = x/4.36 |
| IIa | 8A_Pillar 21_interior_basement | 1.00 | 1.00 | 1800 | −1.70 | 61.10 | 69.10 | 1.34 | ti = x/1.80 |
| I | 9M | ||||||||
| I | 9M_Pillar 1_exterior wall_level 0 | 1.00 | 0.70 | 1800 | −1.70 | 21.90 | 29.90 | 3.91 | ti = x/15.26 |
| II | 10E | ||||||||
| IIa | 10E_Pillar 11_interior_basement | 1.00 | 0.70 | 1800 | −1.70 | 32.80 | 40.80 | 2.30 | ti = x/5.30 |
| General Exposure Class | Vcorr (μm/year) | ||
|---|---|---|---|
| Normal | High humidity | IIa | 3 |
| Medium humidity | IIb | 2 | |
| Ambient | Building | ∅ | Vcorr | t = 80.x /∅.vcorr |
|---|---|---|---|---|
| I | 1D | |||
| I | Stirrups | 6 | 2 | tp = 6.67x |
| I | Longitudinal rebars | 16 | 2 | tp = 2.5x |
| I | 2G | |||
| I | Stirrups | 8 | 2 | tp = 5x |
| I | Longitudinal rebars | 20 | 2 | tp = 2x |
| I | 3T | |||
| I | Stirrups | 6 | 2 | tp = 6.67x |
| I | Longitudinal rebars | 16 | 2 | tp = 2.5x |
| IIa | 4T | |||
| IIa | Stirrups | 6 | 3 | tp = 4.44x |
| IIa | Longitudinal rebars | 20 | 3 | tp = 1.33x |
| I | 5T | |||
| I | Stirrups | 6 | 2 | tp = 6.67x |
| I | Longitudinal rebars | 20 | 2 | tp = 2x |
| I, II | 6D | |||
| IIb | Stirrups | 6 | 2 | tp = 6.67x |
| IIb | Longitudinal rebars | 16 | 2 | tp = 2.5x |
| I | 7R | |||
| I | Stirrups | 6 | 2 | tp = 6.67x |
| I | Longitudinal rebars | 16 | 2 | tp = 2.5x |
| II | 8A | |||
| IIa | Stirrups | 8 | 3 | tp = 3.33x |
| IIa | Longitudinal rebars | 20 | 3 | tp = 1.33x |
| I | 9M | |||
| I | Stirrups | 8 | 2 | tp = 5x |
| I | Longitudinal rebars | 20 | 2 | tp = 2x |
| II | 10E | |||
| IIa | Stirrups | 6 | 3 | tp = 4.44x |
| IIa | Longitudinal rebars | 20 | 3 | tp = 1.33x |
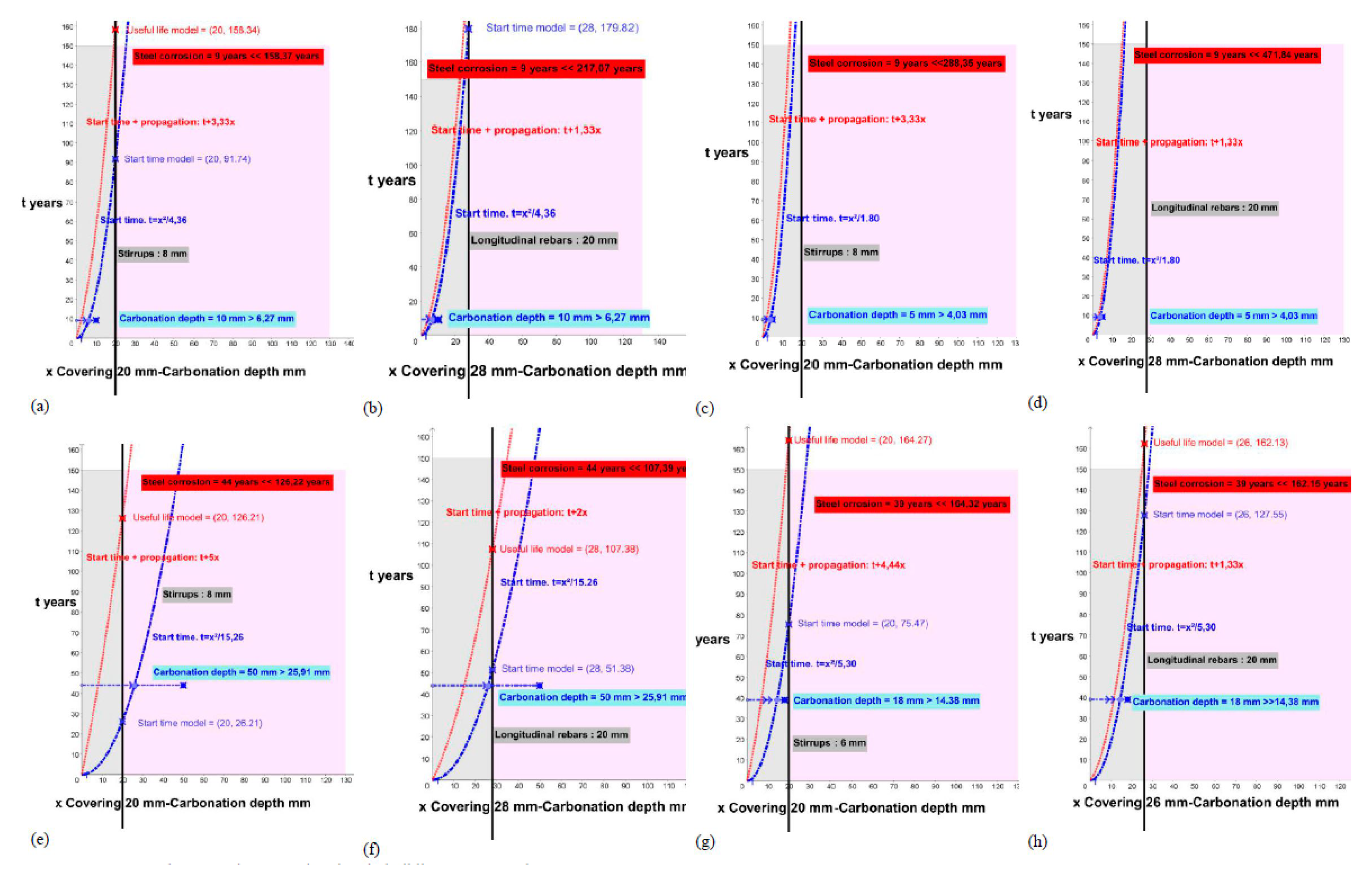
| Environment | Building | f | K | Start Time t | Start Time Model (Covering) | ∅ mm | Propagation Time t | Useful Life Model (T = t + t) (Covering) | Age (Year) |
|---|---|---|---|---|---|---|---|---|---|
| I | 1D-Calle General Pastor, 19, Dolores. | ||||||||
| I | 1D_Pillar 8_interior_Level 0/Stirrups | 12.30 | 7.54 | t = x/56.92 | 7.03 | 6 | t = 6.67x | 140.36 | 24 |
| I | 1D_Pillar 8_interior_Level 0/Longitudinal rebars | 12.30 | 7.54 | t = x/56.92 | 11.88 | 16 | t = 2.5x | 76.88 | 24 |
| I | 1D_Pillar 16_int. wall_Level 0/Stirrups | 18.90 | 4.68 | t = x/21.86 | 18.30 | 6 | t = 6.67x | 151.63 | 24 |
| I | 1D-Pillar 16_int.wall_Level 0/Longitudinal rebars. | 18.90 | 4.68 | t = x/21.86 | 30.93 | 16 | t = 2.5x | 95.93 | 24 |
| I | 2G-Calle San Pedro, 38, Guardamar | ||||||||
| I | 2G_Pillar 1_interior_Level 0/Stirrups | 9.70 | 9.52 | t = x/90.71 | 4.41 | 8 | t = 5x | 104.41 | 9 |
| I | 2G_Pillar 1_interior_Level 0/ Longitudinal rebars | 9.70 | 9.52 | t = x/90.72 | 8.64 | 20 | t = 2x | 64.64 | 9 |
| I | 2G_Pillar 2_ext. wall_Level 0/Stirrups | 12.60 | 7.36 | t = x/54.15 | 7.39 | 8 | t = 5x | 107.39 | 9 |
| I | 2G_Pillar 2_ext. wall_Level 0/ Longitudinal rebars | 12.60 | 7.36 | t = x/54.15 | 14.48 | 20 | t = 2x | 70.48 | 9 |
| I | 3T-Calle Ulpiano,71, Torrevieja | ||||||||
| I | 3T_Pillar 2_interior wall_Level 0/Stirrups | 29.60 | 2.65 | t = x/7 | 57.14 | 6 | t = 6.67x | 190.48 | 25 |
| I | 3T_Pillar 2_int. wall_Level 0/Longitudinal rebars | 29.60 | 2.65 | t = x/7 | 96.57 | 16 | t = 2.5x | 161.57 | 25 |
| IIa | 4T-Av. Holandeses, Torrevieja. | ||||||||
| IIa | 4T_Pillar 2_interior_basement/Stapes | 22.70 | 3.73 | t = x/13.95 | 28.68 | 6 | t = 4.44x | 117.57 | 19 |
| IIa | 4T_Pillar 2_int._basement/Longitudinal rebars | 22.70 | 3.73 | t = x/13.95 | 48.47 | 20 | t = 1.33x | 83.14 | 19 |
| IIa | 4T_Pillar 8_int._basement/Stirrups | 23.80 | 3.52 | t = x/12.37 | 32.33 | 6 | t = 4.44x | 121.22 | 19 |
| IIa | 4T_Pillar 8_int._basement/Longitudinal rebars | 23.80 | 3.52 | t = x/12.37 | 54.63 | 20 | t = 1.33x | 89.30 | 19 |
| I | 5T- Av. Inglaterra, 31, Torrevieja | ||||||||
| I | 5T_Pillar 3 _ext. wall_level 0/Stirrups | 33.00 | 2.28 | t = x/5.22 | 76.70 | 6 | t = 6.67x | 210.03 | 20 |
| I | 5T_Pillar 3_ext wall_Level 0/Longitudinal rebars | 33.00 | 2.28 | t = x/5.22 | 129.62 | 20 | t = 2x | 181.62 | 20 |
| I | 5T_Pillar 14_ext wall_level 0/Stirrups | 46.20 | 1.42 | t = x/2.02 | 198.11 | 6 | t = 6.67x | 331.45 | 20 |
| I | 5T_Pillar 14_ext wall_Level 0/Longitudinal rebars | 46.20 | 1.42 | t = x/2.02 | 334.81 | 20 | t = 2x | 386.81 | 20 |
| I, II | 6D-Av. Crevillente, 4, Dolores | ||||||||
| IIb | 6D_Pillar 1_ext. wall_level 0/ Stirrups | 9.40 | 9.80 | t = x/96.13 | 4.16 | 6 | t = 6.67x | 137.49 | 28 |
| IIb | 6D_Pillar 1_ext. wall_level 0/ Longitudinal rebars | 9.40 | 9.80 | t = x/96.13 | 7.03 | 16 | t = 2.5x | 72.03 | 28 |
| I | 6D_Pillar 5_interior_level 0/ Stirrups | 12.20 | 7.61 | t = x/57.88 | 6.91 | 6 | t = 6.67x | 140.24 | 28 |
| I | 6D_Pillar 5_interior_level 0/Longitudinal rebars | 12.20 | 7.61 | t = x2/57.88 | 11.68 | 16 | t = 2.5x | 76.68 | 28 |
| I | 7R-Pl. Héroes de Africa, Rojales. | ||||||||
| I | 7R_Pillar 1_Interior_level 0/ Stirrups | 9.30 | 9.90 | t = x/98.04 | 4.08 | 6 | t = 6.67x | 137.41 | 28 |
| I | 7R_Pillar 1_interior_level 0/Longitudinal rebars | 9.30 | 9.90 | t = x/98.04 | 6.90 | 16 | t = 2.5x | 71.90 | 28 |
| I | 7R_Pillar 2_Interior_level 0/ Stirrups | 10.90 | 8.52 | t = x/72.57 | 5.51 | 6 | t = 6.67x | 138.85 | 28 |
| I | 7R_Pillar 2int._level 0/Longitudinal rebars | 10.90 | 8.52 | t = x/72.57 | 9.31 | 16 | t = 2.5x | 74.31 | 28 |
| II | 8A-Av. La Peseta, 19, Alicante. | ||||||||
| IIa | 8A_Pillar 1_interior_basement/ Stirrups | 45.30 | 2.09 | t = x/4.36 | 91.70 | 8 | t = 3.33x | 158.37 | 9 |
| IIa | 8A_Pillar_int._basement/Longitudinal rebars | 45.30 | 2.09 | t = x/4.37 | 179.74 | 20 | t = 1.33x | 217.07 | 9 |
| IIa | 8A_Pillar 21_int._basement/ Stirrups | 61.10 | 1.34 | t = x/1.80 | 221.69 | 8 | t = 3.33x | 288.35 | 9 |
| IIa | 8A_Pillar 21_int._basement/Longitudinal rebars | 61.10 | 1.34 | t = x/1.80 | 434.51 | 20 | t = 1.33x | 471.84 | 9 |
| I | 9M-Calle Iberia, 33, Aguilas. Murcia | ||||||||
| I | 9M_Pillar 1_ext. wall_level 0/Stirrups | 21.90 | 3.91 | t = x/15.26 | 26.22 | 8 | t = 5x | 126.22 | 44 |
| I | 9M_Pillar 1_ext. wall_level 0/Longitudinal rebars | 21.90 | 3.91 | t = x/15.26 | 51.39 | 20 | t = 2x | 107.39 | 44 |
| II | 10E-Av. San Bartolomé de T. 8, Elche. | ||||||||
| IIa | 10E_Pillar 11_int._basement/ Stirrups | 32.80 | 2.30 | t = x/5.30 | 75.43 | 6 | t = 4.44x | 164.32 | 39 |
| IIa | 10E_Pillar 11_int._basement/Longitudinal rebars | 32.80 | 2.30 | t = x/5.30 | 127.48 | 20 | t = 1.33x | 162.15 | 39 |
| Environment | Building-Element | f (KN/mm) | Carbonation Depth (mm) | Carbonation Depth Model (mm) | Difference (mm) | Age (years) | Increased Carbonation (%) |
|---|---|---|---|---|---|---|---|
| I | 7R_Pillar 1 | 9.30 | 50 | 52.39 | −2.39 | 28 | 95.4 |
| IIb | 6D_Pillar 1 | 9.40 | 65 | 51.88 | 13.12 | 28 | 125.3 |
| I | 2G_Pillar 1 | 9.70 | 85 | 28.57 | 56.43 | 9 | 297.5 |
| I | 7R_Pillar 2 | 10.90 | 45 | 45.08 | −0.08 | 28 | 100 |
| I | 6D_Pillar 5 | 12.20 | 85 | 40.26 | 44.74 | 28 | 211.1 |
| I | 1D_Pillar 8 | 12.30 | 30 | 36.96 | −6.96 | 24 | 81.2 |
| I | 2G_Pillar 2 | 12.60 | 55 | 22.08 | 32.92 | 9 | 249.1 |
| I | 1D_Pillar 16 | 18.90 | 55 | 22.90 | 32.10 | 24 | 240.2 |
| I | 9M_Pillar 1 | 21.90 | 50 | 25.91 | 24.09 | 44 | 192.9 |
| IIa | 4T_Pillar 2 | 22.70 | 45 | 16.28 | 28.72 | 19 | 276.4 |
| IIa | 4T_Pillar 8 | 23.80 | 40 | 15.33 | 24.67 | 19 | 260.9 |
| I | 3T_Pillar 2 | 29.60 | 20 | 13.23 | 6.77 | 25 | 151.2 |
| IIa | 10E_Pillar 11 | 32.80 | 18 | 14.38 | 3.62 | 39 | 125.2 |
| I | 5T_Pillar 3 | 33.00 | 25 | 10.21 | 14.79 | 20 | 244.9 |
| IIa | 8A_Pillar 1 | 45.30 | 10 | 6.27 | 3.73 | 9 | 159.5 |
| I | 5T_Pillar 14 | 46.2 | 20 | 6.35 | 13.65 | 20 | 315 |
| IIa | 8A_Pillar 21 | 61.10 | 5 | 4.03 | 0.97 | 9 | 124 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saura-Gómez, P.; Rizo-Maestre, C.; Echarri-Iribarren, V. The Useful Life of Reinforced Concrete Structures with Reinforcement Corrosion Due to Carbonation in Non-Aggressive and Normal Exposures in the Spanish Mediterranean. Materials 2022, 15, 745. https://doi.org/10.3390/ma15030745
Saura-Gómez P, Rizo-Maestre C, Echarri-Iribarren V. The Useful Life of Reinforced Concrete Structures with Reinforcement Corrosion Due to Carbonation in Non-Aggressive and Normal Exposures in the Spanish Mediterranean. Materials. 2022; 15(3):745. https://doi.org/10.3390/ma15030745
Chicago/Turabian StyleSaura-Gómez, Pascual, Carlos Rizo-Maestre, and Víctor Echarri-Iribarren. 2022. "The Useful Life of Reinforced Concrete Structures with Reinforcement Corrosion Due to Carbonation in Non-Aggressive and Normal Exposures in the Spanish Mediterranean" Materials 15, no. 3: 745. https://doi.org/10.3390/ma15030745
APA StyleSaura-Gómez, P., Rizo-Maestre, C., & Echarri-Iribarren, V. (2022). The Useful Life of Reinforced Concrete Structures with Reinforcement Corrosion Due to Carbonation in Non-Aggressive and Normal Exposures in the Spanish Mediterranean. Materials, 15(3), 745. https://doi.org/10.3390/ma15030745






