Research Progress on Controlled Low-Strength Materials: Metallurgical Waste Slag as Cementitious Materials
Abstract
1. Introduction
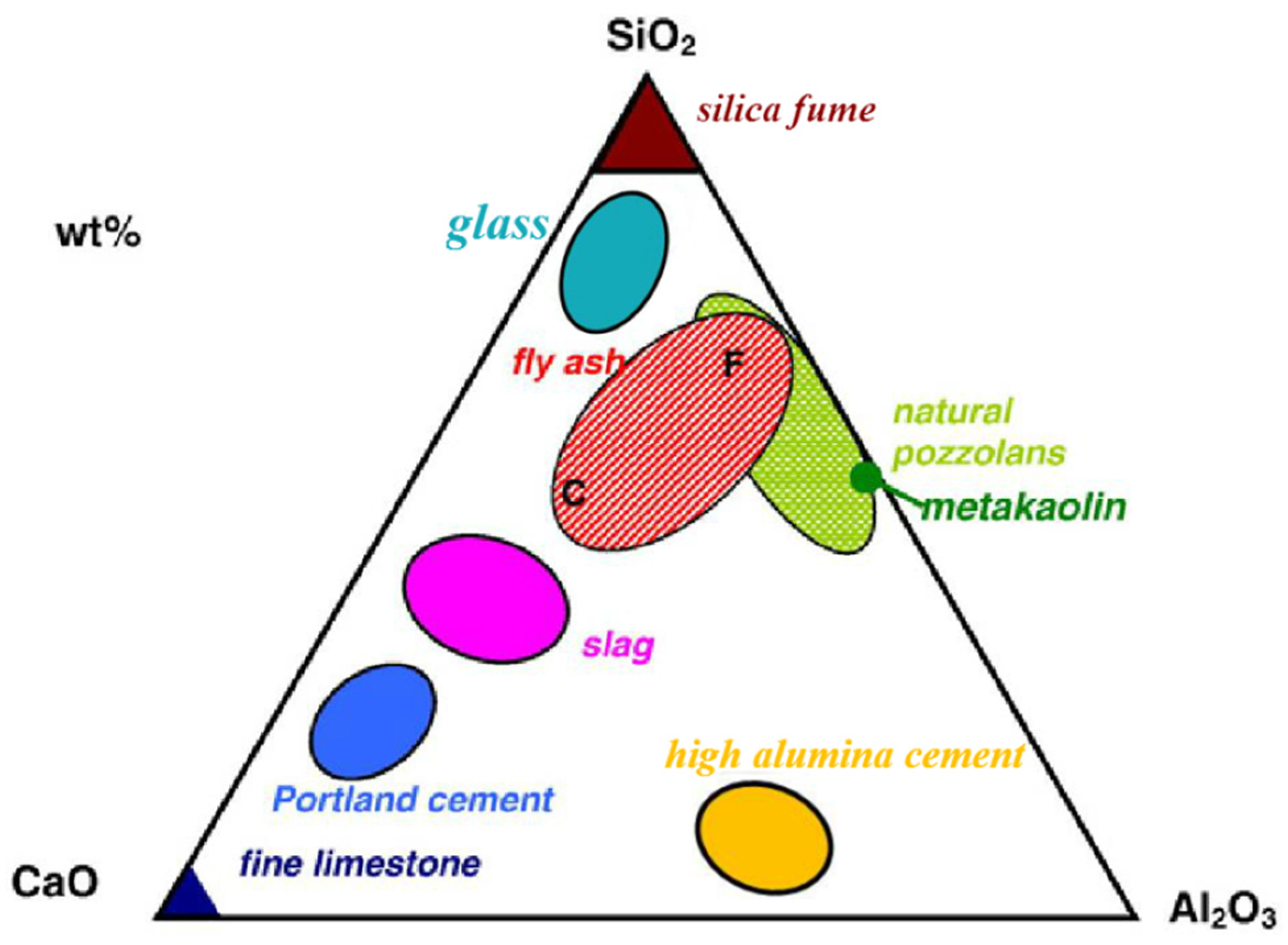
1.1. Supplementary Cementitious Materials
1.2. Controlled Low-Strength Materials (CLSMs)
1.2.1. CLSM Strength
1.2.2. CLSM Constituent Materials
1.2.3. Commonly Used SCMs in CLSMs
1.3. Present Situation of Metallurgical Waste Residue Resource Utilization
2. Production of CLSMs from Metallurgical Waste SCMs
2.1. Blast-Furnace Slag
2.1.1. Overview of Blast-Furnace Slag
2.1.2. Physical and Chemical Properties of Blast-Furnace Slag
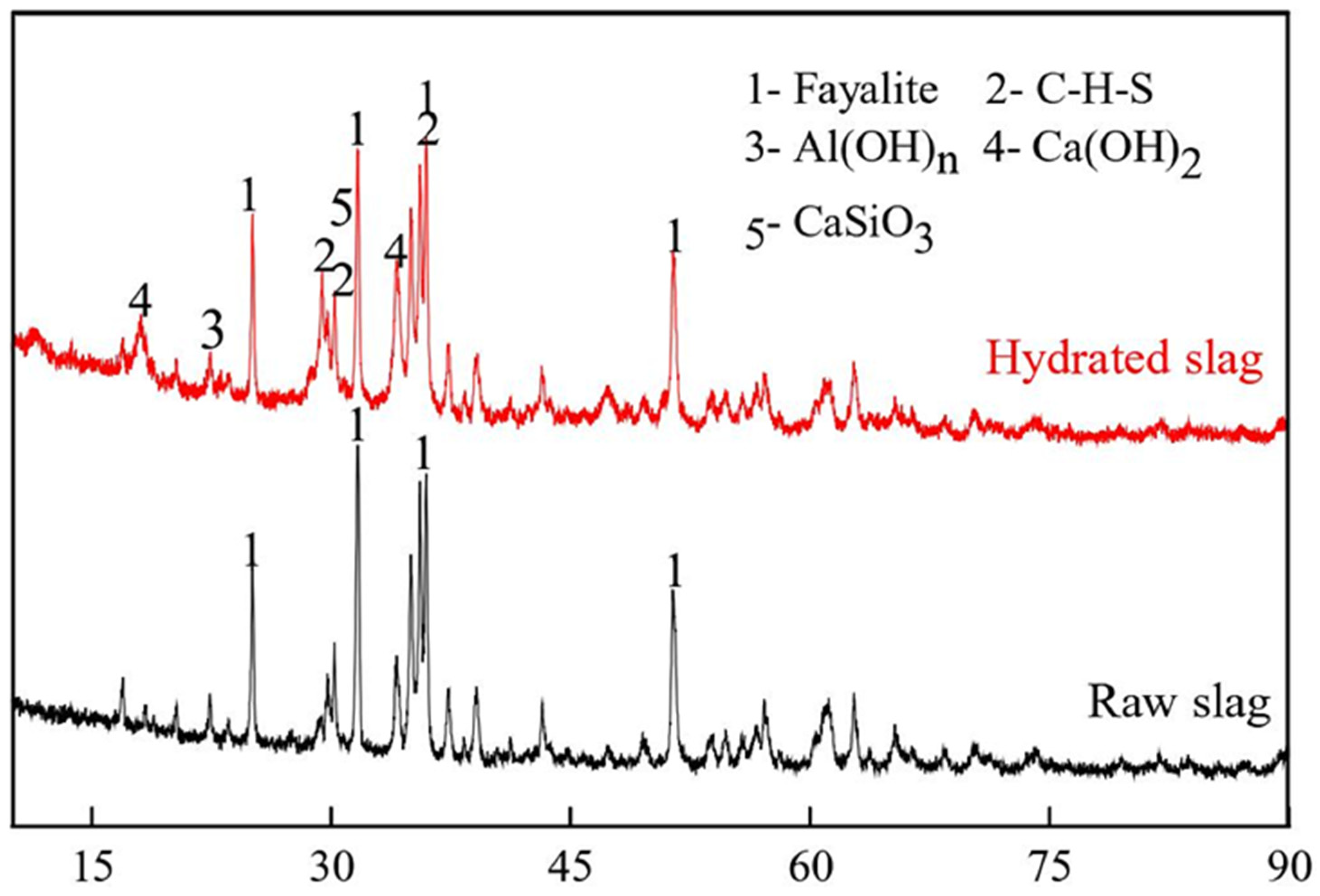
2.1.3. Hydration Activity of Blast-Furnace Slag
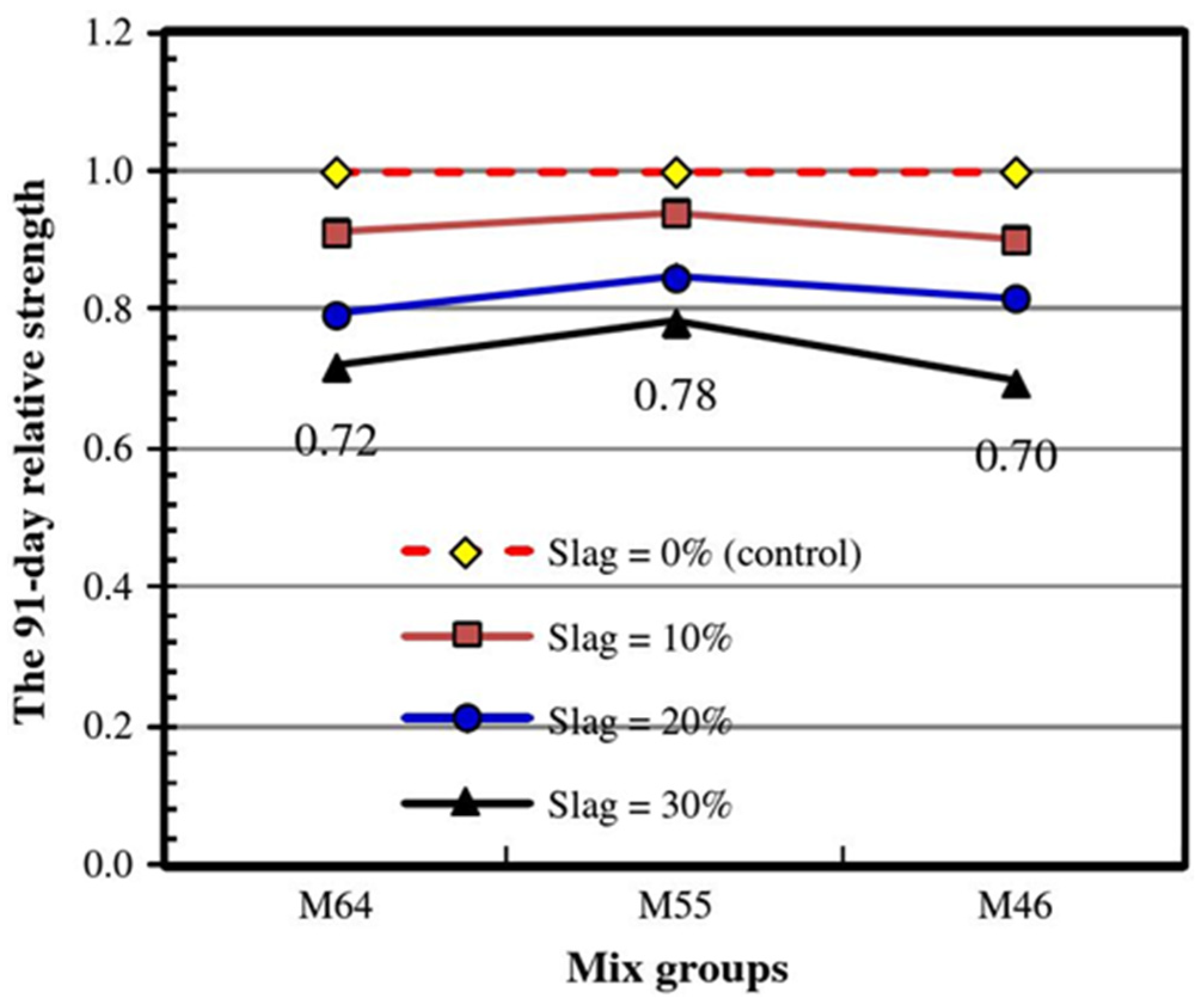
2.1.4. Properties and Mechanism of Slag CLSM
- Mechanical properties and mechanism
- Performance of fresh mixtures
2.2. Steel Slag
2.2.1. Overview of Steel Slag
2.2.2. Physical and Chemical Properties of Steel Slag
2.2.3. Properties and Mechanism of Steel Slag CLSMs
2.2.4. Potential Application of Steel Slag Carbonization in CLSM
2.3. Red Mud
2.3.1. Overview of Red Mud
2.3.2. Performance and Mechanism of Red Mud CLSMs
2.4. Copper Slag
2.4.1. Overview of Copper Slag
2.4.2. Properties and Mechanism of Copper Slag CLSMs
2.5. Other Smelting Waste
2.5.1. Air-Cooled Blast-Furnace Slag
2.5.2. Jarosite Residue
2.5.3. Ferrochrome Slag
2.6. Summary
3. Cement-Free CLSMs
3.1. Alkaline Chemical Solution-Activated Slag
3.1.1. Liquidity
3.1.2. Compressive Strength and Its Mechanism
3.2. Alkaline By-Product Activated Pozzolanic Waste
3.2.1. Phosphogypsum and Waste Lime-Activated Slag
3.2.2. Cement Kiln Dust-Activated Slag
3.2.3. Red Mud as an Alkaline Activator
3.3. Recycled Aggregate-Activated By-Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hannesson, G.; Kuder, K.; Shogren, R.; Lehman, D. The influence of high volume of fly ash and slag on the compressive strength of self-consolidating concrete. Constr. Build. Mater. 2012, 30, 161–168. [Google Scholar] [CrossRef]
- Bouaissi, A.; Li, L.Y.; Moga, L.M.; Sandu, I.G.; Abdullah, M.M.A.B.; Sandu, A.V. A Review on Fly Ash as a Raw Cementitious Material for Geopolymer Concrete. Rev. Chim. 2018, 69, 1661–1667. [Google Scholar] [CrossRef]
- Gartner, E. Industrially interesting approaches to “low-CO2” cements. Cem. Concr. Res. 2004, 34, 1489–1498. [Google Scholar] [CrossRef]
- Liu, S.H.; Leng, F.G.; Li, L.H. Supplementary Cementitious Materials Used in Concrete; China Building Materials Press: Beijing, China, 2010; pp. 80–160. (In Chinese) [Google Scholar]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Turkel, S. Strength properties of fly ash based controlled low strength materials. J. Hazard. Mater. 2007, 147, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, V. Finite element analysis of controlled low strength materials. Front. Struct. Civ. Eng. 2019, 13, 1243–1250. [Google Scholar] [CrossRef]
- Alizadeh, V.; Helwany, S.; Ghorbanpoor, A.; Sobolev, K. Design and application of controlled low strength materials as a structural fill. Constr. Build. Mater. 2014, 53, 425–431. [Google Scholar] [CrossRef]
- ASTM International. Standard Test Method for Flow Consistency of Controlled Low Strength Material (CLSM); ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Qin, Y.G.; Hu, J.H.; Yang, D.J.; Kuang, Y.; Zhao, F.W.; Zhou, T. Optimization of Transport Performance and Strength of the Filling Slurry in Tailings Reservoir Waste by Adding Air Entraining Agent. Minerals 2020, 10, 730. [Google Scholar] [CrossRef]
- Hefni, M.; Hassani, F. Experimental Development of a Novel Mine Backfill Material: Foam Mine Fill. Minerals 2020, 10, 564. [Google Scholar] [CrossRef]
- Han, W.; Kim, S.Y.; Lee, J.S.; Byun, Y.H. Friction behavior of controlled low strength material-soil interface. Geomech. Eng. 2019, 18, 407–415. [Google Scholar] [CrossRef]
- Siddique, R. Utilization of waste materials and by-products in producing controlled low-strength materials. Resour. Conserv. Recycl. 2009, 54, 1–8. [Google Scholar] [CrossRef]
- Wirth, X.; Benkeser, D.; Yeboah, N.N.N.; Shearer, C.R.; Kurtis, K.E.; Burns, S.E. Evaluation of Alternative Fly Ashes as Supplementary Cementitious Materials. ACI Mater. J. 2019, 116, 69–77. [Google Scholar] [CrossRef]
- Kim, S.-C.; Kim, D.-J.; Byun, Y.-H. Effect of Fly Ash on Strength and Stiffness Characteristics of Controlled Low-Strength Material in Shear Wave Monitoring. Materials 2021, 14, 3022. [Google Scholar] [CrossRef]
- Park, J.; Hong, G. Strength Characteristics of Controlled Low-Strength Materials with Waste Paper Sludge Ash (WPSA) for Prevention of Sewage Pipe Damage. Materials 2020, 13, 4238. [Google Scholar] [CrossRef]
- Li, J.S.; Zhou, Y.F.; Wang, Q.M.; Xue, Q.; Poon, C.S. Development of a Novel Binder Using Lime and Incinerated Sewage Sludge Ash to Stabilize and Solidify Contaminated Marine Sediments with High Water Content as a Fill Material. J. Mater. Civ. Eng. 2019, 31, 12. [Google Scholar] [CrossRef]
- Lu, Q.M.; Zhang, S.; Sun, G.Z.; Wang, X.Q.; Fu, S.J. Preparation of Paste Backfill Material from Mix-Calcined Sludge Ash. Waste Biomass Valorization 2021, 12, 1633–1646. [Google Scholar] [CrossRef]
- Kuo, W.-T.; Gao, Z.-C. Engineering Properties of Controlled Low-Strength Materials Containing Bottom Ash of Municipal Solid Waste Incinerator and Water Filter Silt. Appl. Sci. 2018, 8, 1377. [Google Scholar] [CrossRef]
- Naganathan, S.; Razak, H.A.; Hamid, S.N.A. Properties of controlled low-strength material made using industrial waste incineration bottom ash and quarry dust. Mater. Des. 2012, 33, 56–63. [Google Scholar] [CrossRef]
- Naik, T.R.; Kraus, R.N.; Siddique, R. Controlled low-strength materials containing mixtures of coal ash and new pozzolanic material. ACI Mater. J. 2003, 100, 208–215. [Google Scholar]
- Qi, T.Y.; Wang, H.C.; Feng, G.R.; Du, X.J.; Wang, Z.H.; Zhang, S.F. Effects of Corn Stalk Fly Ash (CSFA) on the Mechanical and Deformation Properties of Cemented Coal Gangue Backfill. Adv. Mater. Sci. Eng. 2020, 2020, 7421769. [Google Scholar] [CrossRef]
- Wang, H.C.; Qi, T.Y.; Feng, G.R.; Wen, X.Z.; Wang, Z.H.; Shi, X.D.; Du, X.J. Effect of partial substitution of corn straw fly ash for fly ash as supplementary cementitious material on the mechanical properties of cemented coal gangue backfill. Constr. Build. Mater. 2021, 280, 15. [Google Scholar] [CrossRef]
- Wang, S.; Song, X.P.; Wei, M.L.; Liu, W.; Wang, X.J.; Ke, Y.X.; Tao, T.J. Strength characteristics and microstructure evolution of cemented tailings backfill with rice straw ash as an alternative binder. Constr. Build. Mater. 2021, 297, 13. [Google Scholar] [CrossRef]
- Raghavendra, T.; Sunil, M.; Udayashankar, B.C. Controlled Low-Strength Materials Using Bagasse Ash and Fly Ash. ACI Mater. J. 2016, 113, 447–457. [Google Scholar] [CrossRef]
- Xiao, B.L.; Miao, S.J.; Gao, Q.; Chen, B.Y.; Li, S.H. Hydration Mechanism of Sustainable Clinker-Free Steel Slag Binder and Its Application in Mine Backfill. JOM 2021, 73, 1053–1061. [Google Scholar] [CrossRef]
- Li, X.L.; Li, K.X.; Sun, Q.; Liu, L.; Yang, J.L.; Xue, H.W. Preparation of Cemented Oil Shale Residue-Steel Slag-Ground Granulated Blast Furnace Slag Backfill and Its Environmental Impact. Materials 2021, 14, 2052. [Google Scholar] [CrossRef]
- Mahamaya, M.; Das, S.K. Characterization of ferrochrome slag as a controlled low-strength structural fill material. Int. J. Geotech. Eng. 2020, 14, 312–321. [Google Scholar] [CrossRef]
- Yuan, B.; Yuan, S.S.; Straub, C.; Chen, W. Activation of Binary Binder Containing Fly Ash and Portland Cement Using Red Mud as Alkali Source and Its Application in Controlled Low-Strength Materials. J. Mater. Civ. Eng. 2020, 32, 11. [Google Scholar] [CrossRef]
- Lan, W.T.; Wu, A.X.; Yu, P. Development of a new controlled low strength filling material from the activation of copper slag: Influencing factors and mechanism analysis. J. Clean. Prod. 2020, 246, 10. [Google Scholar] [CrossRef]
- He, Y.; Chen, Q.S.; Qi, C.C.; Zhang, Q.L.; Xiao, C.C. Lithium slag and fly ash-based binder for cemented fine tailings backfill. J. Environ. Manag. 2019, 248, 8. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.T.; Weng, T.L.; Cheng, A.; Chao, S.J.; Hsu, H.M. Properties of Controlled Low Strength Material with Circulating Fluidized Bed Combustion Ash and Recycled Aggregates. Materials 2018, 11, 715. [Google Scholar] [CrossRef]
- Zhou, N.; Ma, H.B.; Ouyang, S.Y.; Germain, D.; Hou, T. Influential Factors in Transportation and Mechanical Properties of Aeolian Sand-Based Cemented Filling Material. Minerals 2019, 9, 116. [Google Scholar] [CrossRef]
- Chompoorat, T.; Thepumong, T.; Nuaklong, P.; Jongvivatsakul, P.; Likitlersuang, S. Alkali-Activated Controlled Low-Strength Material Utilizing High-Calcium Fly Ash and Steel Slag for Use as Pavement Materials. J. Mater. Civ. Eng. 2021, 33, 13. [Google Scholar] [CrossRef]
- Wu, H.; Huang, B.S.; Shu, X.; Yin, J. Utilization of solid wastes/byproducts from paper mills in Controlled Low Strength Material (CLSM). Constr. Build. Mater. 2016, 118, 155–163. [Google Scholar] [CrossRef]
- Singh, J.; Singh, S.P. Geopolymerization of solid waste of non-ferrous metallurgy—A review. J. Environ. Manag. 2019, 251, 109571. [Google Scholar] [CrossRef]
- Pribulová, A.; Futas, P.; Baricová, D. Processing and utilization of metallurgical slags. Prod. Eng. Arch. 2016, 11, 2–5. [Google Scholar] [CrossRef]
- Giannopoulou, I.; Dimas, D.; Maragkos, I.; Panias, D. Utilization of metallurgical solid by-products for the development of inorganic polymeric construction materials. Glob. Nest J. 2009, 11, 127–136. [Google Scholar]
- Gholizadeh Vayghan, A.; Horckmans, L.; Snellings, R.; Peys, A.; Teck, P.; Maier, J.; Friedrich, B.; Klejnowska, K. Use of Treated Non-Ferrous Metallurgical Slags as Supplementary Cementitious Materials in Cementitious Mixtures. Appl. Sci. 2021, 11, 4028. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Zhang, S.Q.; Ni, W.; Yan, Q.H.; Gao, W.; Li, Y.Y. Immobilisation of high-arsenic-containing tailings by using metallurgical slag-cementing materials. Chemosphere 2019, 223, 117–123. [Google Scholar] [CrossRef]
- Shi, C.J.; Qian, J.S. High performance cementing materials from industrial slags—A review. Resour. Conserv. Recycl. 2000, 29, 195–207. [Google Scholar] [CrossRef]
- Abdelli, K.; Tahlaiti, M.; Belarbi, R.; Oudjit, M.N. Influence of the pozzolanic reactivity of the Blast Furnace Slag (BFS) and metakaolin on mortars. Energy Procedia 2017, 139, 224–229. [Google Scholar] [CrossRef]
- Pal, S.; Mukherjee, A.; Pathak, S. Investigation of hydraulic activity of ground granulated blast furnace slag in concrete. Cem. Concr. Res. 2003, 33, 1481–1486. [Google Scholar] [CrossRef]
- Ehrenberg, A.; Sarcos, N.R.; Hart, D.; Bornhoeft, H.; Deubener, J. Influence of the Thermal History of Granulated Blast Furnace Slags on Their Latent Hydraulic Reactivity in Cementitious Systems. J. Sust. Metall. 2020, 6, 207–215. [Google Scholar] [CrossRef]
- Environment, U.; Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar]
- Lin, Y.H.; Xu, D.Q.; Zhao, X.H. Properties and Hydration Mechanism of Soda Residue-Activated Ground Granulated Blast Furnace Slag Cementitious Materials. Materials 2021, 14, 2883. [Google Scholar] [CrossRef] [PubMed]
- Skuza, Z.; Kolmasiak, C.; Prusak, R. Possibilities for the utilization of metallurgical slag in the conditions of the Polish economy. Metalurgija 2009, 48, 125–128. [Google Scholar]
- Richardson, D. Strength and durability of a 70% ground granulated blast furnace slag concrete mix. Mo. Dep. Transp. Organ. Results Final Rep. 2006, 413, 3–5. [Google Scholar]
- Manjunath, R.; Narasimhan, M.C. Alkali-activated concrete systems: A state of art. In New Materials in Civil Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 459–491. [Google Scholar]
- Dong, G. Reaction Degree of Fly Ash and Blast Furnace Slag in Cement Pastes. Ph.D. Thesis, China Building Materials Academy, Beijing, China, 2008. (In Chinese). [Google Scholar]
- Gao, D.Y.; Meng, Y.; Yang, L.; Tang, J.Y.; Lv, M.Y. Effect of ground granulated blast furnace slag on the properties of calcium sulfoaluminate cement. Constr. Build. Mater. 2019, 227, 9. [Google Scholar] [CrossRef]
- Pacierpnik, W.; Nocun-Wczelik, W.; Kapeluszna, E. Application of weathered granulated blast furnace slag as a supplementary cementitious material in concrete. Arch. Civ. Eng. 2020, 66, 381–398. [Google Scholar] [CrossRef]
- Li, Q.T.; Li, Z.G.; Yuan, G.L. Effects of elevated temperatures on properties of concrete containing ground granulated blast furnace slag as cementitious material. Constr. Build. Mater. 2012, 35, 687–692. [Google Scholar] [CrossRef]
- Bheel, N.; Abbasi, S.A.; Awoyera, P.; Olalusi, O.B.; Sohu, S.; Rondon, C.; Echeverria, A.M. Fresh and Hardened Properties of Concrete Incorporating Binary Blend of Metakaolin and Ground Granulated Blast Furnace Slag as Supplementary Cementitious Material. Adv. Civ. Eng. 2020, 2020, 8851030. [Google Scholar] [CrossRef]
- Choi, Y.C.; Park, B. Enhanced autogenous healing of ground granulated blast furnace slag blended cements and mortars. J. Mater. Res. Technol. 2019, 8, 3443–3452. [Google Scholar] [CrossRef]
- Rivera, R.A.; Sanjuan, M.A.; Martin, D.A. Granulated Blast-Furnace Slag and Coal Fly Ash Ternary Portland Cements Optimization. Sustainability 2020, 12, 5783. [Google Scholar] [CrossRef]
- Preetham, H.K.; Nayak, S. Geotechnical Investigations on Marine Clay Stabilized Using Granulated Blast Furnace Slag and Cement. Int. J. Geosynth. Ground Eng. 2019, 5, 12. [Google Scholar] [CrossRef]
- Wang, L.N.; Aslani, F. Electrical resistivity and piezoresistivity of cement mortar containing ground granulated blast furnace slag. Constr. Build. Mater. 2020, 263, 10. [Google Scholar] [CrossRef]
- Hokfors, B.; Bostrom, D.; Viggh, E.; Backman, R. On the phase chemistry of Portland cement clinker. Adv. Cem. Res. 2015, 27, 50–60. [Google Scholar] [CrossRef]
- Wang, J.J.; Xie, J.H.; Wang, C.H.; Zhao, J.B.; Liu, F.; Fang, C. Study on the optimum initial curing condition for fly ash and GGBS based geopolymer recycled aggregate concrete. Constr. Build. Mater. 2020, 247, 21. [Google Scholar] [CrossRef]
- GB/T 18046; Ground Granulated Blast Furnace Slag Used for Cement and Concrete. National Standards of the People’s Republic of China: Beijing, China, 2017. (In Chinese)
- Sasaki, T. Standardization of iron and steel slag products. Nippon Steel Sumitomo Met. Tech. Rep. 2015, 109, 189–194. [Google Scholar]
- KS F 2563; Ground Granulated Blast-Furnace Slag for Use in Concret. Korean Agency for Technology and Standards: Eumseong-gun, Korea, 2019.
- EN 15167-1; Ground Granulated Blast Furnace Slag for Use in Concrete, Mortar and Grout—Part 1: Definitions, Specifications and Conformity Criteria. British Standards Institution: London, UK, 2006.
- ASTM C989/C989M; Standard Specification for Slag Cement for Use in Concrete and Mortars. ASTM International: West Conshohocken, PA, USA, 2018.
- AS 3582.2; Supplementary Cementitious Materials Slag-Ground Granulated Blast-Furnace. Standards Australia: Sydney, Australia, 2016.
- Escalante-Garcia, J.I.; Espinoza-Perez, L.J.; Gorokhovsky, A.; Gomez-Zamorano, L.Y. Coarse blast furnace slag as a cementitious material, comparative study as a partial replacement of Portland cement and as an alkali activated cement. Constr. Build. Mater. 2009, 23, 2511–2517. [Google Scholar] [CrossRef]
- Siddique, R.; Kaur, D. Properties of concrete containing ground granulated blast furnace slag (GGBFS) at elevated temperatures. J. Adv. Res. 2012, 3, 45–51. [Google Scholar] [CrossRef]
- GB/T 203; Granulated Blastfurnace Slag Used for Cement Production. National Standards of the People’s Republic of China: Beijing, China, 2008.
- Frearson, J.P.H. Sulfate resistance of combinations of Portland cement and ground granulated blast furnace slag. J. Am. Concr. Inst. 1986, 83, 341–342. [Google Scholar]
- Huang, H.L.; Ye, G.; Damidot, D. Effect of blast furnace slag on self-healing of microcracks in cementitious materials. Cem. Concr. Res. 2014, 60, 68–82. [Google Scholar] [CrossRef]
- Lovencin, W. Assessment of Design and Properties for Flowable Fill Usage in Highway Pavement Construction for Conditions in Florida. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2007. [Google Scholar]
- Bouikni, A.; Swamy, R.N.; Bali, A. Durability properties of concrete containing 50% and 65% slag. Constr. Build. Mater. 2009, 23, 2836–2845. [Google Scholar] [CrossRef]
- Sheen, Y.N.; Zhang, L.H.; Le, D.H. Engineering properties of soil-based controlled low-strength materials as slag partially substitutes to Portland cement. Constr. Build. Mater. 2013, 48, 822–829. [Google Scholar] [CrossRef]
- Raghavendra, T.; Udayashankar, B.C. Flow and Strength Characteristics of CLSM Using Ground Granulated Blast Furnace Slag. J. Mater. Civ. Eng. 2014, 26, 6. [Google Scholar] [CrossRef]
- Lin, D.-F.; Luo, H.-L.; Wang, H.-Y.; Hung, M.-J. Successful application of CLSM on a weak pavement base/subgrade for heavy truck traffic. J. Perform. Constr. Facil 2007, 21, 70–77. [Google Scholar] [CrossRef]
- Weng, T.L.; Lin, W.T.; Liu, Y.L. Engineering properties of controlled low-strength materials containing co-fired fly ash. Monatsh. Chem. 2017, 148, 1337–1347. [Google Scholar] [CrossRef]
- Du, L.X.; Folliard, K.J.; Trejo, D. Effects of constituent materials and quantities on water demand and compressive strength of controlled low-strength material. J. Mater. Civ. Eng. 2002, 14, 485–495. [Google Scholar] [CrossRef]
- Udayashankar, B.; Raghavendra, T. Proportioning controlled low strength materials using fly ash and ground granulated blast furnace slag. Adv. Mater. Sci. Environ. Energy Technol. III 2014, 250, 13–25. [Google Scholar]
- Wu, J.; Lin, Y. Experimental study of reservoir siltation as CLSM for backfill applications. Geo-Front. Adv. Geotech. Eng. 2011, 1217–1226. [Google Scholar] [CrossRef]
- Tang, C.-W.; Cheng, C.-K. Partial Replacement of Fine Aggregate Using Water Purification Sludge in Producing CLSM. Sustainability 2019, 11, 1351. [Google Scholar] [CrossRef]
- Arivusudar, N.; Babu, S.S. Performance of ground granulated blast-furnace slag based engineered cementitious composites. Cem. Wapno Beton 2020, 25, 95–103. [Google Scholar] [CrossRef]
- Lachemi, M.; Sahmaran, M.; Hossain, K.M.A.; Lotfy, A.; Shehata, M. Properties of controlled low-strength materials incorporating cement kiln dust and slag. Cem. Concr. Comp 2010, 32, 623–629. [Google Scholar] [CrossRef]
- Raghavendra, T.; Siddanagouda, Y.H.; Jawad, F.; Adarsha, C.Y.; Udayashankar, B.C. Performance of Ternary Binder Blend Containing Cement, Waste Gypsum Wall Boards and Blast Furnace Slag in CLSM. Procedia Eng. 2016, 145, 104–111. [Google Scholar] [CrossRef]
- Rashad, A.M. A synopsis manual about recycling steel slag as a cementitious material. J. Mater. Res. Technol. 2019, 8, 4940–4955. [Google Scholar] [CrossRef]
- Shen, D.H.; Wu, C.M.; Du, J.C. Laboratory investigation of basic oxygen furnace slag for substitution of aggregate in porous asphalt mixture. Constr. Build. Mater. 2009, 23, 453–461. [Google Scholar] [CrossRef]
- Tufekci, M.; Demirbas, A.; Genc, H. Evaluation of steel furnace slags as cement additives. Cem. Concr. Res. 1997, 27, 1713–1717. [Google Scholar] [CrossRef]
- Yi, H.; Xu, G.P.; Cheng, H.G.; Wang, J.S.; Wan, Y.F.; Chen, H. An overview of utilization of steel slag. Procedia Environ. Sci. 2012, 16, 791–801. [Google Scholar] [CrossRef]
- Kim, Y.S.; Dinh, B.H.; Do, T.M.; Kang, G.O. Development of thermally enhanced controlled low-strength material incorporating different types of steel-making slag for ground-source heat pump system. Renew. Energy 2020, 150, 116–127. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, J.W.; Yan, P.Y. Cementitious properties of super-fine steel slag. Powder Technol. 2013, 245, 35–39. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, P.; Feng, J. Excitation mechanism of alkaline condition on the hydration activity of steel slag. In Proceedings of the International RILEM Conference on Advances in Construction Materials Through Science and Engineering, Hong Kong, China, 5 September 2011; pp. 643–650. [Google Scholar]
- Muhmood, L.; Vitta, S.; Venkateswaran, D. Cementitious and pozzolanic behavior of electric arc furnace steel slags. Cem. Concr. Res. 2009, 39, 102–109. [Google Scholar] [CrossRef]
- Kang, G.; Cikmit, A.A.; Tsuchida, T.; Honda, H.; Kim, Y.S. Strength development and microstructural characteristics of soft dredged clay stabilized with basic oxygen furnace steel slag. Constr. Build. Mater. 2019, 203, 501–513. [Google Scholar] [CrossRef]
- Seng, S.; Tanaka, H. Properties of cement-treated soils during initial curing stages. Soils Found. 2011, 51, 775–784. [Google Scholar] [CrossRef]
- Sheen, Y.-N.; Huang, L.-J.; Sun, T.-H.; Le, D.-H. Engineering Properties of Self-compacting Concrete Containing Stainless Steel Slags. In Proceedings of the International Conference on Sustainable Development of Civil, Urban and Transportation Engineering (CUTE), Ho Chi Minh City, Vietnam, 11–14 April 2016. [Google Scholar]
- Sheen, Y.; Sun, T.; Chung, W. Compressive strength of controlled low strength materials containing stainless steel slag. J. Chin. Corros. Eng. 2008, 22, 217–230. (In Chinese) [Google Scholar]
- Le, D.-H.; Nguyen, K.-H. An assessment of eco-friendly controlled low-strength material. Procedia Eng. 2016, 142, 260–267. [Google Scholar] [CrossRef][Green Version]
- Sheen, Y.-N.; Huang, L.-J.; Wang, H.-Y.; Le, D.-H. Experimental study and strength formulation of soil-based controlled low-strength material containing stainless steel reducing slag. Constr. Build. Mater. 2014, 54, 1–9. [Google Scholar] [CrossRef]
- Dinh, B.H.; Kim, Y.S.; Kang, G.O. Thermal conductivity of steelmaking slag-based controlled low-strength materials over entire range of degree of saturation: A study for ground source heat pump systems. Geothermics 2020, 88, 10. [Google Scholar] [CrossRef]
- Srivastava, S.; Snellings, R.; Cool, P. Clinker-free carbonate-bonded (CFCB) products prepared by accelerated carbonation of steel furnace slags: A parametric overview of the process development. Constr. Build. Mater. 2021, 303, 11. [Google Scholar] [CrossRef]
- Ma, Z.H.; Liao, H.Q.; Wang, L.; Cheng, F.Q. Effects of iron/silicon/magnesium/aluminum on CaO carbonation of CO2 in steel slag-based building materials during carbonation curing. Constr. Build. Mater. 2021, 298, 12. [Google Scholar] [CrossRef]
- Song, Q.F.; Guo, M.Z.; Wang, L.; Ling, T.C. Use of steel slag as sustainable construction materials: A review of accelerated carbonation treatment. Resour. Conserv. Recycl. 2021, 173, 14. [Google Scholar] [CrossRef]
- Zhong, X.; Li, L.; Jiang, Y.; Ling, T.-C. Elucidating the dominant and interaction effects of temperature, CO2 pressure and carbonation time in carbonating steel slag blocks. Constr. Build. Mater. 2021, 302, 124158. [Google Scholar] [CrossRef]
- Mo, L.W.; Zhang, F.; Deng, M. Mechanical performance and microstructure of the calcium carbonate binders produced by carbonating steel slag paste under CO2 curing. Cem. Concr. Res. 2016, 88, 217–226. [Google Scholar] [CrossRef]
- Ghouleh, Z.; Guthrie, R.I.L.; Shao, Y.X. High-strength KOBM steel slag binder activated by carbonation. Constr. Build. Mater. 2015, 99, 175–183. [Google Scholar] [CrossRef]
- Chen, K.W.; Pan, S.Y.; Chen, C.T.; Chen, Y.H.; Chiang, P.C. High-gravity carbonation of basic oxygen furnace slag for CO2 fixation and utilization in blended cement. J. Clean. Prod. 2016, 124, 350–360. [Google Scholar] [CrossRef]
- Yang, S.; Mo, L.W.; Deng, M. Effects of ethylenediamine tetra-acetic acid (EDTA) on the accelerated carbonation and properties of artificial steel slag aggregates. Cem. Concr. Comp 2021, 118, 14. [Google Scholar] [CrossRef]
- Venkatesh, C.; Chand, M.S.R.; Nerella, R. A State of the Art on Red Mud as a Substitutional Cementitious Material. Ann. Chim.-Sci. Mater. 2019, 43, 99–106. [Google Scholar] [CrossRef]
- Liu, X.M.; Zhang, N.; Sun, H.H.; Zhang, J.X.; Li, L.T. Structural investigation relating to the cementitious activity of bauxite residue—Red mud. Cem. Concr. Res. 2011, 41, 847–853. [Google Scholar] [CrossRef]
- Do, T.M.; Kim, Y.S. Engineering properties of controlled low strength material (CLSM) incorporating red mud. Int. J. Geo-Eng. 2016, 7, 17. [Google Scholar] [CrossRef]
- Do, T.M.; Kim, Y.-S.; Tran, T.Q.; Vu, N. Effect of Lime on Engineering Properties of CLSM Made with Fly Ash-Red Mud-Lime-Gypsum Binder. In Proceedings of the Korean Society of Civil Engineers Convention, Busan, Korean, 18–20 October 2017; pp. 481–482. [Google Scholar]
- van Jaarsveld, J.G.S.; van Deventer, J.S.J.; Lukey, G.C. The characterisation of source materials in fly ash-based geopolymers. Mater. Lett. 2003, 57, 1272–1280. [Google Scholar] [CrossRef]
- He, R.X.; Zhang, S.Y.; Zhang, X.L.; Zhang, Z.H.; Zhao, Y.L.; Ding, H.X. Copper slag: The leaching behavior of heavy metals and its applicability as a supplementary cementitious material. J. Environ. Chem. Eng. 2021, 9, 12. [Google Scholar] [CrossRef]
- Song, J.W.; Feng, S.L.; Xiong, R.R.; Ouyang, Y.; Zeng, Q.L.; Zhu, J.L.; Zhang, C.Y. Mechanical Properties, Pozzolanic Activity and Volume Stability of Copper Slag-Filled Cementitious Materials. Mater. Sci. 2020, 26, 218–224. [Google Scholar] [CrossRef]
- Taha, R.A.; Alnuaimi, A.S.; Al-Jabri, K.S.; Al-Harthy, A.S. Evaluation of controlled low strength materials containing industrial by-products. Build. Environ. 2007, 42, 3366–3372. [Google Scholar] [CrossRef]
- Lim, S.; Lee, W.; Choo, H.; Lee, C. Utilization of high carbon fly ash and copper slag in electrically conductive controlled low strength material. Constr. Build. Mater. 2017, 157, 42–50. [Google Scholar] [CrossRef]
- de Matos, P.R.; Oliveira, J.C.P.; Medina, T.M.; Magalhaes, D.C.; Gleize, P.J.P.; Schankoski, R.A.; Pilar, R. Use of air-cooled blast furnace slag as supplementary cementitious material for self-compacting concrete production. Constr. Build. Mater. 2020, 262, 120102. [Google Scholar] [CrossRef]
- Bouzalakos, S.; Dudeney, A.W.L.; Cheeseman, C.R. Controlled low-strength materials containing waste precipitates from mineral processing. Miner. Eng. 2008, 21, 252–263. [Google Scholar] [CrossRef]
- Chan, B.K.C.; Bouzalakos, S.; Dudeney, A.W.L. Integrated waste and water management in mining and metallurgical industries. Trans. Nonferrous Met. Soc. China 2008, 18, 1497–1505. [Google Scholar] [CrossRef]
- Pariser, H.H.; Backeberg, N.R.; Masson, O.C.M.; Bedder, J.C.M. Changing nickel and chromium stainless steel markets—A review. J. S. Afr. Inst. Min. Metall. 2018, 118, 563–568. [Google Scholar] [CrossRef]
- Fares, A.I.; Sohel, K.M.A.; Al-Jabri, K.; Al-Mamun, A. Characteristics of ferrochrome slag aggregate and its uses as a green material in concrete-A review. Constr. Build. Mater. 2021, 294, 123552. [Google Scholar] [CrossRef]
- Nath, S.K. Geopolymerization behavior of ferrochrome slag and fly ash blends. Constr. Build. Mater. 2018, 181, 487–494. [Google Scholar] [CrossRef]
- Kauppi, M.; Pekka, N. Production, characteristics and use of Ferrochrome slags. In Proceedings of the International Ferro-Alloys Congress XI, New Delhi, India, 18–21 February 2007; pp. 171–179. [Google Scholar]
- Zhou, X.T.; Hao, X.T.; Ma, Q.M.; Luo, Z.Q.; Zhang, M.Q.; Peng, J.H. Effects of compound chemical activators on the hydration of low-carbon ferrochrome slag-based composite cement. J. Environ. Manag. 2017, 191, 58–65. [Google Scholar] [CrossRef]
- Isil, S.K.; Hasan, B. Assessment of Reusing Ferrochrome Slag Wastes in Mortar as SCMs. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 2020, 35, 1043–1052. [Google Scholar] [CrossRef]
- Kim, Y.; Jung, S.; Do, T.; Choi, G. Comparison of Flow Characteristics of Controlled Low Strength Material binded by Cementless Binder s made from Industrial by-products. In Proceedings of the Korean Geo-Environmental Society, Seoul Korean, 26–27 September 2013; pp. 279–282. [Google Scholar]
- Xiaowei, C.; Wen, N.; Chao, R. Hydration mechanism of all solid waste cementitious materials based on steel slag and blast furnace slag. Chin. J. Mater. Res. 2017, 31, 687–694. (In Chinese) [Google Scholar]
- Xu, D.; Ni, W.; Wang, Q.H.; Xu, C.W.; Li, K.Q. Ammonia-soda residue and metallurgical slags from iron and steel industries as cementitious materials for clinker-free concretes. J. Clean. Prod. 2021, 307, 12. [Google Scholar] [CrossRef]
- Lim, S.; Choo, H.; Lee, W.; Lee, C. The Characterization of Controlled Low Strength Material (CLSM) Using High CaO Fly Ash without Chemical Alkaline Activator. J. Korean Geoenviron. Soc. 2016, 17, 17–26. [Google Scholar] [CrossRef]
- Du, L.X.; Arellano, M.; Folliard, K.J.; Nazarian, S.; Trejo, D. Rapid-setting CLSM for bridge approach repair: A case study. ACI Mater. J. 2006, 103, 312–318. [Google Scholar]
- Davidovits, J. Geopolymer, Green Chemistry and Sustainable Development Solutions: Proceedings of the World Congress Geopolymer 2005; Geopolymer Institute: Saint-Quentin, France, 2005. [Google Scholar]
- Lee, N.K.; Kim, H.K.; Park, I.S.; Lee, H.K. Alkali-activated, cementless, controlled low-strength materials (CLSM) utilizing industrial by-products. Constr. Build. Mater. 2013, 49, 738–746. [Google Scholar] [CrossRef]
- Park, S.M.; Lee, N.K.; Lee, H.K. Circulating fluidized bed combustion ash as controlled low-strength material (CLSM) by alkaline activation. Constr. Build. Mater. 2017, 156, 728–738. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Mehrotra, S.P. Influence of granulated blast furnace slag on the reaction, structure and properties of fly ash based geopolymer. J. Mater. Sci. 2010, 45, 607–615. [Google Scholar] [CrossRef]
- Ghanad, D.A.; Soliman, A.M. Bio-based alkali-activated controlled low strength material: Engineering properties. Constr. Build. Mater. 2021, 279, 9. [Google Scholar] [CrossRef]
- Samantasinghar, S.; Singh, S.P. Fresh and Hardened Properties of Fly Ash-Slag Blended Geopolymer Paste and Mortar. Int. J. Concr. Struct. Mater. 2019, 13, 47. [Google Scholar] [CrossRef]
- Mkadmini Hammi, K.; Hammi, H.; Hamzaoui, A.H. Use of Mixture Design Approach for the Optimization and Performance of Cost-Effective Cementitious Quaternary System: Portland Cement-Fly Ash-Silica Fume-Phosphogypsum. Chem. Afr. 2021, 4, 835–848. [Google Scholar] [CrossRef]
- Sun, T.; Hu, T.; Wang, G.M.; Shui, Z.H.; Ge, K.Y.; Dai, Q.T.; Xie, Y.F. Influence of Clinker and SCMs on Soluble Chemicals and Expansion of Phosphogypsum-Based Cementitious Materials. J. Test. Eval. 2020, 48, 1950–1961. [Google Scholar] [CrossRef]
- Wang, T.; Gao, X.J.; Wang, J. Preparation of Foamed Phosphogypsum Lightweight Materials by Incorporating Cementitious Additives. Mater. Sci. 2019, 25, 340–347. [Google Scholar] [CrossRef]
- Lu, W.D.; Ma, B.G.; Su, Y.; He, X.Y.; Jin, Z.H.; Qi, H.H. Low-Energy Consumption Preparation of Fine Waterproof Cementitious Material with High-Volume Phosphogypsum. J. Mater. Civ. Eng. 2020, 32, 11. [Google Scholar] [CrossRef]
- Harrou, A.; Gharibi, E.; Taha, Y.; Fagel, N.; El Ouahabi, M. Phosphogypsum and Black Steel Slag as Additives for Ecological Bentonite-Based Materials: Microstructure and Characterization. Minerals 2020, 10, 1067. [Google Scholar] [CrossRef]
- Chen, X.M.; Gao, J.M.; Zhao, Y.S. Investigation on the hydration of hemihydrate phosphogypsum after post treatment. Constr. Build. Mater. 2019, 229, 9. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q. Hydration Mechanism and Hardening Property of α-Hemihydrate Phosphogypsum. Minerals 2019, 9, 733. [Google Scholar] [CrossRef]
- Rong, K.W.; Lan, W.T.; Li, H.Y. Industrial Experiment of Goaf Filling Using the Filling Materials Based on Hemihydrate Phosphogypsum. Minerals 2020, 10, 324. [Google Scholar] [CrossRef]
- Do, T.M.; Kang, G.O.; Go, G.H.; Kim, Y.S. Evaluation of Coal Ash-Based CLSM Made with Cementless Binder as a Thermal Grout for Borehole Heat Exchangers. J. Mater. Civ. Eng. 2019, 31, 11. [Google Scholar] [CrossRef]
- Kim, Y.S.; Do, T.M.; Kim, H.K.; Kang, G. Utilization of excavated soil in coal ash-based controlled low strength material (CLSM). Constr. Build. Mater. 2016, 124, 598–605. [Google Scholar] [CrossRef]
- Mun, K.J.; Hyoung, W.K.; Lee, C.W.; So, S.Y.; Soh, Y.S. Basic properties of non-sintering cement using phosphogypsum and waste lime as activator. Constr. Build. Mater. 2007, 21, 1342–1350. [Google Scholar] [CrossRef]
- Tan Manh, D.; Kim, Y.-s.; My Quoc, D. Influence of curing conditions on engineering properties of controlled low strength material made with cementless binder. KSCE J. Civ. Eng. 2017, 21, 1774–1782. [Google Scholar] [CrossRef]
- Do, T.M.; Kim, Y.; Dang, M.Q.; Jeong, C.B. Development of controlled low-strength materials derived from coal ash and excavated soil without using Portland cement. In Proceedings of the International Conference of Geotec Hanoi, Hanoi, Vietnam, 24–25 November 2016. [Google Scholar]
- Do, T.M.; Kim, Y.S.; Dang, M.Q. Development of New Cementless Binder for Controlled Low Strength Material (CLSM) utilizing Fly ash, Red mud and Phosphogypsum. In Proceedings of the Korean Society of Civil Engineers Convention, Jeju, Korean, 19–21 October 2016. [Google Scholar]
- Tan Manh, D.; Young Sang, K.; Kang, G.O.; My Quoc, D.; Thien Quoc, T. Thermal Conductivity of Controlled Low Strength Material (CLSM) Made Entirely from By-Products. Key Eng. Mater. 2018, 773, 244–248. [Google Scholar] [CrossRef]
- Do, T.M.; Kim, Y.S.; Ryu, B.C. Improvement of engineering properties of pond ash based CLSM with cementless binder and artificial aggregates made of bauxite residue. Int. J. Geo-Eng. 2015, 6, 10. [Google Scholar] [CrossRef]
- Achtemichuk, S.; Hubbard, J.; Sluce, R.; Shehata, M.H. The utilization of recycled concrete aggregate to produce controlled low-strength materials without using Portland cement. Cem. Concr. Compos. 2009, 31, 564–569. [Google Scholar] [CrossRef]

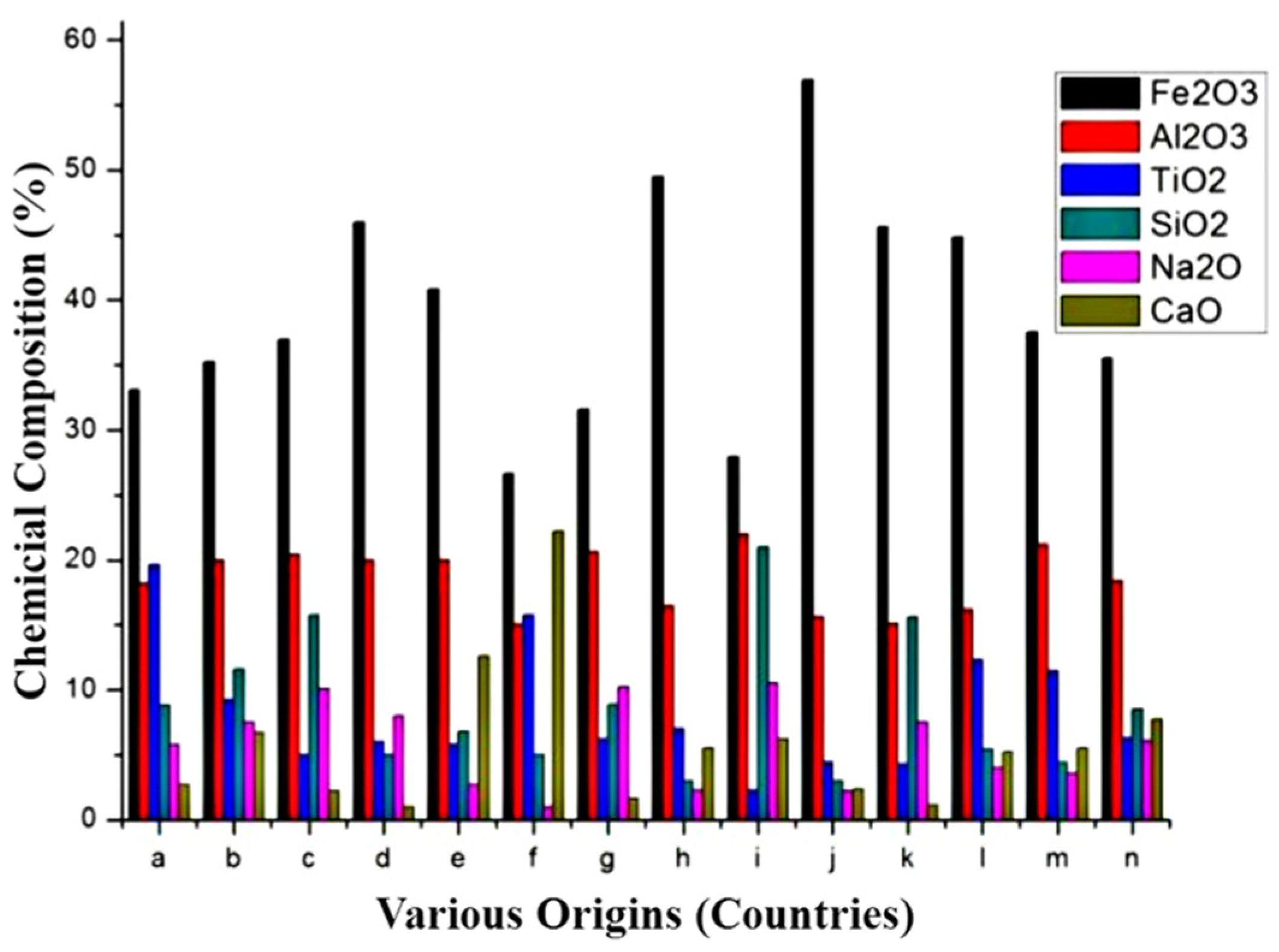

| Source | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | TiO2 | MnO | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Shougang Group | 33.50 | 12.52 | 1.10 | 37.90 | 9.29 | 2.51 | N/A * | N/A | [50] |
| Baosteel | 33.54 | 14.83 | 1.20 | 40.06 | 8.43 | 0.12 | 0.60 | 0.43 | [50] |
| Tangsteel | 30.80 | 14.70 | 2.67 | 38.10 | 8.84 | 2.77 | 0.83 | 0.13 | [50] |
| Datong | 27.90 | 17.30 | 3.60 | 37.90 | 8.37 | 1.90 | 1.87 | 0.22 | [50] |
| Handan | 31.00 | 13.70 | 1.15 | 38.60 | 10.30 | 0.99 | 1.66 | 0.24 | [50] |
| Chengdu | 28.80 | 12.20 | 4.65 | 36.70 | 6.41 | 1.40 | 7.38 | 0.91 | [50] |
| China | 25.56 | 12.85 | 1.11 | 51.65 | 2.95 | 2.8 | 1.17 | N/A | [51] |
| Poland | 38.7 | 7.7 | 0.6 | 40.5 | 6.32 | 0.31 | N/A | N/A | [52] |
| Nippon Steel | 32.51 | 14.37 | 0.15 | 43.98 | 5.17 | 3.03 | N/A | N/A | [53] |
| Pakistan | 37.22 | 10.37 | 1.23 | 35.66 | N/A | 0.34 | N/A | N/A | [54] |
| South Korea | 29.13 | 11.82 | 0.44 | 42.51 | 2.43 | 3.34 | 0.59 | 0.23 | [55] |
| Spain | 35.96 | 10.61 | 0.4 | 42.89 | 7.10 | 2.02 | N/A | N/A | [56] |
| India | 36.9 | 14.1 | 0.11 | 40 | 8 | N/A | N/A | N/A | [57] |
| Australia | 34.5 | 14.5 | N/A | 40.5 | 6.5 | N/A | 1.5 | 0.5 | [58] |
| Standard/Specification | China GB/T 18046-2017 [61] | Japan JIS A 6206:2013 [62] | Korea KS F 2563 (2019 Confirm):2012 [63] | EU EN 15167 -1:2006 [64] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade | S105 | S95 | S75 | 8000 | 6000 | 4000 | 3000 | Class 1 | Class 2 | Class 3 | N/A * | |
| Density/(g/cm3) | ≥2.8 | ≥2.8 | ≥2.8 | N/A | ||||||||
| Specific surface area/(m2/kg) | ≥500 | ≥400 | ≥300 | 700–1000 | 500–700 | 350–500 | 275–350 | 800–1000 | 600–800 | 400–600 | ≥275 | |
| Activity index (%) | 7 d | ≥95 | ≥70 | ≥55 | ≥95 | ≥75 | ≥55 | N/A | ≥95 | ≥75 | ≥55 | ≥45 |
| 28 d | ≥105 | ≥95 | ≥75 | ≥105 | ≥95 | ≥75 | ≥ 60 | ≥105 | ≥95 | ≥75 | ≥70 | |
| 91 d | N/A | N/A | N/A | ≥105 | ≥105 | ≥95 | ≥80 | ≥105 | ≥105 | ≥95 | N/A | |
| SO3 (%) | ≤4.0 | ≤4.0 | ≤4.0 | ≤2.5 | ||||||||
| MgO (%) | N/A | ≤10 | ≤10 | ≤18 | ||||||||
| Glassiness (%) | ≥85 | N/A | N/A | N/A | ||||||||
| Metallurgical Waste Slag | Main Components | Phase or Mineral | Particle Morphology | Cementitious Properties | Reaction Products | Application Mode | Setting Time | Workability | Strength | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| GGBFS | CaO, SiO2, Al2O3, MgO | Glassiness accounts for 80–90% | Smooth and compact surface | Weak hydration when <45 μm; pozzolanic activity | C-S-H | Replace cement | ↑ 1 | ↑ | ↓ 2 | [43,59,72,74] |
| Steel slag powder | CaO, SiO2, Al2O3, Fe2O3, | C2S, C3S, C4AF | N/A * | Weak hydration; pozzolanic activity | C-S-H, Ca(OH)2, Ettringite | Replace aggregate | ↑ | N/A | ↑ | [93,94] |
| Raw steelmaking slag | CaO, SiO2, Al2O3, Fe2O3, | N/A | N/A | Very weak hydration | C-S-H | Replace aggregate | ↓↑ 3 | ↓ | ↑↓ 4 | [89,99] |
| Ground steelmaking slag | CaO, SiO2, Al2O3, Fe2O3, | N/A | N/A | Weak hydration | C-S-H | Replace aggregate | ↓ | ↓ | ↑ | [89,99] |
| SSRS | CaO, SiO2 | N/A | N/A | Weak hydration; pozzolanic activity | C-S-H | Replace cement | ↑ | ↑ | ↓ | [97,98] |
| Red mud | Fe2O3, Al2O3, SiO2, Na2O | N/A | N/A | Alkali stimulates hydration | Ca(OH)2, calcium Aluminates | Replace cement | ↑↓ | ↓ | ↑↓ | [110] |
| Copper slag | Fe2O3, SiO2 | N/A | N/A | Weak pozzolanic activity | N/A | Replace cement | N/A | ↓ | ↓ | [115] |
| Copper slag | Fe2O3, SiO2 | N/A | N/A | N/A | N/A | Replace aggregate | N/A | ↑ | ↓ | [116] |
| Replace fly ash | N/A | ↑ | ↑ | |||||||
| FCS | Al2O3, SiO2, MgO, Cr2O3 | Presence of glass phase | Irregular shape, smooth surface | N/A | N/A | Replace 66% cement and fly ash | N/A | ↓ | ↓ | [28] |
| Replace 55% cement and fly ash | N/A | ↑ | ↓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Su, Y.; Xu, G.; Chen, Y.; You, G. Research Progress on Controlled Low-Strength Materials: Metallurgical Waste Slag as Cementitious Materials. Materials 2022, 15, 727. https://doi.org/10.3390/ma15030727
Liu Y, Su Y, Xu G, Chen Y, You G. Research Progress on Controlled Low-Strength Materials: Metallurgical Waste Slag as Cementitious Materials. Materials. 2022; 15(3):727. https://doi.org/10.3390/ma15030727
Chicago/Turabian StyleLiu, Yiliang, Youpo Su, Guoqiang Xu, Yanhua Chen, and Gaoshuai You. 2022. "Research Progress on Controlled Low-Strength Materials: Metallurgical Waste Slag as Cementitious Materials" Materials 15, no. 3: 727. https://doi.org/10.3390/ma15030727
APA StyleLiu, Y., Su, Y., Xu, G., Chen, Y., & You, G. (2022). Research Progress on Controlled Low-Strength Materials: Metallurgical Waste Slag as Cementitious Materials. Materials, 15(3), 727. https://doi.org/10.3390/ma15030727