Kelvin Probe Microscopy Investigation of Poly-Octylthiophene Aggregates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Sample Characterization and Data Processing
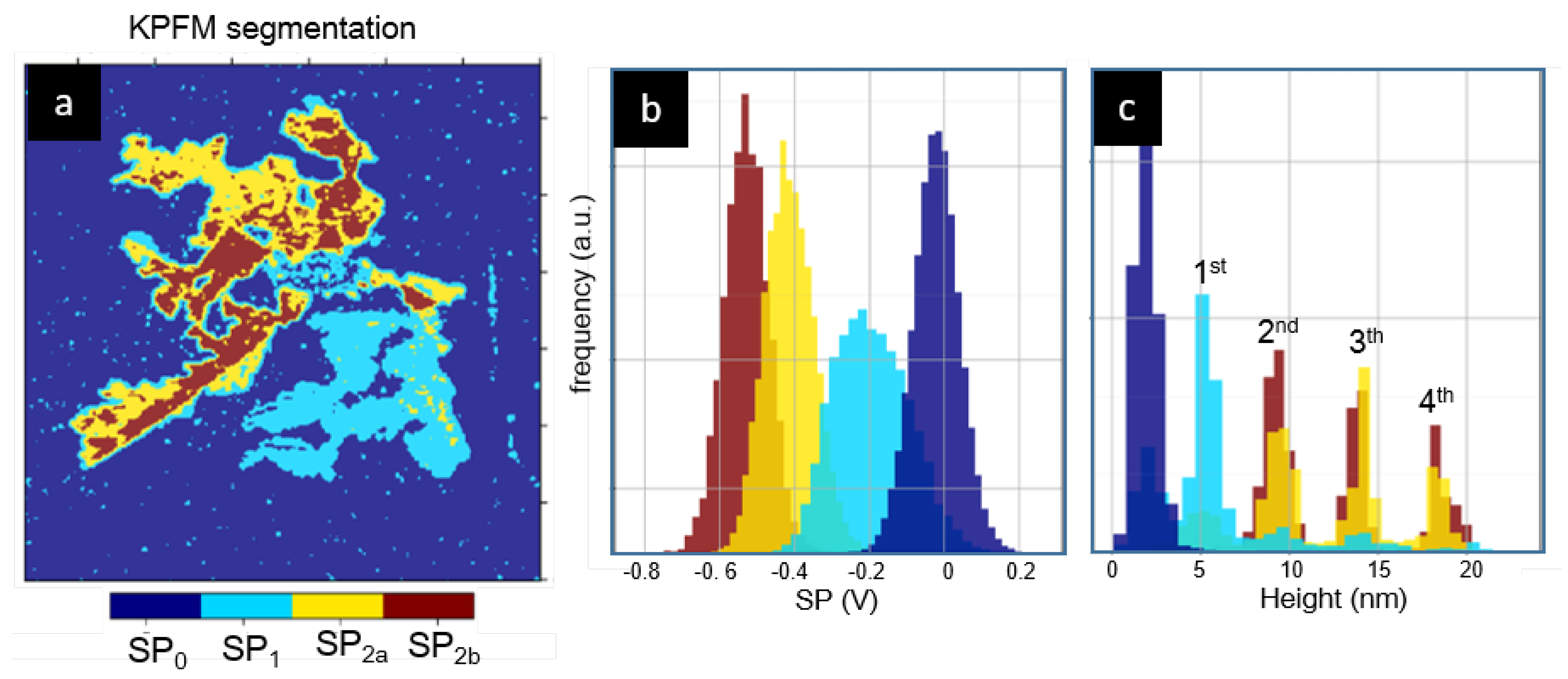
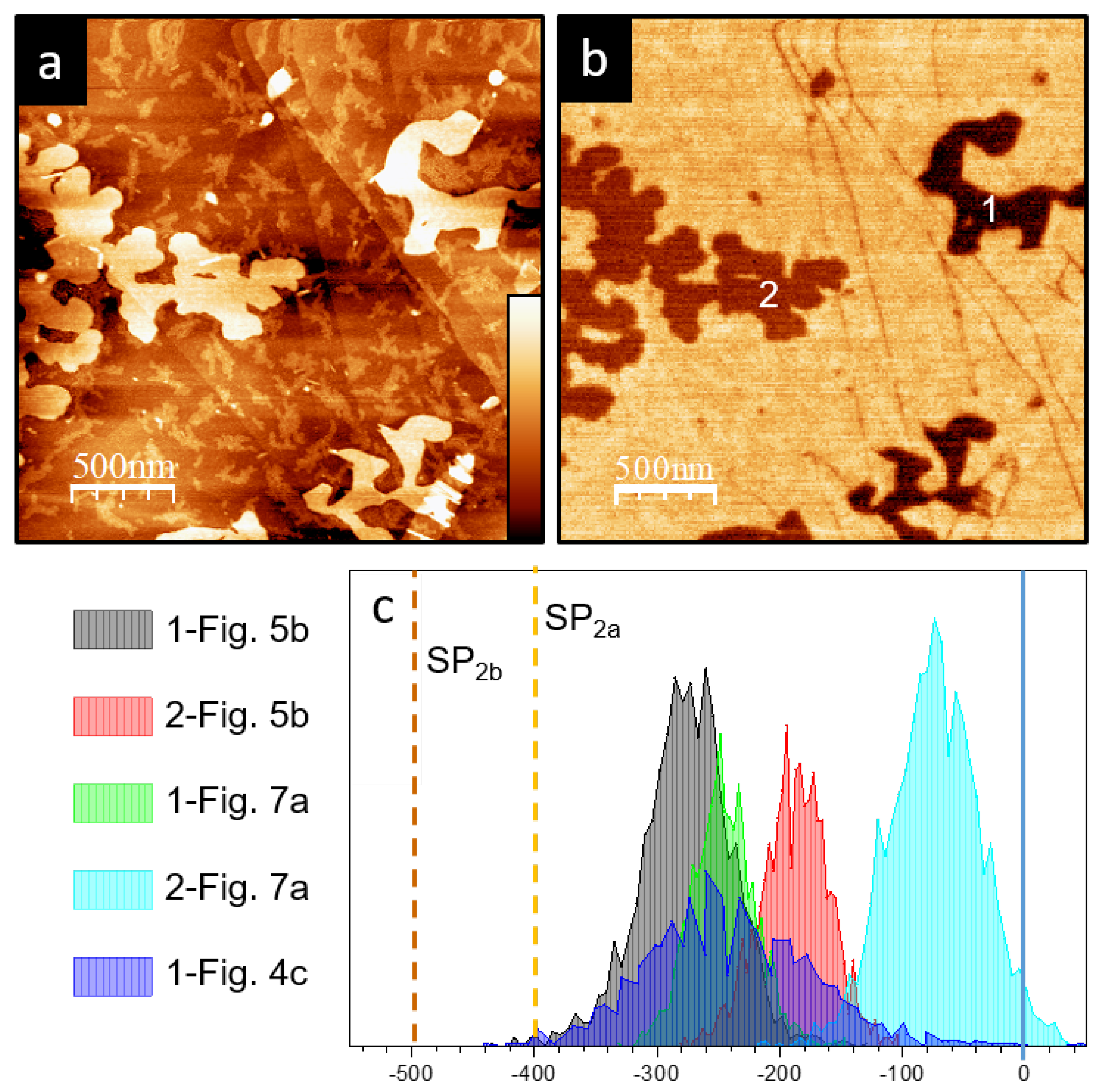
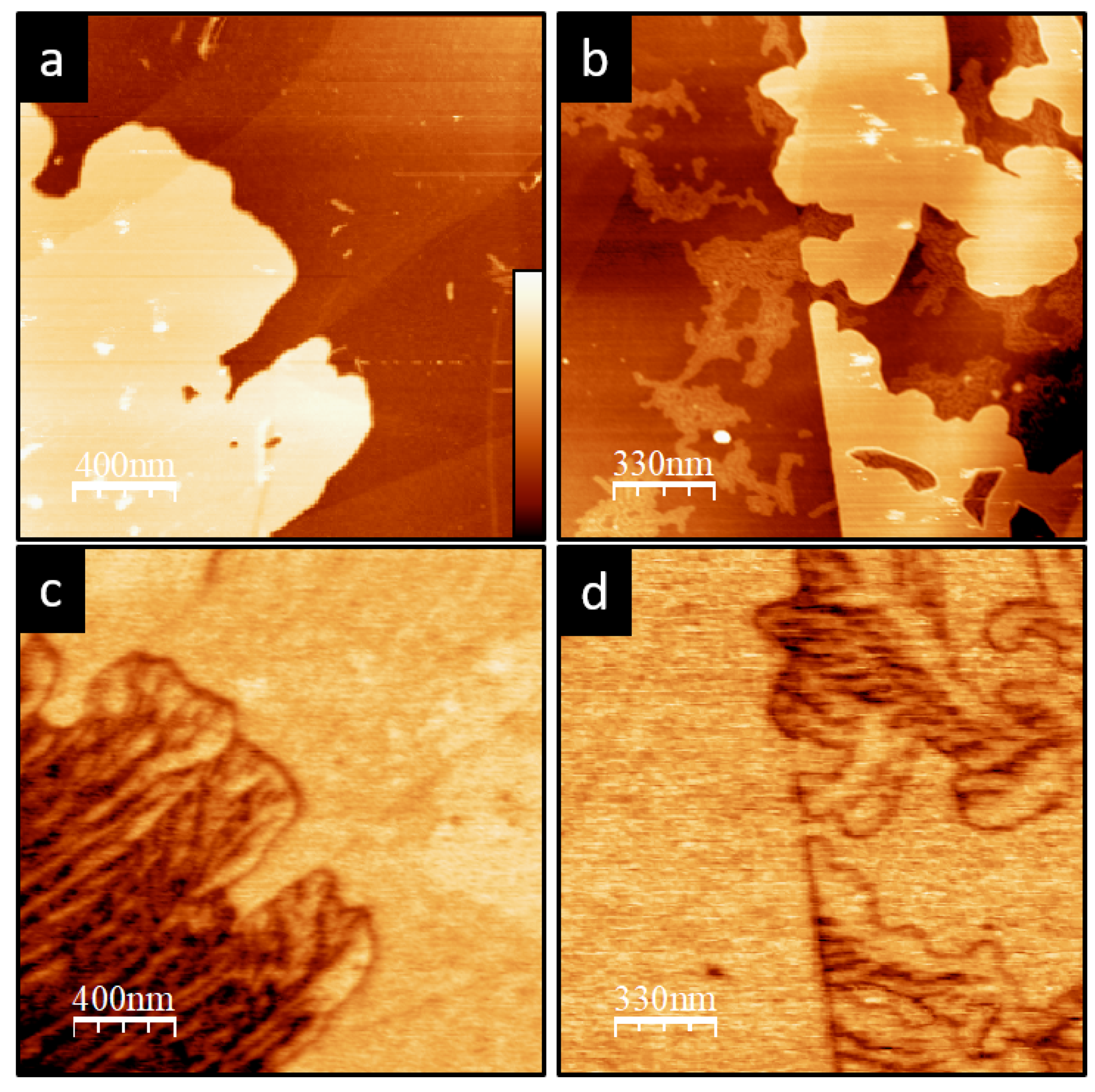
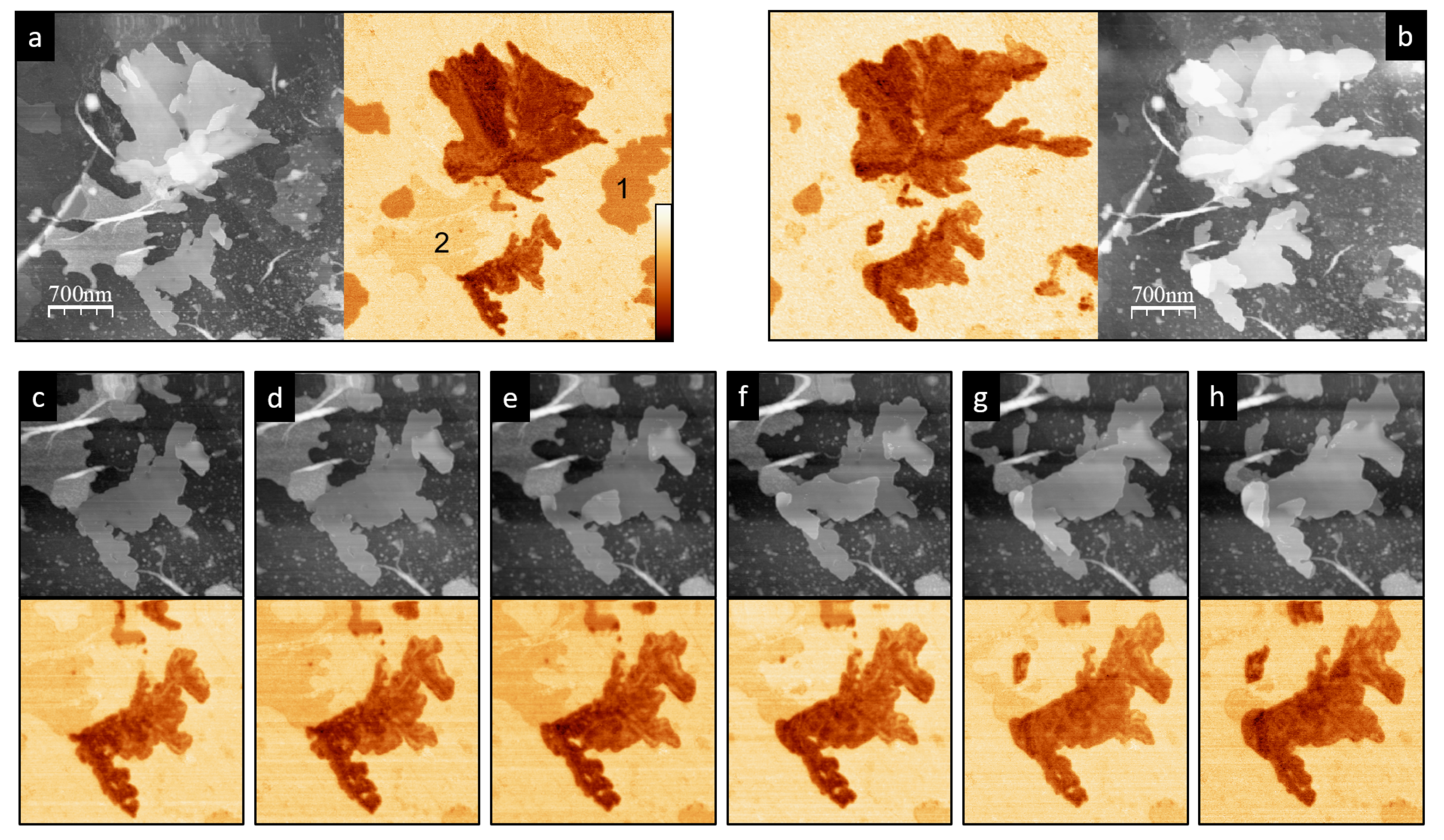
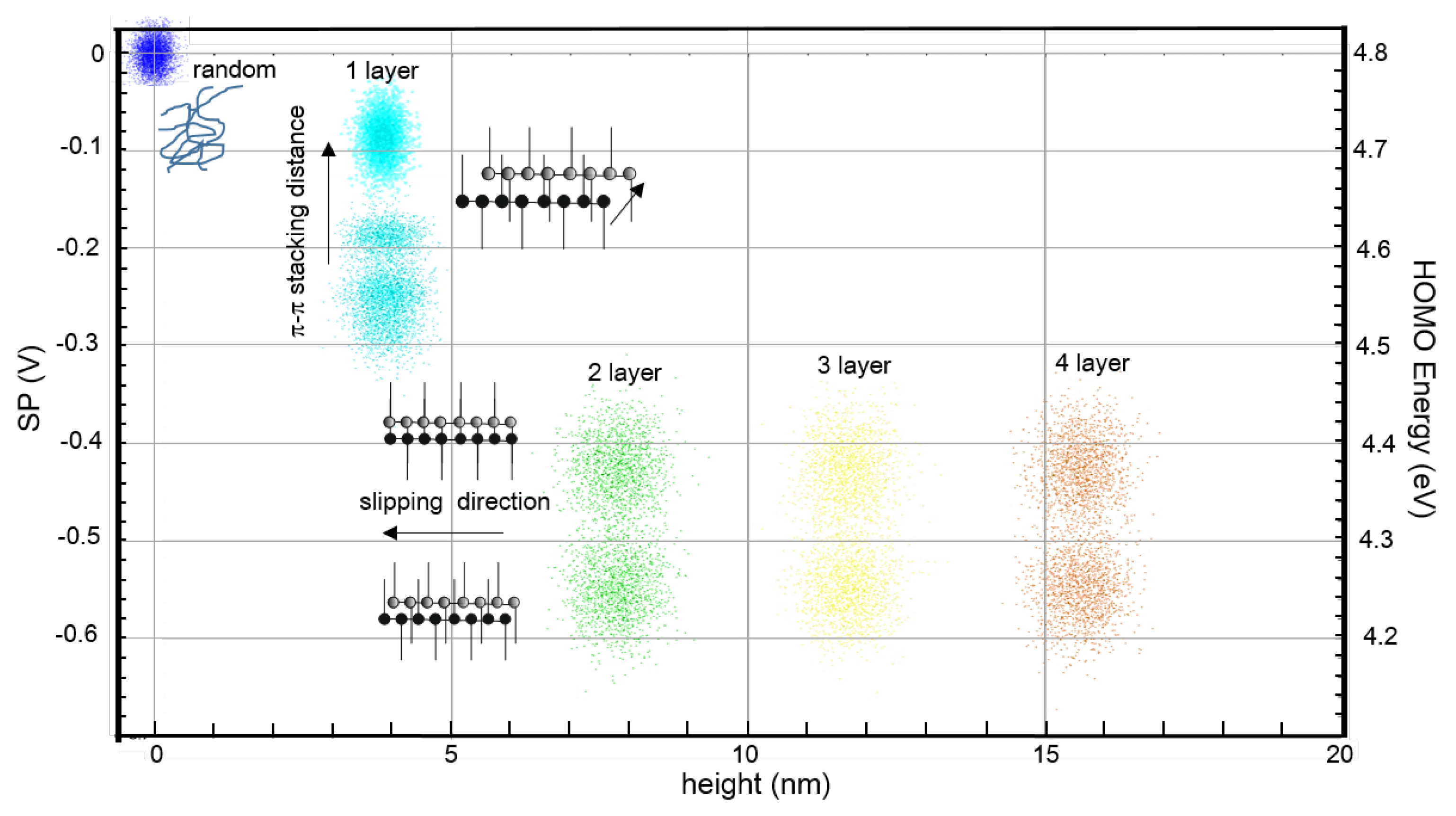
3. Results
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reynolds, J.R.; Thompson, B.C.; Skotheim, T.A. Handbook of Conducting Polymers—2 Volume Set, 4th ed.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Reynolds, J.R.; Thompson, B.C.; Skotheim, T.A. Conjugated Polymers: Perspective, Theory, and New Materials; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar] [CrossRef]
- Zhang, H.; Li, R.; Deng, Z.; Cui, S.; Wang, Y.; Zheng, M.; Yang, W. π-Conjugated oligomers based on aminobenzodifuranone and diketopyrrolopyrrole. Dye. Pigment. 2020, 181, 108552. [Google Scholar] [CrossRef]
- Themed Collection: Conducting polymers for carbon electronics. Chem. Soc. Rev. 2010, 39, 2337. [CrossRef]
- Kasha, M. Characterization of electronic transitions in complex molecules. Discuss. Faraday Soc. 1950, 9, 14–19. [Google Scholar] [CrossRef]
- Yamagata, H.; Pochas, C.M.; Spano, F.C. Designing J- and H-Aggregates through Wave Function Overlap Engineering: Applications to Poly(3-hexylthiophene). J. Phys. Chem. B 2012, 116, 14494–14503. [Google Scholar] [CrossRef]
- Yamagata, H.; Spano, F.C. Interplay between intrachain and interchain interactions in semiconducting polymer assemblies: The HJ-aggregate model. J. Chem. Phys. 2012, 136, 184901. [Google Scholar] [CrossRef] [PubMed]
- Heimel, G.; Salzmann, I.; Duhm, S.; Rabe, J.P.; Koch, N. Intrinsic Surface Dipoles Control the Energy Levels of Conjugated Polymers. Adv. Funct. Mater. 2009, 19, 3874–3879. [Google Scholar] [CrossRef]
- Yamagata, H.; Maxwell, D.S.; Fan, J.; Kittilstved, K.R.; Briseno, A.L.; Barnes, M.D.; Spano, F.C. HJ-Aggregate Behavior of Crystalline 7,8,15,16-Tetraazaterrylene: Introducing a New Design Paradigm for Organic Materials. J. Phys. Chem. C 2014, 118, 28842–28854. [Google Scholar] [CrossRef]
- Ehrenreich, P.; Birkhold, S.T.; Zimmermann, E.; Hu, H.; Kim, K.D.; Weickert, J.; Pfadler, T.; Schmidt-Mende, L. H-aggregate analysis of P3HT thin films-Capability and limitation of photoluminescence and UV/Vis spectroscopy. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- McFarland, F.M.; Brickson, B.; Guo, S. Layered Poly(3-hexylthiophene) Nanowhiskers Studied by Atomic Force Microscopy and Kelvin Probe Force Microscopy. Macromolecules 2015, 48, 3049–3056. [Google Scholar] [CrossRef]
- Baghgar, M.; Barnes, M.D. Work Function Modification in P3HT H/J Aggregate Nanostructures Revealed by Kelvin Probe Force Microscopy and Photoluminescence Imaging. ACS Nano 2015, 9, 7105–7112. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Barnes, M.D. Disentangling “Bright” and “Dark” Interactions in Ordered Assemblies of Organic Semiconductors. Nano Lett. 2017, 17, 6949–6953. [Google Scholar] [CrossRef] [PubMed]
- Agbolaghi, S.; Zenoozi, S. A comprehensive review on poly(3-alkylthiophene)-based crystalline structures, protocols and electronic applications. Org. Electron. 2017, 51, 362–403. [Google Scholar] [CrossRef]
- Ostroverkhova, O. Organic Optoelectronic Materials: Mechanisms and Applications. Chem. Rev. 2016, 116, 13279–13412. [Google Scholar] [CrossRef] [PubMed]
- Prosa, T.J.; Winokur, M.J.; Moulton, J.; Smith, P.; Heeger, A.J. X-ray structural studies of poly(3-alkylthiophenes): An example of an inverse comb. Macromolecules 1992, 25, 4364–4372. [Google Scholar] [CrossRef]
- Seidel, C. Handbook of Conducting Polymers. Volumes 1 and 2. Hg. von Terje A. Skotheim. ISBN 0-8247-7395-0 und 0-8247-7454-X. New York/Basel: Marcel Dekker Inc. 1986. XVIII + XVII, 1417 S., gcb. $ 150.00. Acta Polym. 1987, 38, 707–726. [Google Scholar] [CrossRef]
- Ihn, K.J.; Moulton, J.; Smith, P. Whiskers of poly(3-alkylthiophene)s. J. Polym. Sci. Part B Polym. Phys. 1993, 31, 735–742. [Google Scholar] [CrossRef]
- Abad, J.; Espinosa, N.; Ferrer, P.; García-Valverde, R.; Miguel, C.; Padilla, J.; Alcolea, A.; Castro, G.R.; Colchero, J.; Urbina, A. Molecular structure of poly(3-alkyl-thiophenes) investigated by calorimetry and grazing incidence X-ray scattering. Sol. Energy Mater. Sol. Cells 2012, 97, 109–118. [Google Scholar] [CrossRef]
- Tremel, K.; Ludwigs, S. Morphology of P3HT in Thin Films in Relation to Optical and Electrical Properties. In P3HT Revisited—From Molecular Scale to Solar Cell Devices; Ludwigs, S., Ed.; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2014; pp. 39–82. [Google Scholar] [CrossRef]
- Koch, N. Electronic structure of interfaces with conjugated organic materials. Phys. Status Solidi RRL—Rapid Res. Lett. 2012, 6, 277–293. [Google Scholar] [CrossRef]
- Brinkmann, M.; Rannou, P. Molecular Weight Dependence of Chain Packing and Semicrystalline Structure in Oriented Films of Regioregular Poly(3-hexylthiophene) Revealed by High-Resolution Transmission Electron Microscopy. Macromolecules 2009, 42, 1125–1130. [Google Scholar] [CrossRef]
- Brinkmann, M. Structure and morphology control in thin films of regioregular poly(3-hexylthiophene). J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1218–1233. [Google Scholar] [CrossRef]
- Kline, R.; McGehee, M.; Kadnikova, E.; Liu, J.; Fréchet, J. Controlling the Field-Effect Mobility of Regioregular Polythiophene by Changing the Molecular Weight. Adv. Mater. 2003, 15, 1519–1522. [Google Scholar] [CrossRef]
- Verilhac, J.M.; LeBlevennec, G.; Djurado, D.; Rieutord, F.; Chouiki, M.; Travers, J.P.; Pron, A. Effect of macromolecular parameters and processing conditions on supramolecular organisation, morphology and electrical transport properties in thin layers of regioregular poly(3-hexylthiophene). Synth. Met. 2006, 156, 815–823. [Google Scholar] [CrossRef]
- Snyder, C.R.; Henry, J.S.; DeLongchamp, D.M. Effect of Regioregularity on the Semicrystalline Structure of Poly(3-hexylthiophene). Macromolecules 2011, 44, 7088–7091. [Google Scholar] [CrossRef]
- Chang, J.F.; Sun, B.; Breiby, D.W.; Nielsen, M.M.; Sölling, T.I.; Giles, M.; McCulloch, I.; Sirringhaus, H. Enhanced Mobility of Poly(3-hexylthiophene) Transistors by Spin-Coating from High-Boiling-Point Solvents. Chem. Mater. 2004, 16, 4772–4776. [Google Scholar] [CrossRef]
- Zhang, R.; Li, B.; Iovu, M.C.; Jeffries-EL, M.; Sauvé, G.; Cooper, J.; Jia, S.; Tristram-Nagle, S.; Smilgies, D.M.; Lambeth, D.N.; et al. Nanostructure Dependence of Field-Effect Mobility in Regioregular Poly(3-hexylthiophene) Thin Film Field Effect Transistors. J. Am. Chem. Soc. 2006, 128, 3480–3481. [Google Scholar] [CrossRef]
- Arenas, M.C.; Mendoza, N.; Cortina, H.; Nicho, M.E.; Hu, H. Influence of poly3-octylthiophene (P3OT) film thickness and preparation method on photovoltaic performance of hybrid ITO/CdS/P3OT/Au solar cells. Sol. Energy Mater. Sol. Cells 2010, 94, 29–33. [Google Scholar] [CrossRef]
- Kadem, B.Y.; Al-hashimi, M.K.; Hassan, A.K. The Effect of Solution Processing on the Power Conversion Efficiency of P3HT-based Organic Solar Cells. Energy Procedia 2014, 50, 237–245. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.; Liu, J.; Li, M.; Yu, X.; Xing, R.; Han, Y. Decreased domain size and improved crystallinity by adjusting solvent-polymer interaction parameters in all-polymer solar cells. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 288–296. [Google Scholar] [CrossRef]
- Yang, H.; Shin, T.J.; Yang, L.; Cho, K.; Ryu, C.Y.; Bao, Z. Effect of Mesoscale Crystalline Structure on the Field-Effect Mobility of Regioregular Poly(3-hexyl thiophene) in Thin-Film Transistors. Adv. Funct. Mater. 2005, 15, 671–676. [Google Scholar] [CrossRef]
- Dang, M.T.; Hirsch, L.; Wantz, G.; Wuest, J.D. Controlling the Morphology and Performance of Bulk Heterojunctions in Solar Cells. Lessons Learned from the Benchmark Poly(3-hexylthiophene):[6,6]-Phenyl-C61-butyric Acid Methyl Ester System. Chem. Rev. 2013, 113, 3734–3765. [Google Scholar] [CrossRef]
- Hestand, N.J.; Spano, F.C. Molecular Aggregate Photophysics beyond the Kasha Model: Novel Design Principles for Organic Materials. Acc. Chem. Res. 2017, 50, 341–350. [Google Scholar] [CrossRef]
- Hestand, N.J.; Spano, F.C. Expanded Theory of H- and J-Molecular Aggregates: The Effects of Vibronic Coupling and Intermolecular Charge Transfer. Chem. Rev. 2018, 118, 7069–7163. [Google Scholar] [CrossRef] [PubMed]
- Roehling, J.D.; Arslan, I.; Moulé, A.J. Controlling microstructure in poly(3-hexylthiophene) nanofibers. J. Mater. Chem. 2012, 22, 2498–2506. [Google Scholar] [CrossRef]
- Gao, J.; Stein, B.W.; Thomas, A.K.; Garcia, J.A.; Yang, J.; Kirk, M.L.; Grey, J.K. Enhanced Charge Transfer Doping Efficiency in J-Aggregate Poly(3-hexylthiophene) Nanofibers. J. Phys. Chem. C 2015, 119, 16396–16402. [Google Scholar] [CrossRef]
- Tuncel, D.; Demir, H.V. Conjugated polymer nanoparticles. Nanoscale 2010, 2, 484–494. [Google Scholar] [CrossRef]
- Nagarjuna, G.; Baghgar, M.; Labastide, J.A.; Algaier, D.D.; Barnes, M.D.; Venkataraman, D. Tuning aggregation of poly(3-hexylthiophene) within nanoparticles. ACS Nano 2012, 6, 10750–10758. [Google Scholar] [CrossRef]
- Istif, E.; Hernández-Ferrer, J.; Urriolabeitia, E.P.; Stergiou, A.; Tagmatarchis, N.; Fratta, G.; Large, M.J.; Dalton, A.B.; Benito, A.M.; Maser, W.K. Conjugated Polymer Nanoparticle–Graphene Oxide Charge-Transfer Complexes. Adv. Funct. Mater. 2018, 28, 1707548. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; LeFevre, S.W.; Ryu, C.Y.; Bao, Z. Solubility-driven thin film structures of regioregular poly(3-hexyl thiophene) using volatile solvents. Appl. Phys. Lett. 2007, 90, 172116. [Google Scholar] [CrossRef]
- López-Mata, C.; Nicho, M.E.; Hu, H.; Cadenas-Pliego, G.; García-Hernández, E. Optical and morphological properties of chemically synthesized poly3-octylthiophene thin films. Thin Solid Films 2005, 490, 189–195. [Google Scholar] [CrossRef]
- Nicho, M.E.; Hernández, F.; Hu, H.; Medrano, G.; Güizado, M.; Guerrero, J.A. Physicochemical and morphological properties of spin-coated poly(3-alkylthiophene) thin films. Sol. Energy Mater. Sol. Cells 2009, 93, 37–40. [Google Scholar] [CrossRef]
- Nicho, M.E.; Peña-Salgado, D.; Altuzar-Coello, P. Morphological and physicochemical properties of spin-coated poly(3-octylthiophene)/polystyrene composite thin films. Thin Solid Films 2010, 518, 1799–1803. [Google Scholar] [CrossRef]
- Palermo, V.; Palma, M.; Samorì, P. Electronic Characterization of Organic Thin Films by Kelvin Probe Force Microscopy. Adv. Mater. 2006, 18, 145–164. [Google Scholar] [CrossRef]
- Pérez-García, B.; Abad, J.; Urbina, A.; Colchero, J.; Palacios-Lidón, E. Surface potential domains on lamellar P3OT structures. Nanotechnology 2008, 19, 065709. [Google Scholar] [CrossRef] [PubMed]
- Fischer, F.S.U.; Tremel, K.; Sommer, M.; Crossland, E.J.C.; Ludwigs, S. Directed crystallization of poly(3-hexylthiophene) in micrometre channels under confinement and in electric fields. Nanoscale 2012, 4, 2138–2144. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instruments 2007, 78, 013705. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Lidón, E.; Munuera, C.; Ocal, C.; Colchero, J. Contrast inversion in non-contact Dynamic Scanning Force Microscopy: What is high and what is low? Ultramicroscopy 2010, 110, 789–800. [Google Scholar] [CrossRef]
- O’Neil, P.A. m2py: Materials Morphology Python Package. 2021. Available online: https://github.com/ponl/m2py (accessed on 1 June 2018).
- Tatum, W.K.; Torrejon, D.; O’Neil, P.; Onorato, J.W.; Resing, A.B.; Holliday, S.; Flagg, L.Q.; Ginger, D.S.; Luscombe, C.K. Generalizable Framework for Algorithmic Interpretation of Thin Film Morphologies in Scanning Probe Images. J. Chem. Inf. Model. 2020, 60, 3387–3397. [Google Scholar] [CrossRef] [PubMed]
- Dag, S.; Wang, L.W. Packing Structure of Poly(3-hexylthiophene) Crystal: Ab Initio and Molecular Dynamics Studies. J. Phys. Chem. B 2010, 114, 5997–6000. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.S.; Jo, H.; Kim, J.B.; Seo, I.; Lee, H.H.; Lee, D.R. Structural Transition and Interdigitation of Alkyl Side Chains in the Conjugated Polymer Poly(3-hexylthiophene) and Their Effects on the Device Performance of the Associated Organic Field-Effect Transistor. ACS Appl. Mater. Interfaces 2020, 12, 1142–1150. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bermejo, J.; Colchero, J.; Palacios-Lidon, E. Kelvin Probe Microscopy Investigation of Poly-Octylthiophene Aggregates. Materials 2022, 15, 1212. https://doi.org/10.3390/ma15031212
Bermejo J, Colchero J, Palacios-Lidon E. Kelvin Probe Microscopy Investigation of Poly-Octylthiophene Aggregates. Materials. 2022; 15(3):1212. https://doi.org/10.3390/ma15031212
Chicago/Turabian StyleBermejo, Joaquin, Jaime Colchero, and Elisa Palacios-Lidon. 2022. "Kelvin Probe Microscopy Investigation of Poly-Octylthiophene Aggregates" Materials 15, no. 3: 1212. https://doi.org/10.3390/ma15031212
APA StyleBermejo, J., Colchero, J., & Palacios-Lidon, E. (2022). Kelvin Probe Microscopy Investigation of Poly-Octylthiophene Aggregates. Materials, 15(3), 1212. https://doi.org/10.3390/ma15031212





