On-Demand Hydrogen Generation by the Hydrolysis of Ball-Milled Aluminum–Bismuth–Zinc Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Aluminum Composite Compositions and Their Mechanochemical Preparation
2.3. Hydrolysis Set-Up and Hydrogen Measurements
2.4. Composite Characterization
3. Results and Discussion
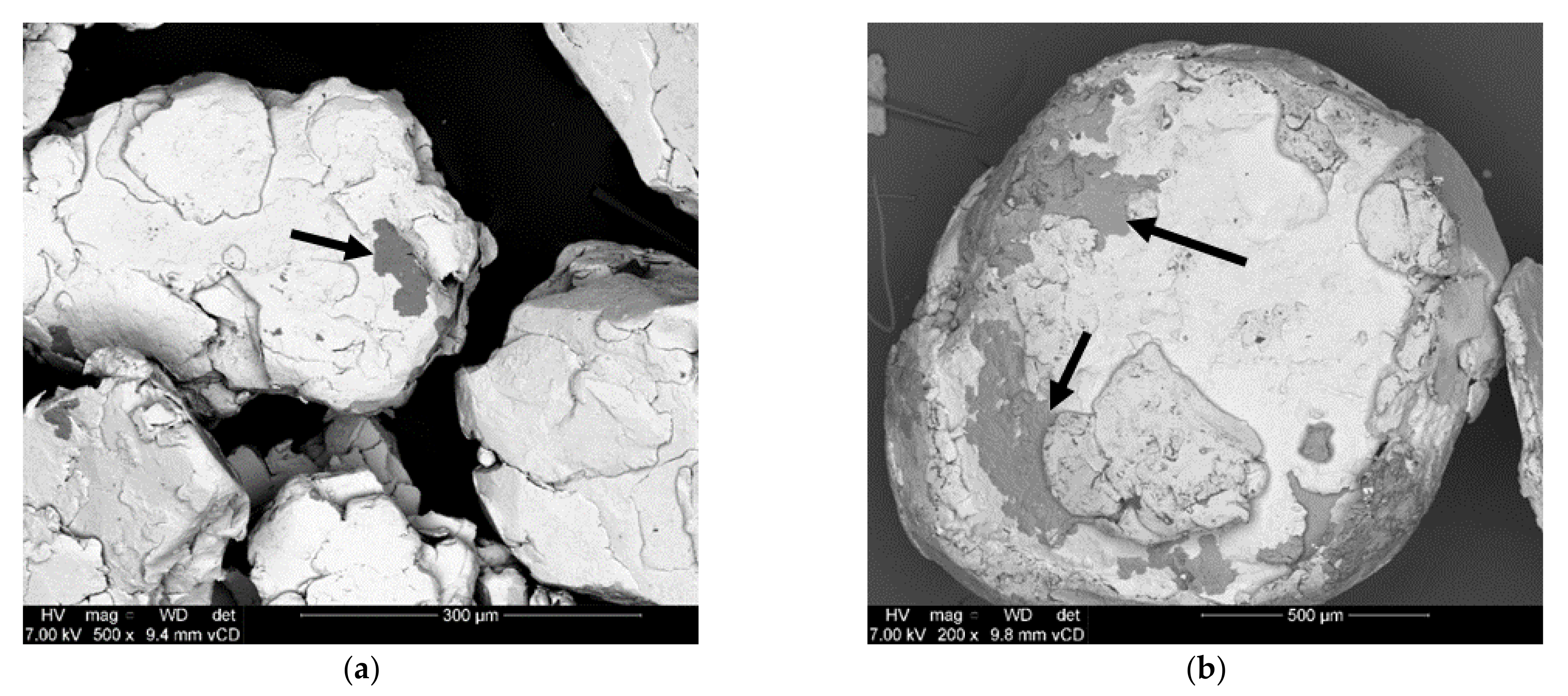
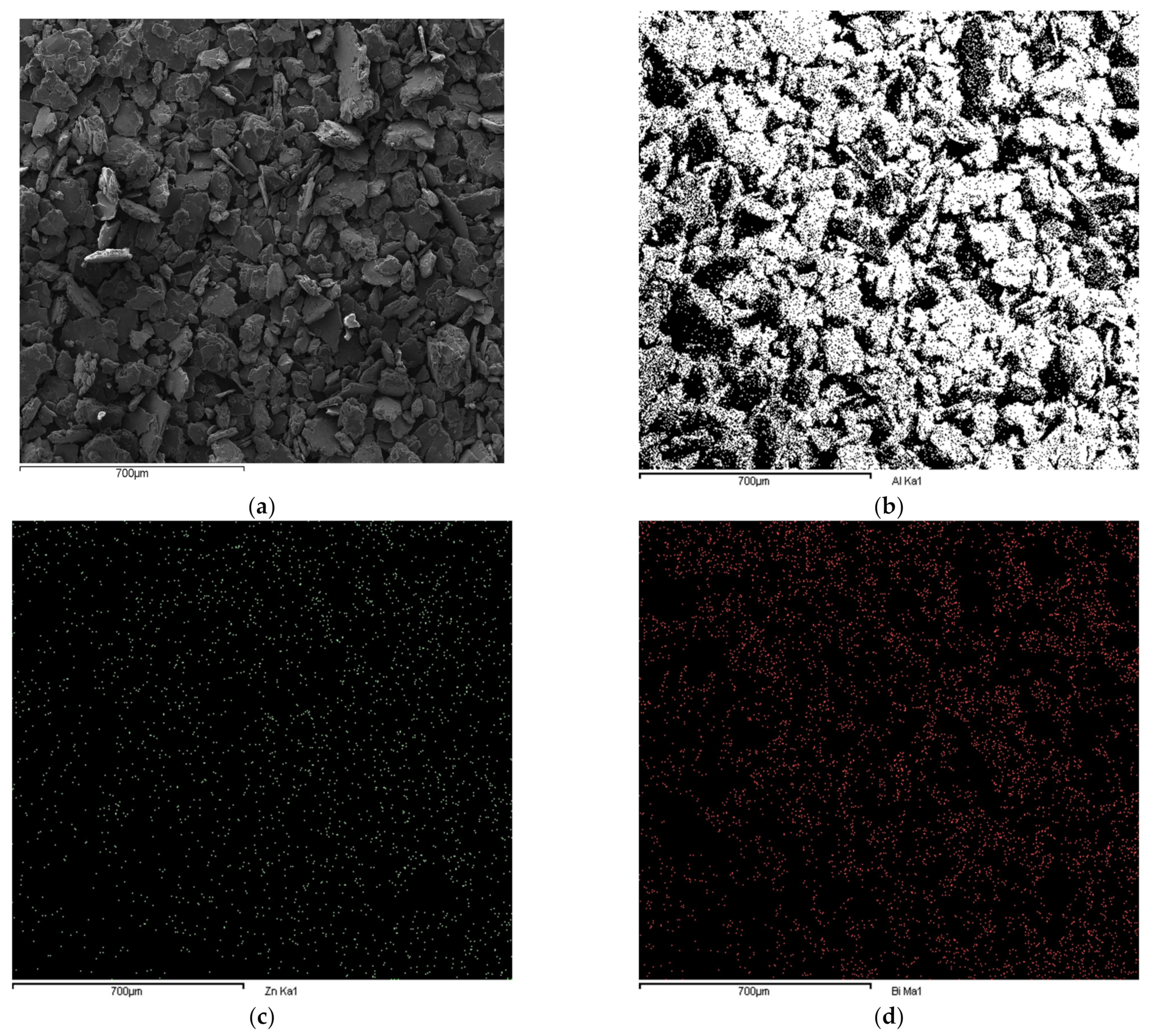
3.1. Effects of Balling on Characteristics of Al Composite Particles
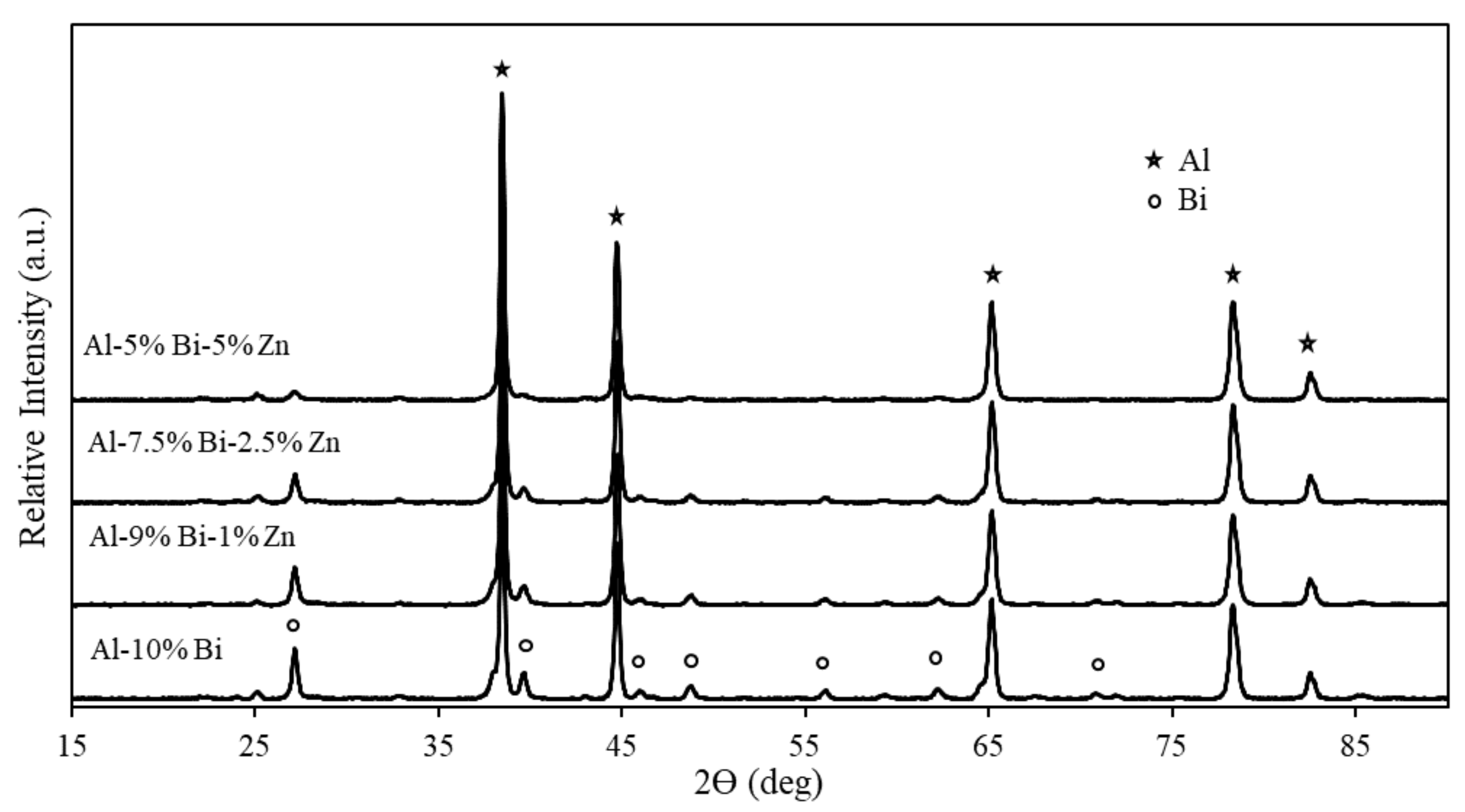
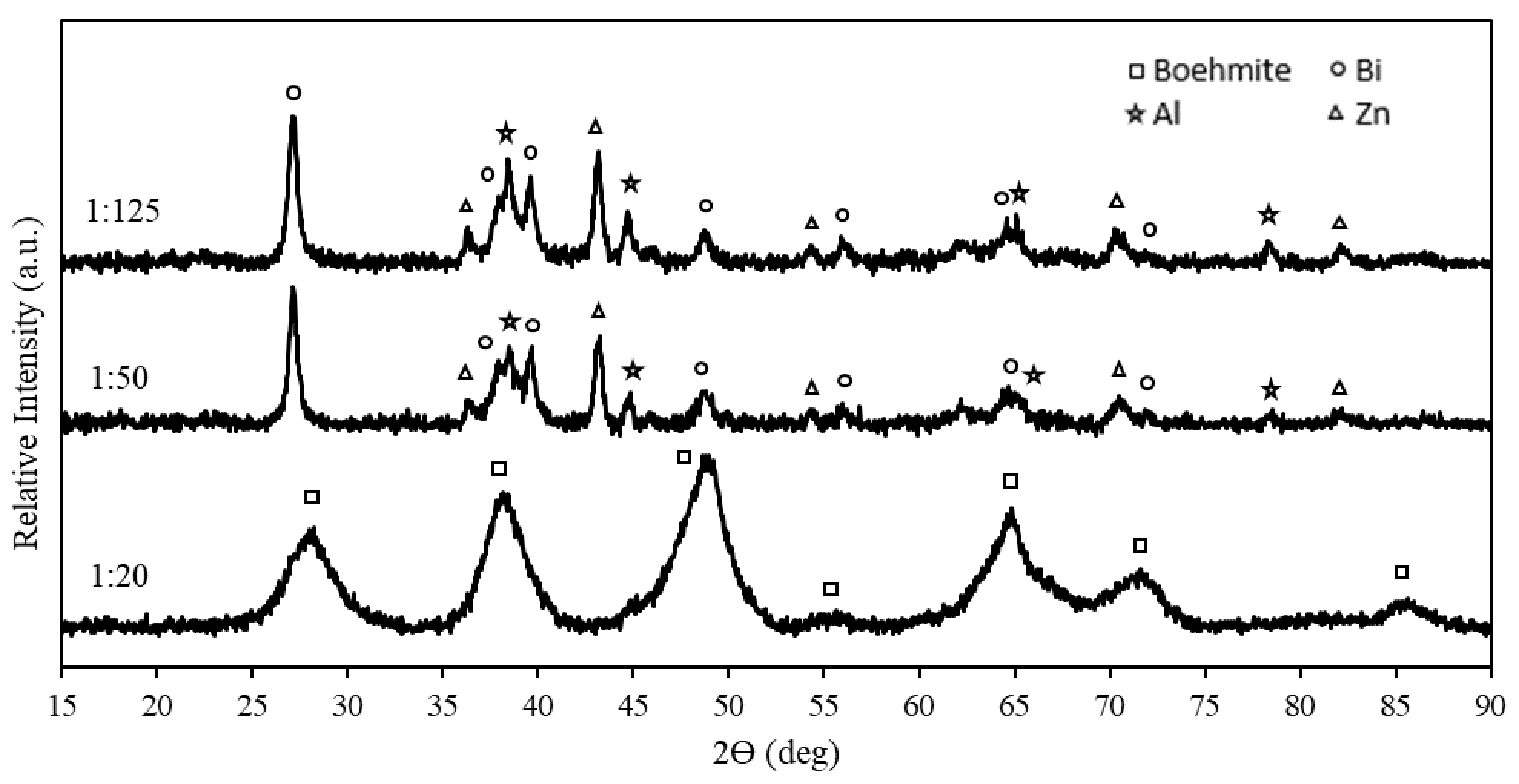
3.2. XRD Analysis of Ternary Al Composites
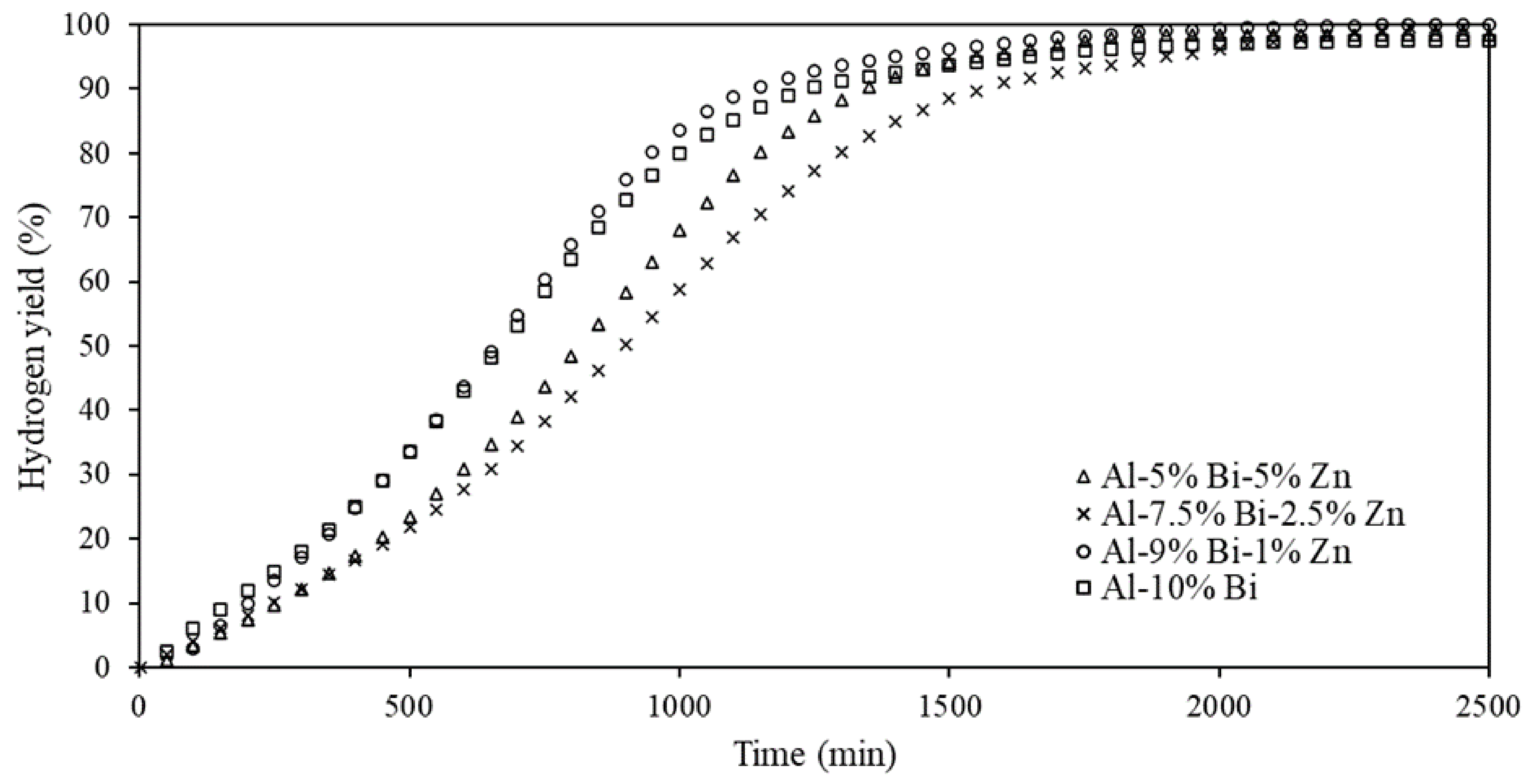
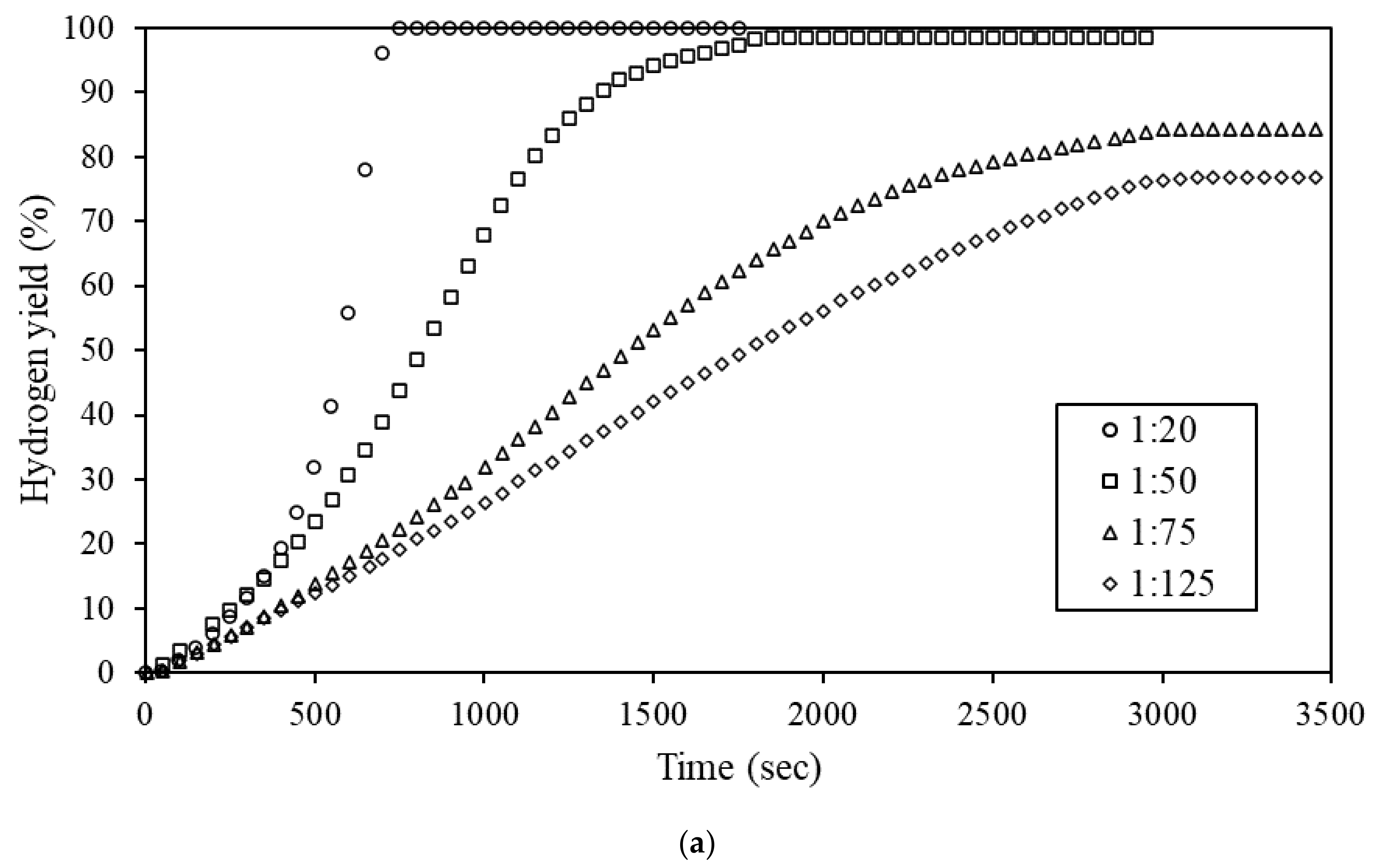
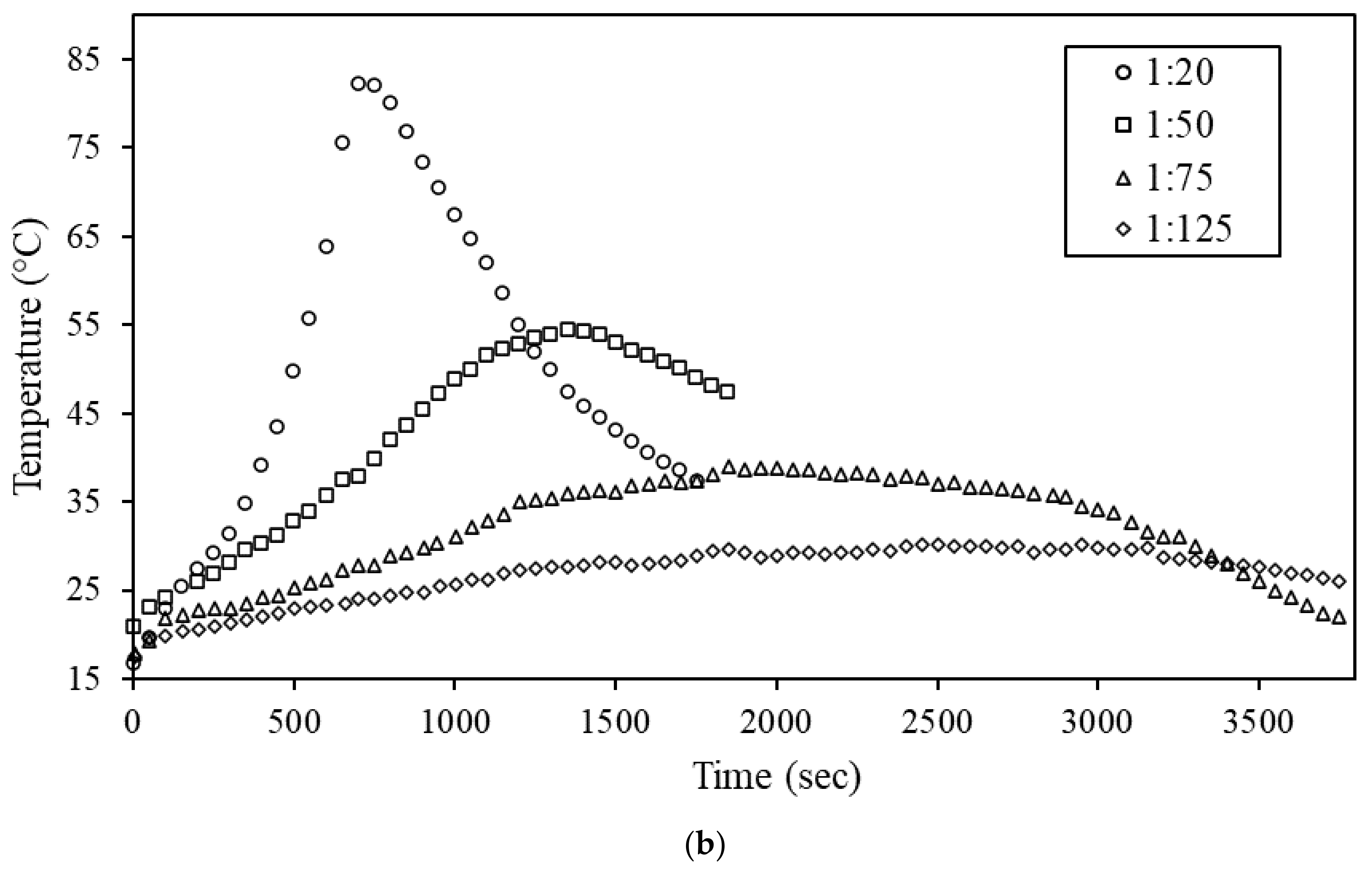
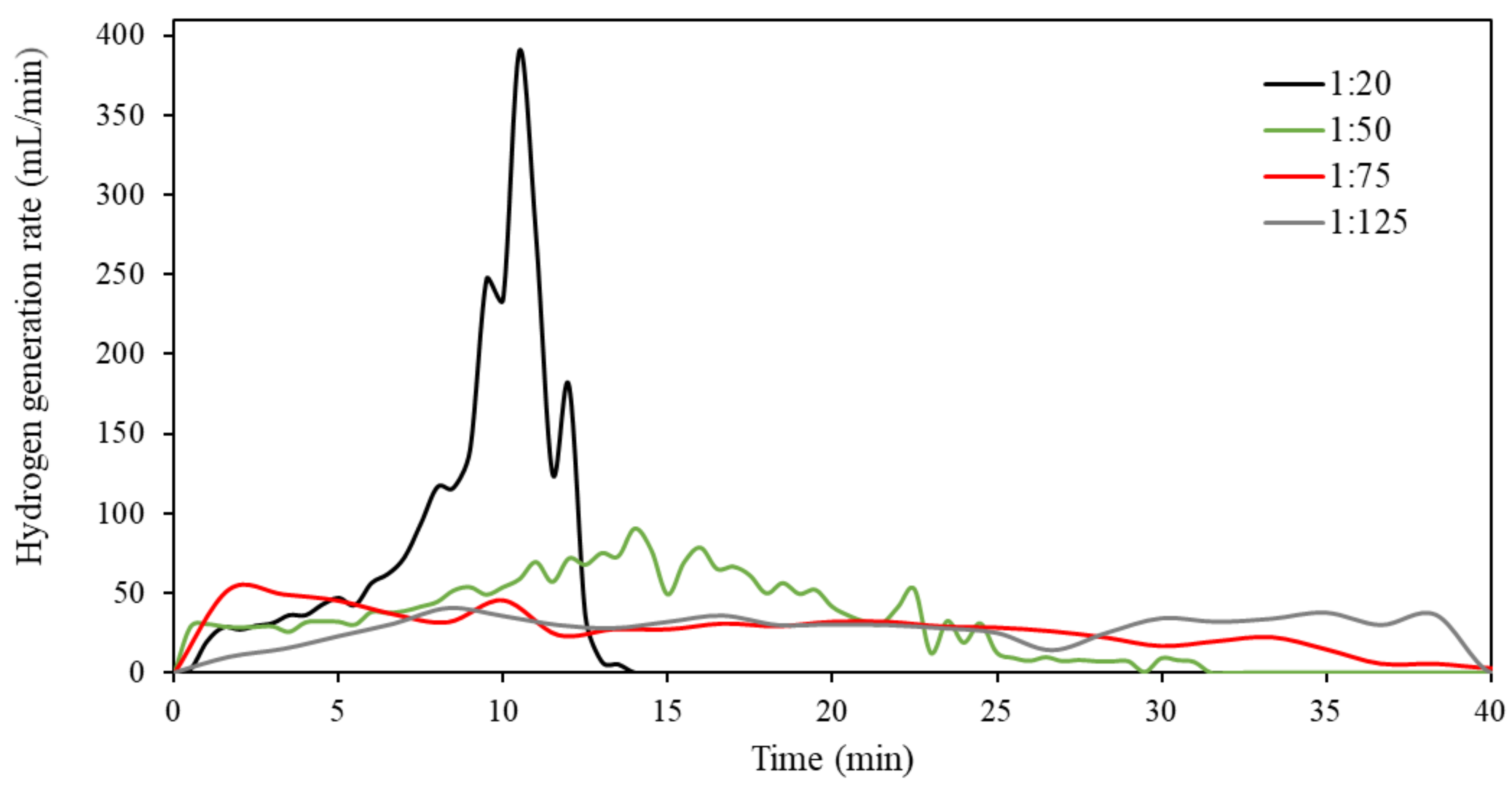
3.3. Hydrolysis of Ternary Al-Bi-Zn Composites
3.4. Effects of Mass Ratio on the Hydrolysis Reaction
3.5. Analysis of Hydrolysis Residues
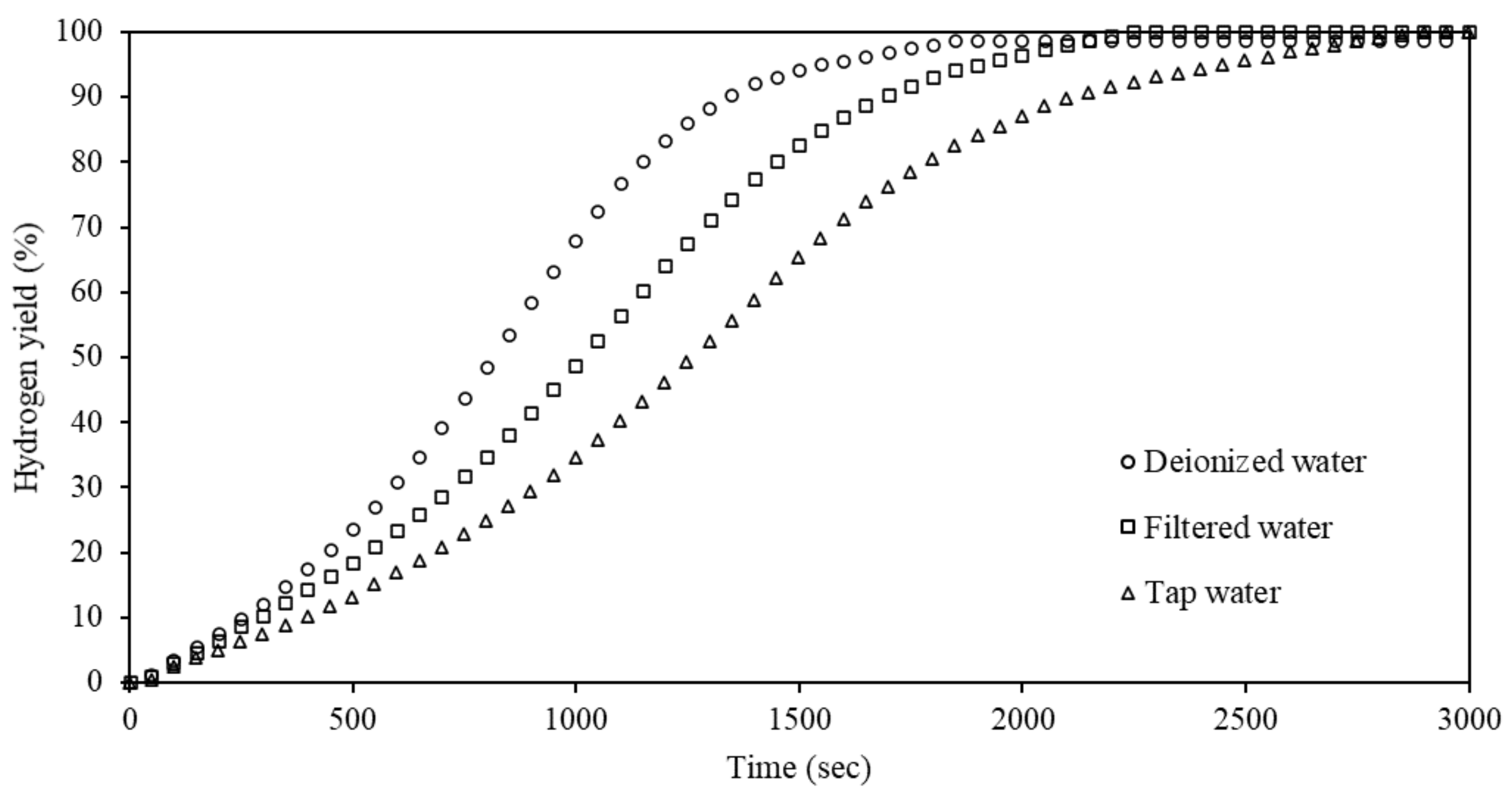
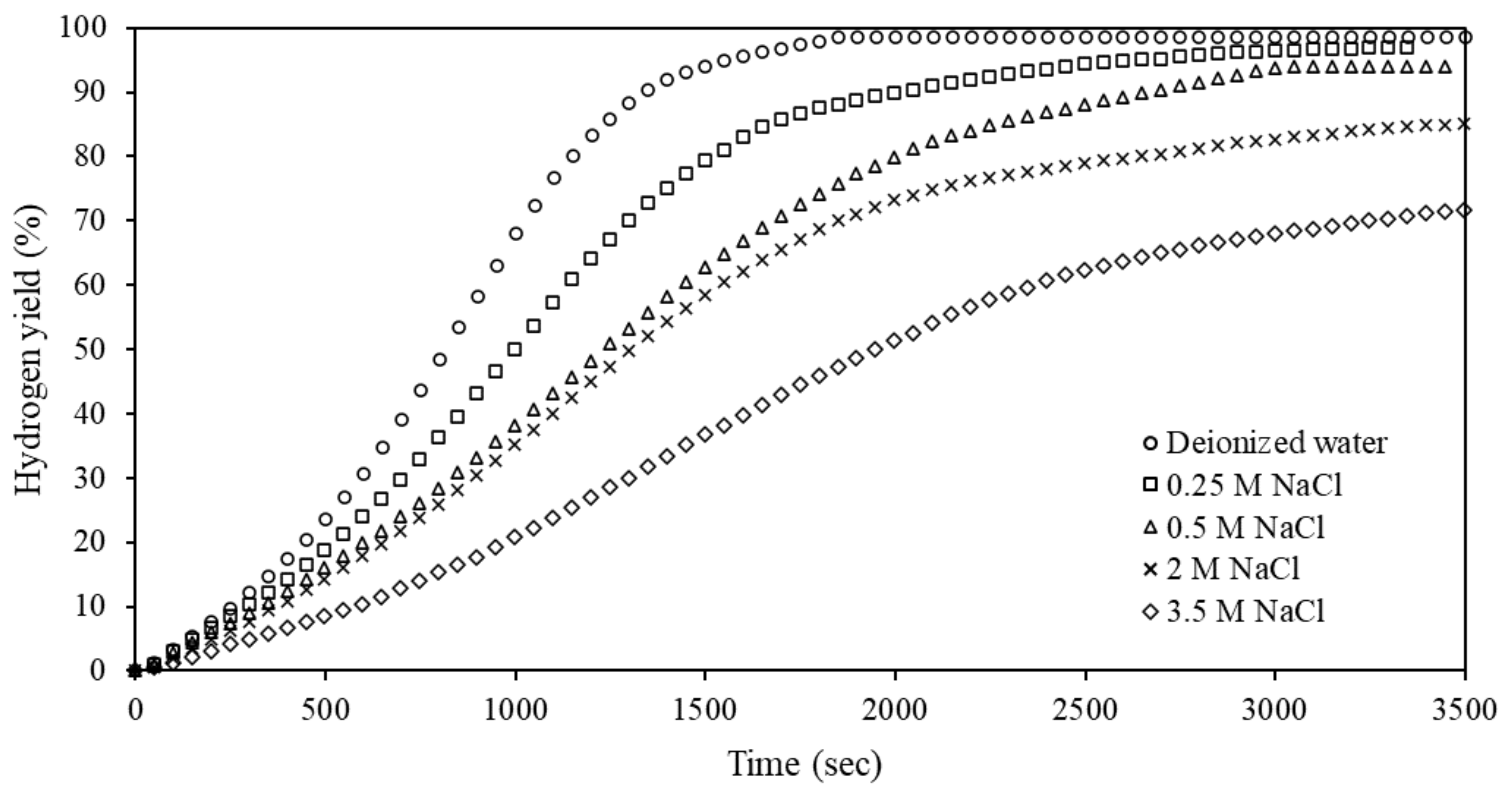
3.6. Effects of Water Quality on Hydrolysis
3.7. Proposed Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olivares-Ramírez, J.; De Jesus, A.M.; Jiménez-Sandoval, O.; Pless, R. Hydrogen Generation by Treatment of Aluminium Metal with Aqueous Solutions: Procedures and Uses. Hydrog. Energy-Chall. Perspect. 2012, 2012, 55–76. [Google Scholar] [CrossRef] [Green Version]
- Edwards, P.P.; Kuznetsov, V.; David, W.I. Hydrogen energy. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2007, 365, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Sivasubramanian, P.; Ramasamy, R.P.; Freire, F.J.; Holland, C.E.; Weidner, J.W. Electrochemical hydrogen production from thermochemical cycles using a proton exchange membrane electrolyzer. Int. J. Hydrogen Energy 2007, 32, 463–468. [Google Scholar] [CrossRef]
- Ronaszegi, K.; Fraga, E.S.; Darr, J.; Shearing, P.R.; Brett, D.J.L. Application of Photo-Electrochemically Generated Hydrogen with Fuel Cell Based Micro-Combined Heat and Power: A Dynamic System Modelling Study. Molecules 2019, 25, 123. [Google Scholar] [CrossRef] [Green Version]
- Karn, R.; Misra, M.; Srivastava, O. Semiconductor-septum photoelectrochemical cell for solar hydrogen production. Int. J. Hydrogen Energy 2000, 25, 407–413. [Google Scholar] [CrossRef]
- Amao, Y.; Tomonou, Y.; Okura, I. Highly efficient photochemical hydrogen production system using zinc porphyrin and hydrogenase in CTAB micellar system. Sol. Energy Mater. Sol. Cells 2003, 79, 103–111. [Google Scholar] [CrossRef]
- Guan, Y.; Deng, M.; Yu, X.; Zhang, W. Two-stage photo-biological production of hydrogen by marine green alga Platymonas subcordiformis. Biochem. Eng. J. 2004, 19, 69–73. [Google Scholar] [CrossRef]
- Puga, A. Photocatalytic production of hydrogen from biomass-derived feedstocks. Coord. Chem. Rev. 2016, 315, 1–66. [Google Scholar] [CrossRef]
- Sengodan, S.; Lan, R.; Humphreys, J.; Du, D.; Xu, W.; Wang, H.; Tao, S. Advances in reforming and partial oxidation of hydrocarbons for hydrogen production and fuel cell applications. Renew. Sustain. Energy Rev. 2018, 82, 761–780. [Google Scholar] [CrossRef]
- Steinfeld, A. Solar thermochemical production of hydrogen—A review. Sol. Energy 2005, 78, 603–615. [Google Scholar] [CrossRef]
- Du Preez, S.P.; Bessarabov, D. Hydrogen generation of mechanochemically activated Al Bi In composites. Int. J. Hydrogen Energy 2017, 42, 16589–16602. [Google Scholar] [CrossRef]
- Du Preez, S.P. Hydrogen Generation by Means of Hydrolysis Using Activated Al-In-Bi-Sn Composites for Electrochemical Energy Applications. Int. J. Electrochem. Sci. 2017, 12, 8663–8682. [Google Scholar] [CrossRef]
- Du Preez, S.P.; Bessarabov, D. Hydrogen generation by the hydrolysis of mechanochemically activated aluminum-tin-indium composites in pure water. Int. J. Hydrogen Energy 2018, 43, 21398–21413. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Ouma, C.N.M.; du Preez, S.P.; Modisha, P.; Engelbrecht, N.; Bessarabov, D.G.; Ghimire, A. Application of nanoparticles in biofuels: An overview. Fuel 2019, 237, 380–397. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Daramola, M.O.; Mogwase, B.; Engelbrecht, N.; Yoro, K.O.; du Preez, S.P.; Mhlongo, S.; Ezeokoli, O.T.; Ghimire, A.; Ayeni, A.O.; et al. Revising the dark fermentative H2 research and development scenario–An overview of the recent advances and emerging technological approaches. Biomass-Bioenergy 2020, 140, 105673. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Engelbrecht, N.; Du Preez, S.P.; Bessarabov, D. Thermophilic Biogas Upgrading via ex Situ Addition of H2 and CO2 Using Codigested Feedstocks of Cow Manure and the Organic Fraction of Solid Municipal Waste. ACS Omega 2020, 5, 17367–17376. [Google Scholar] [CrossRef] [PubMed]
- Du Preez, S.P.; Beukes, J.P.; Paktunc, D.; van Zyl, P.; Jordaan, A. Recycling pre-oxidized chromite fines in the oxidative sintered pellet production process. J. S. Afr. Inst. Min. Met. 2019, 119, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Abdin, Z.; Zafaranloo, A.; Rafiee, A.; Mérida, W.; Lipiński, W.; Khalilpour, K.R. Hydrogen as an energy vector. Renew. Sustain. Energy Rev. 2020, 120, 109620. [Google Scholar] [CrossRef]
- Tzimas, E.; Filiou, C.; Peteves, S.; Veyret, J. Hydrogen Storage: State-of-the-Art and Future Perspective; EUR 20995EN; EU Commission: Petten, The Netherlands, 2003. [Google Scholar]
- Najjar, Y.S. Hydrogen safety: The road toward green technology. Int. J. Hydrogen Energy 2013, 38, 10716–10728. [Google Scholar] [CrossRef]
- Dodds, P.E.; Staffell, I.; Hawkes, A.D.; Li, F.; Grünewald, P.; McDowall, W.; Ekins, P. Hydrogen and fuel cell technologies for heating: A review. Int. J. Hydrogen Energy 2015, 40, 2065–2083. [Google Scholar] [CrossRef] [Green Version]
- Scott, M.; Powells, G. Sensing hydrogen transitions in homes through social practices: Cooking, heating, and the decomposition of demand. Int. J. Hydrogen Energy 2020, 45, 3870–3882. [Google Scholar] [CrossRef]
- Du Preez, S.P.; Jones, D.; Bessarabov, D.; Falch, A.; Quaresma, C.M.D.N.; Dunnill, C. Development of a Pt/stainless steel mesh catalyst and its application in catalytic hydrogen combustion. Int. J. Hydrogen Energy 2019, 44, 27094–27106. [Google Scholar] [CrossRef]
- Du Preez, S.; Jones, D.; Warwick, M.; Falch, A.; Sekoai, P.; Quaresma, C.M.D.N.; Bessarabov, D.; Dunnill, C. Thermally stable Pt/Ti mesh catalyst for catalytic hydrogen combustion. Int. J. Hydrogen Energy 2020, 45, 16851–16864. [Google Scholar] [CrossRef]
- Kozhukhova, A.; du Preez, S.; Shuro, I.; Bessarabov, D. Development of a low purity aluminum alloy (Al6082) anodization process and its application as a platinum-based catalyst in catalytic hydrogen combustion. Surf. Coat. Technol. 2020, 404, 126483. [Google Scholar] [CrossRef]
- Kozhukhova, A.E.; du Preez, S.P.; Bessarabov, D.G. Catalytic Hydrogen Combustion for Domestic and Safety Applications: A Critical Review of Catalyst Materials and Technologies. Energies 2021, 14, 4897. [Google Scholar] [CrossRef]
- Kozhukhova, A.; du Preez, S.; Malakhov, A.; Bessarabov, D. A Thermally Conductive Pt/AAO Catalyst for Hydrogen Passive Autocatalytic Recombination. Catalysts 2021, 11, 491. [Google Scholar] [CrossRef]
- Trimm, D.L.; Önsan, Z.I. Onboard Fuel Conversion For Hydrogen-Fuel-Cell-Driven Vehicles. Catal. Rev. 2001, 43, 31–84. [Google Scholar] [CrossRef]
- Manoharan, Y.; Hosseini, S.E.; Butler, B.; Alzhahrani, H.; Senior, B.T.F.; Ashuri, T.; Krohn, J. Hydrogen Fuel Cell Vehicles; Current Status and Future Prospect. Appl. Sci. 2019, 9, 2296. [Google Scholar] [CrossRef] [Green Version]
- Baroutaji, A.; Wilberforce, T.; Ramadan, M.; Olabi, A.G. Comprehensive investigation on hydrogen and fuel cell technology in the aviation and aerospace sectors. Renew. Sustain. Energy Rev. 2019, 106, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Mosca, L.; Palo, E.; Colozzi, M.; Iaquaniello, G.; Salladini, A.; Taraschi, S. Hydrogen in chemical and petrochemical industry. In Current Trends and Future Developments on (Bio-)Membranes; Iulianelli, A., Basile, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 387–410. [Google Scholar]
- Demirel, Y.; Matzen, M.; Alhajji, M. Technoeconomics and Sustainability of Renewable Methanol and Ammonia Productions Using Wind Power-based Hydrogen. J. Adv. Chem. Eng. 2015, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Giddey, S.; Badwal, S.; Kulkarni, A. Review of electrochemical ammonia production technologies and materials. Int. J. Hydrogen Energy 2013, 38, 14576–14594. [Google Scholar] [CrossRef]
- Kumar, D.; Muthukumar, K. An overview on activation of aluminium-water reaction for enhanced hydrogen production. J. Alloy. Compd. 2020, 835, 155189. [Google Scholar] [CrossRef]
- Chen, Y.-K.; Teng, H.-T.; Lee, T.-Y.; Wang, H.-W. Rapid hydrogen generation from aluminum–water system by adjusting water ratio to various aluminum/aluminum hydroxide. Int. J. Energy Environ. Eng. 2014, 5, 87. [Google Scholar] [CrossRef] [Green Version]
- Haupin, W.E. Electrochemistry of the Hall-Heroult process for aluminum smelting. J. Chem. Educ. 1983, 60, 279. [Google Scholar] [CrossRef]
- Elitzur, S.; Rosenband, V.; Gany, A. Urine and aluminum as a source for hydrogen and clean energy. Int. J. Hydrogen Energy 2016, 41, 11909–11913. [Google Scholar] [CrossRef]
- Wang, H.; Chang, Y.; Dong, S.; Lei, Z.; Zhu, Q.; Luo, P.; Xie, Z. Investigation on hydrogen production using multicomponent aluminum alloys at mild conditions and its mechanism. Int. J. Hydrogen Energy 2013, 38, 1236–1243. [Google Scholar] [CrossRef]
- Zou, M.-S.; Guo, X.-Y.; Huang, H.-T.; Yang, R.-J.; Zhang, P. Preparation and characterization of hydro-reactive Mg–Al mechanical alloy materials for hydrogen production in seawater. J. Power Sources 2012, 219, 60–64. [Google Scholar] [CrossRef]
- Xiao, F.; Guo, Y.; Li, J.; Yang, R. Hydrogen generation from hydrolysis of activated aluminum composites in tap water. Energy 2018, 157, 608–614. [Google Scholar] [CrossRef]
- Huang, X.; Gao, T.; Pan, X.; Wei, D.; Lv, C.; Qin, L.; Huang, Y. A review: Feasibility of hydrogen generation from the reaction between aluminum and water for fuel cell applications. J. Power Sources 2013, 229, 133–140. [Google Scholar] [CrossRef]
- El-Meligi, A. Hydrogen production by aluminum corrosion in hydrochloric acid and using inhibitors to control hydrogen evolution. Int. J. Hydrogen Energy 2011, 36, 10600–10607. [Google Scholar] [CrossRef]
- Czech, E.; Troczynski, T. Hydrogen generation through massive corrosion of deformed aluminum in water. Int. J. Hydrogen Energy 2010, 35, 1029–1037. [Google Scholar] [CrossRef]
- Parmuzina, A.; Kravchenko, O. Activation of aluminium metal to evolve hydrogen from water. Int. J. Hydrogen Energy 2008, 33, 3073–3076. [Google Scholar] [CrossRef]
- Du Preez, S.; Bessarabov, D. The effects of bismuth and tin on the mechanochemical processing of aluminum-based composites for hydrogen generation purposes. Int. J. Hydrogen Energy 2019, 44, 21896–21912. [Google Scholar] [CrossRef]
- Ilyukhina, A.; Kravchenko, O.; Bulychev, B. Studies on microstructure of activated aluminum and its hydrogen generation properties in aluminum/water reaction. J. Alloy. Compd. 2017, 690, 321–329. [Google Scholar] [CrossRef]
- Baniamerian, M.; Moradi, S. Al–Ga doped nanostructured carbon as a novel material for hydrogen production in water. J. Alloy. Compd. 2011, 509, 6307–6310. [Google Scholar] [CrossRef]
- Kravchenko, O.; Semenenko, K.; Bulychev, B.; Kalmykov, K. Activation of aluminum metal and its reaction with water. J. Alloy. Compd. 2005, 397, 58–62. [Google Scholar] [CrossRef]
- Tan, S.-C.; Gui, H.; Yang, X.-H.; Yuan, B.; Zhan, S.-H.; Liu, J. Comparative study on activation of aluminum with four liquid metals to generate hydrogen in alkaline solution. Int. J. Hydrogen Energy 2016, 41, 22663–22667. [Google Scholar] [CrossRef]
- Wang, C.; Qiu, C.; Wei, H.; Zou, H.; Lin, K.; Guo, Y.; Yang, S.; Liu, X. Mild hydrogen production from the hydrolysis of Al–Bi–Zn composite powder. Int. J. Hydrogen Energy 2021, 46, 9314–9323. [Google Scholar] [CrossRef]
- Irankhah, A.; Fattahi, S.M.S.; Salem, M. Hydrogen generation using activated aluminum/water reaction. Int. J. Hydrogen Energy 2018, 43, 15739–15748. [Google Scholar] [CrossRef]
- Razavi-Tousi, S.; Szpunar, J. Effect of addition of water-soluble salts on the hydrogen generation of aluminum in reaction with hot water. J. Alloy. Compd. 2016, 679, 364–374. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, F.; Sun, L.; Chen, J.; Guo, X.; Yan, E.; Yu, F.; Chu, H.; Peng, H.; Zou, Y.; et al. A novel Al BiOCl composite for hydrogen generation from water. Int. J. Hydrogen Energy 2019, 44, 6655–6662. [Google Scholar] [CrossRef]
- Hsieh, C.P.; Ho, C.Y.; Hsu, L.C.; Chang, Y.-J. Synergistic effect on hydrolytic sodium borohydride adding waste Al for hydrogen generation. Int. J. Hydrogen Energy 2020, 45, 10334–10341. [Google Scholar] [CrossRef]
- Newell, A.; Thampi, K.R. Novel amorphous aluminum hydroxide catalysts for aluminum–water reactions to produce H2 on demand. Int. J. Hydrogen Energy 2017, 42, 23446–23454. [Google Scholar] [CrossRef]
- López-Miranda, J.; Rosas, G. Hydrogen generation by aluminum hydrolysis using the Fe2Al5 intermetallic compound. Int. J. Hydrogen Energy 2016, 41, 4054–4059. [Google Scholar] [CrossRef]
- Fan, M.-Q.; Xu, F.; Sun, L.-X.; Zhao, J.-N.; Jiang, T.; Li, W.-X. Hydrolysis of ball milling Al–Bi–hydride and Al–Bi–salt mixture for hydrogen generation. J. Alloy. Compd. 2008, 460, 125–129. [Google Scholar] [CrossRef]
- Jia, Y.; Shen, J.; Meng, H.; Dong, Y.; Chai, Y.; Wang, N. Hydrogen generation using a ball-milled Al/Ni/NaCl mixture. J. Alloy. Compd. 2014, 588, 259–264. [Google Scholar] [CrossRef]
- Zhang, F.; Edalati, K.; Arita, M.; Horita, Z. Fast hydrolysis and hydrogen generation on Al-Bi alloys and Al-Bi-C composites synthesized by high-pressure torsion. Int. J. Hydrogen Energy 2017, 42, 29121–29130. [Google Scholar] [CrossRef]
- Chen, J.; Xu, F.; Sun, L.; Zhang, K.; Xia, Y.; Guo, X.; Zhang, H.; Yu, F.; Yan, E.; Peng, H. Effect of doped Ni-Bi-B alloy on hydrogen generation performance of Al-In Cl3. J. Energy Chem. 2019, 39, 268–274. [Google Scholar] [CrossRef] [Green Version]
- Kaya, M.F.; Kahveci, O.; Erol, H.; Akkaya, A. Effect of low B addition on Al-Zn alloy’s hydrogen production performance. Int. J. Hydrogen Energy 2021, 46, 15192–15202. [Google Scholar] [CrossRef]
- Liu, K.; Luo, P.; Deng, Y.; Zuo, Y.; Xu, X.; Yi, S.; Dong, S. Hydrogen production from hydrolysis of Al–Ga–In–SnCl2 composites. Mater. Res. Express 2019, 6, 085515. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, X.; Sun, L.; Yu, F.; Li, P.; Chen, J.; Wu, Y.; Cao, L.; Xu, C.; Yang, X.; et al. Hydrogen generation of a novel Al NaMgH3 composite reaction with water. Int. J. Hydrogen Energy 2017, 42, 30535–30542. [Google Scholar] [CrossRef]
- Sheng, P.; Zhang, S.; Guan, C.; Qian, W.; Gao, X.; Wang, Y. Preparation and characterization of the Al-Ga-In-Sn-KCl composites for hydrogen generation. Energy Storage 2021, 3, 241. [Google Scholar] [CrossRef]
- Katsoufis, P.; Doukas, E.; Politis, C.; Avgouropoulos, G.; Lianos, P. Enhanced rate of hydrogen production by corrosion of commercial aluminum. Int. J. Hydrogen Energy 2020, 45, 10729–10734. [Google Scholar] [CrossRef]
- McCormick, P.G.; Froes, F.H. The fundamentals of mechanochemical processing. JOM 1998, 50, 61–65. [Google Scholar] [CrossRef]
- Ghenciu, A.F. Review of fuel processing catalysts for hydrogen production in PEM fuel cell systems. Curr. Opin. Solid State Mater. Sci. 2002, 6, 389–399. [Google Scholar] [CrossRef]
- Kreuer, K. On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells. J. Membr. Sci. 2001, 185, 29–39. [Google Scholar] [CrossRef]
- Guo, Y.-F.; Chen, H.-C.; Wang, F.-C. The development of a hybrid PEMFC power system. Int. J. Hydrogen Energy 2015, 40, 4630–4640. [Google Scholar] [CrossRef]
- Wang, F.-Q.; Wang, H.-H.; Wang, J.; Lu, J.; Luo, P.; Chang, Y.; Ma, X.-G.; Dong, S.-J. Effects of low melting point metals (Ga, In, Sn) on hydrolysis properties of aluminum alloys. Trans. Nonferrous Met. Soc. China 2016, 26, 152–159. [Google Scholar] [CrossRef]
- Mahmoodi, K.; Alinejad, B. Enhancement of hydrogen generation rate in reaction of aluminum with water. Int. J. Hydrogen Energy 2010, 35, 5227–5232. [Google Scholar] [CrossRef]
- Fan, M.-Q.; Xu, F.; Sun, L.-X. Studies on hydrogen generation characteristics of hydrolysis of the ball milling Al-based materials in pure water. Int. J. Hydrogen Energy 2007, 32, 2809–2815. [Google Scholar] [CrossRef]
- Liu, S.; Fan, M.-Q.; Wang, C.; Huang, Y.-X.; Chen, D.; Bai, L.-Q.; Shu, K.-Y. Hydrogen generation by hydrolysis of Al–Li–Bi–NaCl mixture with pure water. Int. J. Hydrogen Energy 2012, 37, 1014–1020. [Google Scholar] [CrossRef]
- Fan, M.; Sun, L.; Xu, F. Experiment assessment of hydrogen production from activated aluminum alloys in portable generator for fuel cell applications. Energy 2010, 35, 2922–2926. [Google Scholar] [CrossRef]
- Fan, M.-Q.; Xu, F.; Sun, L.-X. Hydrogen Generation by Hydrolysis Reaction of Ball-Milled Al−Bi Alloys. Energy Fuels 2007, 21, 2294–2298. [Google Scholar] [CrossRef]
- Benjamin, J. Mechanical alloying. Sci. Am. 1976, 234, 40–48. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Haines, W. The effect of temperature upon the ductility of zinc. Proc. R. Soc. Lond. Ser. A Contain. Pap. A Math. Phys. Character 1911, 85, 526–532. [Google Scholar]
- Razavi-Tousi, S.S.; Szpunar, J. Effect of structural evolution of aluminum powder during ball milling on hydrogen generation in aluminum–water reaction. Int. J. Hydrogen Energy 2013, 38, 795–806. [Google Scholar] [CrossRef]
- Razavi-Tousi, S.S.; Szpunar, J.A. Role of Ball Milling of Aluminum Powders in Promotion of Aluminum-Water Reaction to Generate Hydrogen. Met. Mater. Trans. E 2014, 1, 247–256. [Google Scholar] [CrossRef] [Green Version]
- Du Preez, S.; Bessarabov, D. On-demand hydrogen generation by the hydrolysis of ball-milled aluminum composites: A process overview. Int. J. Hydrogen Energy 2021, 46, 35790–35813. [Google Scholar] [CrossRef]
- Reboul, M.C.; Gimenez, P.; Rameau, J.J. A Proposed Activation Mechanism for Al Anodes. Corrosion 1984, 40, 366–371. [Google Scholar] [CrossRef]
- Tavoosi, M.; Enayati, M.; Karimzadeh, F. Softening behaviour of nanostructured Al–14wt% Zn alloy during mechanical alloying. J. Alloy. Compd. 2008, 464, 107–110. [Google Scholar] [CrossRef]
- Mazilkin, A.; Straumal, B.; Rabkin, E.; Baretzky, B.; Enders, S.; Protasova, S.; Kogtenkova, O.; Valiev, R. Softening of nanostructured Al–Zn and Al–Mg alloys after severe plastic deformation. Acta Mater. 2006, 54, 3933–3939. [Google Scholar] [CrossRef]
- Papworth, A.; Fox, P. The disruption of oxide defects within aluminium alloy castings by the addition of bismuth. Mater. Lett. 1998, 35, 202–206. [Google Scholar] [CrossRef]
- Zhang, F.; Edalati, K.; Arita, M.; Horita, Z. Hydrolytic hydrogen production on Al–Sn–Zn alloys processed by high-pressure torsion. Materials 2018, 11, 1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayraktar, Ş.; Hekimoğlu, A.P. Effect of Zinc Content and Cutting Tool Coating on the Machinability of the Al-(5–35) Zn Alloys. Met. Mater. Int. 2020, 26, 477–490. [Google Scholar] [CrossRef]
- Khireche, S.; Boughrara, D.; Kadri, A.; Hamadou, L.; Benbrahim, N. Corrosion mechanism of Al, Al–Zn and Al–Zn–Sn alloys in 3 wt.% NaCl solution. Corros. Sci. 2014, 87, 504–516. [Google Scholar] [CrossRef]
- Fan, M.Q.; Sun, L.X.; Xu, F. Hydrogen production for micro-fuel-cell from activated Al–Sn–Zn–X (X: Hydride or halide) mixture in water. Renew. Energy 2011, 36, 519–524. [Google Scholar] [CrossRef]
- Guseva, O.; Schmutz, P.; Suter, T.; von Trzebiatowski, O. Modelling of anodic dissolution of pure aluminium in sodium chloride. Electrochim. Acta 2009, 54, 4514–4524. [Google Scholar] [CrossRef]
- Foundation, R.S. SK Nuclear. Available online: https://sk.ru/news/m/wiki/14838/download.aspx (accessed on 15 November 2021).
- Elitzur, S.; Rosenband, V.; Gany, A. Electric energy storage using aluminum and water for hydrogen production on-demand. Int. J. Appl. 2015, 5, 112–121. [Google Scholar]
- Godart, P.; Hart, D. Aluminum-powered climate change resiliency: From aluminum debris to electricity and clean water. Appl. Energy 2020, 275, 115316. [Google Scholar] [CrossRef]
- Shkolnikov, E.I.; Zhuk, A.Z.; Vlaskin, M.S. Aluminum as energy carrier: Feasibility analysis and current technologies overview. Renew. Sustain. Energy Rev. 2011, 15, 4611–4623. [Google Scholar] [CrossRef]

| Composites | Activation Metals (wt%) | |
|---|---|---|
| Bi | Zn | |
| Al-5% Bi-5% Zn | 5 | 5 |
| Al-7.5% Bi-2.5% Zn | 7.5 | 2.5 |
| Al-2.5% Bi-7.5% Zn | 2.5 | 7.5 |
| Al-9% Bi-1% Zn | 9 | 1 |
| Al-1% Bi-9% Zn | 1 | 9 |
| Al-10% Bi | 10 | 0 |
| Al-2.5% Bi-2.5% Zn | 2.5 | 2.5 |
| Composite | Activation Metal | Yield (% or mL/g) | Reaction Time (s or Min) | Ref. | |
|---|---|---|---|---|---|
| Bi | Zn | ||||
| 1 | 9 | 1 | 99.9% | 2300 s | This work |
| 2 | 7.5 | 2.5 | 99.5% | 2400 s | |
| 3 | 5 | 5 | 98.5% | 1850 s | |
| 4 * | 12 | 7 | 98% | 280 min | [50] |
| 5 * | 11 | 8 | 94% | 300 min # | |
| 6 * | 10 | 9 | 94% | 300 min # | |
| 7 * | 9 | 10 | 92% | 320 min # | |
| 8 | 10 | 10 | 830 mL/g | 5 min | [72] |
| 9 | 10 (Sn) | 10 | 680 mL/g | 14 min | [89] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davies, J.; du Preez, S.P.; Bessarabov, D.G. On-Demand Hydrogen Generation by the Hydrolysis of Ball-Milled Aluminum–Bismuth–Zinc Composites. Materials 2022, 15, 1197. https://doi.org/10.3390/ma15031197
Davies J, du Preez SP, Bessarabov DG. On-Demand Hydrogen Generation by the Hydrolysis of Ball-Milled Aluminum–Bismuth–Zinc Composites. Materials. 2022; 15(3):1197. https://doi.org/10.3390/ma15031197
Chicago/Turabian StyleDavies, Jamey, Stephanus P. du Preez, and Dmitri G. Bessarabov. 2022. "On-Demand Hydrogen Generation by the Hydrolysis of Ball-Milled Aluminum–Bismuth–Zinc Composites" Materials 15, no. 3: 1197. https://doi.org/10.3390/ma15031197
APA StyleDavies, J., du Preez, S. P., & Bessarabov, D. G. (2022). On-Demand Hydrogen Generation by the Hydrolysis of Ball-Milled Aluminum–Bismuth–Zinc Composites. Materials, 15(3), 1197. https://doi.org/10.3390/ma15031197








