Toxicological Assessment of an Acrylic Removable Orthodontic Appliance Using 2D and 3D In Vitro Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
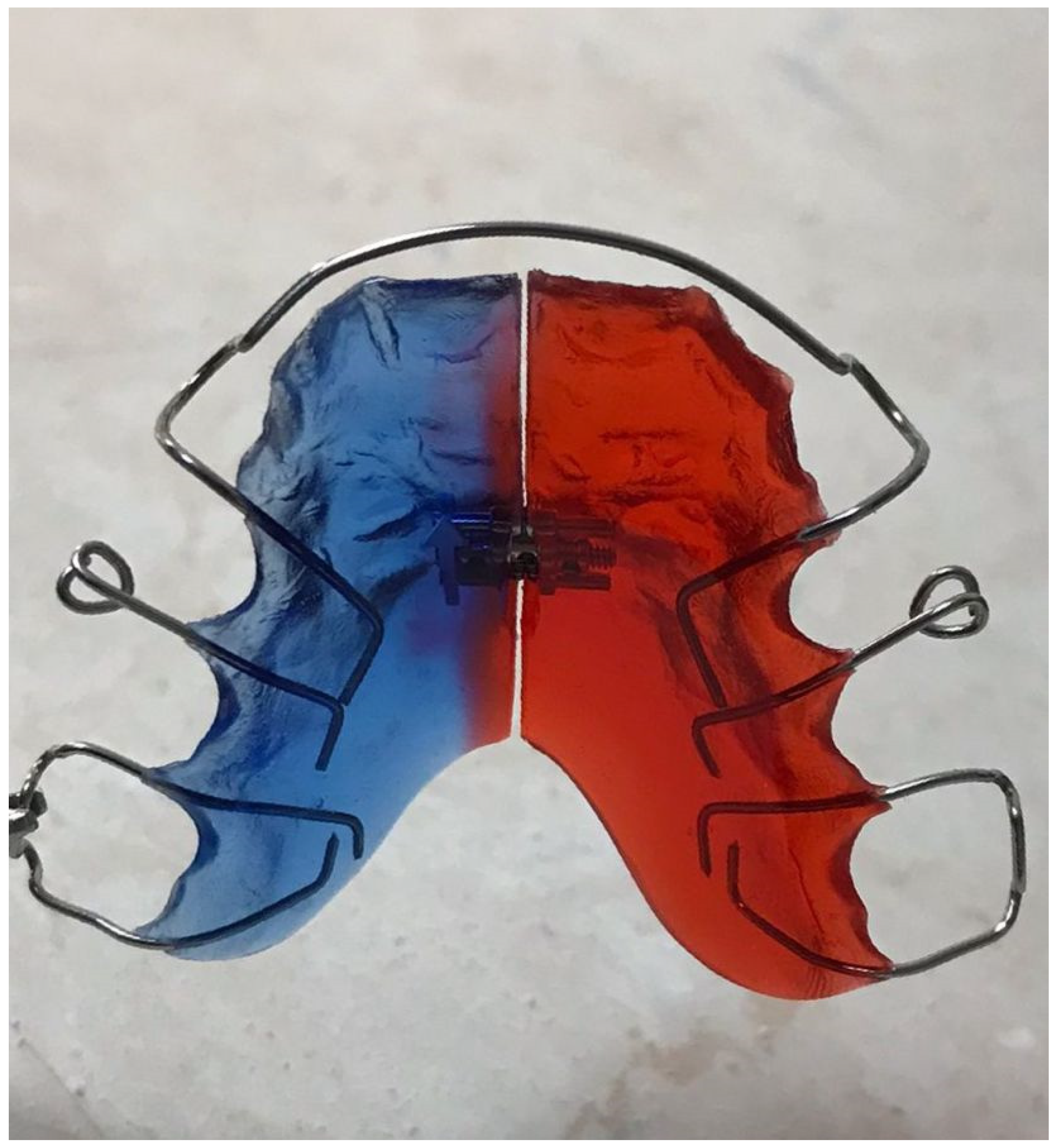
2.2. Development of Acrylic Removable Appliance (ARA)
2.3. Preparation of the Artificial Saliva
2.4. Maintaining the Acrylic Removable Appliance in Artificial Saliva
2.5. Cell Culture
2.6. Cytotoxicity Assessment
2.7. Cell Morphology and Confluence Evaluation
2.8. Biocompatibility Testing in 3D Reconstructed Human Epidermis
3. Results
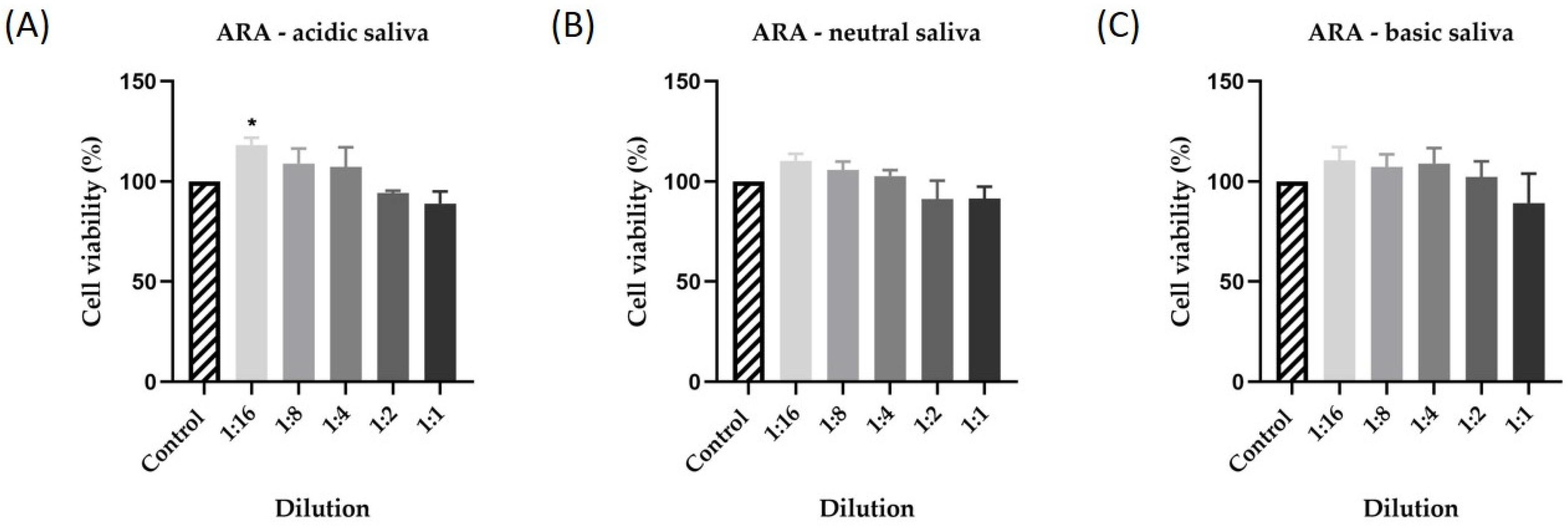
3.1. Cytotoxicity Assessment
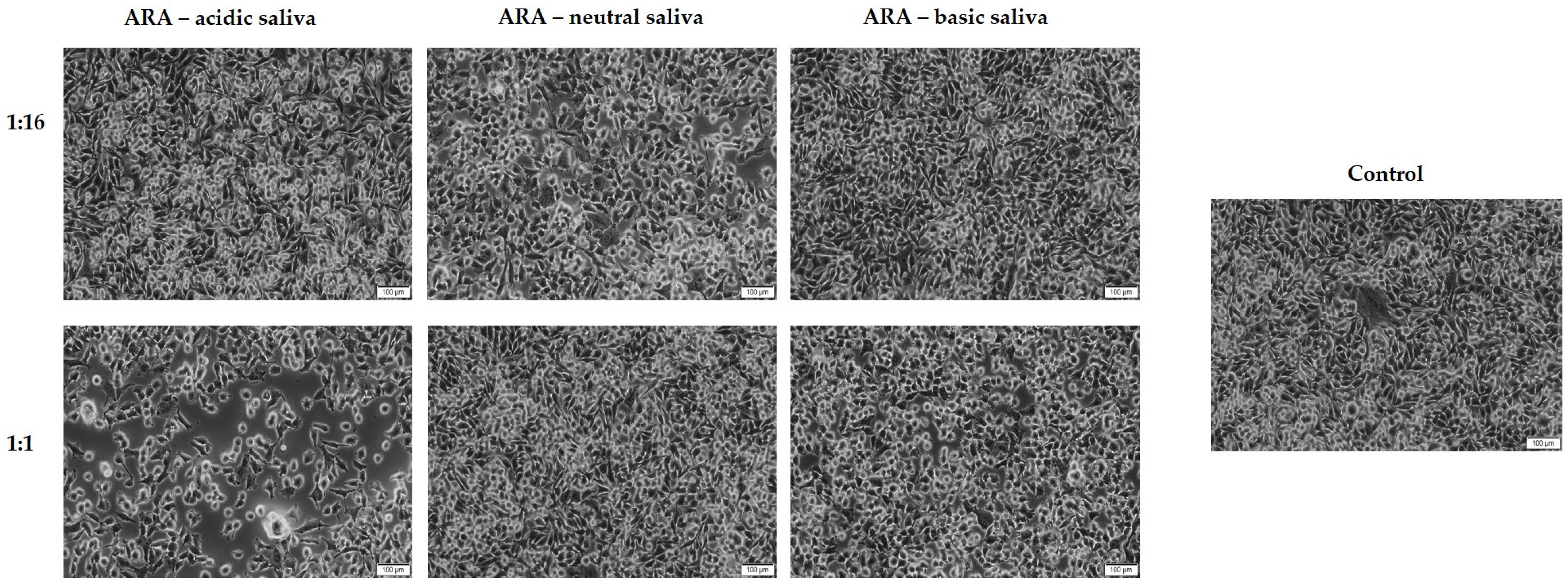
3.2. Cell Morphology and Confluence Evaluation
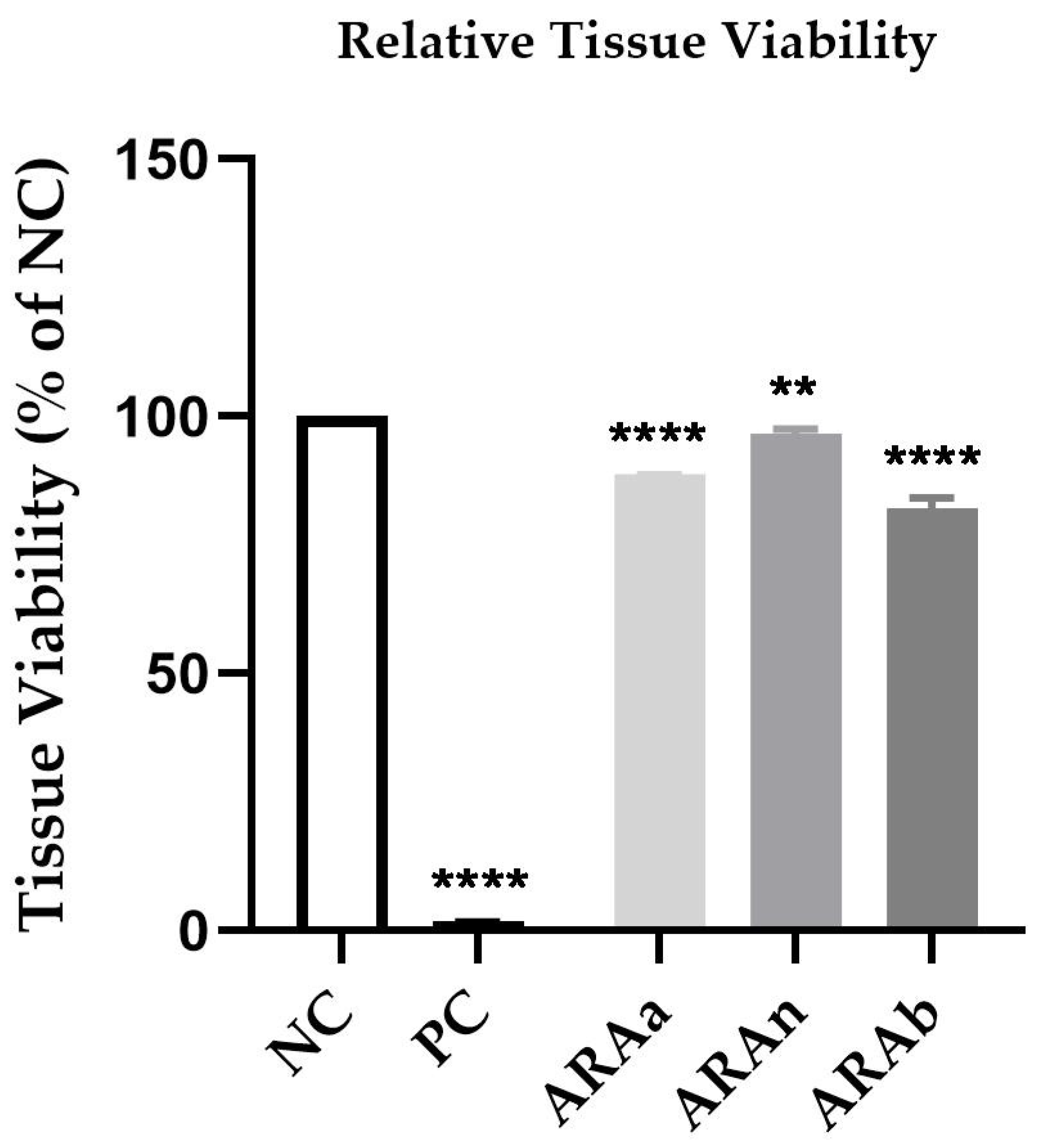
3.3. Biocompatibility Testing in Reconstructed Human Epidermis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elyaskhil, M.; Shafai, N.A.A.; Mokhtar, N. Effect of malocclusion severity on oral health related quality of life in Malay adolescents. Health Qual. Life Outcomes 2021, 19, 71. [Google Scholar] [CrossRef] [PubMed]
- Tefera, A.T.; Bekele, B.G.; Derese, K.; Andualem, G. Prevalence of Occlusal Features and Their Relation to Sociodemographic Variables in Northwest Ethiopia: A Cross-Sectional Study. Clin. Cosmet. Investig. Dent. 2021, 13, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Mistry, P.; Moles, D.R.; O’Neill, J.; Noar, J. The occlusal effects of digit sucking habits amongst school children in Northamptonshire (UK). J. Orthod. 2010, 37, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Li, D.-D.; Yang, Y.-Q.; McGrath, C.P.J.; Mattheos, N. What are patients’ expectations of orthodontic treatment: A systematic review. BMC Oral Health 2016, 16, 19. [Google Scholar] [CrossRef] [Green Version]
- Katepogu, P.; Balagangadhar; Patil, C.D.; Jakati, S.V. A Comparative Evaluation of Frictional Resistance of Conventional, Teflon and Epoxy Coated Stainless Steel Archwires in Metal, Ceramic Brackets—An In vitro Study. J. Adv. Med. Med. Res. 2020, 32, 307–315. [Google Scholar] [CrossRef]
- Alajmi, S.; Shaban, A.; Al-Azemi, R. Comparison of Short-Term Oral Impacts Experienced by Patients Treated with Invisalign or Conventional Fixed Orthodontic Appliances. Med. Princ. Pract. 2019, 29, 382–388. [Google Scholar] [CrossRef]
- Huh, H.; Chaudhry, K.; Stevens, R.; Subramani, K. Practice of lingual orthodontics and practitioners’ opinion and experience with lingual braces in the United States. J. Clin. Exp. Dent. 2021, 13, 789–794. [Google Scholar] [CrossRef]
- Tamer, I.; Oztas, E.; Marsan, G. Orthodontic Treatment with Clear Aligners and The Scientific Reality Behind Their Marketing: A Literature Review. Turk. J. Orthod. 2019, 32, 241–246. [Google Scholar] [CrossRef]
- Elkholy, F.; Schmidt, S.; Amirkhani, M.; Schmidt, F.; Lapatki, B.G. Mechanical Characterization of Thermoplastic Aligner Materials: Recommendations for Test Parameter Standardization. J. Health Eng. 2019, 2019, 8074827. [Google Scholar] [CrossRef] [Green Version]
- Weir, T. Clear aligners in orthodontic treatment. Aust. Dent. J. 2017, 62, 58–62. [Google Scholar] [CrossRef] [Green Version]
- Papadimitriou, A.; Mousoulea, S.; Gkantidis, N.; Kloukos, D. Clinical effectiveness of Invisalign® orthodontic treatment: A systematic review. Prog. Orthod. 2018, 19, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosny, M.A.A.; Alasmari, F.S.; Alsaidi, N.M.; Alsharif, H.M.; Alshareef, S.A.; Aldwyyan, N.F.; Alahmadi, R.Y.; Almutairi, R.A.; Almutairi, B.M.; Alhemaidi, G.S.; et al. Indications, advantages, disadvantages and effectiveness of Invisalign aligners. Int. J. Community Med. Public Health 2021, 8, 5064. [Google Scholar] [CrossRef]
- Baseer, M.A.; Almayah, N.A.; Alqahtani, K.M.; Alshaye, M.I.; Aldhahri, M.M. Oral Impacts Experienced by Orthodontic Patients Undergoing Fixed or Removable Appliances Therapy in Saudi Arabia: A Cross-Sectional Study. Patient Prefer. Adherence 2021, 15, 2683–2691. [Google Scholar] [CrossRef] [PubMed]
- Longoni, J.N.; Lopes, B.M.V.; Freires, I.A.; Dutra, K.L.; Franco, A.; Paranhos, L.R. Self-ligating versus conventional metallic brackets on Streptococcus mutans retention: A systematic review. Eur. J. Dent. 2017, 11, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Gorbunkova, A.; Pagni, G.; Brizhak, A.; Farronato, G.; Rasperini, G. Impact of Orthodontic Treatment on Periodontal Tissues: A Narrative Review of Multidisciplinary Literature. Int. J. Dent. 2016, 2016, 4723589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- E Kettle, J.; Hyde, A.C.; Frawley, T.; Granger, C.; Longstaff, S.J.; E Benson, P. Managing orthodontic appliances in everyday life: A qualitative study of young people’s experiences with removable functional appliances, fixed appliances and retainers. J. Orthod. 2020, 47, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Faltermeier, A.; Rosentritt, M.; Müssig, D. Acrylic removable appliances: Comparative evaluation of different postpolymerization methods. Am. J. Orthod. Dentofac. Orthop. 2007, 131, 301.e16–301.e22. [Google Scholar] [CrossRef]
- Hassan, R.; Aslam Khan, M.U.; Abdullah, A.M.; Abd Razak, S.I. A Review on Current Trends of Polymers in Orthodontics: BPA-Free and Smart Materials. Polymers 2021, 13, 1409. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, H.; Wu, J.; Yuan, L.; Wang, Y.; Du, X.; Wang, R.; Marwa, P.W.; Petlulu, P.; Chen, X.; et al. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ. Res. 2019, 176, 108575. [Google Scholar] [CrossRef]
- Siracusa, J.S.; Yin, L.; Measel, E.; Liang, S.; Yu, X. Effects of bisphenol A and its analogs on reproductive health: A mini review. Reprod. Toxicol. 2018, 79, 96–123. [Google Scholar] [CrossRef]
- Naderi, M.; Kwong, R.W. A comprehensive review of the neurobehavioral effects of bisphenol S and the mechanisms of action: New insights from in vitro and in vivo models. Environ. Int. 2020, 145, 106078. [Google Scholar] [CrossRef] [PubMed]
- Dumitrascu, M.C.; Mares, C.; Petca, R.-C.; Sandru, F.; Popescu, R.-I.; Mehedintu, C.; Petca, A. Carcinogenic effects of bisphenol A in breast and ovarian cancers (Review). Oncol. Lett. 2020, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Sabour, A.; El Helou, M.; Roger-Leroi, V.; Bauer, C. Release and toxicity of bisphenol-A (BPA) contained in orthodontic adhesives: A systematic review. Int. Orthod. 2021, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.S. Prosthodontic Applications of Polymethyl Methacrylate (PMMA): An Update. Polymers 2020, 12, 2299. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, T.; Bao, S. Water Absorption Rate Prediction of PMMA and Its Composites Using BP Neural Network. MATEC Web Conf. 2016, 67, 6017. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.; Asghar, M.; Din, S.U.; Zafar, M.S. Thermoset polymethacrylate-based materials for dental applications. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 273–308. [Google Scholar]
- Pituru, S.M.; Greabu, M.; Totan, A.; Imre, M.; Pantea, M.; Spinu, T.; Tancu, A.M.C.; Popoviciu, N.O.; Stanescu, I.-I.; Ionescu, E. A Review on the Biocompatibility of PMMA-Based Dental Materials for Interim Prosthetic Restorations with a Glimpse into their Modern Manufacturing Techniques. Materials 2020, 13, 2894. [Google Scholar] [CrossRef]
- Saruta, J.; Ozawa, R.; Hamajima, K.; Saita, M.; Sato, N.; Ishijima, M.; Kitajima, H.; Ogawa, T. Prolonged Post-Polymerization Biocompatibility of Polymethylmethacrylate-Tri-n-Butylborane (PMMA-TBB) Bone Cement. Materials 2021, 14, 1289. [Google Scholar] [CrossRef]
- Vasiliu, M.P.; Sachelarie, L.; Romila, L.E.; Folescu, E.; Atanase, L.; Zaharia, A. Surface State Studies and Biocompatibility of PMMA. J. Biomim. Biomater. Biomed. 2016, 28, 57–65. [Google Scholar] [CrossRef]
- Ozkir, S.E.; Yilmaz, B.; Unal, S.M.; Culhaoglu, A.; Kurkcuoglu, I. Effect of heat polymerization conditions and microwave on the flexural strength of polymethyl methacrylate. Eur. J. Dent. 2018, 12, 116–119. [Google Scholar] [CrossRef]
- Sifakakis, I.; Eliades, T. Adverse Reactions to Orthodontic Materials. Aust. Dent. J. 2017, 62, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Maia, L.H.E.G.; Filho, H.L.D.L.; Araújo, M.V.A.; Ruellas, A.C.D.O.; Araújo, M.T.D.S. Incorporation of metal and color alteration of enamel in the presence of orthodontic appliances. Angle Orthod. 2012, 82, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Keinan, D.; Mass, E.; Zilberman, U. Absorption of Nickel, Chromium, and Iron by the Root Surface of Primary Molars Covered with Stainless Steel Crowns. Int. J. Dent. 2010, 2010, 326124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourhajibagher, M.; Salehi-Vaziri, A.; Noroozian, M.; Akbar, H.; Bazarjani, F.; Ghaffari, H.; Bahador, A. An orthodontic acrylic resin containing seaweed Ulva lactuca as a photoactive phytocompound in antimicrobial photodynamic therapy: Assessment of anti-biofilm activities and mechanical properties. Photodiagnosis Photodyn. Ther. 2021, 35, 102295. [Google Scholar] [CrossRef] [PubMed]
- Dinu, S.; Buzatu, R.; Macasoi, I.; Popa, M.; Vlad, C.S.; Marcovici, I.; Pinzaru, I.; Dehelean, C.A.; Moacă, E.-A.; Barbu-Tudoran, L.; et al. Toxicological Profile of Biological Environment of Two Elastodontic Devices. Processes 2021, 9, 2116. [Google Scholar] [CrossRef]
- Hut, E.-F.; Radulescu, M.; Pilut, N.; Macasoi, I.; Berceanu, D.; Coricovac, D.; Pinzaru, I.; Cretu, O.; Dehelean, C. Two Antibiotics, Ampicillin and Tetracycline, Exert Different Effects in HT-29 Colorectal Adenocarcinoma Cells in Terms of Cell Viability and Migration Capacity. Curr. Oncol. 2021, 28, 2466–2480. [Google Scholar] [CrossRef]
- Skin Irritation for Medical Device Extracts (ISO 10993–23:2021). MatTek Life Sciences. Available online: https://www.mattek.com/application/medical-device-extracts-skin-irritation-iso-10993/ (accessed on 15 December 2021).
- Pinzaru, I.; Tanase, A.; Enatescu, V.; Coricovac, D.; Bociort, F.; Marcovici, I.; Watz, C.; Vlaia, L.; Soica, C.; Dehelean, C. Proniosomal Gel for Topical Delivery of Rutin: Preparation, Physicochemical Characterization and In Vitro Toxicological Profile Using 3D Reconstructed Human Epidermis Tissue and 2D Cells. Antioxidants 2021, 10, 85. [Google Scholar] [CrossRef]
- Vaida, L.; Mutiu, G.; Tara, I.G.; Bodog, F. An Algorithm of Ethical Approach to The Orthodontic Patient. Iran. J. Public Health 2015, 44, 1296–1298. [Google Scholar]
- Sulewska, M.E.; Baczewska, A.; Bugała-Musiatowicz, B.; Waszkiewicz-Sewastianik, E.; Pietruski, J.K.; Pietruska, M. Long-Term Assessment of Periodontal Tissues after Corticotomy-Assisted Orthodontic Arch Expansion. J. Clin. Med. 2021, 10, 5588. [Google Scholar] [CrossRef]
- Sharma, M.R.; Chaturvedi, T. An Overview of Biocompatibility of Orthodontic Materials. J. Indian Orthod. Soc. 2008, 42, 27–32. [Google Scholar] [CrossRef]
- Cardoso, R.M.; Godinho, J.; Jardim, L. Bond strength of orthodontic brackets to polymethylmethacrylate: Effect of the surface treatment and adhesive system. Rev. Port. Estomatol. Med. Dent. Cir. Maxilofac. 2021, 62, 2. [Google Scholar] [CrossRef]
- Dentaurum. Orthodontics Catalog, 22nd ed.; Dentaurum: Ispringen, Germany, 2020; pp. 377–385. [Google Scholar]
- Nik, T.H.; Shahroudi, A.S.; Eraghihzadeh, Z.; Aghajani, F. Comparison of residual monomer loss from cold-cure orthodontic acrylic resins processed by different polymerization techniques. J. Orthod. 2014, 41, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthi, S.; Padmanabhan, S.; Chitharanjan, A.B. Allergy and orthodontics. J. Orthod. Sci. 2012, 1, 83–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viwattanatipa, N.; Pataijindachote, J.; Juntavee, N. Corrosion Analysis of Orthodontic Wires: An Interaction Study of Wire Type, pH and Immersion Time. Adv. Dent. Oral Health 2018, 10, 555780. [Google Scholar] [CrossRef]
- Wendl, B.; Wiltsche, H.; Lankmayr, E.; Winsauer, H.; Walter, A.; Muchitsch, A.; Jakse, N.; Wendl, M.; Wendl, T. Metal release profiles of orthodontic bands, brackets, and wires: An in vitro study. J. Orofac. Orthop. 2017, 78, 494–503. [Google Scholar] [CrossRef]
- Schiff, N.; Dalard, F.; Lissac, M.; Morgon, L.; Grosgogeat, B. Corrosion resistance of three orthodontic brackets: A comparative study of three fluoride mouthwashes. Eur. J. Orthod. 2005, 27, 541–549. [Google Scholar] [CrossRef] [Green Version]
- Jacoby, L.S.; Junior, V.D.S.R.; Campos, M.; de Menezes, L.M. Cytotoxic outcomes of orthodontic bands with and without silver solder in different cell lineages. Am. J. Orthod. Dentofac. Orthop. 2017, 151, 957–963. [Google Scholar] [CrossRef]
- Wataha, J.C.; Hanks, C.T.; Sun, Z. Effect of cell line on in vitro metal ion cytotoxicity. Dent. Mater. 1994, 10, 156–161. [Google Scholar] [CrossRef] [Green Version]
- Bonatto, L.D.R.; Goiato, M.C.; Silva, E.; Oliveira, S.H.P.; Haddad, M.F.; Chaves-Neto, A.H.; Brito, V.G.B.; dos Santos, D.M. Biocompatibility of primers and an adhesive used for implant-retained maxillofacial prostheses: An in vitro analysis. J. Prosthet. Dent. 2017, 117, 799–805. [Google Scholar] [CrossRef] [Green Version]
- Çakırbay Tanış, M.; Akay, C.; Sevim, H. Cytotoxicity of long-term denture base materials. Int. J. Artif. Organs 2018, 41, 677–683. [Google Scholar] [CrossRef]
- Lung, C.; Darvell, B. Minimization of the inevitable residual monomer in denture base acrylic. Dent. Mater. 2005, 21, 1119–1128. [Google Scholar] [CrossRef]
- Huang, H.-H. Corrosion resistance of stressed NiTi and stainless steel orthodontic wires in acid artificial saliva. J. Biomed. Mater. Res. 2003, 66A, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.-T.; Ding, S.-J.; He, H.; Chou, M.Y.; Huang, T.-H. Cytotoxicity of Orthodontic Wire Corroded in Fluoride Solution In Vitro. Angle Orthod. 2007, 77, 349–354. [Google Scholar] [CrossRef]
- Galeotti, A.; Uomo, R.; Spagnuolo, G.; Paduano, S.; Cimino, R.; Valletta, R.; D’Antò, V. Effect of pH on in vitro biocompatibility of orthodontic miniscrew implants. Prog. Orthod. 2013, 14, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baruffaldi, D.; Palmara, G.; Pirri, C.; Frascella, F. 3D Cell Culture: Recent Development in Materials with Tunable Stiffness. ACS Appl. Bio Mater. 2021, 4, 2233–2250. [Google Scholar] [CrossRef]
- Vannet, B.V.; Mohebbian, N.; Wehrbein, H. Toxicity of used orthodontic archwires assessed by three-dimensional cell culture. Eur. J. Orthod. 2006, 28, 426–432. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Kim, S.; Park, K. In vitro–in vivo correlation: Perspectives on model development. Int. J. Pharm. 2011, 418, 142–148. [Google Scholar] [CrossRef] [Green Version]
- Bechir, F.; Pacurar, M.; Tohati, A.; Bataga, S.M. Comparative Study of Salivary pH, Buffer Capacity, and Flow in Patients with and without Gastroesophageal Reflux Disease. Int. J. Environ. Res. Public Health 2021, 19, 201. [Google Scholar] [CrossRef]
- Fróis, A.; Evaristo, M.; Santos, A.C.; Louro, C.S. Salivary pH Effect on Orthodontic Appliances: In Vitro Study of the SS/DLC System. Coatings 2021, 11, 1302. [Google Scholar] [CrossRef]
- Lee, M.; Hwang, J.-H.; Lim, K.-M. Alternatives to In Vivo Draize Rabbit Eye and Skin Irritation Tests with a Focus on 3D Reconstructed Human Cornea-Like Epithelium and Epidermis Models. Toxicol. Res. 2017, 33, 191–203. [Google Scholar] [CrossRef] [Green Version]
- Hayden, P.J.; Bachelor, M.; Ayehunie, S.; Letasiova, S.; Kaluzhny, Y.; Klausner, M.; Kandárová, H. Application of MatTek In Vitro Reconstructed Human Skin Models for Safety, Efficacy Screening, and Basic Preclinical Research. Appl. In Vitro Toxicol. 2015, 1, 226–233. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinu, S.; Craciunescu, E.L.; Macasoi, I.; Chioran, D.; Rivis, M.; Vlad, D.; Milutinovici, R.A.; Marcovici, I.; Dolghi, A.; Moaca, A.; et al. Toxicological Assessment of an Acrylic Removable Orthodontic Appliance Using 2D and 3D In Vitro Methods. Materials 2022, 15, 1193. https://doi.org/10.3390/ma15031193
Dinu S, Craciunescu EL, Macasoi I, Chioran D, Rivis M, Vlad D, Milutinovici RA, Marcovici I, Dolghi A, Moaca A, et al. Toxicological Assessment of an Acrylic Removable Orthodontic Appliance Using 2D and 3D In Vitro Methods. Materials. 2022; 15(3):1193. https://doi.org/10.3390/ma15031193
Chicago/Turabian StyleDinu, Stefania, Emanuela Lidia Craciunescu, Ioana Macasoi, Doina Chioran, Mircea Rivis, Daliborca Vlad, Raluca Adriana Milutinovici, Iasmina Marcovici, Alina Dolghi, Alina Moaca, and et al. 2022. "Toxicological Assessment of an Acrylic Removable Orthodontic Appliance Using 2D and 3D In Vitro Methods" Materials 15, no. 3: 1193. https://doi.org/10.3390/ma15031193
APA StyleDinu, S., Craciunescu, E. L., Macasoi, I., Chioran, D., Rivis, M., Vlad, D., Milutinovici, R. A., Marcovici, I., Dolghi, A., Moaca, A., Dinu, D. C., Dehelean, C., & Popa, M. (2022). Toxicological Assessment of an Acrylic Removable Orthodontic Appliance Using 2D and 3D In Vitro Methods. Materials, 15(3), 1193. https://doi.org/10.3390/ma15031193











