Theoretical Mechanism on the Cellulose Regeneration from a Cellulose/EmimOAc Mixture in Anti-Solvents
Abstract
:1. Introduction
2. Computational Methods
2.1. Molecular Dynamics Simulations
2.2. Quantum Chemistry Calculations
3. Results and Discussion
3.1. The Cellulose Regeneration in Different Anti-Solvents
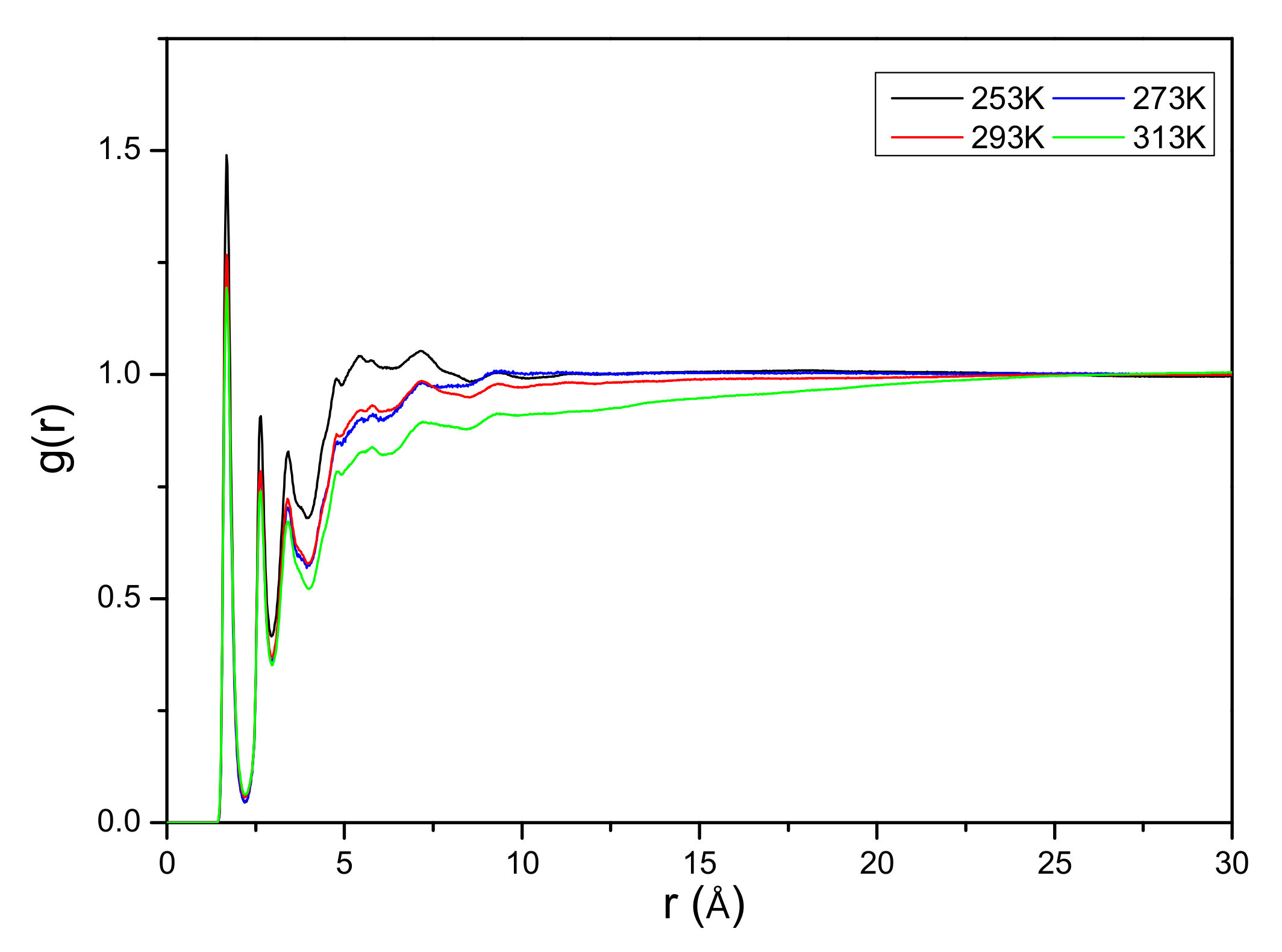
3.2. The Effects of Temperature on Cellulose Regeneration
3.3. The Effect of Water Concentration on Cellulose Regeneration
3.4. DFT Study on the Interaction between ILs and Anti-Solvents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.; Song, J.; Han, B. Catalytic Transformation of Lignocellulose into Chemicals and Fuel Products in Ionic Liquids. Chem. Rev. 2017, 117, 6834–6880. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Focher, B.; Palma, M.T.; Canetti, M.; Torri, G.; Cosentino, C.; Gastaldi, G. Structural differences between non-wood plant celluloses: Evidence from solid state NMR, vibrational spectroscopy and X-ray diffractometry. Ind. Crops Prod. 2001, 13, 193–208. [Google Scholar] [CrossRef]
- Muhammad, N.; Man, Z.; Bustam, M. Ionic liquid—A future solvent for the enhanced uses of wood biomass. Eur. J. Wood Wood Prod. 2011, 70, 125–133. [Google Scholar] [CrossRef]
- O’Sullivan, A.C. Cellulose: The structure slowly unravels. Cellulose 1997, 4, 173–207. [Google Scholar] [CrossRef]
- Zhu, C.; Xin, H.; Hong, J.; Wang, H.; Wang, C.; Ma, L.; Liu, Q. Hydrocarbon Distribution of Cellulose Hydrogenolysis over Ru–MoOx/C Combined with HZSM-5. ACS Sustain. Chem. Eng. 2021, 9, 14704–14721. [Google Scholar] [CrossRef]
- Gupta, K.M.; Jiang, J. Cellulose dissolution and regeneration in ionic liquids: A computational perspective. Chem. Eng. Sci. 2015, 121, 180–189. [Google Scholar] [CrossRef]
- Rogers, R.D.; Seddon, K.R. Ionic Liquids—Solvents of the Future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef]
- Freudenmann, D.; Wolf, S.; Wolff, M.; Feldmann, C. Ionic Liquids: New Perspectives for Inorganic Synthesis? Angew. Chem. Int. Ed. 2011, 50, 11050–11060. [Google Scholar] [CrossRef]
- Zakrzewska, M.E.; Bogel-Łukasik, E.; Bogel-Łukasik, R. Solubility of Carbohydrates in Ionic Liquids. Energy Fuels 2010, 24, 737–745. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of Cellose with Ionic Liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic liquid processing of cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, J.; Yu, J.; Zhang, X.; He, J.; Zhang, J. Application of ionic liquids for dissolving cellulose and fabricating cellulose-based materials: State of the art and future trends. Mater. Chem. Front. 2017, 1, 1273–1290. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Bhanage, B.M. Factors governing dissolution process of lignocellulosic biomass in ionic liquid: Current status, overview and challenges. Bioresour. Technol. 2015, 178, 2–18. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, D.; Duan, C.; Liu, C. Probing anion–cellulose interactions in imidazolium-based room temperature ionic liquids: A density functional study. Carbohydr. Res. 2010, 345, 2201–2205. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, J.; Zhang, H.; He, J.; Ren, Q.; Guo, M. Homogeneous Acetylation of Cellulose in a New Ionic Liquid. Biomacromolecules 2004, 5, 266–268. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Zhang, J.; He, J. 1-Allyl-3-methylimidazolium Chloride Room Temperature Ionic Liquid: A New and Powerful Nonderivatizing Solvent for Cellulose. Macromolecules 2005, 38, 8272–8277. [Google Scholar] [CrossRef]
- Hauru, L.K.J.; Hummel, M.; King, A.W.T.; Kilpeläinen, I.; Sixta, H. Role of Solvent Parameters in the Regeneration of Cellulose from Ionic Liquid Solutions. Biomacromolecules 2012, 13, 2896–2905. [Google Scholar] [CrossRef]
- Sayyed, A.J.; Deshmukh, N.A.; Pinjari, D.V. A critical review of manufacturing processes used in regenerated cellulosic fibres: Viscose, cellulose acetate, cuprammonium, LiCl/DMAc, ionic liquids, and NMMO based lyocell. Cellulose 2019, 26, 2913–2940. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhang, L. Recent advances in regenerated cellulose materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Yousefi, H.; Nishino, T.; Faezipour, M.; Ebrahimi, G.; Shakeri, A. Direct Fabrication of all-Cellulose Nanocomposite from Cellulose Microfibers Using Ionic Liquid-Based Nanowelding. Biomacromolecules 2011, 12, 4080–4085. [Google Scholar] [CrossRef]
- Gupta, K.M.; Hu, Z.; Jiang, J. Cellulose regeneration from a cellulose/ionic liquid mixture: The role of anti-solvents. RSC Adv. 2013, 3, 12794–12801. [Google Scholar] [CrossRef]
- Shamsuri, A.A.; Abdan, K.; Jamil, S.N.A.M. Properties and applications of cellulose regenerated from cellulose/imidazolium-based ionic liquid/co-solvent solutions: A short review. e-Polymers 2021, 21, 869–880. [Google Scholar] [CrossRef]
- Ju, Z.; Xiao, W.; Yao, X.; Tan, X.; Simmons, B.A.; Sale, K.L.; Sun, N. Theoretical study on the microscopic mechanism of lignin solubilization in Keggin-type polyoxometalate ionic liquids. Phys. Chem. Chem. Phys. 2020, 22, 2878–2886. [Google Scholar] [CrossRef]
- Ju, Z.; Zhang, Y.; Zhao, T.; Xiao, W.; Yao, X. Mechanism of Glucose–Fructose Isomerization over Aluminum-Based Catalysts in Methanol Media. ACS Sustain. Chem. Eng. 2019, 7, 14962–14972. [Google Scholar] [CrossRef]
- Miyamoto, H.; Umemura, M.; Aoyagi, T.; Yamane, C.; Ueda, K.; Takahashi, K. Structural reorganization of molecular sheets derived from cellulose II by molecular dynamics simulations. Carbohydr. Res. 2009, 344, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Medronho, B.; Lindman, B. Brief overview on cellulose dissolution/regeneration interactions and mechanisms. Adv. Colloid Interface Sci. 2015, 222, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Chaban, V.V.; Prezhdo, O.V. Ionic and Molecular Liquids: Working Together for Robust Engineering. J. Phys. Chem. Lett. 2013, 4, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Chaban, V.V.; Voroshylova, I.V.; Kalugin, O.N.; Prezhdo, O.V. Acetonitrile Boosts Conductivity of Imidazolium Ionic Liquids. J. Phys. Chem. B 2012, 116, 7719–7727. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Zhang, S.; Yao, Y.; Yao, X.; Xu, J.; Lu, X. Dissolving process of a cellulose bunch in ionic liquids: A molecular dynamics study. Phys. Chem. Chem. Phys. 2015, 17, 17894–17905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, H.; Wu, J.; Zhang, J.; He, J.; Xiang, J. NMR spectroscopic studies of cellobiose solvation in EmimAc aimed to understand the dissolution mechanism of cellulose in ionic liquids. Phys. Chem. Chem. Phys. 2010, 12, 1941–1947. [Google Scholar] [CrossRef]
- Martínez, J.M.; Martínez, L. Packing optimization for automated generation of complex system’s initial configurations for molecular dynamics and docking. J. Comput. Chem. 2003, 24, 819–825. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, S.; Wang, W. A Refined Force Field for Molecular Simulation of Imidazolium-Based Ionic Liquids. J. Phys. Chem. B 2004, 108, 12978–12989. [Google Scholar] [CrossRef]
- Kirschner, K.N.; Yongye, A.B.; Tschampel, S.M.; González-Outeiriño, J.; Daniels, C.R.; Foley, B.L.; Woods, R.J. GLYCAM06: A generalizable biomolecular force field. Carbohydrates. J. Comput. Chem. 2008, 29, 622–655. [Google Scholar] [CrossRef] [Green Version]
- Berendsen, H.J.C.; Grigera, J.R.; Straatsma, T.P. The missing term in effective pair potentials. J. Phys. Chem. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.J.; Fraaije, J.G. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Diez, V.; DeWeese, A.; Kalb, R.S.; Blauch, D.N.; Socha, A.M. Cellulose Dissolution and Biomass Pretreatment Using Quaternary Ammonium Ionic Liquids Prepared from H-, G-, and S-Type Lignin-Derived Benzaldehydes and Dimethyl Carbonate. Ind. Eng. Chem. Res. 2019, 58, 16009–16017. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Mao, Q.; Ren, Y.; Luo, K.H.; van Duin, A.C.T. Dynamics and kinetics of reversible homo-molecular dimerization of polycyclic aromatic hydrocarbons. J. Chem. Phys. 2017, 147, 244305. [Google Scholar] [CrossRef] [Green Version]
- Simeon, T.M.; Ratner, M.A.; Schatz, G.C. Nature of Noncovalent Interactions in Catenane Supramolecular Complexes: Calibrating the MM3 Force Field with ab Initio, DFT, and SAPT Methods. J. Phys. Chem. A 2013, 117, 7918–7927. [Google Scholar] [CrossRef] [Green Version]
- Fu, R.; Lu, T.; Chen, F.-W. Comparing Methods for Predicting the Reactive Site of Electrophilic Substitution. Acta Phys. Chim. Sin. 2014, 30, 628–639. [Google Scholar]
- Wang, Z.; Liu, Y.; Zheng, B.; Zhou, F.; Jiao, Y.; Liu, Y.; Ding, X.; Lu, T. A theoretical investigation on Cu/Ag/Au bonding in XH2P⋯MY(X = H, CH3, F, CN, NO2; M = Cu, Ag, Au; Y = F, Cl, Br, I) complexes. J. Chem. Phys. 2018, 148, 194106. [Google Scholar] [CrossRef]
- Luzar, A.; Chandler, D. Hydrogen-bond kinetics in liquid water. Nature 1996, 379, 55–57. [Google Scholar] [CrossRef]
- Dong, K.; Liu, X.; Dong, H.; Zhang, X.; Zhang, S. Multiscale Studies on Ionic Liquids. Chem. Rev. 2017, 117, 6636–6695. [Google Scholar] [CrossRef]
- Fan, Z.; Chen, J.; Guo, W.; Ma, F.; Sun, S.; Zhou, Q. Anti-solvents tuning cellulose nanoparticles through two competitive regeneration routes. Cellulose 2018, 25, 4513–4523. [Google Scholar] [CrossRef]
- Shibata, M.; Teramoto, N.; Nakamura, T.; Saitoh, Y. All-cellulose and all-wood composites by partial dissolution of cotton fabric and wood in ionic liquid. Carbohydr. Polym. 2013, 98, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Wang, L.; Zhang, L. Influence of coagulation temperature on pore size and properties of cellulose membranes prepared from NaOH–urea aqueous solution. Cellulose 2007, 14, 205–215. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, L.; Sun, Y.; Lin, Y.; Zhang, X.; Nishiyama, Y. Supramolecular Structure and Properties of High Strength Regenerated Cellulose Films. Macromol. Biosci. 2009, 9, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.M.; Hu, Z.; Jiang, J. Molecular insight into cellulose regeneration from a cellulose/ionic liquid mixture: Effects of water concentration and temperature. RSC Adv. 2013, 3, 4425–4433. [Google Scholar] [CrossRef]
- Parthasarathi, R.; Balamurugan, K.; Shi, J.; Subramanian, V.; Simmons, B.A.; Singh, S. Theoretical Insights into the Role of Water in the Dissolution of Cellulose Using IL/Water Mixed Solvent Systems. J. Phys. Chem. B 2015, 119, 14339–14349. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Zhang, Y.; Jiang, K.; Wang, J.; Zhang, S. Why Only Ionic Liquids with Unsaturated Heterocyclic Cations Can Dissolve Cellulose: A Simulation Study. ACS Sustain. Chem. Eng. 2017, 5, 3417–3428. [Google Scholar] [CrossRef]
- Stange, P.; Fumino, K.; Ludwig, R. Ion Speciation of Protic Ionic Liquids in Water: Transition from Contact to Solvent-Separated Ion Pairs. Angew. Chem. Int. Ed. 2013, 52, 2990–2994. [Google Scholar] [CrossRef]
- Marcus, Y.; Hefter, G. Ion Pairing. Chem. Rev. 2006, 106, 4585–4621. [Google Scholar] [CrossRef]
- Glendening, E.D.; Landis, C.R.; Weinhold, F. NBO 6.0: Natural bond orbital analysis program. J. Comput. Chem. 2013, 34, 1429–1437. [Google Scholar] [CrossRef]







| Entry | Emim | OAc | ||||
|---|---|---|---|---|---|---|
| Ecoul | EL–J | Etotal | Ecoul | EL–J | Etotal | |
| 0% | −3796.20 | −7644.57 | −11,440.70 | −23,310.90 | −136.08 | −23,446.98 |
| 20% | −3462.37 | −6302.39 | −9764.80 | −17,095.40 | −416.39 | −17,511.8 |
| 40% | −3150.73 | −5363.59 | −8514.32 | −13,431.40 | −673.23 | −14,104.60 |
| 60% | −1840.56 | −3099.88 | −4940.40 | −7357.76 | −669.81 | −8027.57 |
| 80% | −899.75 | −1378.85 | −2278.60 | −3532.38 | −400.68 | −3933.06 |
| Structure | Donor (i) | Acceptor (j) | E(2) kcal/mol | ε(i)-ε(j) | F(i,j) |
|---|---|---|---|---|---|
| CIP | LP O26 | σ* C3-H6 | 54.43 | 0.85 | 0.194 |
| CIP + 1W | LP O26 | σ* C3-H6 | 18.63 | 0.80 | 0.111 |
| LP O26 | σ* O27-H29 | 16.71 | 1.25 | 0.129 | |
| CIP + 2W | LP O26 | σ* C3-H6 | 16.07 | 0.84 | 0.105 |
| LP O26 | σ* O30-H31 | 18.47 | 1.28 | 0.138 | |
| LP O25 | σ* O27-H29 | 13.25 | 1.28 | 0.117 | |
| CIP + 3W | LP O26 | σ* C3-H6 | 7.42 | 0.86 | 0.057 |
| LP O26 | σ* O33-H34 | 16.42 | 0.79 | 0.110 | |
| LP O26 | σ* O30-H31 | 1.95 | 0.87 | 0.038 | |
| LP O25 | σ* O30-H32 | 5.86 | 0.88 | 0.066 | |
| CIP + 4W | LP O26 | σ* C3-H6 | 6.24 | 0.88 | 0.067 |
| LP O26 | σ* O27-H29 | 4.81 | 1.27 | 0.070 | |
| LP O26 | σ* O36-H37 | 1.29 | 1.27 | 0.036 | |
| LP O25 | σ* O33-H34 | 10.85 | 1.21 | 0.103 | |
| LP O25 | σ* O30-H32 | 30.99 | 0.89 | 0.150 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, Z.; Yu, Y.; Feng, S.; Lei, T.; Zheng, M.; Ding, L.; Yu, M. Theoretical Mechanism on the Cellulose Regeneration from a Cellulose/EmimOAc Mixture in Anti-Solvents. Materials 2022, 15, 1158. https://doi.org/10.3390/ma15031158
Ju Z, Yu Y, Feng S, Lei T, Zheng M, Ding L, Yu M. Theoretical Mechanism on the Cellulose Regeneration from a Cellulose/EmimOAc Mixture in Anti-Solvents. Materials. 2022; 15(3):1158. https://doi.org/10.3390/ma15031158
Chicago/Turabian StyleJu, Zhaoyang, Yihang Yu, Shaokeng Feng, Tingyu Lei, Minjia Zheng, Liyong Ding, and Mengting Yu. 2022. "Theoretical Mechanism on the Cellulose Regeneration from a Cellulose/EmimOAc Mixture in Anti-Solvents" Materials 15, no. 3: 1158. https://doi.org/10.3390/ma15031158
APA StyleJu, Z., Yu, Y., Feng, S., Lei, T., Zheng, M., Ding, L., & Yu, M. (2022). Theoretical Mechanism on the Cellulose Regeneration from a Cellulose/EmimOAc Mixture in Anti-Solvents. Materials, 15(3), 1158. https://doi.org/10.3390/ma15031158






