Tin Diselenide (SnSe2) Van der Waals Semiconductor: Surface Chemical Reactivity, Ambient Stability, Chemical and Optical Sensors
Abstract
:1. Introduction
2. Materials and Methods
3. Results
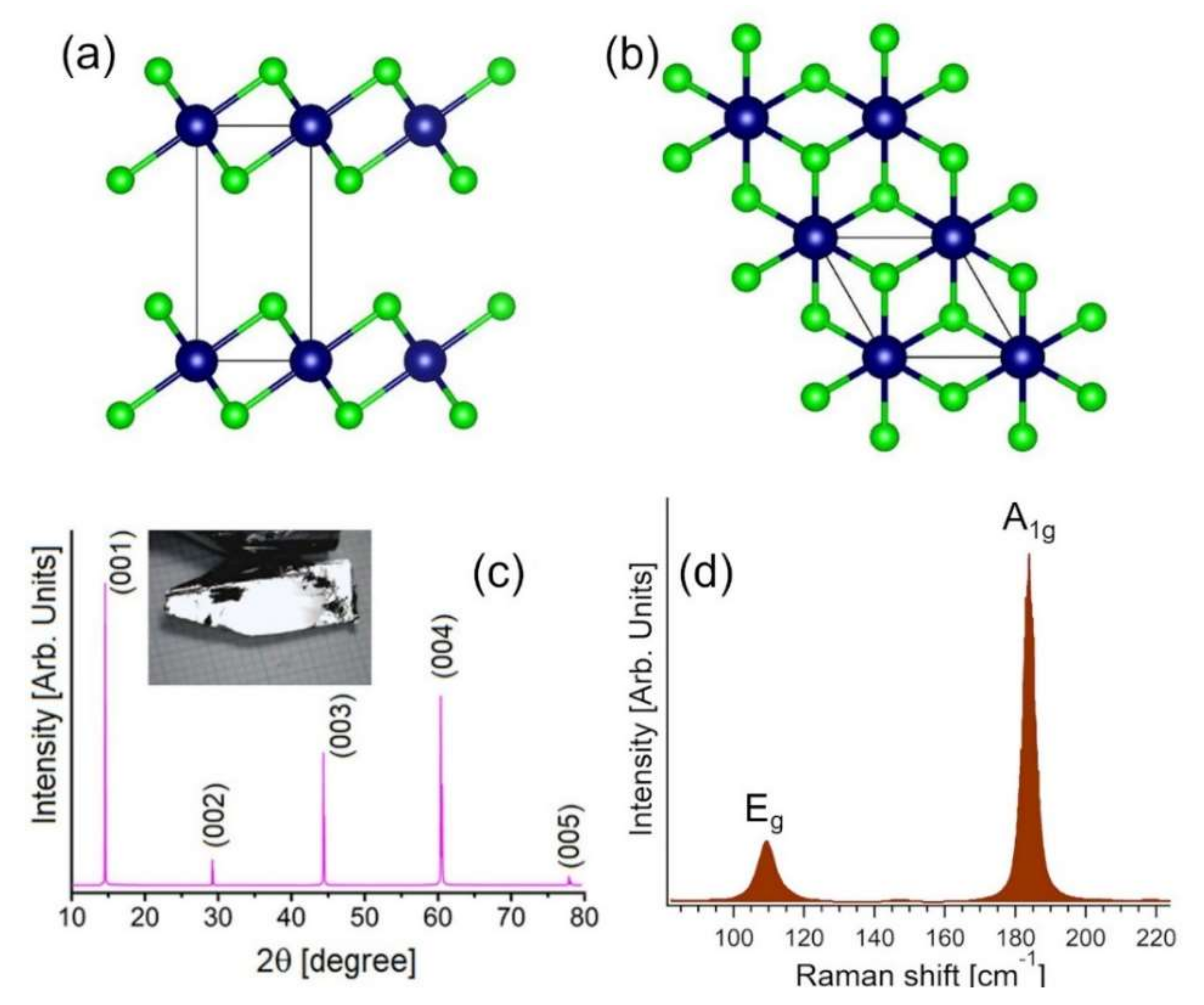
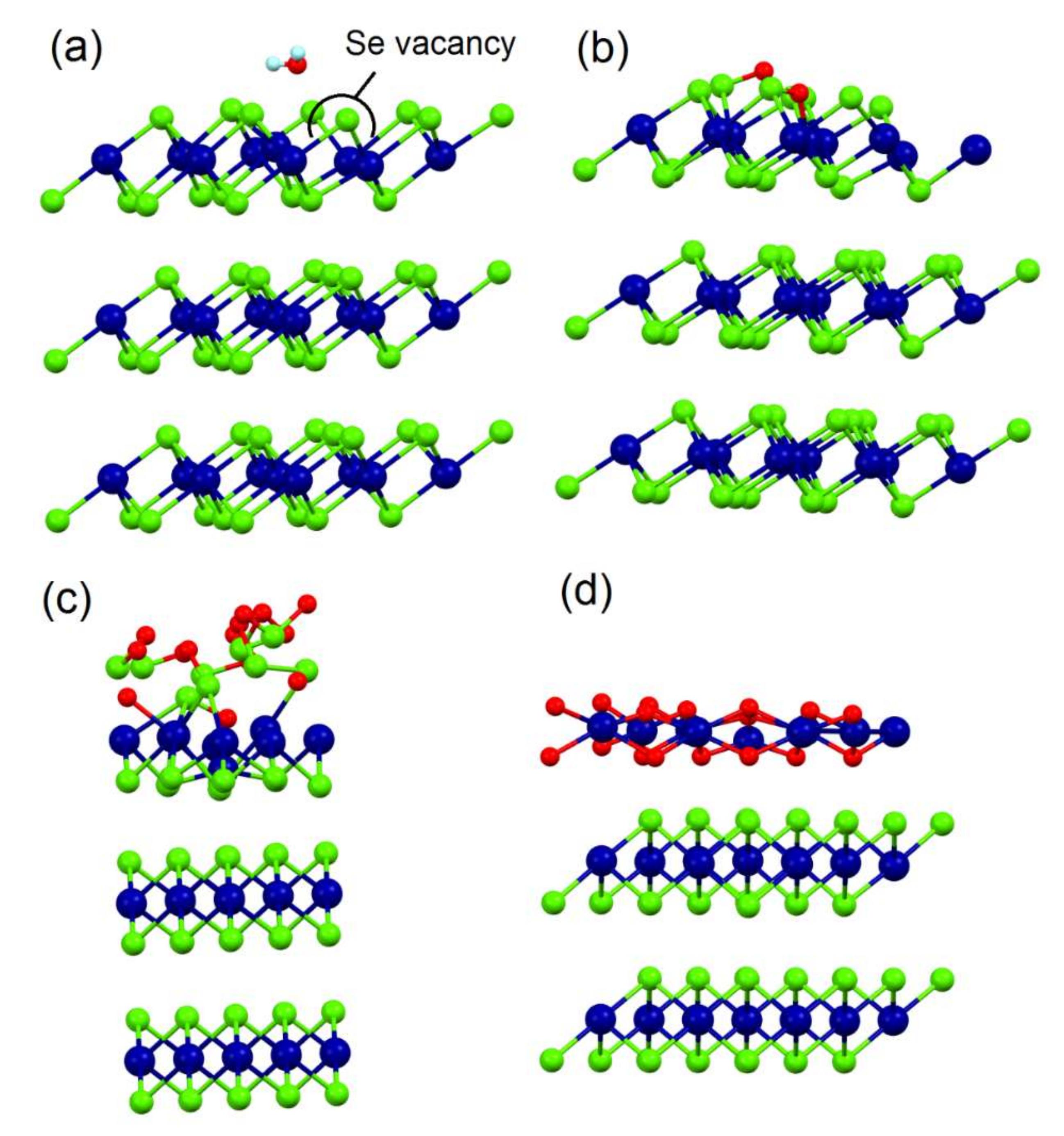
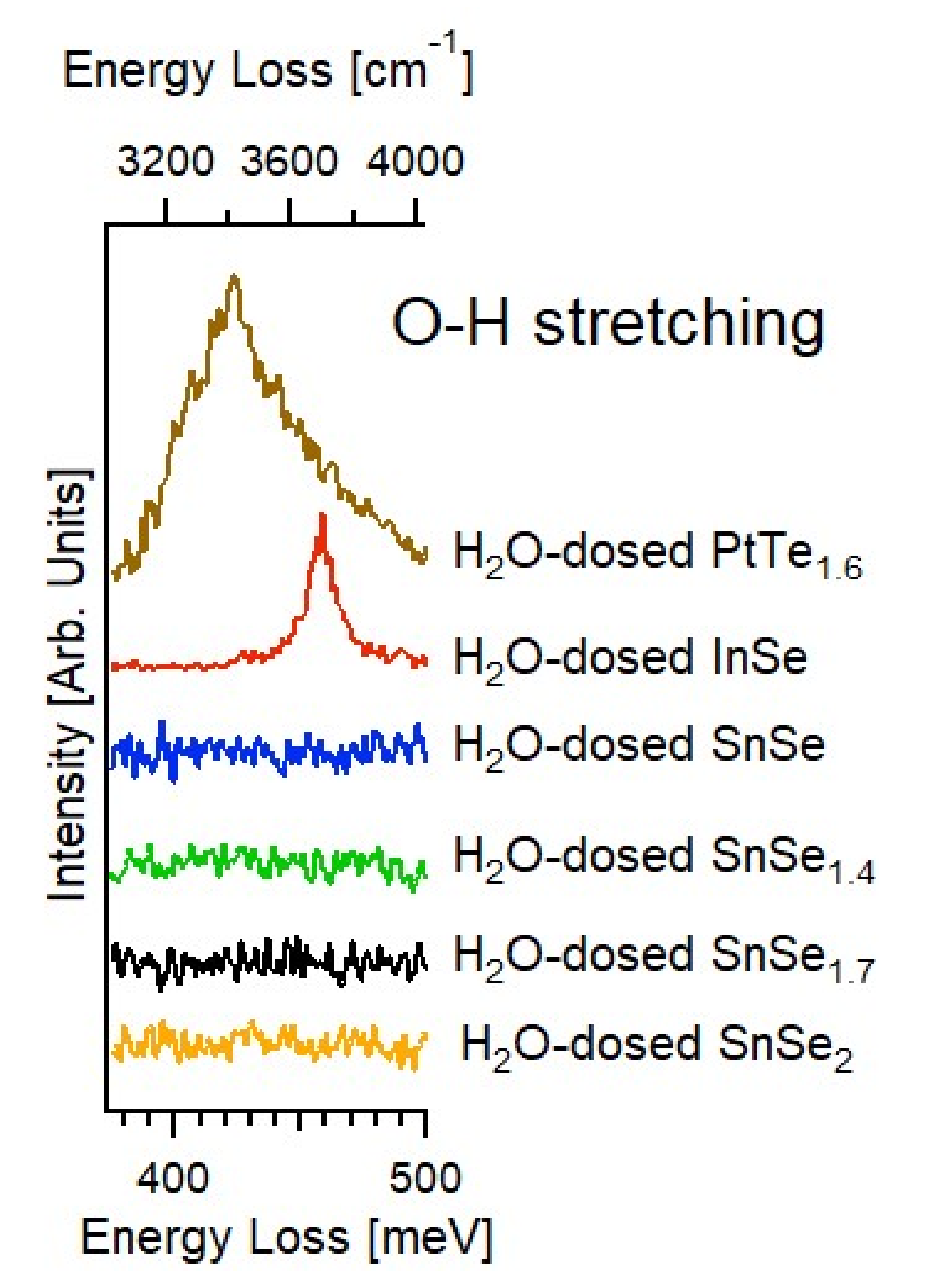
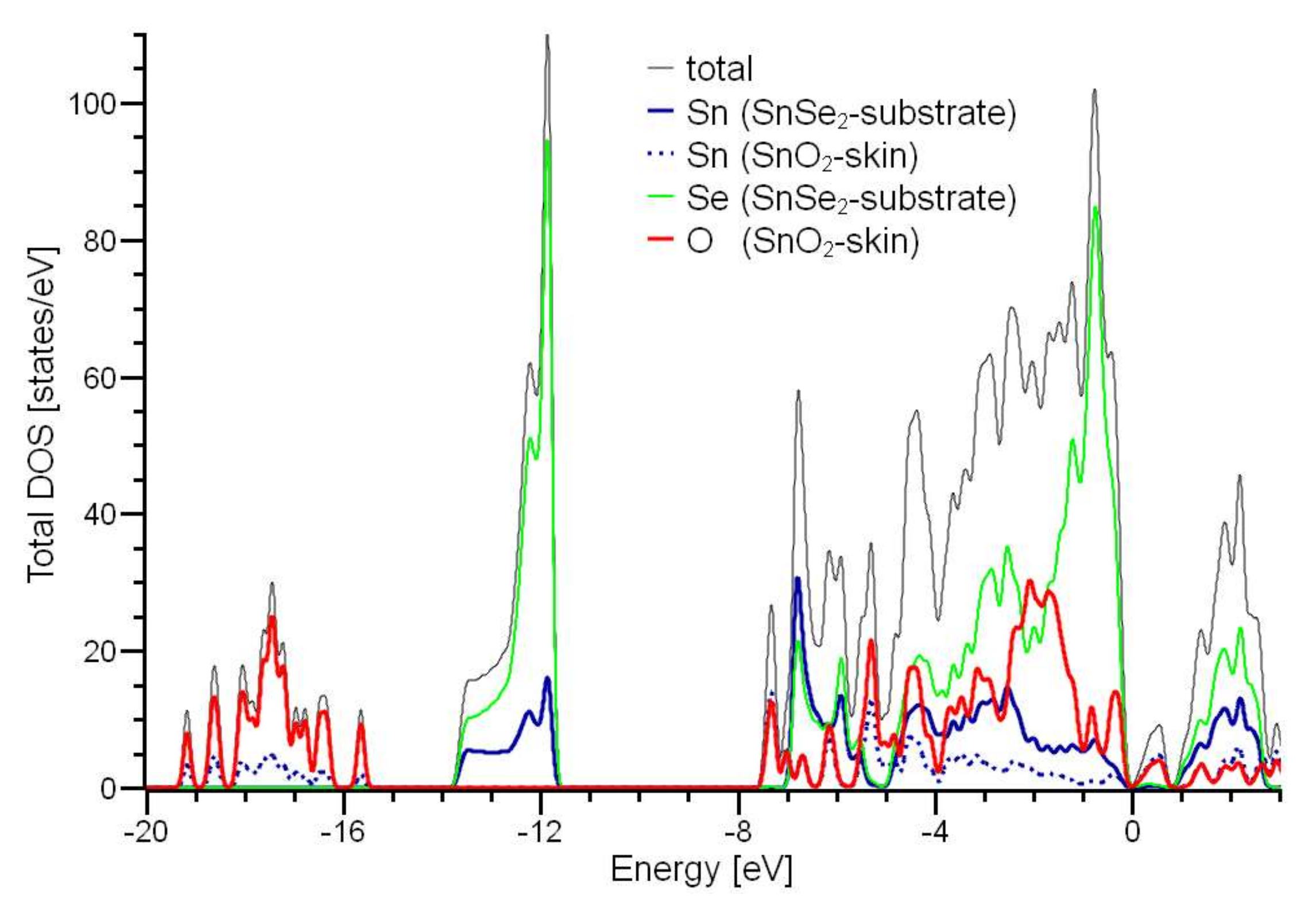
3.1. Chemisorption of O2 and H2O on Bulk SnSe2
3.2. Experimental Validation of the Theoretical Model
3.3. Gas Sensing
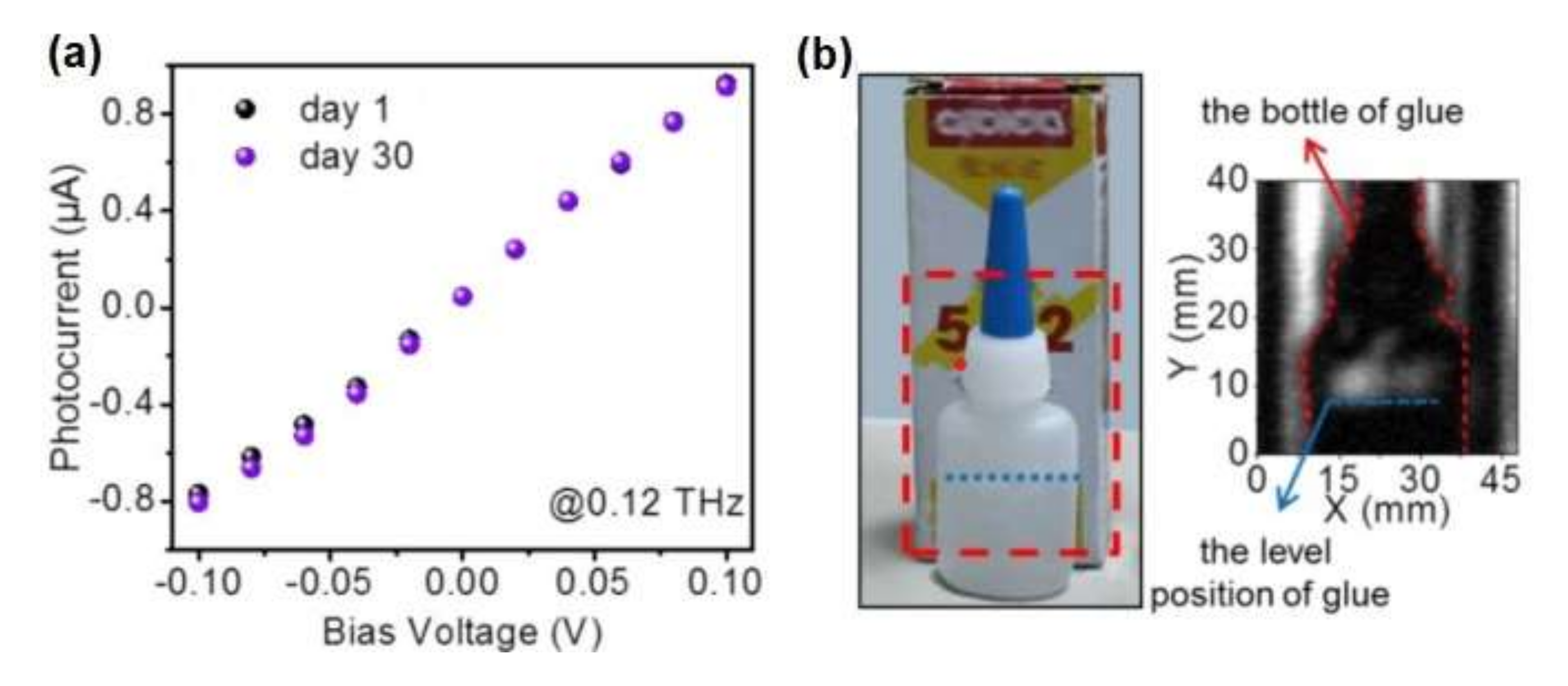
3.4. SnSe2-Based Sensors for Large-Area Imaging with Millimetre Waves
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Novoselov, K. Nobel Lecture: Graphene: Materials in the Flatland. Rev. Mod. Phys. 2011, 83, 837–849. [Google Scholar] [CrossRef]
- Geim, A.K. Nobel Lecture: Random walk to graphene. Rev. Mod. Phys. 2011, 83, 851–862. [Google Scholar] [CrossRef] [Green Version]
- Tao, Y.; Han, B.H.; Sui, Z.Y.; Tao, Y.; Han, B.H. Advanced porous graphene materials: From in-plane pore generation to energy storage applications. J. Mater. Chem. A 2020, 8, 6125–6143. [Google Scholar] [CrossRef]
- Yang, L.; Yi, N.; Zhu, J.; Cheng, Z.; Yin, X.; Zhang, X.; Zhu, H.; Cheng, H. Novel gas sensing platform based on a stretchable laser-induced graphene pattern with self-heating capabilities. J. Mater. Chem. A 2020, 8, 6487–6500. [Google Scholar] [CrossRef]
- Ahmadi, M.; Zabihi, O.; Jeon, S.; Yoonessi, M.; Dasari, A.; Ramakrishna, S.; Naebe, M. 2D transition metal dichalcogenide nanomaterials: Advances, opportunities, and challenges in multi-functional polymer nanocomposites. J. Mater. Chem. A 2020, 8, 845–883. [Google Scholar] [CrossRef]
- Jalil, A.; Zhuo, Z.; Sun, Z.; Wu, F.; Wang, C.; Wu, X. A phosphorene-like InP3 monolayer: Structure, stability, and catalytic properties toward the hydrogen evolution reaction. J. Mater. Chem. A 2020, 8, 1307–1314. [Google Scholar] [CrossRef]
- Wu, J.; Liu, J.; Cui, J.; Yao, S.; Ihsan-Ul-Haq, M.; Mubarak, N.; Quattrocchi, E.; Ciucci, F.; Kim, J.-K. Dual-phase MoS2 as a high-performance sodium-ion battery anode. J. Mater. Chem. A 2020, 8, 2114–2122. [Google Scholar] [CrossRef]
- Zeng, L.; Li, X.; Chen, S.; Wen, J.; Huang, W.; Chen, A. Unique hollow Ni-Fe@MoS2 nanocubes with boosted electrocat-alytic activity for N2 reduction to NH3. J. Mater. Chem. A 2020, 8, 7339–7349. [Google Scholar] [CrossRef]
- Zu, G.; Guo, G.; Li, H.; Lu, Y.; Wang, R.; Hu, Y.; Wang, L.; Wang, J. Revealing the failure mechanism of transition-metal chalcogenides towards the copper current collector in secondary batteries. J. Mater. Chem. A 2020, 8, 6569–6575. [Google Scholar] [CrossRef]
- Hu, G.; Fung, V.; Sang, X.; Unocic, R.R.; Ganesh, P. Superior electrocatalytic hydrogen evolution at engineered non-stoichiometric two-dimensional transition metal dichalcogenide edges. J. Mater. Chem. A 2019, 7, 18357–18364. [Google Scholar] [CrossRef]
- Li, J.; Hong, W.; Jian, C.; Cai, Q.; He, X.; Liu, W. High-performance hydrogen evolution at a MoSe2-Mo2C seamless heterojunction enabled by efficient charge transfer. J. Mater. Chem. A 2020, 8, 6692–6698. [Google Scholar] [CrossRef]
- Hu, H.; Shi, Z.; Khan, K.; Cao, R.; Liang, W.; Tareen, A.K.; Zhang, Y.; Huang, W.; Guo, Z.; Luo, X.; et al. Recent advances in doping engineering of black phosphorus. J. Mater. Chem. A 2020, 8, 5421–5441. [Google Scholar] [CrossRef]
- Ozawa, A.; Yamamoto, M.; Tanabe, T.; Hosokawa, S.; Yoshida, T. Black phosphorus synthesized by solvothermal reaction from red phosphorus and its catalytic activity for water splitting. J. Mater. Chem. A 2020, 8, 7368–7376. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, H.; Li, H.; Guo, Z.; Tian, B.; Cui, Y.; Li, Z.; Li, G.; Zhang, H.; Wu, Y. Recent advances in black phosphorus/carbon hybrid composites: From improved stability to applications. J. Mater. Chem. A 2020, 8, 4647–4676. [Google Scholar] [CrossRef]
- Ghosh, B.; Kumar, P.; Thakur, A.; Chauhan, Y.S.; Bhowmick, S.; Agarwal, A. Anisotropic plasmons, excitons, and electron energy loss spectroscopy of phosphorene. Phys. Rev. B 2017, 96, 035422. [Google Scholar] [CrossRef] [Green Version]
- Sadhukhan, K.; Agarwal, A. Anisotropic plasmons, Friedel oscillations, and screening in 8-Pmmn borophene. Phys. Rev. B 2017, 96, 035410. [Google Scholar] [CrossRef] [Green Version]
- Ottaviano, L.; Palleschi, S.; Perrozzi, F.; D’Olimpio, G.; Priante, F.; Donarelli, M.; Benassi, P.; Nardone, M.; Gonchigsuren, M.; Gombosuren, M.; et al. Mechanical exfoliation and layer number identification of MoS2 revisited. 2D Mater. 2017, 4, 045013. [Google Scholar] [CrossRef]
- Yi, M.; Shen, Z. A review on mechanical exfoliation for the scalable production of graphene. J. Mater. Chem. A 2015, 3, 11700–11715. [Google Scholar] [CrossRef]
- Gibaja, C.; Assebban, M.; Torres, I.; Fickert, M.; Sanchis-Gual, R.; Brotons-Alcázar, I.; Paz, W.; Palacios, J.J.; Michel, E.G.; Abellán, G.; et al. Liquid phase exfoliation of antimonene: Systematic optimization, characterization and electrocatalytic properties. J. Mater. Chem. A 2019, 7, 22475–22486. [Google Scholar] [CrossRef]
- Adilbekova, B.; Lin, Y.; Yengel, E.; Faber, H.; Harrison, G.T.; Firdaus, Y.; El Labban, A.; Anjum, D.H.; Tung, V.; Anthopoulos, T.D. Liquid phase exfoliation of MoS2 and WS2 in aqueous ammonia and their application in highly efficient organic solar cells. J. Mater. Chem. C 2020, 8, 5259–5264. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Z.; Li, X.; Mullen, J.T.; Kim, K.W. Intrinsic transport properties of electrons and holes in monolayer transition-metal dichalcogenides. Phys. Rev. B 2014, 90, 045422. [Google Scholar] [CrossRef] [Green Version]
- Edmonds, M.; Tadich, A.; Carvalho, A.; Ziletti, A.; O’Donnell, K.M.; Koenig, S.P.; Coker, D.F.; Özyilmaz, B.; Neto, A.H.C.; Fuhrer, M. Creating a Stable Oxide at the Surface of Black Phosphorus. ACS Appl. Mater. Interfaces 2015, 7, 14557–14562. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, A.; Ibrahim, J.; Leonelli, R.; Francoeur, S. Oxidation dynamics of ultrathin GaSe probed through Raman spectroscopy. Appl. Phys. Lett. 2017, 110, 241901. [Google Scholar] [CrossRef]
- Shi, L.; Li, Q.; Ouyang, Y.; Wang, J. Effect of illumination and Se vacancies on fast oxidation of ultrathin gallium selenide. Nanoscale 2018, 10, 12180–12186. [Google Scholar] [CrossRef]
- Lan, Y.; Chen, X.; Hu, C.; Cheng, Y.; Chen, Q. Penta-PdX2 (X = S, Se, Te) monolayers: Promising anisotropic thermoelectric materials. J. Mater. Chem. A 2019, 7, 11134–11142. [Google Scholar] [CrossRef]
- Wu, D.; Jia, C.; Shi, F.; Zeng, L.; Lin, P.; Dong, L.; Shi, Z.; Tian, Y.; Li, X.; Jie, J. Mixed-dimensional PdSe2/SiNWA hetero-structure based photovoltaic detectors for self-driven, broadband photodetection, infrared imaging and humidity sensing. J. Mater. Chem. A 2020, 8, 3632–3642. [Google Scholar] [CrossRef]
- Yu, B.; Qi, F.; Zheng, B.; Hou, W.; Zhang, W.; Li, Y.; Chen, Y. Self-assembled pearl-bracelet-like CoSe2-SnSe2/CNT hollow architecture as highly efficient electrocatalysts for hydrogen evolution reaction. J. Mater. Chem. A 2018, 6, 1655–1662. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, L.; Zhuo, M.; Ni, W.; Wang, H.; Ma, J. Layered tin sulfide and selenide anode materials for Li- and Na-ion batteries. J. Mater. Chem. A 2018, 6, 12185–12214. [Google Scholar] [CrossRef]
- Huang, Y.; Ling, C.; Liu, H.; Wang, S. Tuning electronic and magnetic properties of SnSe2 armchair nanoribbons via edge hydrogenation. J. Mater. Chem. C 2014, 2, 10175–10183. [Google Scholar] [CrossRef]
- Li, W.; Xiong, L.; Li, N.; Pang, S.; Xu, G.; Yi, C.; Wang, Z.; Gu, G.; Li, K.; Li, W.; et al. Tunable 3D light trapping architectures based on self-assembled SnSe2 nanoplate arrays for ultrasensitive SERS detection. J. Mater. Chem. C 2019, 7, 10179–10186. [Google Scholar] [CrossRef]
- Shafique, A.; Samad, A.; Shin, Y.-H. Ultra low lattice thermal conductivity and high carrier mobility of monolayer SnS2and SnSe2: A first principles study. Phys. Chem. Chem. Phys. 2017, 19, 20677–20683. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Fu, Z.-G.; Li, S.; Cao, Y.; Bian, Q.; Sun, H.; Zhang, Z.; Gedeon, H.; Zhang, X.; Liu, L.; et al. Strongly Compressed Few-Layered SnSe2 Films Grown on a SrTiO3 Substrate: The Coexistence of Charge Ordering and Enhanced Interfacial Superconductivity. Nano Lett. 2019, 19, 5304–5312. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Liu, E.; Fu, Y.; Chen, Z.; Pan, C.; Wang, C.; Wang, M.; Wang, Y.; Xu, K.; Cai, S.; et al. Gate-Induced Interfacial Superconductivity in 1T-SnSe2. Nano Lett. 2018, 18, 1410–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Yao, Z.; Lim, J.-M.; Hersam, M.C.; Wolverton, C.; Dravid, V.P.; He, K. Lithium-Ion Batteries: Atomic-Scale Observation of Electrochemically Reversible Phase Transformations in SnSe2 Single Crystals. Adv. Mater. 2018, 30, 1870393. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Xia, C.; Zhu, J.; Ahmed, B.; Liang, H.; Velusamy, D.B.; Schwingenschlögl, U.; Alshareef, H.N. SnSe22D Anodes for Advanced Sodium Ion Batteries. Adv. Energy Mater. 2016, 6, 1601188. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, N.; Li, C.; Song, H.; Zhang, Q.; Hu, X.; Gan, L.; Li, H.; Lü, J.; Luo, J.; et al. Vertical heterostructures based on SnSe2/MoS2 for high performance photodetectors. 2D Mater. 2017, 4, 025048. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, J.; Zhao, M. Spontaneous full photocatalytic water splitting on 2D MoSe2/SnSe2 and WSe2/SnSe2 vdW heterostructures. Nanoscale 2019, 11, 14836–14843. [Google Scholar] [CrossRef]
- Tan, P.; Chen, X.; Wu, L.; Shang, Y.Y.; Liu, W.; Pan, J.; Xiong, X. Hierarchical flower-like SnSe2 supported Ag3PO4 nanoparticles: Towards visible light driven photocatalyst with enhanced performance. Appl. Catal. B: Environ. 2017, 202, 326–334. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Wang, Z.; Xu, X.; Duan, S.; Du, C. Tin diselenide-based saturable absorbers for eye-safe pulse lasers. Nanotechnology 2019, 30, 265703. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Lim, K.H.; Xing, C.; Li, M.; Zhang, T.; Tang, P.; Arbiol, J.; Llorca, J.; Ng, K.M.; et al. Tin Diselenide Molecular Precursor for Solution-Processable Thermoelectric Materials. Angew. Chem. Int. Ed. 2018, 57, 17063–17068. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zheng, Y.; Luo, Z.; Hao, S.; Du, C.; Liang, Q.; Li, Z.; Khor, K.A.; Hippalgaonkar, K.; Xu, J.; et al. n-Type SnSe2 Oriented-Nanoplate-Based Pellets for High Thermoelectric Performance. Adv. Energy Mater. 2018, 8, 1702167. [Google Scholar] [CrossRef]
- Lee, Y.K.; Luo, Z.-Z.; Cho, S.P.; Kanatzidis, M.G.; Chung, I. Surface Oxide Removal for Polycrystalline SnSe Reveals Near-Single-Crystal Thermoelectric Performance. Joule 2019, 3, 719–731. [Google Scholar] [CrossRef] [Green Version]
- Lamuta, C.; Campi, D.; Pagnotta, L.; Dasadia, A.; Cupolillo, A.; Politano, A. Determination of the mechanical properties of SnSe, a novel layered semiconductor. J. Phys. Chem. Solids 2018, 116, 306–312. [Google Scholar] [CrossRef]
- Yamashita, A.; Ogiso, O.; Matsumoto, R.; Tanaka, M.; Hara, H.; Tanaka, H.; Takeya, H.; Lee, C.-H.; Takano, Y. Influence of Oxidation in Starting Material Sn on Electric Transport Properties of SnSe Single Crystals. J. Phys. Soc. Jpn. 2018, 87, 065001. [Google Scholar] [CrossRef]
- Chung, K.-M.; Wamwangi, D.M.; Woda, M.; Wuttig, M.; Bensch, W. Investigation of SnSe, SnSe2, and Sn2Se3 alloys for phase change memory applications. J. Appl. Phys. 2008, 103, 083523. [Google Scholar] [CrossRef]
- Guo, C.; Tian, Z.; Xiao, Y.; Mi, Q.; Xue, J. Field-effect transistors of high-mobility few-layer SnSe2. Appl. Phys. Lett. 2016, 109, 203104. [Google Scholar] [CrossRef] [Green Version]
- Palosz, B.; Salje, E. Lattice parameters and spontaneous strain in AX2 polytypes: CdI2, PbI2 SnS2 and SnSe2. J. Appl. Crystallogr. 1989, 22, 622–623. [Google Scholar] [CrossRef]
- Evans, B.L.; Hazelwood, R.A. Optical and electrical properties of SnSe2. J. Phys. D: Appl. Phys. 1969, 2, 1507–1516. [Google Scholar] [CrossRef]
- Mead, D.G.; Irwin, J.C. Raman spectra of SnS2 and SnSe2. Solid State Commun. 1976, 20, 885–887. [Google Scholar] [CrossRef]
- Smith, A.J.; Meek, P.E.; Liang, W.Y. Raman scattering studies of SnS2 and SnSe2. J. Phys. C Solid State Phys. 1977, 10, 1321–1323. [Google Scholar] [CrossRef]
- Taube, A.; Łapińska, A.; Judek, J.; Zdrojek, M. Temperature dependence of Raman shifts in layered ReSe2 and SnSe2 semiconductor nanosheets. Appl. Phys. Lett. 2015, 107, 013105. [Google Scholar] [CrossRef]
- Henderson, M. The interaction of water with solid surfaces: Fundamental aspects revisited. Surf. Sci. Rep. 2002, 46, 1–308. [Google Scholar] [CrossRef]
- Inamdar, A.N.; Som, N.N.; Pratap, A.; Jha, P.K. Hydrogen evolution and oxygen evolution reactions of pristine and alkali metal doped SnSe2 monolayer. Int. J. Hydrogen Energy 2020, 45, 18657–18665. [Google Scholar] [CrossRef]
- Deng, J.; Mo, Y.; Liu, J.; Guo, R.; Zhang, Y.; Xue, W.; Zhang, Y. In Vitro Study of SnS2, BiOCl and SnS2-Incorporated BiOCl Inorganic Nanoparticles Used as Doxorubicin Carrier. J. Nanosci. Nanotechnol. 2016, 16, 5740–5745. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.; Egdell, R.; Flavell, W.; Helbig, R. Observation of surface optical phonons on SnO2 (110). Vacuum 1983, 33, 835–838. [Google Scholar] [CrossRef]
- Nehasil, V.; Janeček, P.; Korotchenkov, G.; Matolin, V. Investigation of behaviour of Rh deposited onto polycrystalline SnO2 by means of TPD, AES and EELS. Surf. Sci. 2003, 532–535, 415–419. [Google Scholar] [CrossRef]
- Tanuma, S.; Powell, C.J.; Penn, D.R. Calculations of electron inelastic mean free paths (IMFPS). IV. Evaluation of calculated IMFPs and of the predictive IMFP formula TPP-2 for electron energies between 50 and 2000 eV. Surf. Interface Anal. 1993, 20, 77–89. [Google Scholar] [CrossRef]
- Cox, D.F.; Hoflund, G.B. An electronic and structural interpretation of tin oxide ELS spectra. Surf. Sci. 1985, 151, 202–220. [Google Scholar] [CrossRef]
- Wu, S.; Liu, C.; Wu, Z.; Miao, L.; Gao, J.; Hu, X.; Chen, J.; Zheng, Y.; Wang, X.; Shen, C.; et al. Realizing tremendous electrical transport properties of polycrystalline SnSe2 by Cl-doped and anisotropy. Ceram. Int. 2019, 45, 82–89. [Google Scholar] [CrossRef]
- Nagaraju, G.; Cha, S.M.; Sekhar, S.C.; Yu, J.S. Metallic Layered Polyester Fabric Enabled Nickel Selenide Nanostructures as Highly Conductive and Binderless Electrode with Superior Energy Storage Performance. Adv. Energy Mater. 2017, 7. [Google Scholar] [CrossRef]
- Al-Hada, N.M.; Kamari, H.M.; Baqer, A.A.; Shaari, A.H.; Saion, E. Thermal Calcination-Based Production of SnO2 Nanopowder: An Analysis of SnO2 Nanoparticle Characteristics and Antibacterial Activities. Nanomaterials 2018, 8, 250. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Li, M.; Xiao, X.; Huang, X.; Jiang, Y.; Fan, X.; Chen, L. In situ synthesis of ultrasmall SnO2 quantum dots on nitrogen-doped reduced graphene oxide composite as high performance anode material for lithium-ion batteries. J. Alloys Compd. 2017, 727, 1–7. [Google Scholar] [CrossRef]
- Bachvarova-Nedelcheva, A.; Iordanova, R.; Kostov, K.; Yordanov, S.; Ganev, V. Structure and properties of a non-traditional glass containing TeO2, SeO2 and MoO3. Opt. Mater. 2012, 34, 1781–1787. [Google Scholar] [CrossRef]
- Wakita, T.; Paris, E.; Kobayashi, K.; Terashima, K.; Hacisalihoǧlu, M.Y.; Ueno, T.; Bondino, F.; Magnano, E.; Píš, I.; Olivi, L.; et al. The electronic structure of Ag1−xSn1 + xSe2 (x = 0.0, 0.1, 0.2, 0.25 and 1.0). Phys. Chem. Chem. Phys. 2017, 19, 26672–26678. [Google Scholar] [CrossRef] [PubMed]
- Cimino, A.; Gazzoli, D.; Valigi, M. XPS quantitative analysis and models of supported oxide catalysts. J. Electron Spectrosc. Relat. Phenom. 1999, 104, 1–29. [Google Scholar] [CrossRef]
- Park, J.H.; Vishwanath, S.; Wolf, S.; Zhang, K.; Kwak, I.; Edmonds, M.; Breeden, M.; Liu, X.; Dobrowolska, M.; Furdyna, J.; et al. Selective Chemical Response of Transition Metal Dichalcogenides and Metal Dichalcogenides in Ambient Conditions. ACS Appl. Mater. Interfaces 2017, 9, 29255–29264. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, V.; D’Olimpio, G.; Kuo, C.-N.; Lue, C.S.; Boukhvalov, D.W.; Cantalini, C.; Politano, A. Self-Assembled SnO2/SnSe2 Heterostructures: A Suitable Platform for Ultrasensitive NO2 and H2 Sensing. ACS Appl. Mater. Interfaces 2020, 12, 34362–34369. [Google Scholar] [CrossRef]
- Tian, K.; Wang, X.-X.; Yu, Z.-Y.; Li, H.-Y.; Guo, X. Hierarchical and Hollow Fe2O3 Nanoboxes Derived from Metal–Organic Frameworks with Excellent Sensitivity to H2S. ACS Appl. Mater. Interfaces 2017, 9, 29669–29676. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y.; Sun, Q.; Zheng, S.; Liu, L.; Li, J.; Hao, J. Boosted interfacial charge transfer in SnO2/SnSe2 hetero-structures: Toward ultrasensitive room-temperature H2S detection. Inorg. Chem. Front. 2021, 8, 2068–2077. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Z.; Zong, X. Flexible and highly sensitive H2S gas sensor based on in-situ polymerized SnO2/rGO/PANI ternary nanocomposite with application in halitosis diagnosis. Sens. Actuators B Chem. 2019, 289, 32–41. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Dai, Y.; Li, G.; Wang, S.; Li, T.; Zhang, T.; Qin, S. In situ growth of NiO@SnO2 hierarchical nanostructures for high performance H2S sensing. ACS Appl. Mater. Interfaces 2019, 11, 44829–44836. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Ouyang, Q.; Zhu, C.; Zhang, X.; Chen, Y. Porous MoO3/SnO2 Nanoflakes with n–n Junctions for Sensing H2S. ACS Appl. Nano Mater. 2019, 2, 2418–2425. [Google Scholar] [CrossRef]
- Eom, N.S.A.; Cho, H.-B.; Song, Y.; Go, G.M.; Lee, J.; Choa, Y.-H. Room-temperature H2S gas sensing by selectively synthesized Cux(x=1, 2)O: SnO2 thin film nanocomposites with oblique & vertically assembled SnO2 ceramic nanorods. Sens. Actuators B Chem. 2018, 273, 1054–1061. [Google Scholar]
- Shen, Y.; Yamazaki, T.; Liu, Z.; Meng, D.; Kikuta, T.; Nakatani, N.; Saito, M.; Mori, M. Microstructure and H2 gas sensing properties of undoped and Pd-doped SnO2 nanowires. Sens. Actuators B Chem. 2009, 135, 524–529. [Google Scholar] [CrossRef]
- Ghimbeu, C.M.; Lumbreras, M.; Siadat, M.; van Landschoot, R.C.; Schoonman, J. Electrostatic sprayed SnO2 and Cu-doped SnO2 films for H2S detection. Sens. Actuators B Chem. 2008, 133, 694–698. [Google Scholar] [CrossRef]
- Guo, X.; Ding, Y.; Liang, C.; Du, B.; Zhao, C.; Tan, Y.; Shi, Y.; Zhang, P.; Yang, X.; He, Y. Humidity-activated H2S sensor based on SnSe2/WO3 composite for evaluating the spoilage of eggs at room temperature. Sens. Actuators B Chem. 2022, 357, 131424. [Google Scholar] [CrossRef]
- Song, Z.; Wei, Z.; Wang, B.; Luo, Z.; Xu, S.; Zhang, W.; Yu, H.; Li, M.; Huang, Z.; Zang, J.; et al. Sensitive Room-Temperature H2S Gas Sensors Employing SnO2 Quantum Wire/Reduced Graphene Oxide Nanocomposites. Chem. Mater. 2016, 28, 1205–1212. [Google Scholar] [CrossRef]
- Moreira, L.C.; Cheng, W.-Y.; Fuh, H.-R.; Chien, W.-C.; Yan, W.; Fei, H.; Xu, H.; Zhang, D.; Chen, Y.; Zhao, Y.; et al. High Selectivity Gas Sensing and Charge Transfer of SnSe2. ACS Sens. 2019, 4, 2546–2552. [Google Scholar] [CrossRef]
- Pan, Q.; Li, T.; Zhang, D. Ammonia gas sensing properties and density functional theory investigation of coral-like Au-SnSe2 Schottky junction. Sens. Actuators B Chem. 2021, 332, 129440. [Google Scholar] [CrossRef]
- Rani, S.; Kumar, M.; Singh, Y.; Tomar, M.; Sharma, A.; Gupta, V.; Singh, V.N. NO2 Gas Sensor Based on SnSe/SnSe2p-n Hetrojunction. J. Nanosci. Nanotechnol. 2021, 21, 4779–4785. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Chen, C.-W.; Manikandan, A.; Lee, S.-H.; Wang, Z.M.; Chueh, Y.-L. Phase-engineered SnSex toward SnSe2/SnSe heterostructure with improved thermal conductance by a low-temperature plasma-assisted chemical vapor reaction. Nano Energy 2018, 44, 419–429. [Google Scholar] [CrossRef]
- Liu, W.; Gu, D.; Li, X. AuPt Bimetal-Functionalized SnSe2 Microflower-Based Sensors for Detecting Sub-ppm NO2 at Low Temperatures. ACS Appl. Mater. Interfaces 2021, 13, 20336–20348. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Dai, J.; Chen, Q.; Huang, X.; Huang, W. Solution-Processed p-SnSe/n-SnSe2 Hetero-Structure Layers for Ultrasensitive NO2 Detection. Chem. Eur. J. 2020, 26, 3870–3876. [Google Scholar] [CrossRef]
- Li, X.; Liu, W.; Huang, B.; Liu, H.; Li, X. Layered SnSe2 microflakes and SnSe2/SnO2 heterojunctions for low-temperature chemiresistive-type gas sensing. J. Mat. Chem. C 2020, 8, 15804–15815. [Google Scholar] [CrossRef]
- Song, J.C.W.; Rudner, M.S.; Marcus, C.M.; Levitov, L.S. Hot Carrier Transport and Photocurrent Response in Graphene. Nano Lett. 2011, 11, 4688–4692. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Ebrish, M.A.; Olson, E.J.; Koester, S.J. SnSe2 field-effect transistors with high drive current. Appl. Phys. Lett. 2013, 103, 263104. [Google Scholar] [CrossRef]
- Mics, Z.; Tielrooij, K.-J.; Parvez, K.; Jensen, S.A.; Ivanov, I.; Feng, X.; Müllen, K.; Bonn, M.; Turchinovich, D. Thermodynamic picture of ultrafast charge transport in graphene. Nat. Commun. 2015, 6, 7655. [Google Scholar] [CrossRef] [Green Version]
- Bandurin, D.A.; Gayduchenko, I.; Cao, Y.; Moskotin, M.; Principi, A.; Grigorieva, I.V.; Goltsman, G.; Fedorov, G.; Svintsov, D. Dual origin of room temperature sub-terahertz photoresponse in graphene field effect transistors. Appl. Phys. Lett. 2018, 112, 141101. [Google Scholar] [CrossRef] [Green Version]
- Viti, L.; Hu, J.; Coquillat, D.; Politano, A.; Knap, W.; Vitiello, M.S. Efficient Terahertz detection in black-phosphorus nano-transistors with selective and controllable plasma-wave, bolometric and thermoelectric response. Sci. Rep. 2016, 6, 20474. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Guo, W.; Xu, H.; Zhang, L.; Chen, G.; D’Olimpio, G.; Kuo, C.-N.; Lue, C.S.; Wang, L.; Politano, A.; et al. Ultra-sensitive Ambient-Stable SnSe2-based Broadband Photodetectors for Room-Temperature IR/THz Energy conversion and Imaging. 2D Mater. 2020, 7, 035026. [Google Scholar] [CrossRef]
- Island, J.O.; Steele, G.A.; Van Der Zant, H.S.J.; Castellanos-Gomez, A. Environmental instability of few-layer black phosphorus. 2D Mater. 2015, 2, 011002. [Google Scholar] [CrossRef] [Green Version]

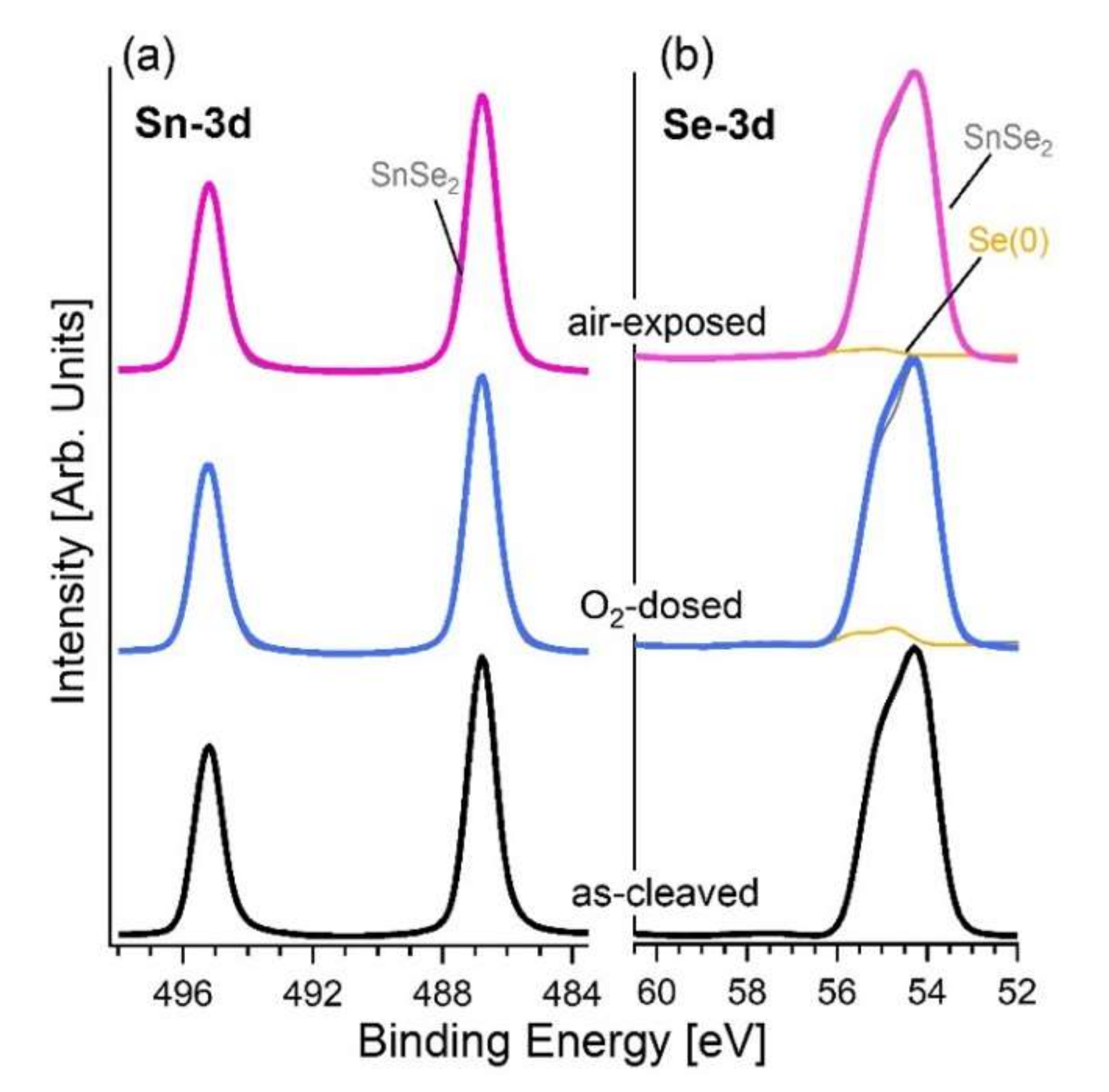
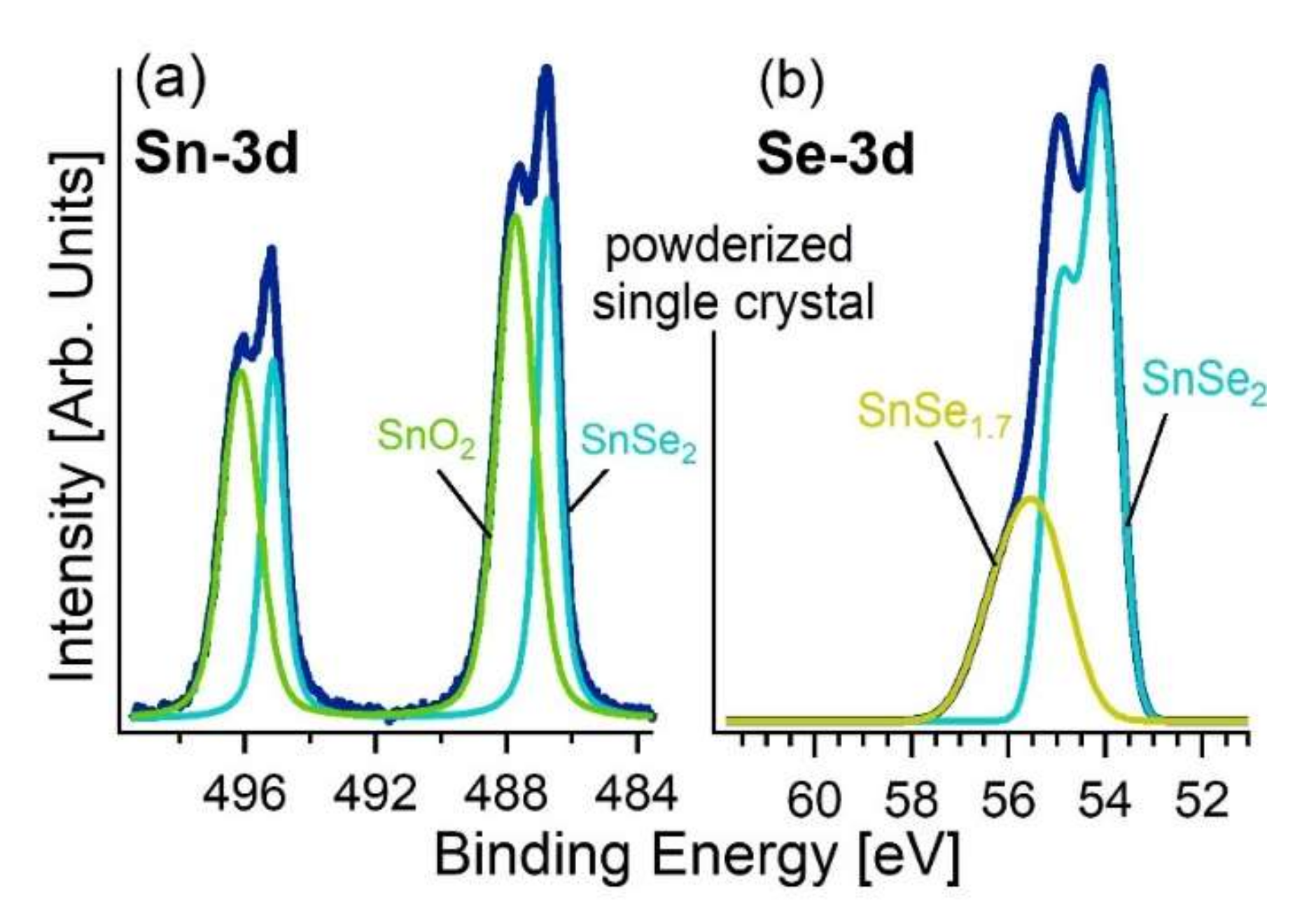

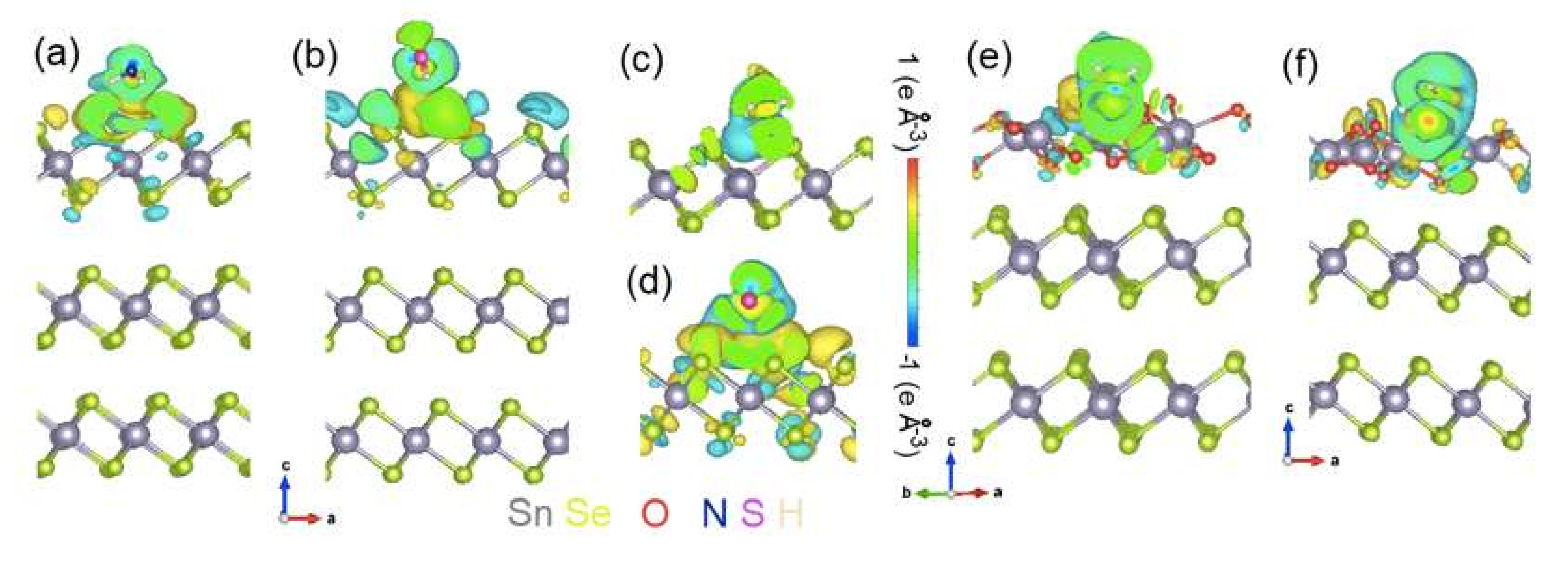
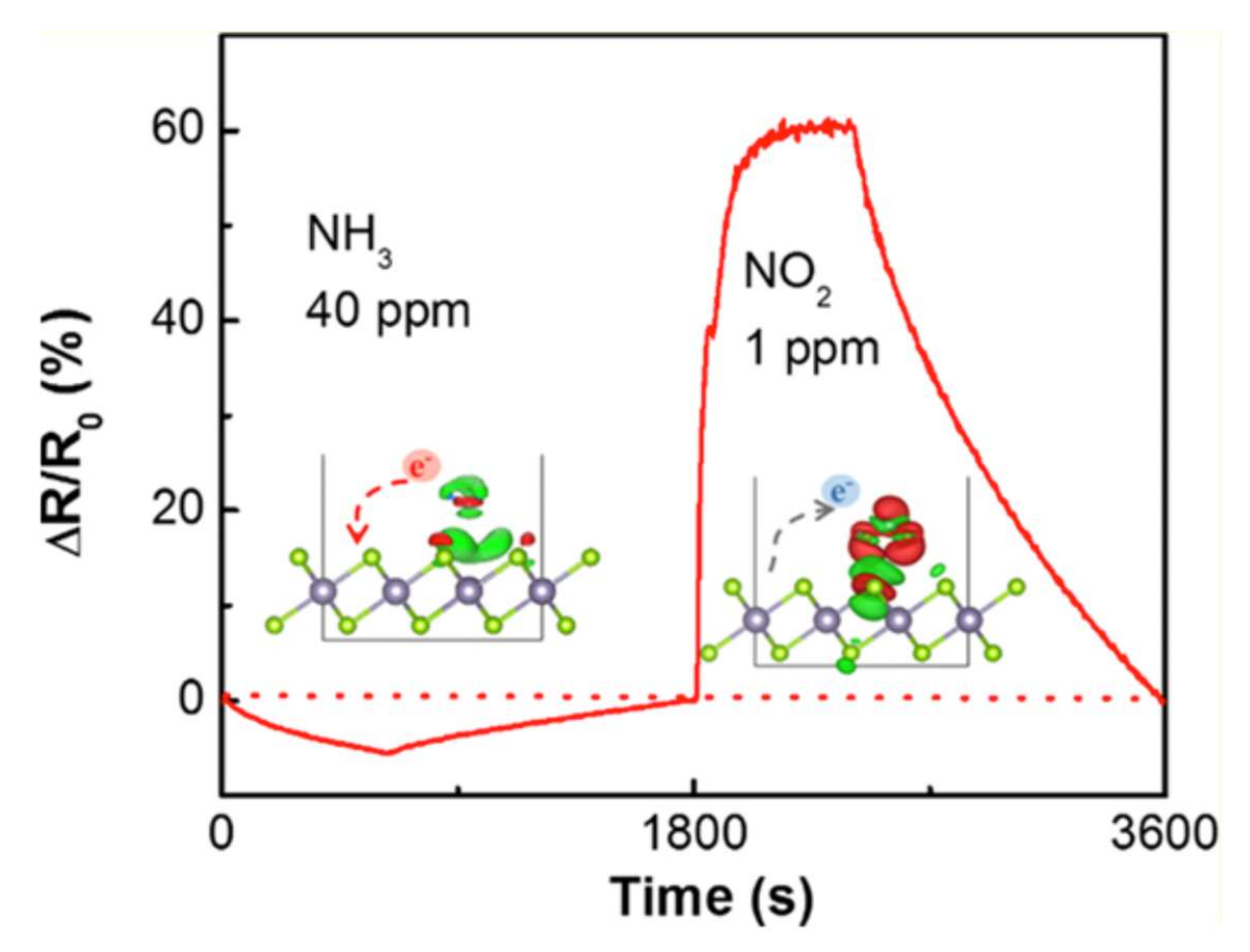

| Surface | Adsorbant | Physisorption | Decomposition | |
|---|---|---|---|---|
| ΔHads (kJ/mol) | ΔG (kJ/mol) | ΔHdec (kJ/mol) | ||
| SnSe2 | O2 | −17.5 | −3.2 | −42.3 (−161.6/~ −40.2) |
| H2O | −13.3 | +18.0 | +220.9 | |
| SnSe1.88 | O2 | −37.6 | −26.3 | −135.7 (−99.1/−406.7) |
| H2O | −27.9 | +3.4 | +175.6 | |
| SnSe | O2 | −11.6 | −0.2 | −236.1 (−323.1/+95.4) |
| H2O | −8.1 | +23.2 | +82.2 | |
| SnO2 skin | H2O | −119.7 | −106.7 | −121.3 |
| System | Surface | ΔHphys (kJ/mol) | ΔGphys (kJ/mol) | ΔHdec (kJ/mol) |
|---|---|---|---|---|
| Bulk | SnSe2 SnSe1.88 | −17.5 −37.6 | +5.24 −14.88 | −42.3 −135.7 |
| Bilayer | SnSe2 SnSe1.88 | +38.9 −47.6 | +61.6 −24.9 | −76.3 −115.5 |
| Monolayer | SnSe2 SnSe1.88 | +53.1 −59.3 | +75.8 −36.6 | −56.3 +183.5 |
| Substrate | Analyte | ΔG (kJ/mol) | Δe− |
|---|---|---|---|
| Bulk SnSe2 (SnSe1.88) | H2 H2O H2S NH3 NO2 | +6.79 (−0.11) +45.63 (−30.97) +38.70 (−21.32) +24.50 (−12.10) +58.87 (−36.75) | +0.09 (+0.10) −0.17 (−0.20) −0.12 (−0.14) +0.06 (+0.12) −0.07 (−0.10) |
| Bilayer SnSe2 (SnSe1.88) | H2 H2O H2S NH3 NO2 | +8.91 (−0.10) +41.23 (−26.8) +36.14 (−29.65) +18.91 (−10.01) +46.75 (−25.44) | +0.08 (+0.10) −0.15 (−0.18) −0.10 (−0.13) +0.10 (+0.15) −0.10 (−0.11) |
| Monolayer SnSe2 (SnSe1.88) | H2 H2O H2S NH3 NO2 | +2.89 (−4.63) +28.97 (−7.11) +18.35 (−0.12) −4.91 (−12.10) +0.12 (−3.45) | +0.10 (+0.09) −0.15 (−0.20) −0.12 (−0.09) +0.16 (+0.15) −0.09 (−0.10) |
| SnO2/SnSe2 | H2 H2O H2S NH3 NO2 | −135.61 −60.80 −71.28 −96.20 −127.35 | +0.15 −0.43 −0.36 +0.44 −0.25 |
| Gas | Sensing Materials | OperationalTemperature (°C) | Concentration (ppm) | Response | Reference |
|---|---|---|---|---|---|
| H2 | SnO2/SnSe2-x | 150 | 100 | 3 | [68] |
| H2 | SnO2 | 150 | 1000 | 5.5 | [75] |
| H2S | SnO2 | 100 | 10 | 1–6 | [76] |
| H2S | SnSe2 | RT | 10 | 10–15 | [77] |
| H2S | SnO2/SnSe2 | RT | 10 | 32 | [70] |
| H2S | SnO2 | RT | 50 | 33 | [78] |
| NH3 | SnO2/SnSe2 | RT | 100 | 2 | [70] |
| NH3 | SnSe2 | RT | 40 | 2.7 | [79] |
| NH3 | Au-SnSe2 | RT | 5 | 5.3 | [80] |
| NO2 | SnO2/SnSe2 | RT | 10 | 3.5 | [70] |
| NO2 | SnO2/SnSe2-x | 150 | 1 | 3.2 | [68] |
| NO2 | SnSe2 | RT | 1 | 6 | [79] |
| NO2 | SnSe2 | RT | 5 | 112 | [81] |
| NO2 | SnSe2/SnSe | RT | 1 | 75 | [82] |
| NO2 | Au/SnSe2 | 130 | 8 | 3 | [83] |
| NO2 | Pt-SnSe2 | 130 | 8 | 3.9 | [83] |
| NO2 | SnSe/SnSe2 | RT | 5 | 12 | [84] |
| NO2 | SnSe2 | RT | 8 | 1.4 | [85] |
| NO2 | SnO2 | 100 | 10 | 1 | [76] |
| Gas | Sensitivity [ppm]−1 | Limit of Detection |
|---|---|---|
| H2 | 0.43 ± 0.02 [68] | 5 ppm at 150 °C [68] |
| H2S | 2.13 ± 0.01 [77] | 10 ppb at RT [70] |
| NH3 | 0.11 ± 0.01 [80] | 250 ppb at RT [80] |
| NO2 | 1.06 ± 0.03 [68] | 400 ppb at 150 °C [68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D'Olimpio, G.; Farias, D.; Kuo, C.-N.; Ottaviano, L.; Lue, C.S.; Boukhvalov, D.W.; Politano, A. Tin Diselenide (SnSe2) Van der Waals Semiconductor: Surface Chemical Reactivity, Ambient Stability, Chemical and Optical Sensors. Materials 2022, 15, 1154. https://doi.org/10.3390/ma15031154
D'Olimpio G, Farias D, Kuo C-N, Ottaviano L, Lue CS, Boukhvalov DW, Politano A. Tin Diselenide (SnSe2) Van der Waals Semiconductor: Surface Chemical Reactivity, Ambient Stability, Chemical and Optical Sensors. Materials. 2022; 15(3):1154. https://doi.org/10.3390/ma15031154
Chicago/Turabian StyleD'Olimpio, Gianluca, Daniel Farias, Chia-Nung Kuo, Luca Ottaviano, Chin Shan Lue, Danil W. Boukhvalov, and Antonio Politano. 2022. "Tin Diselenide (SnSe2) Van der Waals Semiconductor: Surface Chemical Reactivity, Ambient Stability, Chemical and Optical Sensors" Materials 15, no. 3: 1154. https://doi.org/10.3390/ma15031154
APA StyleD'Olimpio, G., Farias, D., Kuo, C.-N., Ottaviano, L., Lue, C. S., Boukhvalov, D. W., & Politano, A. (2022). Tin Diselenide (SnSe2) Van der Waals Semiconductor: Surface Chemical Reactivity, Ambient Stability, Chemical and Optical Sensors. Materials, 15(3), 1154. https://doi.org/10.3390/ma15031154








