Novel Fabrication of Silver-Coated Copper Nanowires with Organic Compound Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method for Synthesis of Copper Nanowires
2.3. Method for Fabrication of Cu-Ag Nanowires
2.4. Characterization
3. Results and Discussion
3.1. The Effect of Concentration of N2H4
3.2. The Effect of Temperature on Morphology of Copper Nanowires
3.3. The Effect of Concentration of EDA
3.4. The Effect of Concentration of NaOH
3.5. The Effect of Concentration of Copper Precursor
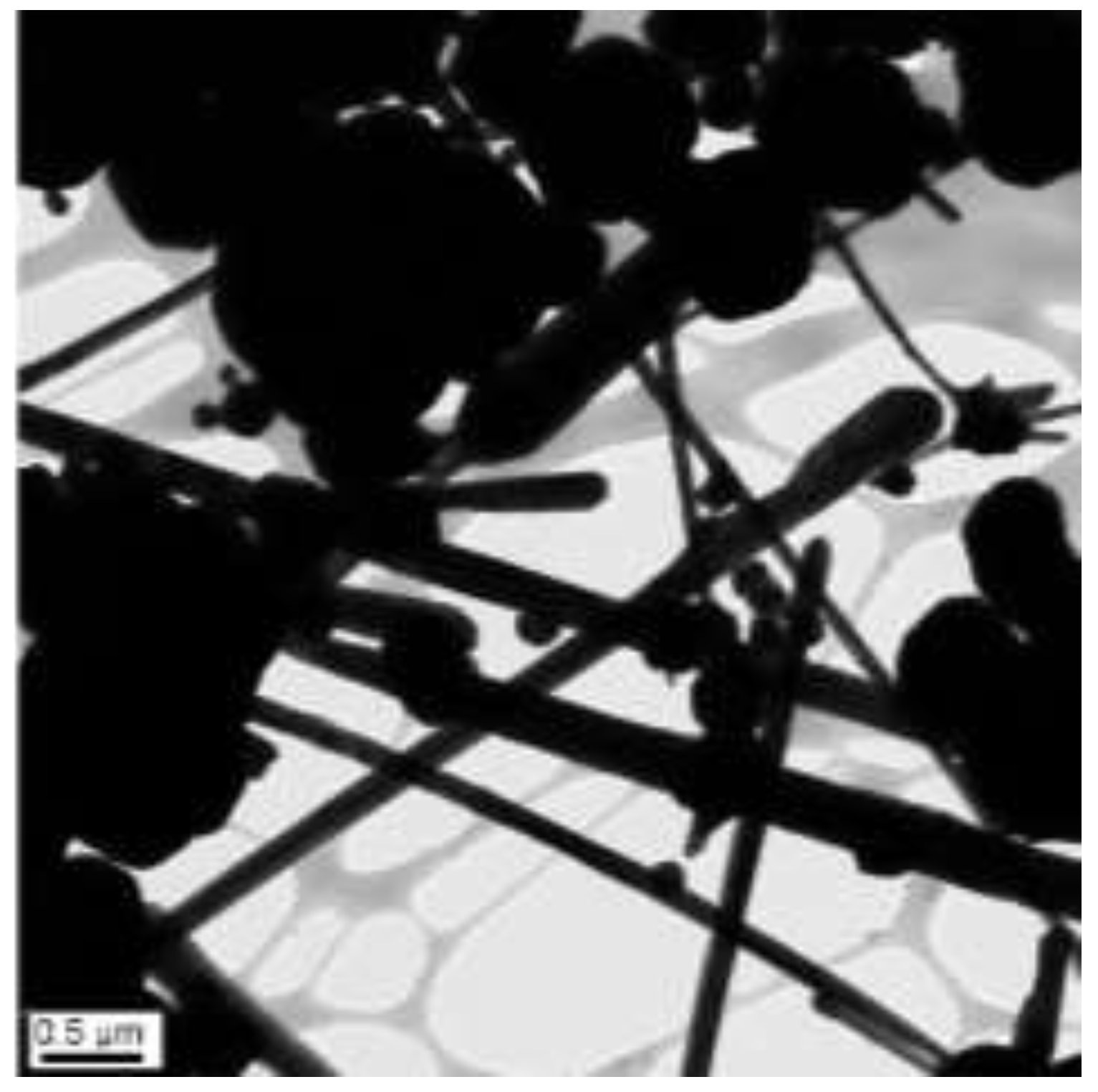
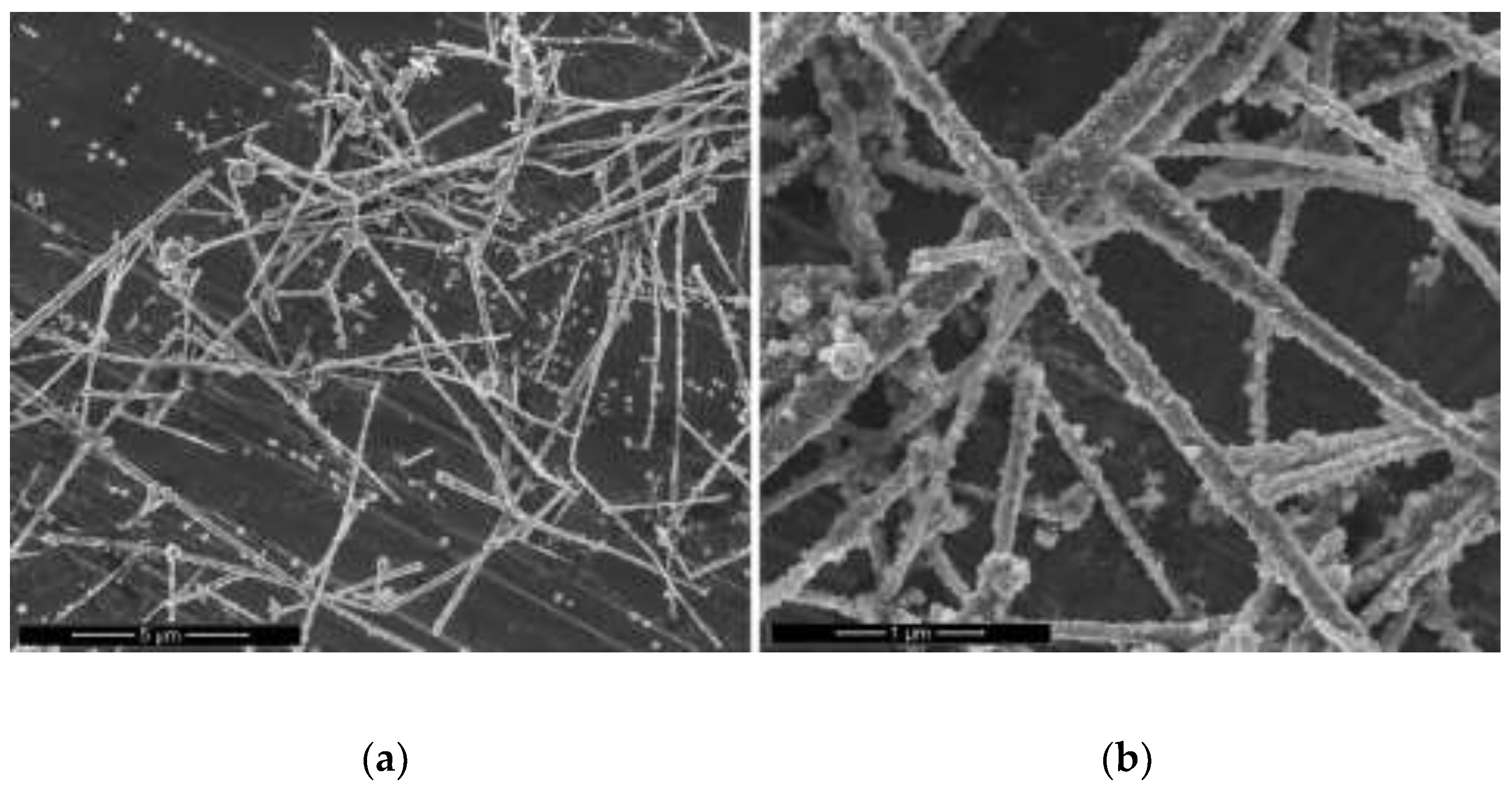
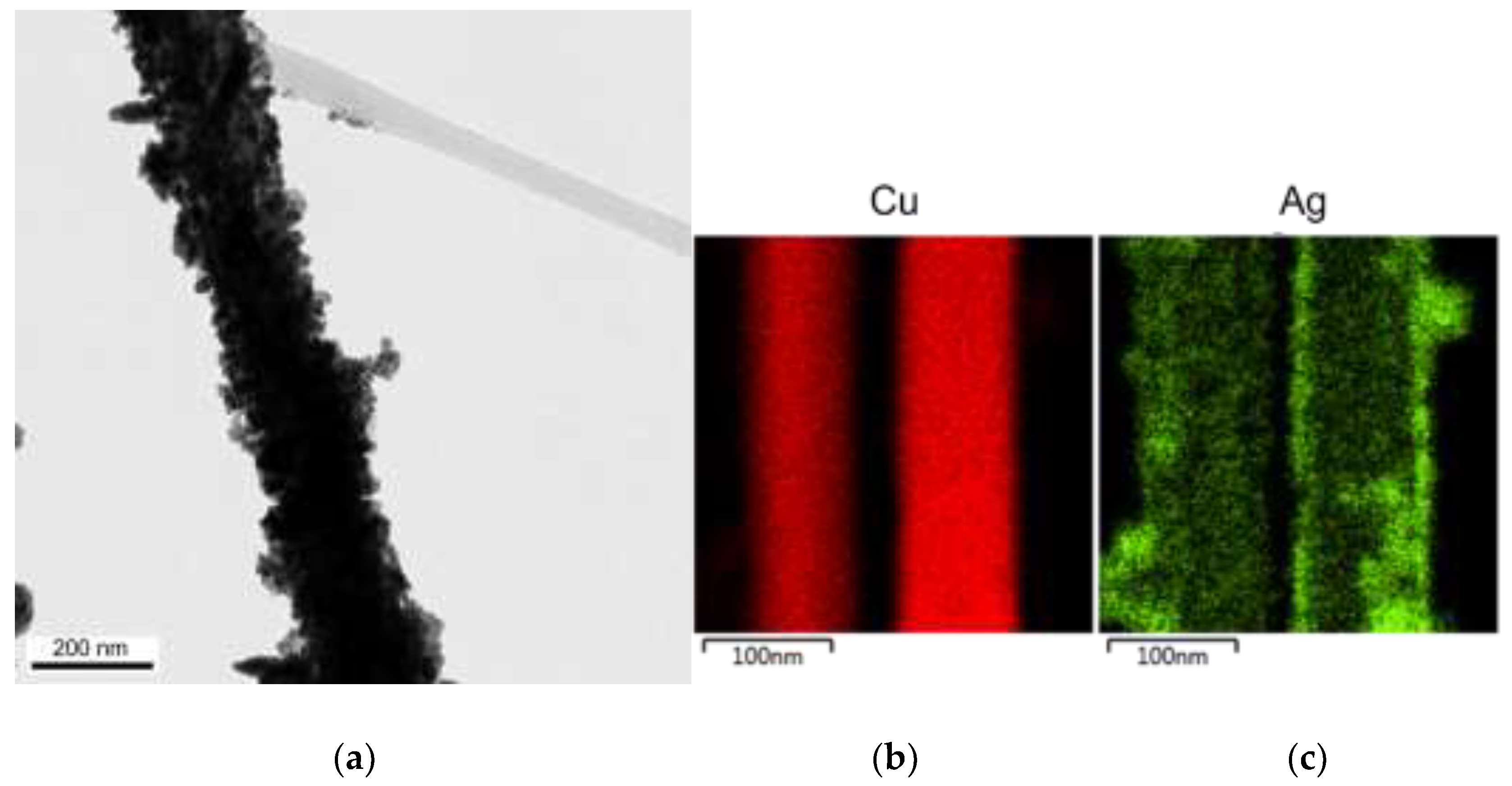
3.6. Fabrication of Cu-Ag Nanowires
Ag+ + e− → Ag E0 = +0.7996 V
Cu2+ + 2Ag+ → Cu2+ + 2Ag ↓ ∆E0 = +0.4577 V
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, H.; Lin, N.; Chen, Y.; Wang, Z.; Xie, Q.; Zheng, T.; Gao, N.; Li, S.; Kang, J.; Cai, D.; et al. Copper nanowires as fully transparent conductive electrodes. Sci. Rep. 2013, 3, 2323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, C.; An, J.; Choi, B.D.; Kim, K.; Jung, S.W.; Baeg, K.J.; Kim, M.G.; Ok, K.M.; Hong, J. Controlled aqueous synthesis of ultra-long copper nanowires for stretchable transparent conducting electrode. J. Mater. Chem. C 2016, 4, 1441–1447. [Google Scholar] [CrossRef]
- Naghdi, S.; Rhee, K.Y.; Hui, D.; Park, S.J. A review of conductive metal nanomaterials as conductive, transparent, and flexible coatings, thin films, and conductive fillers: Different deposition methods and applications. Coatings 2018, 8, 278. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Wang, S.; Wern, C.; Yi, S. The green synthesis of 2D copper nanosheets and their light absorption. Materials 2021, 14, 1926. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, R.; Wen, M.; Weng, D.; Cui, X.; Sun, J.; Li, H.; Lu, Y. Synthesis of ultralong copper nanowires for high-performance transparent electrodes. J. Am. Chem. Soc. 2012, 134, 14283–14286. [Google Scholar] [CrossRef]
- Rathmell, A.R.; Wiley, B.J. The synthesis and coating of long, thin copper nanowires to make flexible, transparent conducting films on plastic substrates. Adv. Mater. 2011, 23, 4798–4803. [Google Scholar] [CrossRef]
- Hsu, P.C.; Wang, S.; Wu, H.; Narasimhan, V.K.; Kong, D.; Lee, H.R.; Cui, Y. Performance enhancement of metal nanowire transparent conducting electrodes by mesoscale metal wires. Nat. Commun. 2013, 4, 4–2522. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Wu, H.; Cui, Y. Metal nanogrids, nanowires, and nanofibers for transparent electrodes. MRS Bull. 2011, 36, 760–765. [Google Scholar] [CrossRef] [Green Version]
- Cui, F.; Yu, Y.; Dou, L.; Sun, J.; Yang, Q.; Schildknecht, C.; Schierle-Arndt, K.; Yang, P. Synthesis of ultrathin copper nanowires using tris (trimethylsilyl) silane for high-performance and low-haze transparent conductors. Nano Lett. 2015, 15, 7610–7615. [Google Scholar] [CrossRef]
- Ding, S.; Tian, Y.; Jiu, J.; Suganuma, K. Highly conductive and transparent copper nanowire electrodes on surface coated flexible and heat-sensitive substrates. RSC Adv. 2018, 8, 2109–2115. [Google Scholar] [CrossRef] [Green Version]
- Duong, T.H.; Kim, H.C. A high productivity and speedy synthesis process for copper nanowires via an ethylenediamine-mediated method. Int. Nano Lett. 2017, 7, 165–169. [Google Scholar] [CrossRef] [Green Version]
- Ye, S.; Rathmell, A.R.; Stewart, I.E.; Ha, Y.C.; Wilson, A.R.; Chen, Z.; Wiley, B.J. A rapid synthesis of high aspect ratio copper nanowires for high-performance transparent conducting films. Chem. Commun. 2014, 50, 2562–2564. [Google Scholar] [CrossRef]
- Choi, H.; Park, S.H. Seedless growth of free-standing copper nanowires by chemical vapor deposition. J. Am. Chem. Soc. 2004, 126, 6248–6249. [Google Scholar] [CrossRef]
- Kim, C.; Gu, W.; Briceno, M.; Robertson, I.M.; Choi, H.; Kim, K. Copper Nanowires with a five-twinned structure grown by chemical vapor deposition. Adv. Mater. 2008, 20, 1859–1863. [Google Scholar] [CrossRef]
- Haase, D.; Hampel, S.; Leonhardt, A.; Thomas, J.; Mattern, N.; Büchner, B. Facile one-step-synthesis of carbon wrapped copper nanowires by thermal decomposition of Copper (II)–acetylacetonate. Surf. Coat. Technol. 2007, 201, 9184–9188. [Google Scholar] [CrossRef]
- Gelves, G.A.; Murakami, Z.T.; Krantz, M.J.; Haber, J.A. Multigram synthesis of copper nanowires using ac electrodeposition into porous aluminium oxide templates. J. Mater. Chem. 2006, 16, 3075–3083. [Google Scholar] [CrossRef]
- Pate, J.; Zamora, F.; Watson, S.M.; Wright, N.G.; Horrocks, B.R.; Houlton, A. Solution-based DNA-templating of sub-10 nm conductive copper nanowires. J. Mater. Chem. C 2014, 2, 9265–9273. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Lye, M.L.; Zeng, H.C. Large-scale synthesis of high-quality ultralong copper nanowires. Langmuir 2005, 21, 3746–3748. [Google Scholar] [CrossRef]
- Rathmell, A.R.; Bergin, S.M.; Hua, Y.L.; Li, Z.Y.; Wiley, B.J. The growth mechanism of copper nanowires and their properties in flexible, transparent conducting films. Adv. Mater. 2010, 22, 3558–3563. [Google Scholar] [CrossRef]
- Kumar, D.R.; Woo, K.; Moon, J. Promising wet chemical strategies to synthesize Cu nanowires for emerging electronic applications. Nanoscale 2015, 7, 17195–17210. [Google Scholar]
- Li, S.; Chen, Y.; Huang, L.; Pan, D. Large-scale synthesis of well-dispersed copper nanowires in an electric pressure cooker and their application in transparent and conductive networks. Inorg. Chem. 2014, 53, 4440–4444. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Lee, C.; Cho, S.; Yoo, J.; Piao, Y.; Kim, Y.S. Facile synthesis of oxidation-resistant copper nanowires toward solution-processable, flexible, foldable, and free-standing electrodes. Small 2014, 10, 5047–5052. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.; Kwon, S.; Kim, N.R.; Shin, K.; Lee, H.M. Ethylenediamine-enhanced oxidation resistivity of a copper surface during water-based copper nanowire synthesis. J. Phys. Chem. C 2016, 120, 3334–3340. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, S.; Lin, Y.; Yang, Z.; Liu, L. Cu–Ag core–shell nanowires for electronic skin with a petal molded microstructure. J. Mater. Chem. C 2015, 3, 9594–9602. [Google Scholar] [CrossRef]
- Xu, L.; Yang, Y.; Hu, Z.W.; Yu, S.H. Comparison study on the stability of copper nanowires and their oxidation kinetics in gas and liquid. ACS Nano 2016, 10, 3823–3834. [Google Scholar] [CrossRef]
- Luo, X.; Gelves, G.A.; Sundararaj, U.; Luo, J.L. Silver-coated copper nanowires with improved anti-oxidation property as conductive fillers in low-density polyethylene. Can. J. Chem. Eng. 2013, 91, 630–637. [Google Scholar] [CrossRef]
- Chen, Z.; Rathmell, A.R.; Ye, S.; Wilson, A.R.; Wiley, B.J. Optically transparent water oxidation catalysts based on copper nanowires. Angew. Chem. 2013, 125, 13953–13956. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Ruditskiy, A.; Xia, Y. Galvanic replacement: A simple and versatile route to hollow nanostructures with tunable and well-controlled properties. Adv. Mater. 2013, 25, 6313–6333. [Google Scholar] [CrossRef]
- Niu, Z.; Cui, F.; Yu, Y.; Becknell, N.; Sun, Y.; Khanarian, G.; Kim, D.; Dou, L.; Dehestani, A.; Schierle-Arndt, K.; et al. Ultrathin epitaxial Cu@ Au core–shell nanowires for stable transparent conductors. J. Am. Chem. Soc. 2017, 139, 7348–7354. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Ye, S.; Wilson, A.R.; Ha, Y.C.; Wiley, B.J. Optically transparent hydrogen evolution catalysts made from networks of copper–platinum core–shell nanowires. Energy Environ. Sci. 2014, 7, 1461–1467. [Google Scholar] [CrossRef]
- Jiang, Z.; Tian, Y.; Ding, S.; Wen, J.; Wang, C. Facile synthesis of Cu–Ag hybrid nanowires with strong surface-enhanced Raman scattering sensitivity. CrystEngComm 2016, 18, 1200–1206. [Google Scholar] [CrossRef]
- Ye, S.; Stewart, I.E.; Chen, Z.; Li, B.; Rathmell, A.R.; Wiley, B.J. How copper nanowires grow and how to control their properties. Acc. Chem. Res. 2016, 49, 442–451. [Google Scholar] [CrossRef]
- Stewart, I.E.; Ye, S.; Chen, Z.; Flowers, P.F.; Wiley, B.J. Synthesis of Cu–Ag, Cu–Au, and Cu–Pt core–shell nanowires and their use in transparent conducting films. Chem. Mater. 2015, 27, 7788–7794. [Google Scholar] [CrossRef]

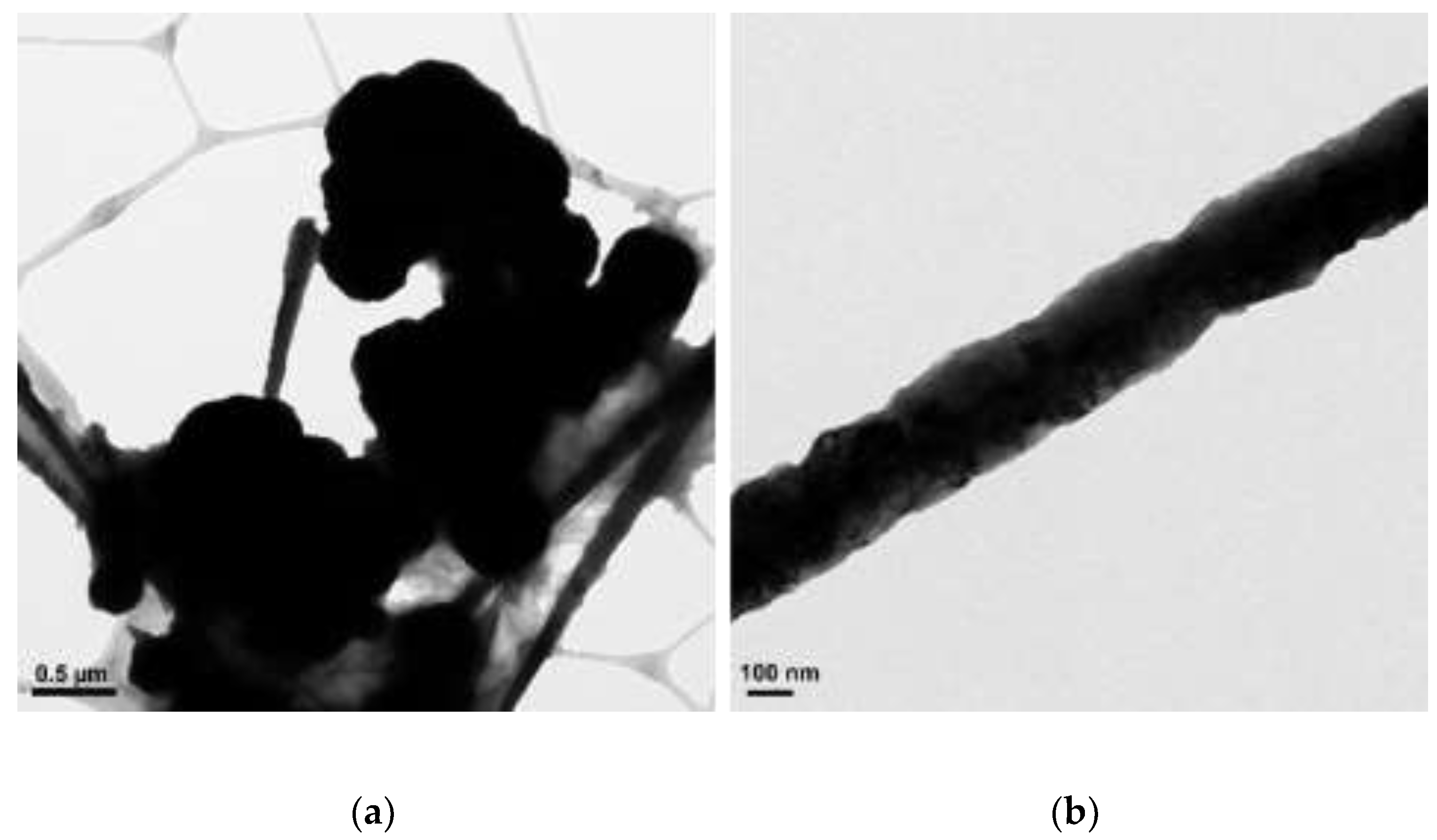
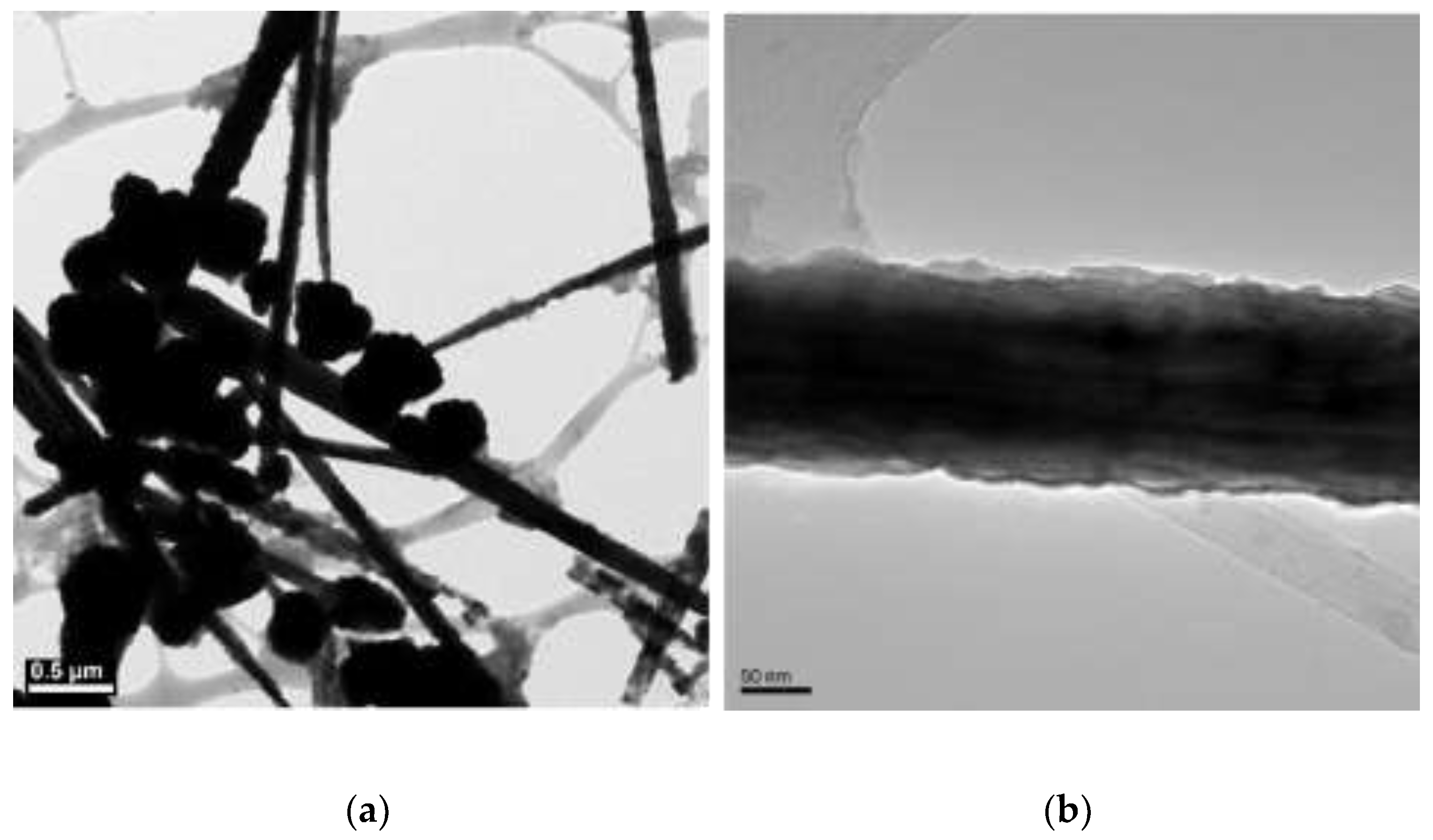
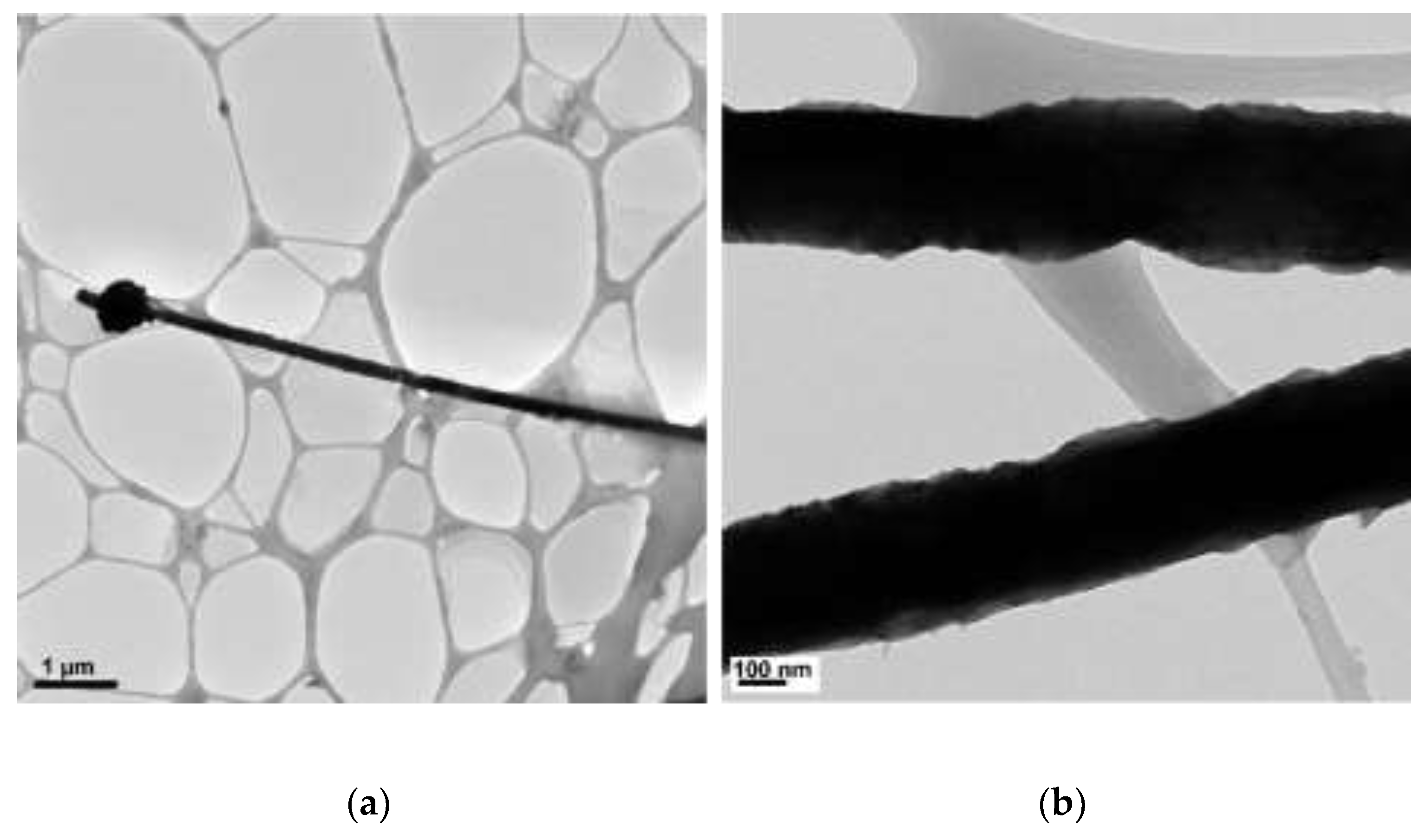

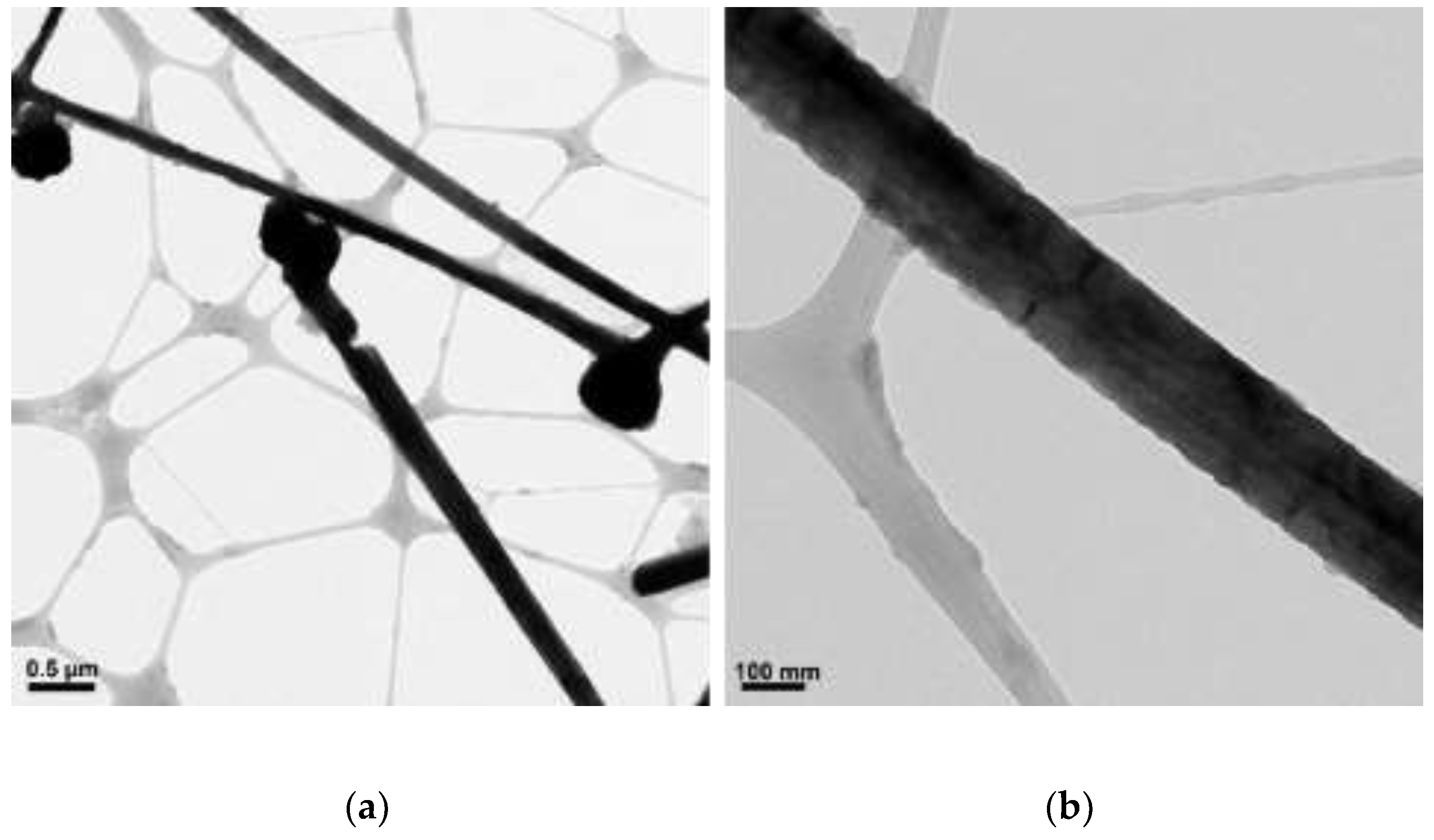
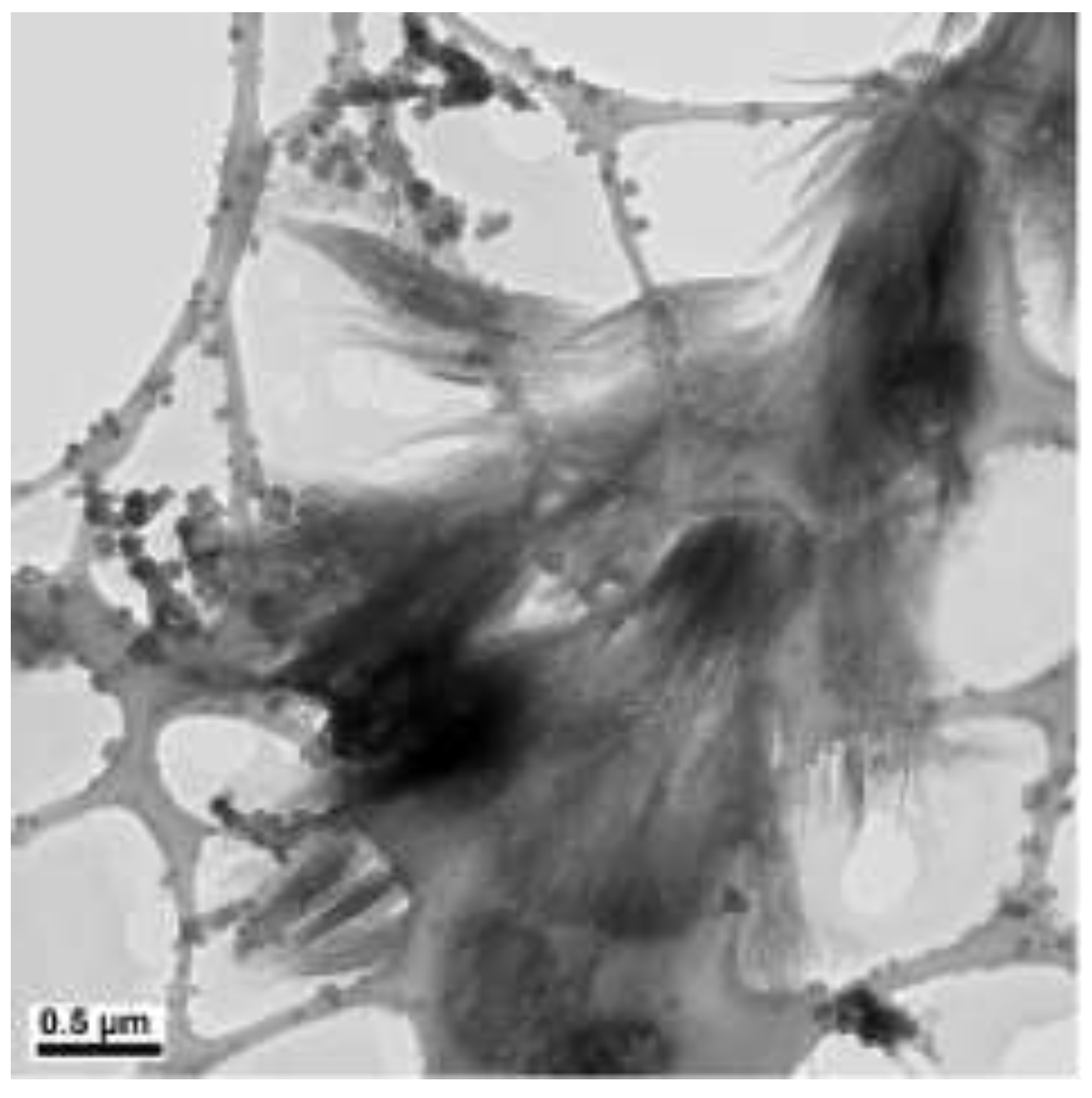
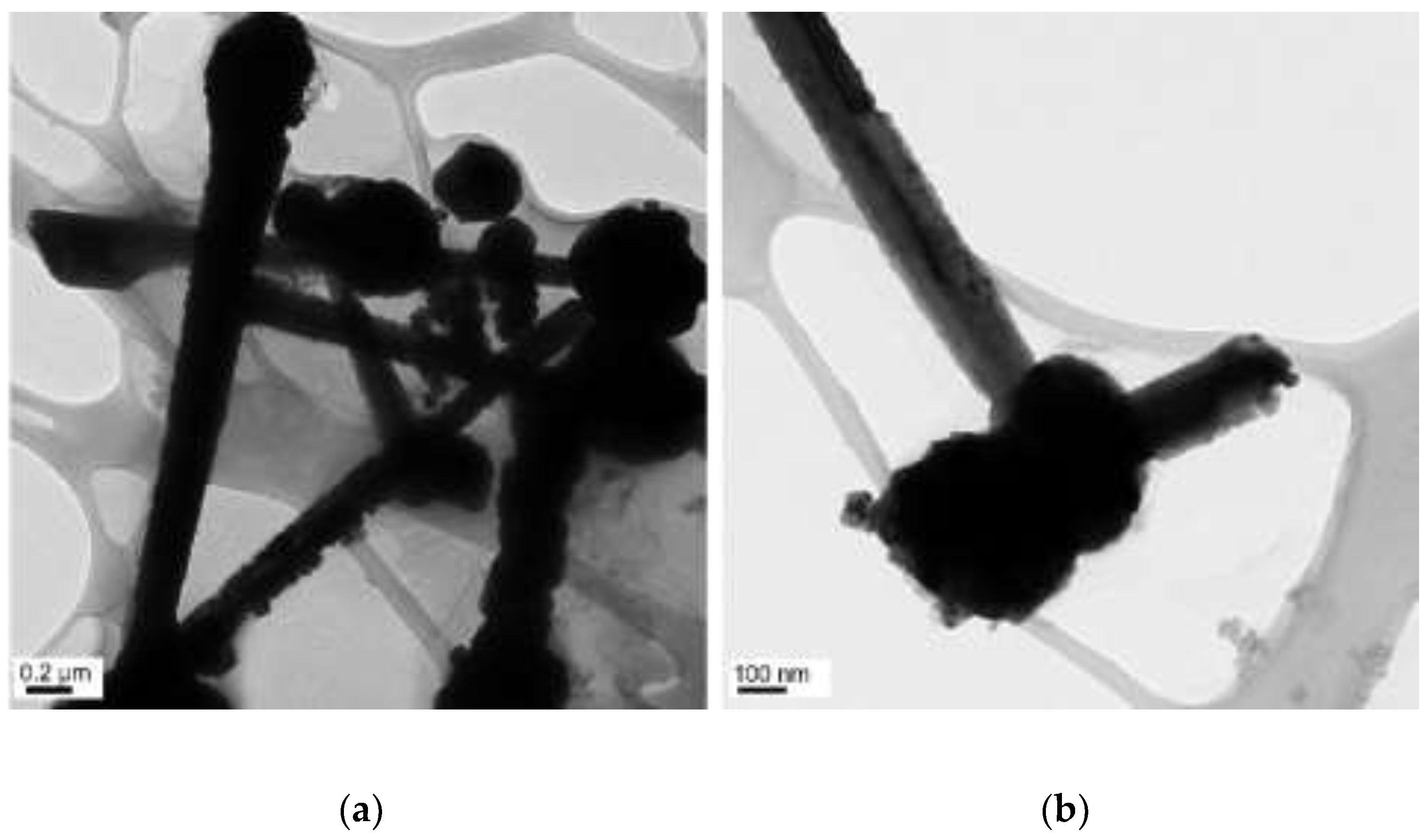

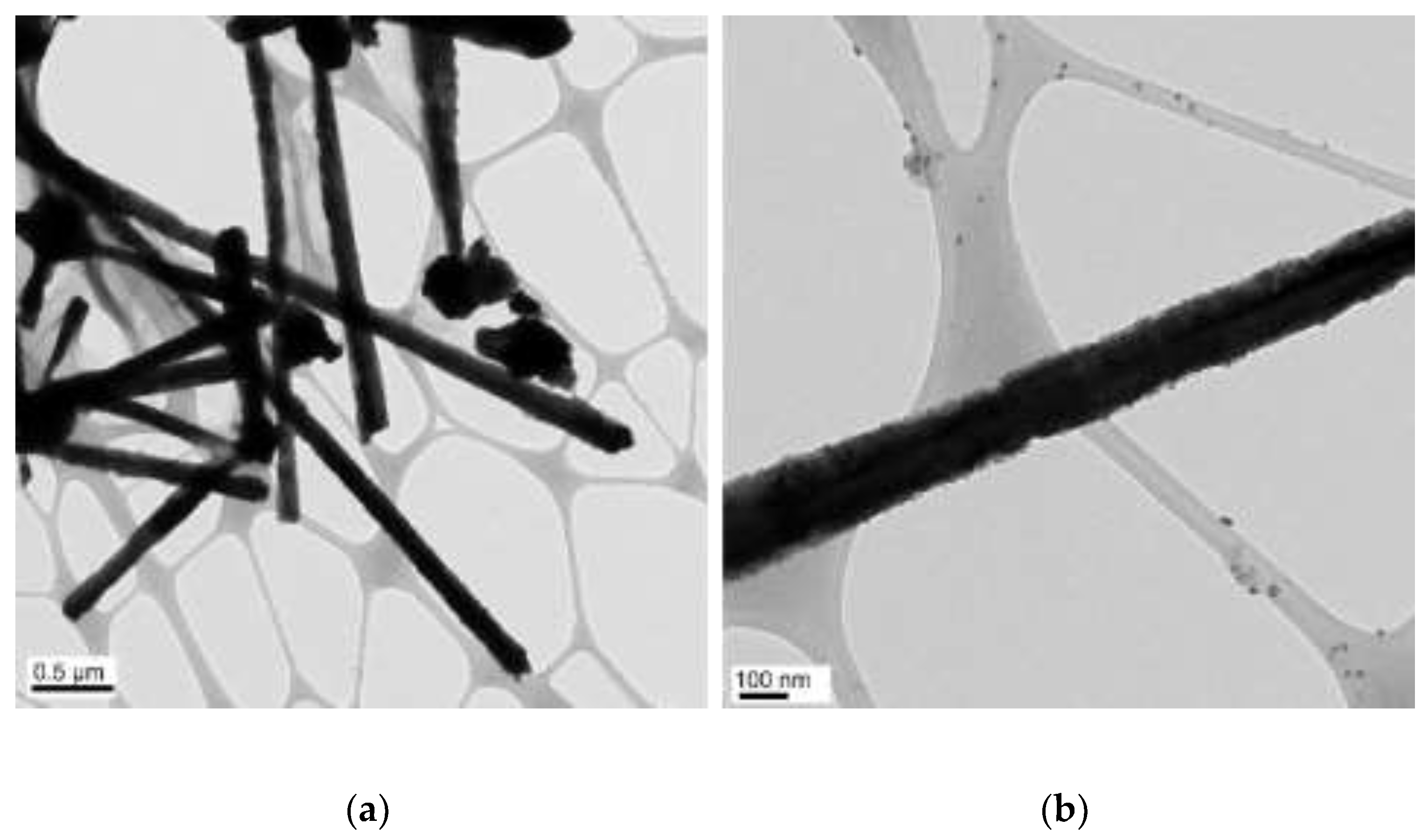



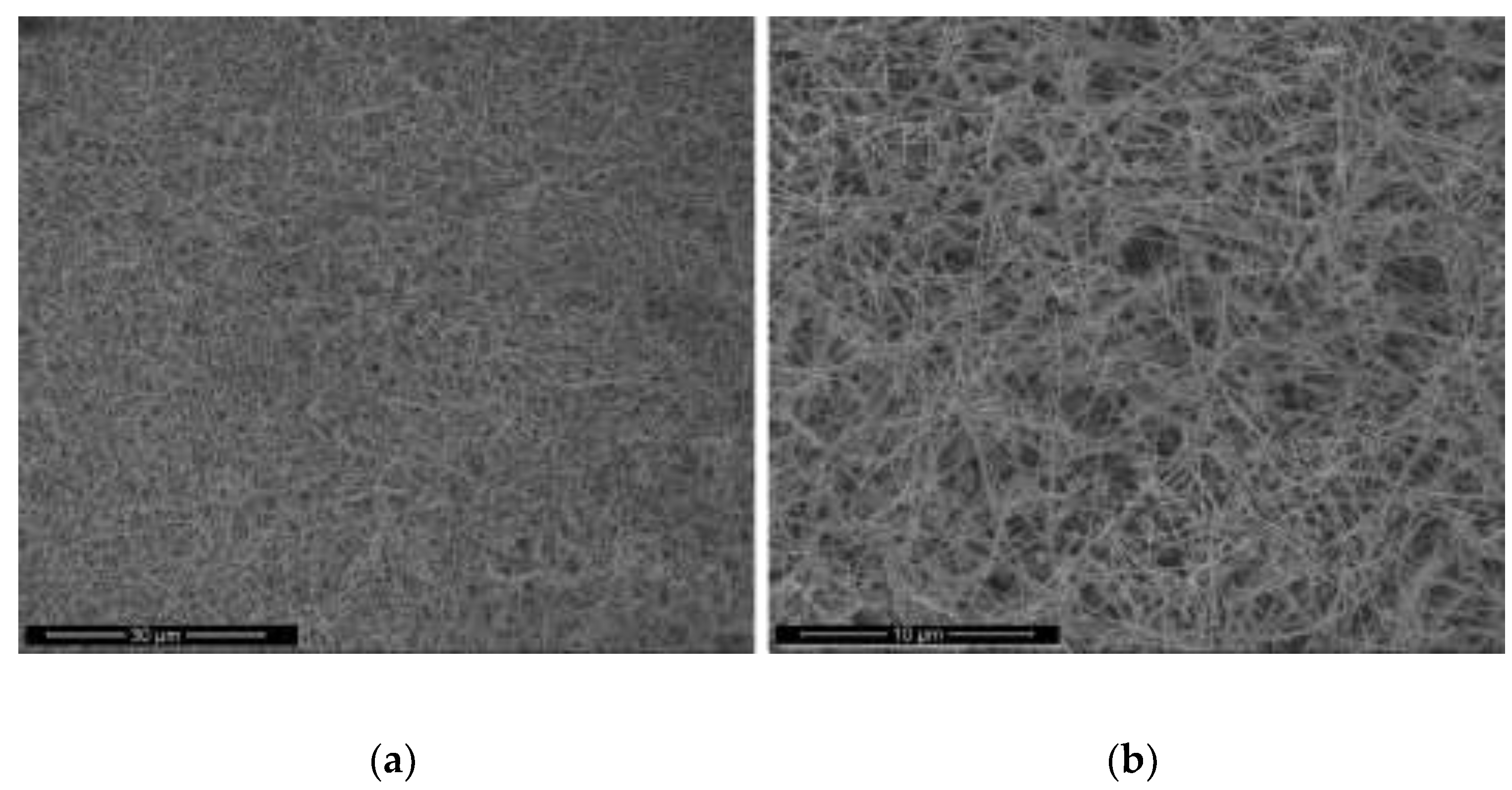

| N2H4 (µL) | Length (µm) | Diameter (nm) |
|---|---|---|
| 15 | 4–13 | 250–550 |
| 30 | 4–15 | 450–750 |
| 240 | Copper nanoparticles of the diameter of 800 nm | |
| Reaction Temperature (°C) | Length (µm) | Diameter (nm) |
|---|---|---|
| 40 | more than 3.5 | 130–250 |
| 50 | more than 5 | 130–280 |
| 60 | more than 8 | 200–310 |
| 70 | more than 5 | 210–260 |
| 80 | more than 6 | 120–280 |
| 90 | The oxidation of copper nanowires | |
| EDA | Length (µm) | Diameter (nm) |
|---|---|---|
| 15 | more than 4 | 150–220 |
| 50 | more than 2 | 210–270 |
| 60 | more than 6 | 130–170 |
| NaOH (M) | Length (µm) | Diameter (nm) |
|---|---|---|
| 5 | more than 3 | 130–250 |
| 7 | more than 12 | 80–160 |
| 9 | more than 18 | 25–45 |
| 12 | more than 12 | 100–500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Wern, C.; Yi, S. Novel Fabrication of Silver-Coated Copper Nanowires with Organic Compound Solution. Materials 2022, 15, 1135. https://doi.org/10.3390/ma15031135
Lee S, Wern C, Yi S. Novel Fabrication of Silver-Coated Copper Nanowires with Organic Compound Solution. Materials. 2022; 15(3):1135. https://doi.org/10.3390/ma15031135
Chicago/Turabian StyleLee, Suhyun, Chien Wern, and Sung Yi. 2022. "Novel Fabrication of Silver-Coated Copper Nanowires with Organic Compound Solution" Materials 15, no. 3: 1135. https://doi.org/10.3390/ma15031135
APA StyleLee, S., Wern, C., & Yi, S. (2022). Novel Fabrication of Silver-Coated Copper Nanowires with Organic Compound Solution. Materials, 15(3), 1135. https://doi.org/10.3390/ma15031135







