1. Introduction
Since renewables are fluctuating energy sources, stationary energy storage media in the form of batteries and electrolyzers are necessary to enable grid stability and boost the total efficiency of renewable energy utilization. Especially lithium-ion batteries (LIB) with high specific energy densities (100–300 Wh/kg), acceptable cycling life, and high charge/discharge rates are suitable for use in electromobility. Due to the limited occurrence of lithium resources and the steadily increasing price of lithium, supplementary systems with comparable performance must be found [
1]. The most promising alternatives is sodium-based electrochemistry, which has lower energy densities, but still shows sufficient performance for applications in stationary energy storage. Furthermore, sodium is advantageous due to its high abundance in the earth’s crust and low price [
1,
2,
3]. The most advanced Na-ion batteries are the Na-S and the Na-NiCl
2 (ZEBRA). Both cells operate at high temperatures of about 300 °C to ensure that the electrode materials are in a molten state [
4,
5]. However, the progress of ambient temperature sodium-ion batteries (SIBs) utilizing liquid electrolytes is hindered by dendrite formation, which is more severe in comparison to lithium electrochemistry [
6]. For this reason, the focus of current research regarding sodium ion batteries is increasingly concentrated on dendrite-resistant solid state batteries (SSB), which have high energy density and can be used at ambient temperatures [
7].
Similar to the high-temperature batteries, solid-state batteries also require a dense, ion-conducting solid electrolyte membrane for the reliable and functional separation of the anode and cathode. This can either be designed as a self-supporting component or as a coating on one of the electrodes. Sintered ceramic solid electrolytes based on oxides (Na-β’’-Al
2O
3) or phosphates (Na
1+xZr
2(SiO
4)
x(PO
4)
3-x—NASICON) are considered suitable to suppress dendrite growth and exhibit high electrochemical stability [
8]. The Na-β’’-Al
2O
3 solid electrolyte has been used for decades in high-temperature sodium batteries. Na
2O × 5 Al
2O
3, the so-called β’’-aluminate, was invented in 1962 by Thèry et al. [
9]. Regarding the review of Fergus at room temperature, an ionic conductivity σ starting ranging between 0.017 and 0.05 S/cm can be expected for a single-crystalline β/β’’ aluminate and polycrystalline β/β’’ aluminate values between 3 × 10
−4 and 3 × 10
−3 S/cm [
10]. Sintering temperatures for components such as thin membranes or tubes higher than 1600 °C are necessary to produce a dense ceramic [
11]. The high sintering temperature is the main drawback of its manufacturing, causing sodium loss and ageing of ceramic furnace linings as well as setter materials.
In 1976, Hong et al. discovered the composition, Na
1+xZr
2(SiO
4)
x(PO
4)
3-x (NZSP), which had an ionic conductivity of 0.2 S/cm at 300 °C. By substituting different elements in this composition without changing the crystal structure, higher ionic conductivities for many compositions could be realized. These materials are grouped under the term “NASICON,” which stands for “Na superionic conductor.” The ionic conductivity of this class of solid electrolytes varies between 1 × 10
−4 and 1 × 10
−3 S/cm at 25 °C [
12]. Although numerous compositions and stoichiometric variations of the basic NASICON has been synthesized and characterized, their sintering process remains challenging, and several attempts have been investigated to reduce the sintering temperatures by using field-assisted sintering (FAST), a cold sintering Process (CSP), or the additions of sintering additives for liquid phase sintering (LPS) [
11,
13,
14]. For conventional sintering processes, temperatures above 1200 °C (up to 1300 °C) together with several hours of soaking time are necessary to achieve dense microstructures. Such conditions lead to evaporation of Sodium and Phosphor from the specimens, resulting in changed compositions, unwanted phases, and reduced conductivities [
15].
One attempt to reduce the sintering temperature of NASICON has been undertaken by using a glass processing route followed by the crystallization process. Susman et al. reported a so-called NASIGLAS material with reduced ZrO
2 content and a modified composition Na
1+xZr
2-x/3Si
xP
3-xO
12−2x/3 with x = 3, which can be manufactured as glass and has a conductivity of approximately 1.5 × 10
−3 S cm
−1 at 300 °C and 1.3 × 10
−7 S cm
−1 at 25 °C [
16]. Glasses with lower x-values (x = 2.25–3) synthesized by Niyompan et al. showed similar conductivities at 300 °C and also a tendency for crystallization under thermal treatment [
17]. However, the high content of crystalline, highly conductive NASICON phase by a subsequent crystallization step of NASIGLAS could not be demonstrated up to date.
In 1969, Maksimov et al. discovered the structure Na
5RSi
4O
12 (R = La-Lu and Y) with a rhombohedral crystal structure (R3c
−) [
18]. Shannon et al. [
19] performed a systematical study on the conductivity of hydrothermally sintered Na
5RSi
4O
12 compositions for R = Er, Y, Ho, Dy, Gd, Eu, and Sm and found an increasing ionic conductivity for increasing the radii of trivalent cations from Sc
3+ to Sm
3+ ranging from 3 × 10
−3 S/cm (Sc
3+) to 0.1 S/cm (Sm
3+) at 200 °C. Beyeler et al. [
20] performed room temperature conductivity measurements on the single crystal Na
5YSi
4O
12 and obtained an ionic conductivity parallel to the c axis of 3.2 × 10
−3 S/cm. Perpendicular to the c axis, even higher conductivity values of 8 × 10
−3 S/cm were measured. However, an ionic conductivity of 1 × 10
−3 S/cm was obtained for the Na
5RSi
4O
12 phase in polycrystalline microstructures at room temperature [
20]. The crystallization of the Na
5RSi
4O
12 phase from glasses with composition Na
3.2R
0.9Si
2.1P
0.1O
8.7 with R=Er, Y, Gd, and Sm has been investigated by Banks et al. [
21] and led to the formation of sodium conducting glass-ceramics. The possibility of synthesizing inorganic solid electrolytes as glass-ceramics, which have conductivities comparable to NASICON and Na-β’-Al
2O
3, provides an interesting alternative to these established solid electrolytes and their complex manufacturing processes.
Since 1985, Yamashita’s group has worked on manufacturing and optimizing the properties of Na
5YSi
4O
12-based electrolytes [
22]. By tuning Na
3+3x−1Y
1-xP
ySi
3-yO
9 composition, it was possible to increase the volume content of the conductive N5 phase [
23]. In 2012 Okura et al. [
24] demonstrated the predominant crystallization of the N5 phase from quenched glass samples with proper Na
3+3x-yY
1-xP
ySi
3-yO
9 (NAPSY) stoichiometry. However, the presence of additional non-conducting phases such as N8 (Na
8.1YSi
6O
18), N3 (Na
3YSi
2O
7), and N9 (Na
9YSi
6O
18) have been observed. Despite the crystallization ability of the high content of the N5 phase, the presence of the isolating N3 and N9 phases press the value of the total conductivity to 0.066 S cm
−1 at 300 °C [
24]. These phases, as well as a residual glass phase at grain boundaries, have ionic conductivity 1 to 3 orders of magnitude lower than the N5 target phase [
25]. A recently published review article by Okura and Yamashita [
26] provides a good guideline for R&D activities on NAPSY based sodium-ion superconductors produced by recrystallization from the glass phase. Similar to the β-aluminates and NASICON, oxide glass-ceramics are also resistant to high temperatures and sustain contact with metallic sodium [
27]. According to Danzer, the mechanics of this material’s sintered powder compacts have been investigated by the 3-Ball-on-ball method, and a bending strength up to 60 MPa was determined [
28].
Previously, Wagner at al. demonstrated the possibility of manufacturing thin tape casted substrates from NAPSY glass-ceramics with a parent composition corresponding to the N5-type phase [
29]. It was found that the crystallization of P-containing N5 phase in NAPSY led to highly ionic conducting grains and the substantial enhancement of total ionic conductivity even in the presence of isolating secondary phases [
30]. According to the early work of Shannon et al. [
19], Sm and Gd provide the option for enhancing the ionic conductivity of electrolytes with high N5 phase content. The purpose of this work is to elicit the potential of ionic conductivity enhancement at ambient conditions in sintered glass-ceramic microstructures of the stoichiometric N5-composition with rare earth cations with larger radii in comparison to Y
3+ (Gd
3+ and Sm
3+).
2. Materials and Methods
Na
2CO
3 (AnalaR NORMAPUR, VWR), SiO
2 (Millisil W8, Quarzwerke Frechen, Frechen, Germany), and rare earth metal oxides Y
2O
3 (Grade C, H.C. Starck, Goslar, Germany), Gd
2O
3 (Alfa Aesar, Kandel, Germany), and Sm
2O
3 (Alfa Aesar, Kandel, Germany) have been used as raw materials to synthesize amorphous glass with composition corresponding to Na
5RSi
2O
12 stoichiometry. The powders with molar ratio Na
2CO
3:SiO
2:R
2O
3 = 35.7:7.2:57.1 were mixed with a dry tumble mixer (TURBULA, Willy A. Bachofen AG, Muttenz, Switzerland) for 30 min. The well-mixed powders were melted in a platinum crucible at 1350 °C and quenched on a brass block to room temperature. The resulting glass frit was transparent and colorless for yttria and gadolinia and had a yellowish color for the samaria sample. The obtained glass samples were milled down and crystallized to the N5 phase (N5-Y for Na
5YSi
4O
12, Na
5GdSi
4O
12 for N5-Gd, and Na
5SmSi
4O
12 for N5-Sm) using corresponding heat treatments summarized in
Table 1. The temperatures for the pre-crystallization of N5-Sm and N5-Gd glasses were defined based on preliminary caloric analyses carried out on the milled powders. Accordingly, annealing temperatures were selected that exceeded the crystallization temperature of the glasses but stayed at least 100 K below their corresponding melting point. The corresponding analysis of crystallization behavior of N5-Y glass and the definition of favorable N5 phase crystallization conditions are described in our previous studies on this material [
28].
After crystallization, the frit was crushed in a mortar and milled for 1 h in ethanol using an attritor (NETZSCH-Feinmahltechnik GmbH, Selb, Germany). The particle size distributions were determined by laser diffraction in a range between 20 nm and 2000 µm (Mastersizer 2000, Malvern Instruments Ltd., Malvern, UK). The characteristic particle sizes of the glass powders after milling were for d
10 = 0.6–0.7 µm, d
50 = 1.6–1.8 µm, and d
90 = 3.7 µm for N5-Y and N5-Gd and d
90 = 4.6 µm for N5-Sm. The powders were dried and pelletized with 25.5 MPa to discs with a thickness of 1–2 mm and a diameter of 20 mm. The green density of pellets was measured from their weight and discs dimensions. The discs were sintered in air on platinum foils under an ambient atmosphere with a heating rate of 5 K/min. The sintering conditions for different compositions are summarized in
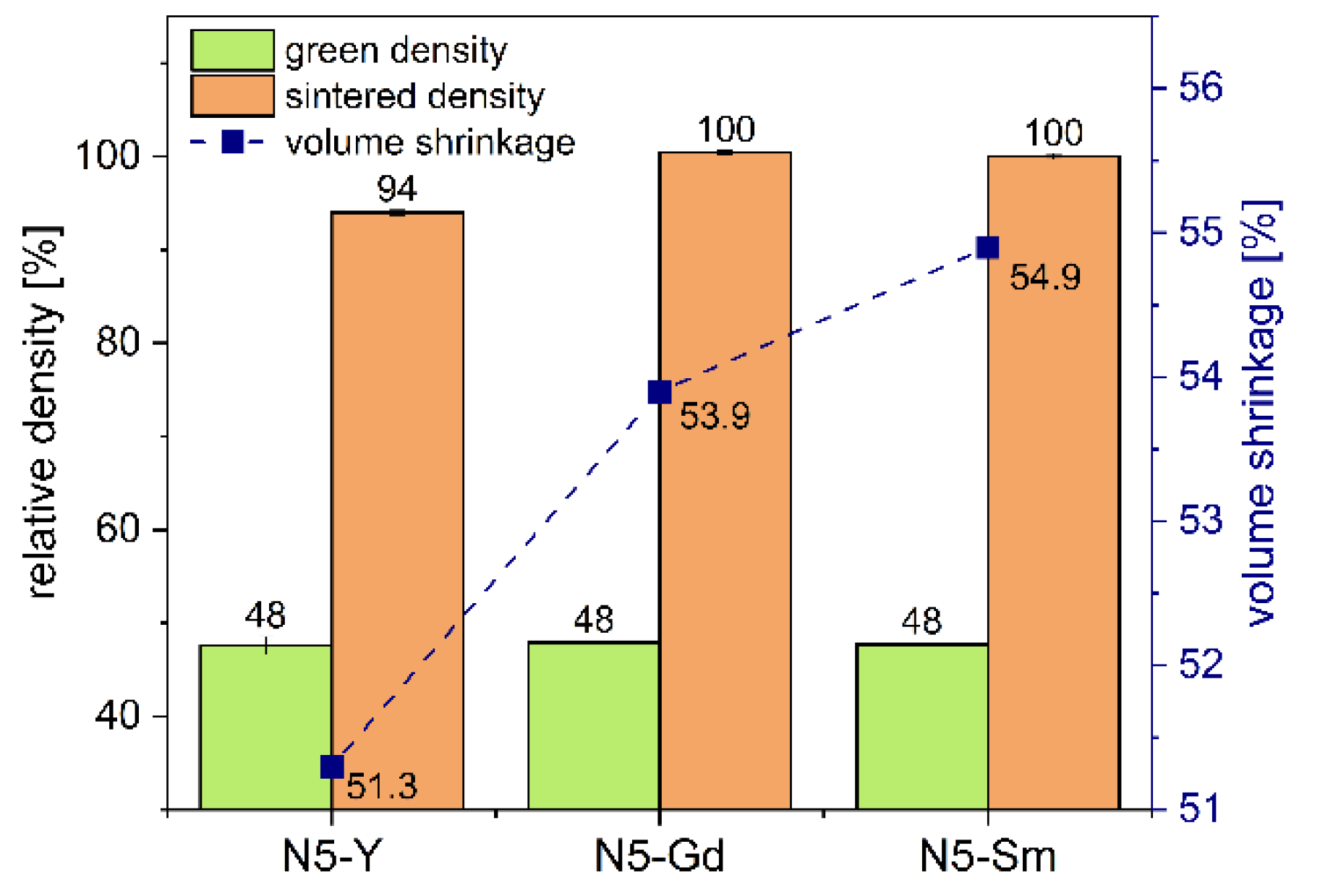
Table 2. They represent the results of a series of tests conducted with the aim of achieving the highest possible densities in the microstructures.
The shrinkage of the powder has been analyzed by optical dilatometry using a hot stage microscope (Hesse Instruments, Osterode am Harz, Germany) which analyzes the changes of the projected and photographed shadows of the sample according to DIN 51730. This method is well described by Pascual and Durán [
31].
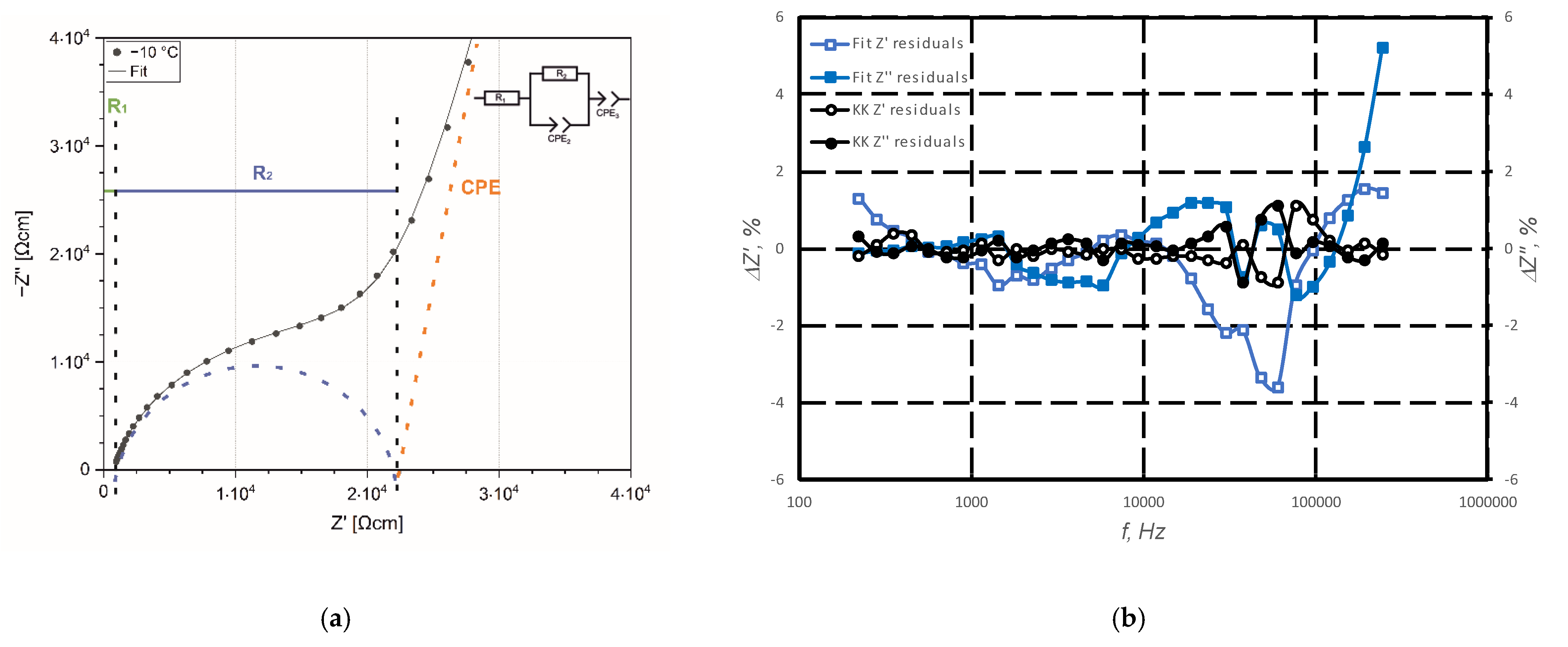
Samples for analysis of the ionic conductivity (by electrochemical impedance spectroscopy—EIS) were sputtered with gold (acting as ion blocking electrode), placed in ECC Std test cells (EL-CELL GmbH, Hamburg, Germany), and put in the temperature chamber (Binder GmbH, Tuttlingen, Germany). Impedance measurements were carried out by a potentiostat VMP3 (BioLogic Science Instruments, Seyssinet-Pariset, France) with an amplitude of ac perturbating voltage signal of 25 mV in a frequency range between 100 mHz and 1 MHz. Impedance spectra were recorded in a temperature range from −10 to 30 °C in 10 K steps. The measurement program used was EC Lab V11.36. Prior to each measurement, the cell was tempered for 1.5 h to reach the appropriate temperature inside. The impedance spectra were evaluated using RelaxIS Software vers. 3.0.17.10 from RHD GmbH (Wedemark, Germany).
Density measurements of the sintered pellets were performed according to the Archimedean principle in ethanol with a Sartorius CP2245 balance and a density determination kit. The theoretical density of the fully crystallized material was measured from milled samples by helium pycnometry.
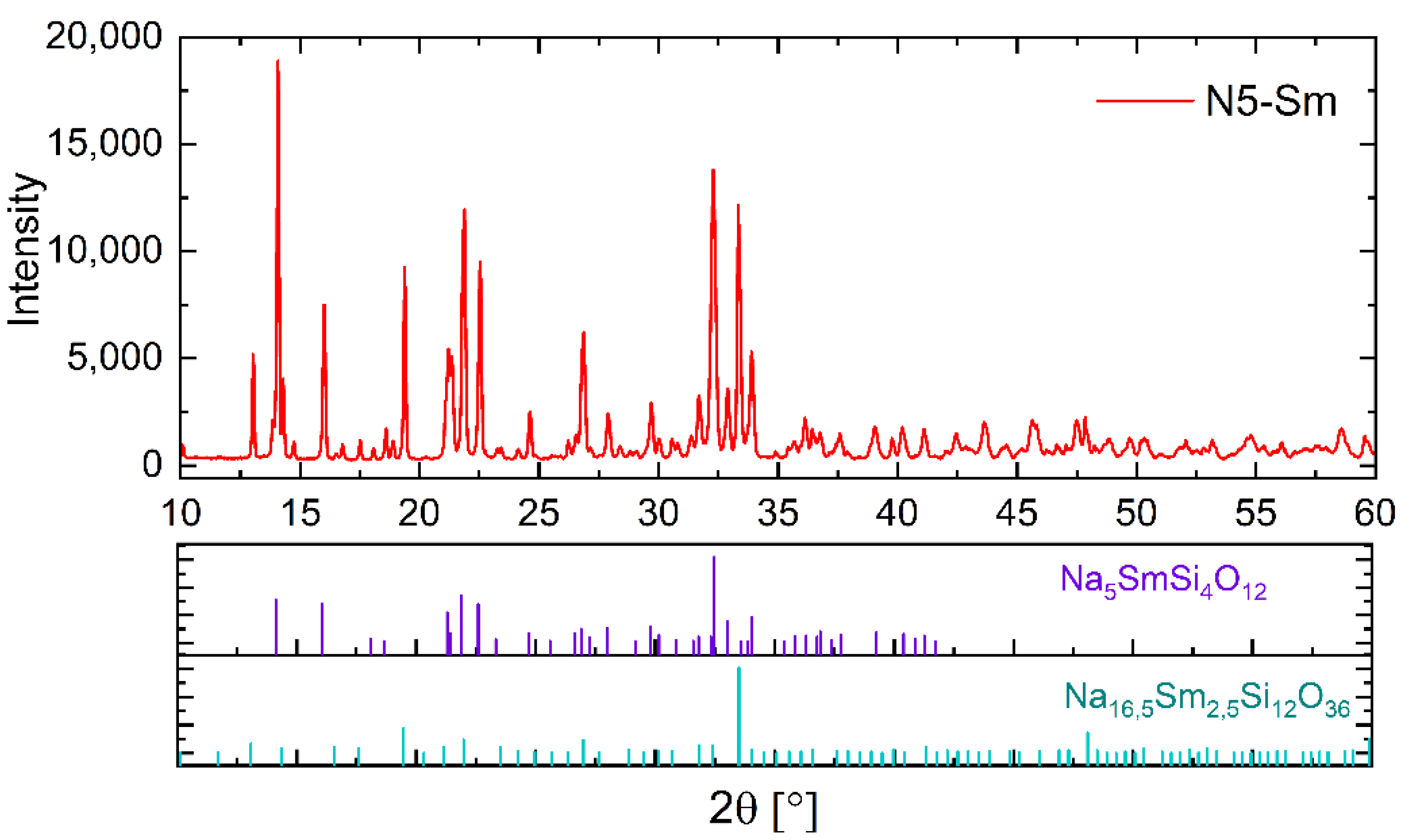
For the quantitative XRD analysis, powder compacts were heat-treated under the same conditions as those used for impedance measurements. Afterwards, the samples were ground down to a particle size < 63 µm and mixed with 33.333 wt.% pure Si powder as internal reference for calculation of the amorphous phase content. The diffractograms were recorded by a X-ray-diffractometer (D8 Advance, Bruker AXS) with Cu Kα-radiation and a LynxEye position sensitive detector (PSD). The samples were measured in a range of 10–90° (2θ, with a step width of 0.03° and a measuring time of 3 s (PSD). The qualitative analysis was performed with the software DIFFRAC.EVA (Version 5.2., Ltd., Bruker AXS, Karlsruhe, Germany) with an ICDD PDF(2021)-database. The quantitative analysis was performed using the Topas 6.0 software package. For the quantitative analysis of Na
5YSi
4O
12, the structural data set [
32] was used. In the case of Na
5SmSi
4O
12 and Na
5GdSi
4O
12, the Y data were used with an adjustment of the lattice parameters. The same procedure was used for the cubic Na
16(Na
0.5Y
0.5)Y
2Si
12O
36 phase, which was described by Többens et al. [
33]. The uncertainty of the values determined for the crystalline phases is limited to ca. 1 wt.% and for the residual amorphous phase to 3–5 wt.%.
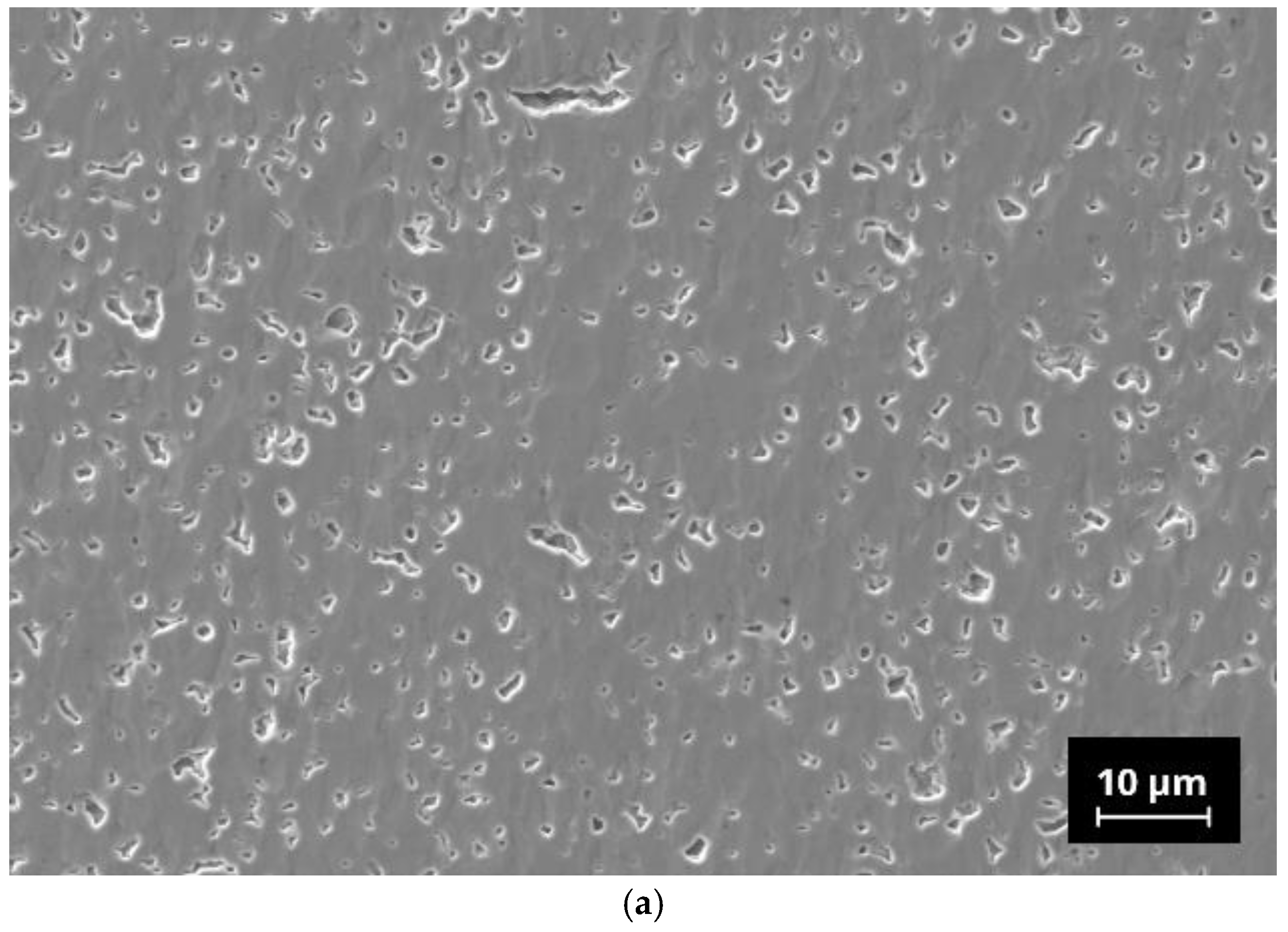
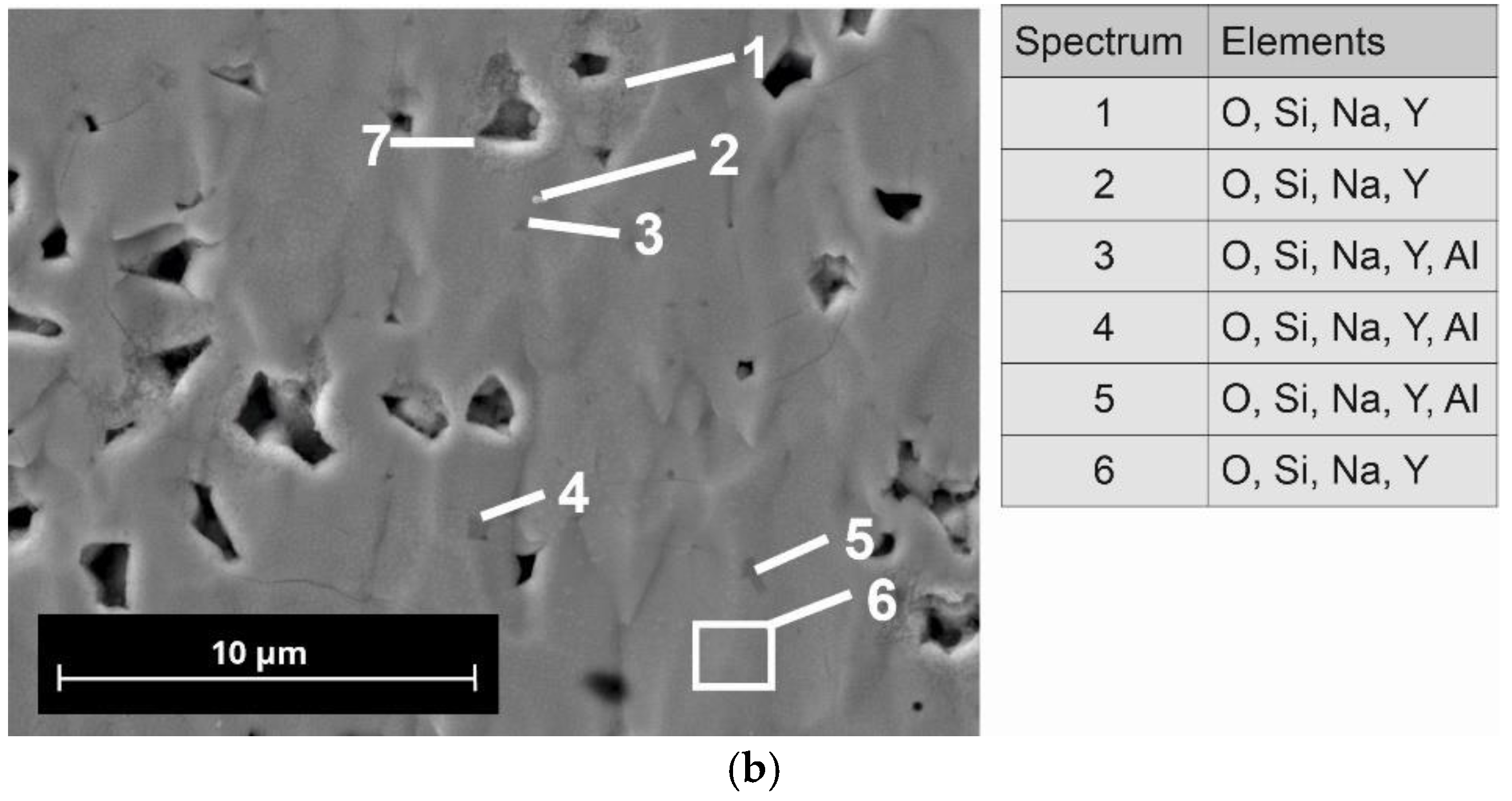
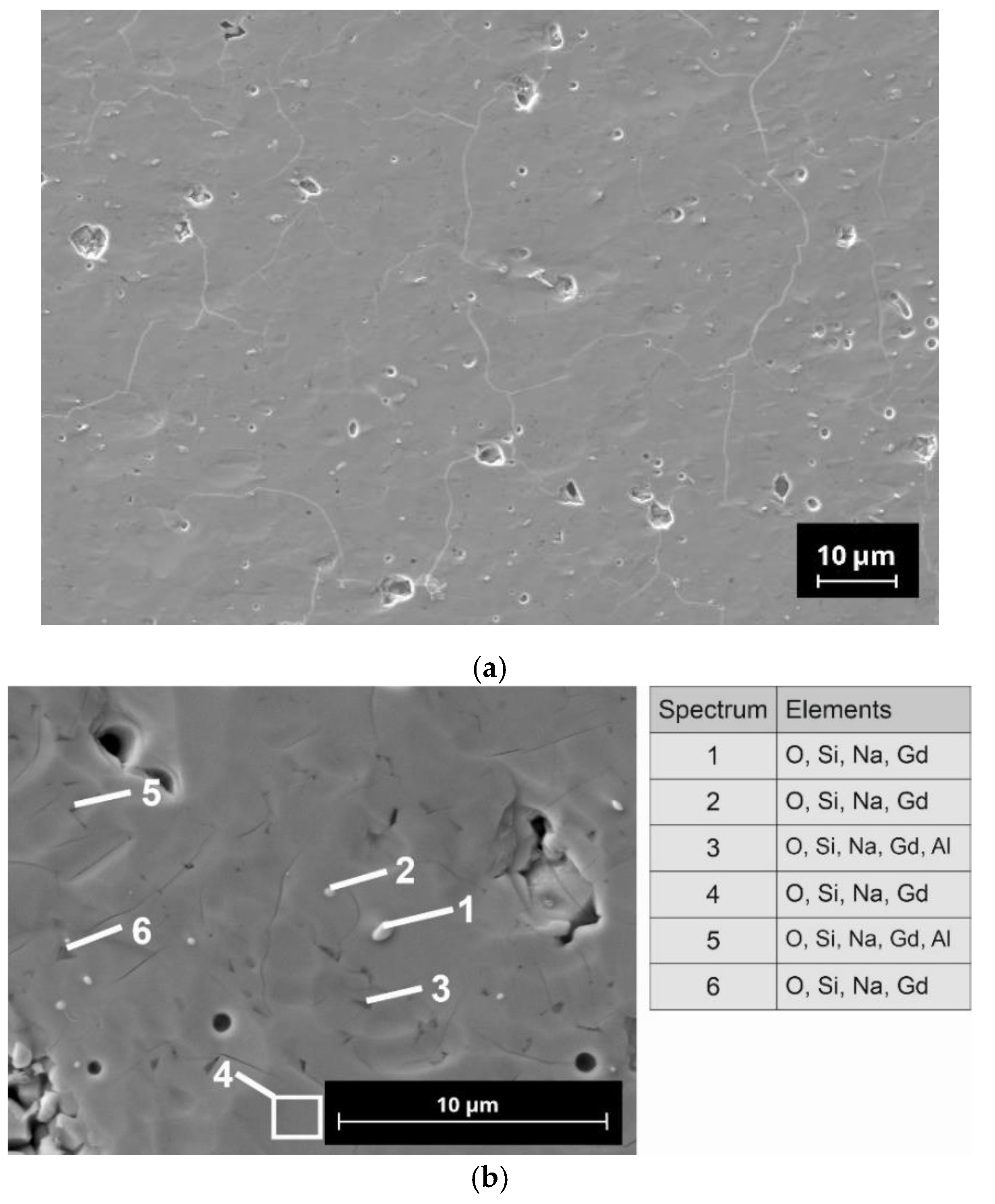
Microstructural analysis was performed with polished cross-sectioned samples using a field emission scanning electron microscope (FE-SEM, NVision 40; Carl Zeiss SMT, Oberkochen, Germany). For the preparation of the samples, a combination of mechanical grinding steps and final ion-polishing by argon-ions was used (RS 101, Bal-Tec, Balzers, Liechtenstein). The micrographs shown in this paper were recorded in the atomic-number-specific back-scattered electron (BSE) contrast. The FE-SEM is equipped with an energy dispersive X-ray analysis system (Inca x-sight; Oxford Instruments, Abingdon, UK), which allows a quantitative detection of elements.
4. Discussion
The glass ceramic route has been chosen for the synthesis of the parental compositions of the solid electrolytes. According to our previous studies on the Y-substituted type of this material (N5-Y), it has been found that a pre-crystallization of the parental glass powder prior to the sintering of compact specimens is advantageous in achieving dense microstructures [
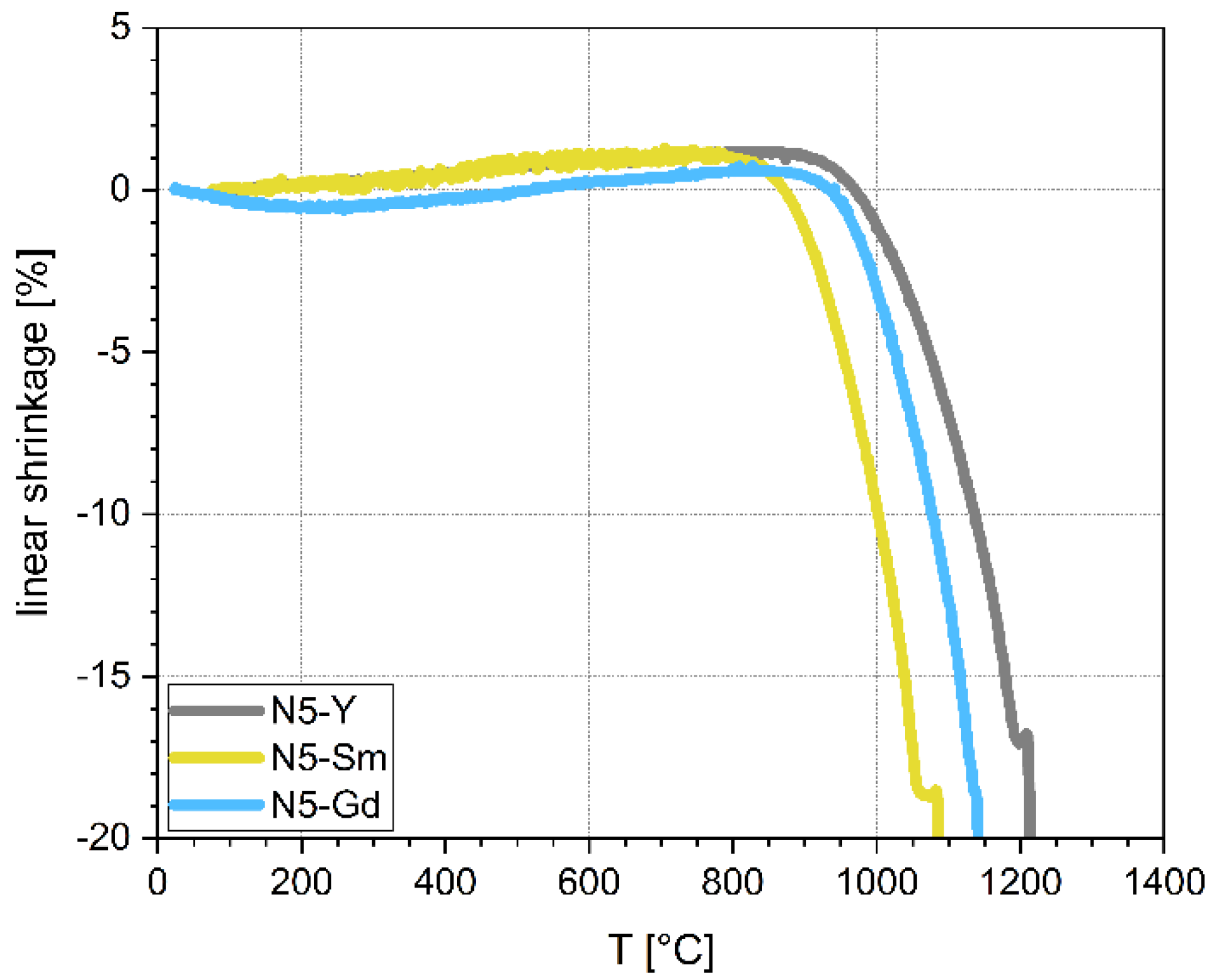
28]. The sintering of glass-based powder compacts is driven by the viscous flow at temperatures above the glass transition temperature (Tg). In the case of the chosen N5-Y composition, the crystallization occurs within the same temperature windows where the densification takes place and consequently results in only porous microstructures. Preliminary studies with the Sm- and Gd-substituted materials revealed a similar behavior that made it impossible to sinter dense microstructures directly from glass-based powder compacts. From the optical dilatometry it is possible to deduce characteristic temperatures for sintering individual compositions (
Table 1). With respect to the ionic radii of the substituted elements Y, Gd, and Sm, the sintering temperature decreases with an increasing ionic radius. As one explanatory approach, this behavior could be attributed to the widening of the crystal lattice of the N5-type phases with increasing ionic radius and an enhanced sintering activity. Also, the temperature windows, in which the densification takes place, become narrower for an increasing ionic radius (N5-Y: Δ
T = 237 K, N5-Gd: Δ
T = 215 K; N5-Sm Δ
T = 192 K). Additionally, we see that the maximum sintering temperature and the melting point lie quite close, especially for N5-Y and N5-Gd. Therefore, the sintering temperatures need to be chosen somewhat below the melting point to avoid any melting and consequently the destruction of the conductive N5-phases. In the case of N5-Y, a soaking time of 2 h at a constant sintering temperature still leaves a residual porosity in the microstructure. In contrast, for N5-Gd a dwell time of 30 min at the same sintering temperature is sufficient to achieve a full densification. According to the lower melting point of N5-Sm, the sintering temperature was also reduced by 70 K and a dwell time of 30 min is enough to densify the powder compacts.
Regarding the formation of the multiphase microstructures, in the case of N5-Gd and N5-Sm, the considerably higher fractions of N16.5 phases compared to N5-Y are worth discussing. On the one hand, this might be attributed to the shorter holding times at the sintering temperature. It is known from the Yttrium substituted system that the non-conducting phases N3 and N9 are formed first at sintering temperatures below 900 °C [
28,
34]. At higher temperatures the conducting N5-phase crystallizes from the parental glass matrix, and a transformation from the formerly formed non-conducting phases is likely. This behavior has been observed for P
2O
5-containing compositions. Additionally, in the case of our ternary composition, N5-Y, after sintering, the microstructure contains only a low amount of the N16.5 phase. If it can be assumed that N16.5 represents the cubic high temperature phase of the N5-phase, it is possible that the sintering conditions chosen for N5-Gd and N5-Sm are suitable to stabilize this phase. Therefore, further experiments must clarify if lower sintering temperatures or additional thermal treatments at lower temperatures can initiate phase transformations in the direction of N5.
One the other hand, the specimens N5-Sm and N5-Gd contain numerous rare-earth-rich precipitations distributed homogenously in the microstructures. Because the respective N5 phases are richer in rare earth ions than the N16.5 phases, it is also conceivable that these rare earth ion-rich precipitates prevent the formation of higher proportions of N5 phases. A reason for the formation of these precipitates cannot be given at this stage. We are aware of the fact, that for N5-Gd and N5-Sm further optimization of the sintering conditions in the direction of longer sintering profiles or adjusted temperatures is necessary to realize higher contents of the N5-phases. However, in view of the holding times of several hours during sintering processes often mentioned in the literature in the case of NaSICON or β-Al2O3, distinct advantages can be assumed for this class of materials regarding the reduced evaporation of sodium.
When examining the phase composition of the investigated samples, in addition to the conductive N5 phase, another, rarely described N16.5 phase has been detected [
33]. In our previous studies and in those of Yamashita and Okura, this phase has not been detected so far [
28,
30,
34]. This may be related to the fact that in the literature, mainly compositions of this material class have been investigated, which deviate from the exact N5 stoichiometry. Yamashita and Okura, who have contributed the predominant part of knowledge on this class of materials, have mainly investigated compositions with nominal stoichiometries of Na
3+3x-yY
1-xSi
3-yP
yO
9 with x = 0.2…0.5 and y = 0…0.45. In addition, our group was focused on a composition having an x-value of 0.25 which corresponds to the stoichiometric N5-type composition with an additional amount of 3 mol% of P
2O
5 as an sintering additive [
28,
30,
42]. It is to be noted that N5-Y, with approx. 4 wt.%, contains a significantly lower proportion of the N16.5 phase than the compositions N5-Gd and N5-Sm. As we do not know the thermophysical properties of the N16.5-type phase, it is also possible that the increased amounts of this phase in N5-Gd and N5-Sm are responsible for their increased sintering activity in comparison to N5-Y. We assume that due to the similar composition of the N5 and the N16.5 phases in the present SEM images, no visual differentiation is possible. In the case of N5-Y, the porosity is very homogenously distributed, indicating only a neglecting coarsening effect of the grains. The EDX analysis reveals the presence of aluminum impurities whose origin we must assign to the abrasion of the alumina containers used for ball milling. However, as the same preparation procedure has been applied to all three compositions, we do not assume that the aluminum impurities are responsible for the formation of the high N16.5 contents in case of N5-Gd and N5-Sm. The inhomogeneous distribution of the aluminum indicates the formation of additional phases which cannot be identified by XRD because their content is below the detection limit of this method.
The molar lattice volume increases and the melting point of N5 phase decreases in the series from Y to Sm. This correlates well with the increasing radii of trivalent cations from 0.900 Å for Y
3+ to 0.938 Å for Gd
3+ and to 0.958 Å for Sm
3+ [
43]. However, the observed dependence of ionic conductivity for the N5 phase with Y, Gd, and Sm cations, extracted from the impedance measurements up to 100 kHz shows a different trend with decreasing conductivities. The investigations of microstructure of the samples reveal the increasing content of N16.5 phase from Y to Sm in investigated Na
5RSi
4O
12 compositions. There are two possibilities explaining this observation:
Composition with Na5YSi4O12 has the highest bulk ionic conductivity and lowest activation energy due to higher Na+-ion mobility in the lattice in comparison to other compositions;
The measured conductivity at 100 kHz still includes grain boundary contributions, visible at higher frequencies, which are strongly affected by the presence of the N16.5 phase at grain boundaries.
A remaining issue is judging the ion-conducting properties of the individual N16.5-phases. If N16.5 represents a cubic high-temperature phase of the N5-modification it could be possible that its specific ionic conductivity is even higher than that of the N5-modification. If the phase has a special ordering of Y
3+- and Na
+-ions and a slightly lower Na content, as described by Többens, then the conductivity could be lower [
33]. In the case of the N5-Y composition, the effect of the N16.5 phase on the overall conductivity would be quite low as the content is lower than 5 vol.%. In contrast, the N5-Sm and N5-Gd contain considerable amounts of the respective N16.5-phases, therefore leading to a significant increase of the overall conductivity in this case. The ionic conductivities reported by Shannon and Okura are listed in
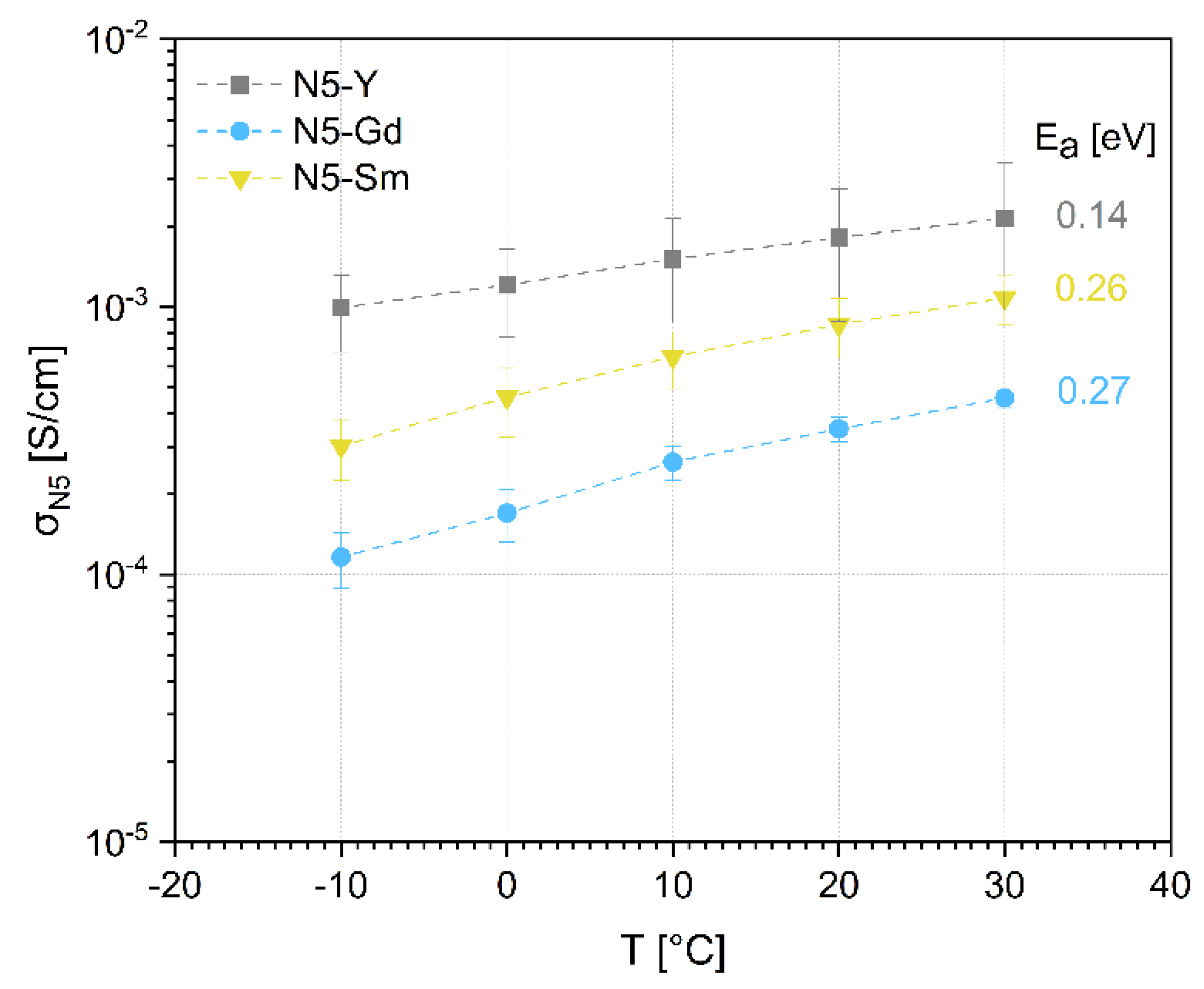
Table 8 together with the reported crystallographic phases. In both cases, only the corresponding N5-type phases are described, and no uniform sequence of conductivities corresponding to the ionic radii of the rare earth elements used is given. However, both Sm-based compositions have higher conductivities than the Y-based composition. In the case of the Gd-based compositions, one sample has a higher conductivity than the Y-based sample and one sample has almost the same conductivity. In contrast, our data in
Figure 8 attribute the highest conductivity to N5-Y, followed by N5-Gd and N5-Sm. This behavior has led us to assume that the N16.5 phases in N5-Gd and N5-Sm must have a considerably lower conductivity in comparison to the N5 phase, even if the ionic conductivity of this phase cannot be excluded. Additionally, Banks et al. showed temperature-dependent conductivity data for a set of glass-ceramic Na
5RSi
4O
12 (R=Er, Y, Gd, Sm) compositions but reported no information of the phase content, therefore the data are not taken into account [
21]. One must also be aware that the assumed tortuosity with a value of 1 brings some uncertainty, which varies with the content of both phases. However, the SEM images still do not allow a visual distinction between the N5 and the N16.5 phase, so quantifying this parameter from performed image analysis is impossible.
For activation energies, some discrepancy to earlier published work is found. The early investigations of Shannon et al. [
19] have shown that Na
+-ionic conductivity increases by substitution of Y by Gd and Gd by Sm in Na
5RSi
2O
12 single crystals all demonstrating similar activation energies of 0.27…0.29 eV. This is also in agreement with the increasing lattice parameter and cell volumes of the phases (see
Table 3). The activation energy of 0.14 eV for the Y-substituted composition must be rated as unusually low. Shannon at el. gives a value of 0.31 eV for hydrothermally synthesized Na
5YSi
4O
12 and Yamashita et al. a value of 0.26 eV for an analogous composition prepared by spray drying and calcination [
19,
44]. Our investigations show a different trend for ionic conductivity regarding the size of the ionic radii. One possible explanation can be that conductivities, estimated in our work, contain grain and grain boundary contributions. The grain boundary contribution depends on the N16.5 phase and increases from the N5-Y to the N5-Sm sample. This can also explain the increase of activation energy and decrease of observed total conductivity because the grain boundaries not only have higher resistivity but also higher activation energy for ionic conductivity. Even if the indications for the contribution of both grain and grain boundary resistivities to the measured values of σ
N5 in
Figure 8 are strong, the performed measurements and investigations do not allow to make a decisive conclusion about the true reasons for the observed behavior and high-frequency measurements are necessary to clarify the open questions.